Abstract
Triple-negative breast cancer (TNBC) is characterized by extensive tumor heterogeneity at both the pathologic and molecular levels, particularly accelerated aggressiveness, and terrible metastasis. It is responsible for the increased mortality of breast cancer patients. Due to the negative expression of estrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2, the progress of targeted therapy has been hindered. Higher immune response in TNBCs than for other breast cancer types makes immunotherapy suitable for TNBC therapy. At present, promising treatments in immunotherapy of TNBC include immune checkpoints (ICs) blockade therapy, adoptive T-cell immunotherapy, and tumor vaccine immunotherapy. In addition, nanomedicines exhibit great potential in cancer therapy through the enhanced permeability and retention (EPR) effect. Immunotherapy-involved combination therapy may exert synergistic effects by combining with other treatments, such as traditional chemotherapy and new treatments, including photodynamic therapy (PTT), photodynamic therapy (PDT), and sonodynamic therapy (SDT). This review focuses on introducing the principles and latest development as well as progress in using nanocarriers as drug-delivery systems for the immunotherapy of TNBC.
1. Immunotherapy in Triple-Negative Breast Cancer (TNBC)
Cancer constitutes the largest public health problem in the world. According to the data released by the American Cancer Society (ACS), there are about 4950 patients diagnosed with cancer and 1662 dying of it every day; Among them, breast cancer is the most popular female cancer type, which is estimated to be nearly 30% of the new cases and the death rate of which is as high as 15% in 2020 [1]. According to the expression of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2), breast cancer is defined as three major subtypes: hormone-receptor (HR) positive, HER2-enriched, and triple-negative breast cancer (TNBC). TNBC accounts for 15–20% of all breast cancers, particularly those in young women [2]. Compared with the other subtypes, TNBC does not respond well to hormonal therapy or medicines targeting HER2 protein receptors. It shows higher drug resistance and tumor heterogeneity and aggressiveness, and is often accompanied by lung or brain metastasis. Lacking therapeutic target is the main reason challenging the effective treatment of TNBC. Once the tumor metastasizes, the median overall survival of TNBC patients is only 12 to 18 months due to the limited therapeutic window [3]. Additionally, TNBC contains more immune cells [4], and is easily recognized by the immune system due to its high genetic instability and tumor mutational burden, making it one of the tumor types suitable for immunotherapy intervention [5]. Although there are limited options for its treatment, TNBC is the most immunogenic subtype of breast cancers. The robust antitumor responses of immunotherapy in hematologic and solid malignancies bring hope to TNBC patients [6].
Changes in the tumor microenvironment (TME), including tumor cell proliferation, tumor metastasis, tumor recurrence, and tumor resistance, play a critical role in the progression of tumors as well as in their response to treatment and prognosis. In fact, the success of immunotherapy links the TME with immunity [7,8]. In particular, tumor immune microenvironment (TIME) composed of various immune cells has also attracted much attention and exhibits significant importance to immunotherapy. Immune cells, scattered in the tumor center and infiltrating edge or adjacent tertiary lymphoid tissue, can be roughly divided into immunosuppressive cells and immune effector cells [9,10]. Similar to many other cancers, the antitumor immune killing effect in TNBC is performed by cytotoxic T cells CD8+ and helper T cells CD4+ [10]. Regulatory T cells (Tregs) is a major group of immunosuppressive cells, characterized by elevated Foxp3 expression and tumor-associated macrophages [11]. They inhibit the immune function mediated by CD8+ and CD4+ T cells by secreting TGF-β, IL-10, and IL-35 in the process of immune escape. Meanwhile, they can kill T cells directly through producing granzymes and perforin [12]. When Tregs dominate the immune function of tumor, immune escape would occur [13].
Immune checkpoints (ICs) are molecules playing a protective role similar to brakes in the immune system. It can prevent inflammation damage and autoimmune diseases caused by excessive activation of T cells (Figure 1). Tumor cells use human immune system to over-express immune checkpoint molecules to inhibit the response of the human immune system and to escape immune surveillance and killing [14,15]. In particular, programmed cell death protein-1 (PD-1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), which weaken the immune function mediated by T cells, are of great importance to tumor immunosuppression [16]. More potential checkpoints have been discovered, and their value in immunotherapy is gradually being explored. Meanwhile, adoptive T-cell immunotherapy and tumor vaccine are also constantly being studied as promising treatments.
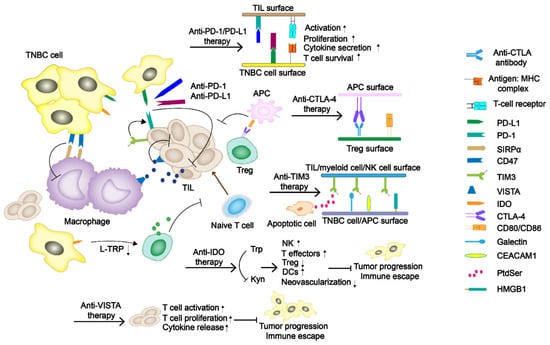
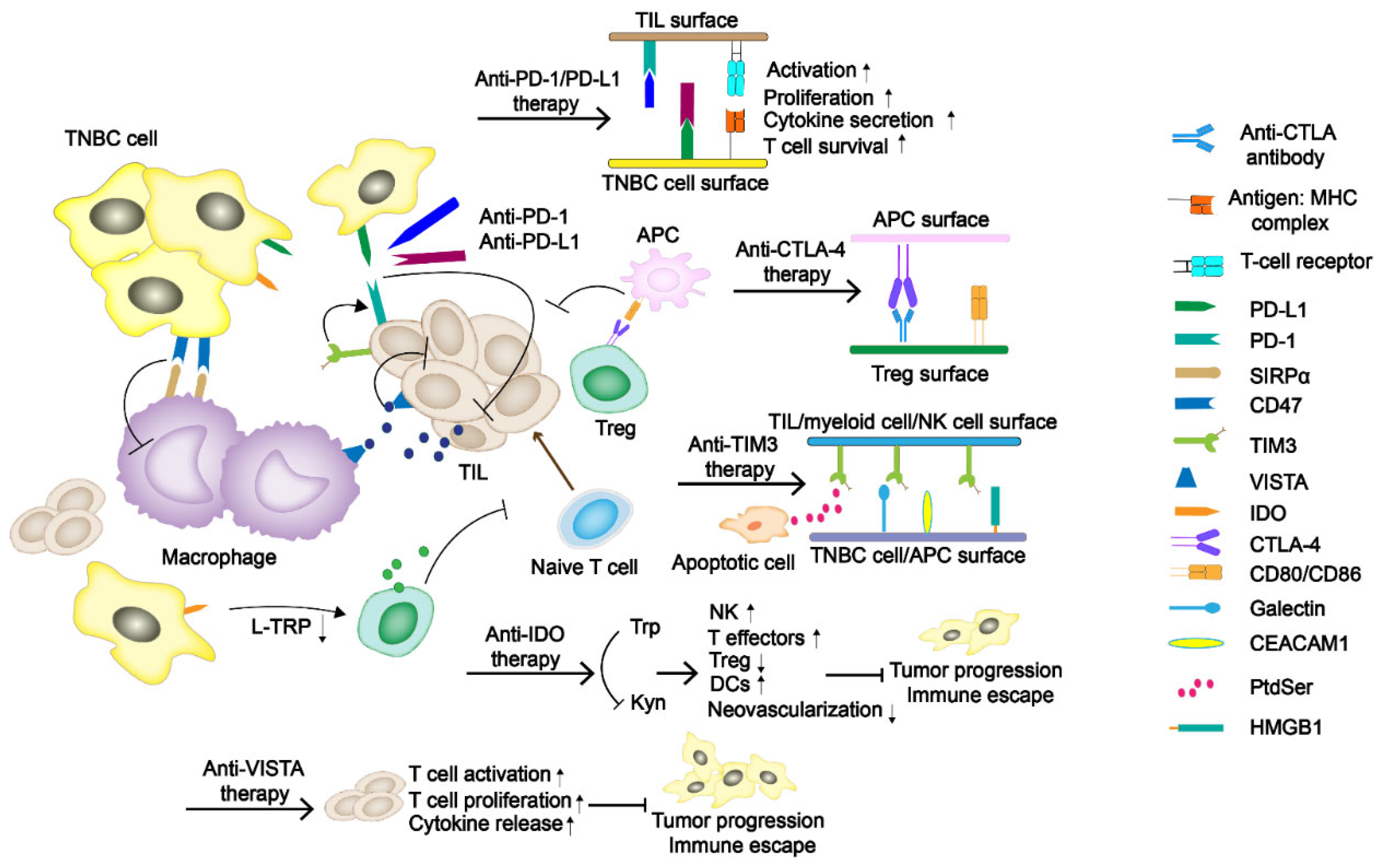
Figure 1.
The regulatory mechanism of immune checkpoints (ICs) in TNBC tumor progress. The programmed cell death-1/programmed death-ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) have been the primary immune checkpoint blockades. Some potentially new immune ICs, such as T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), indoleamine 2,3-dioxygenase (IDO), as well as V domain Ig suppressor of T-cell activation (VISTA), are also demonstrated in the figure.
1.1. Immune Checkpoint Blockades PD-1/PD-L1 and CTLA-4
Immune checkpoint blockades PD-1/PD-L1 and CTLA-4 are currently the primary and most widely studied immunotherapy agents (Figure 1). PD-1 is a member of the CD28 superfamily and is mainly expressed in activated T and B lymphocytes, natural killer (NK) cells, and myeloid cells. The structure of PD-1 includes an extracellular immunoglobulin variable region (IGV), a hydrophobic transmembrane domain, and an intracellular domain. The tail of the intracellular region contains the immune receptor tyrosine-based inhibitory motif (ITIM) and the immune receptor tyrosine-based switch motif (ITSM) [17]. PD-L1 and PD-L2 are two ligands of PD-1. The inhibitory signals often appear upon PD-1/PD-L1 binding, tyrosine phosphorylation in ITSM causes dephosphorylation of downstream protein kinases Syk and PI3K, hinders downstream pathway activation, and inhibits transcription and translation of genes and cytokines required for T-cell activation. Studies have shown that PD-L1 expression is positive in about 20% of the TNBC patients, which is significantly higher than that in non-TNBC patients [18]. CTLA-4 is a transmembrane protein exclusively expressed on T cells and Tregs in tumor infiltrating lymphocytes (TILs). It has a similar domain structure to CD28 (sharing 31% identity) and binds to B7.1 (CD 80) and B7.2 (CD 86) with higher affinity [19]. Moreover, its engagement on Tregs could strengthen the suppressive effect.
Currently, PD-1/PD-L1 inhibitors are mainly monoclonal antibodies (mAbs) and some small molecules [20]. For example, pembrolizumab and emiplimab are humanized IgG4κ monoclonal antibodies binding to PD-1 [21], nivolumab is an all humanized genetically engineered monoclonal antibody against PD-1 [22,23], and atezolizumab and durvalumab are both humanized IgG1κ type monoclonal antibodies against PD-L1 [24]. There are many ongoing clinical trials in the treatment of TNBCs with mAbs (Table 1). Immune checkpoint blockade therapy has been proved for the treatment of multiple types of cancer; however, none of them has been approved for the treatment of TNBC so far. Some problems came along with the clinical trials. Demaria et al. [25] concluded that CTLA-4 antibody monotherapy could not inhibit the growth of metastatic tumor in mouse breast cancer cell 4T1. In the randomized phase II trial NCT02519322, grade III adverse events occurred in 8% of patients treated with nivolumab monotherapy and as high as 73% of patients treated with both nivolumab and ipilimumab [26]. In the phase II clinical trial NCT02536794, durvalumab combined with tremelimumab were administered, but was finally terminated due to objective response rate (ORR) did not match the required criteria [27]. Fortunately, the KEYNOTE-012 trial in Phase Ib and KEYNOTE-086 trial in phase II both revealed that pembrolizumab had controllable safety and persistent antitumor activity in TNBC with PD-L1 positive expression [28,29].

Table 1.
Status of clinical trials with immune checkpoint blockade reagents for the treatment of TNBC [30].
1.2. T-Cell Immunoglobulin Domain and Mucin Domain-3 (TIM-3)
TIM-3, also known as HAVcr2 or CD366, is a type I cell-surface glycoprotein consisted of an amino-terminal immunoglobulin variable domain (V domain) with five noncanonical cysteines, a mucin-like stalk, a transmembrane domain, and an intracellular cytoplasmic tail [31]. It contains four different ligands, including galectin-9 (Gal-9), phosphatidylserine (PtdSer), carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM-1), and high mobility group protein B-1 (HMGB-1) [32]. TIM-3 is associated with tumor immune regulation and autoimmune diseases. Byun et al. [33] demonstrated that TIM-3 expression is a positive prognostic factor in TNBC. Due to the diversity of receptors causing the binding form to change under different situations, whether TIM-3 acts as a costimulatory receptor or a co-inhibitory receptor has not been fully determined. However, a recent study shows that TIM-3 mostly plays its role as an inhibitory receptor [34].
1.3. Indoleamine 2,3-Dioxygenase (IDO)
IDO is a rate-limiting enzyme in the catabolism of essential amino acid tryptophan (Trp) to kynurenine (Kyn). The downstream signal transduction of IDO includes the changes of general control non-derepressible-2 (GCN2), mammalian target of rapamycin (mTOR) and aryl hydrocarbon receptor (AhR) [35]. Research has proved that with the decrease of Trp followed by uncharged Trp tRNA accumulation, the GCN2 would be activated. Then, phosphorylation of eukaryotic initiation factor-2α (eIF2α) induced by GNC2 would inhibit the proliferation of effector T cells. Moreover, its metabolite Kyn can bind to AhR, leading to an increase in the number of Tregs. The suppression of mTOR and the increase of IL-6 secretion are both reasons why IDO exerts its immunosuppressive effect [36]. In breast cancer therapy, it is closely related to poor prognosis and increased microvessel density [37]. Asghar et al. [38] conducted a study on 100 female breast cancer patients in Pakistani (including triple-negative and non-triple-negative ones), linking the expression of IDO with median overall survival, proving that IDO plays a pivotal role in TNBCs. The overall survival of patients with low IDO expression is about 91 ± 41.9 months, which is much higher than the intermediate and high levels, 50 ± 4.4 and 24 ± 10.1 months, respectively. Because of the potential effect of IDO in immunotherapy, some of the IDO inhibitors, including Epacadostat, BMS986205, PF-06840003, Navoximod, Indoximod, NLG802, and LY3381916, are under the latest ongoing clinical trials [39].
1.4. V Domain Ig Suppressor of T-Cell Activation (VISTA)
VISTA, also referred to as PD-1H, is a newly discovered negative immune checkpoint related to immunotherapy resistance. VISTA is a type I transmembrane protein consisting of an N-terminal IgV domain, a stalk of about 30 amino acids, a transmembrane domain, and a cytoplasmic tail of 95 amino acids. Its molecule shares sequence homology with PD-L1 and PD-L2 [40,41]. Similar to PD-1, VISTA also serves as a negative regulatory agent for T cells by suppressing their activation, proliferation, and cytokine release. In breast cancer, it is expressed on TILs, macrophages, and other immune cells. Xue et al. [42] found that VISTA expression was higher in CD68+ tumor-associated macrophages (32.58%), CD4+ T cells (4.97%), CD8+ cytotoxic T cells (4.48%), and CD20+ B cells (1.46%). Gao et al. [43] proved that treating prostate cancer with ipilimumab can lead to a compensatory up-regulation of VISTA, indicating that VISTA may be related to the development of resistance to immune checkpoint blocking therapy. However, Cao et al. [44] evaluated the expression of VISTA in a cohort of 254 untreated TNBC patients, and found that VISTA was expressed in 87.8% (223/254) and 18.5% (47/254) of the immune cells and tumor cells, respectively. Meanwhile, the expression of VISTA in ICs is positively correlated with some TILs, especially CD4+ TIL. The information confirms the regulatory role of VISTA in antitumor immunity, but it has not been developed as a negative immune checkpoint so far.
1.5. Adoptive T-Cell Immunotherapy
The adoptive T-cell immunotherapy starts with isolating T cells, which are afterwards genetically modified to express CRA, followed by proliferation, then are reinjected to patients. The injected T cells combine with specific antigens, and ultimately eliminate targeted tumor cells [45]. Moreover, using genetic engineering technology, two types of engineered T cells, T cells with T-cell receptor (TCR) and Chimeric antigen receptor T-cell (CAR-T), were given higher recognition. CAR-T immunotherapy, started in the late 1980s, has been proved to play a key role in CD19 positive hematological malignancies [46]. CARs are synthesized receptors which consist of extra- and intracellular domains: the extracellular part is single chain fragment variable (scFv) domain composed of specific antibody, while the intracellular domain contains CD3ζ and CD3ζ co-stimulate with CD28, ICOS, 4-1BB (CD137), CD27, or OX40 signals domain. The main advantage of CAR-T technique is that it can recognize cancer cells without the presence of major histocompatibility complex (MHC) antigen.
To develop an effective CARs therapy for TNBCs, it is necessary to select a desired tumor cell-surface antigen, which can be express stably in most tumor cells, and has high tumor specificity [47]. For this purpose, Song et al. [48] designed a new folate receptor α (FRα)-specific CAR-T, which was composed of MOv19 scFv and CD8a hinge in extracellular region, and of CD27 costimulatory domain and CD3ζ signaling domain in the intracellular region. The FRα-specific CAR-T cells show a more robust immune effect in TNBCs with FRα protein overexpression. Zhou et al. [49] generated the MUC28z CAR-T cell consisted of TAB004 scFv coupled with CD28 and CD3ζ, and demonstrated that MUC28z CAR-T cells have high tumor antigen specificity, and refrained recognition of normal tissues. Moreover, it is found that MUC28z CAR-T cells can lyse TNBCs and reduce the tumor growth both in vitro and in vivo. Moreover, the growth factor receptor (EGFR) is a potential tumor surface antigen, and EGFR-specific CAR-T has strong cytotoxicity. However, the emergence of drug resistance is an urgent problem. Lin et al. [50] combined EGFR-specific CAR-T with THZ1, a CDK7 inhibitor, which together demonstrated a good effect on TNBCs proliferation, tumor metastasis and suppressed immune resistance in mice. Identically, Stuber et al. [51] combined ROR1-specific CAR-T cells to SD-208, a TGF-β inhibitor, to weaken the immunosuppressive effects in therapy.
1.6. Tumor Vaccine Immunotherapy
In contrast to the traditional concept of vaccination, the definition of vaccine in modern medicine is not only limited to prevention of diseases, but expanded to target disease-specific antigens for the treatment of ongoing diseases. In the development of immune vaccines, tumor-associated antigens and delivery technology are the main considerations. The neoantigens is produced when gene coding contained non-synonymous mutations and only expressed in tumors [52]. However, tumor-associated antigens can be expressed in tumors and normal tissues at the same time. Studies have shown that neoantigens have a stronger affinity to human leukocyte antigen (HLA) and T-cell receptors, and are not limited by central tolerance and autoimmune problems [53] Therefore, it has been regarded as one of the most potential tumor treatment targets since its discovery, and is the key to the development of personalized immunization vaccines.
Immunization vaccine also offers a viable option in the treatment of TNBCs. In the study by Liu et al. [54], an mRNA-based vaccine encoding tumor antigen MUC1 was delivered to dendritic cells (DCs) in lymph nodes using a nano-delivery system, and anti-CTLA-4 antibodies were used in combination to exert antitumor effects. Among them, the nano-delivery system can enhance the stability, persistence and expression level of the vaccine, and the vaccine that enters the body plays a role by activating and expanding tumor-specific T cells. Compared with other groups, the combination treatment group has the strongest effect of inhibiting tumor growth. Pack et al. [55] isolated tumor membrane vesicles (TMV) form 4T1 tumor, and glycosylphosphatidylinositol (GPI) anchored form of immunostimulatory B7-1 (CD80) and IL-12 molecules were combined to these TMVs to prepare TMV vaccine. Compared with monotherapy, tumor-bearing mice administrated with combined treatment of vaccine and anti-CTLA-4 antibody exhibited significantly improved survival rate and reduced lung metastasis.
1.7. Immunotherapy-Involved Combination Therapies
In the treatment with immune checkpoint inhibitors (ICIs), though mAbs show certain therapeutic effect, the response rate is generally low. For instance, the response rate of pembrolizumab monotherapy in TNBC is only 5.3% [29]. To solve this problem, combination therapy, combining mAbs with various chemotherapeutics, such as nab-paclitaxel, epirubicin, cyclophosphamide, with radiotherapy, or with some cytokines, has been intensively used in clinic trials. Table 1 summarizes such clinical trials that are being recruited or in progress. For example, the ongoing clinical phase II trial NCT02730130 was designed to assess the efficacy and safety of pembrolizumab with radiotherapy. Results show that the obtained ORR and progression-free survival (PFS) were dramatically higher than those for pembrolizumab monotherapy, increased from 3% to 17.6% and 1.9 to 2.6 months, respectively [56]. As for the phase III clinical trial KEYNOTE-355 using combined pembrolizumab, nab-paclitaxel, carboplatin, and paclitaxel/gemcitabine the combination therapy resulted in a remarkable and clinically meaningful improvement in median PFS (4.1 months longer) [57]. Similarly, phase III trial Impassion130 showed that atezolizumab plus nab-paclitaxel would induce significantly longer PFS in TNBCs [58]. As for CTLA-4, Bernier et al. [59] demonstrated that combining DZ-2384 with CTLA-4 antibody could slow down tumor growth and increase overall survival rate. Li et al. [60] demonstrated that compared with monotherapy in mice, matrix metalloproteinase inhibitor plus CTLA-4 antibody could delay tumor growth and reduce distant metastases.
It is worth noticing that the combination therapy has been designed mostly for locally advanced or metastatic TNBCs. Neoadjuvant therapy, which has been used in the treatment of melanoma or colon cancer, is a promising strategy for early TNBC. The KEYNOTE-522 trial (NCT03036488, phase III) using a combination of pembrolizumab (MK-3475) and chemotherapy as adjuvant therapy for participants with early-stage TNBCs show that the rate of pathological complete response is significantly higher than in the placebo-chemotherapy group, even for patients with low PD-L1 expression [61].
2. Nanocarriers for the Immunotherapeutic Treatment of TNBC
The emergence of immunotherapy has shed light to the treatment of TNBC. However, due to the complex tumor microenvironment and complicated immunosuppressive mechanism, conventional drug administration methods are still limited to relatively low immune response and high adverse side effects. Thus, rational design of functional drug-delivery systems is necessary to improve drug targeting, control drug release, and obtain favorable pharmacokinetic behavior, enhanced drug absorption and more drug passing through biological barriers. In particular, using nanocarriers for the delivery of immune-responsive drugs can take advantage of the enhanced permeability and retention (EPR) effect, making more drug accumulate at the tumor site and a longer time circulation period [62,63,64]. The unique EPR effect and active targeting modification of drug-delivery systems play significant roles in the therapies for tumor in deep positions or metastasized. Therefore, nanocarrier-based immunotherapy may provide TNBC patients with safer and more effective treatment [65,66].
2.1. Nanomaterials for Delivering the Immunotherapeutic Agents of TNBC
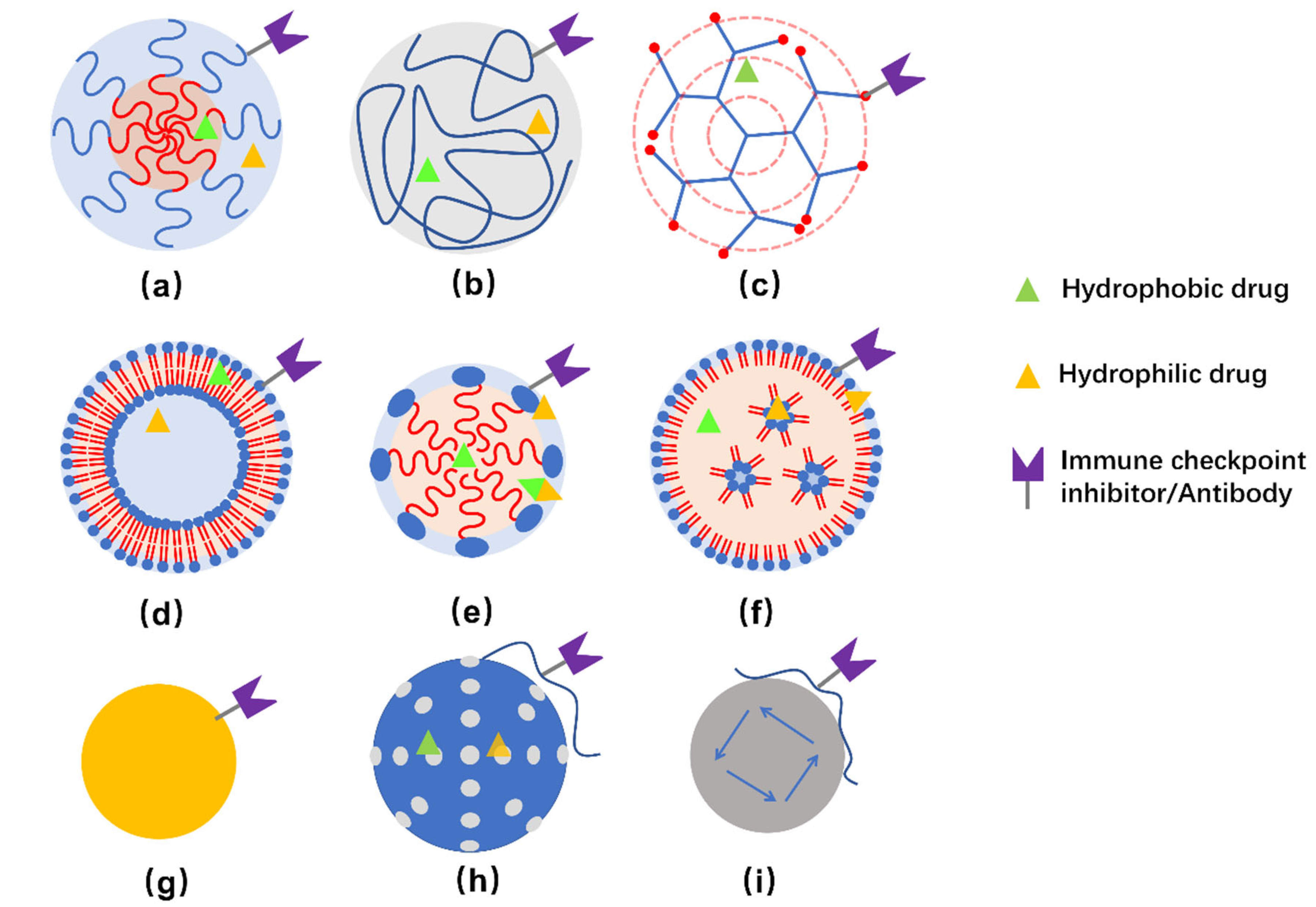
Commonly studied NPs for delivering immunotherapeutic drugs to treat TNBC include polymeric micelles, dendrimers, liposomes, inorganic NPs, and so on [67]. Illustration of some representative NPs are presented in Figure 2.
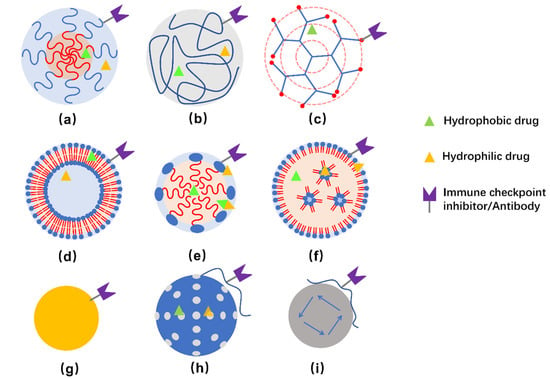
Figure 2.
Schemes of structure of polymeric nanocarriers (a–c), lipid-based nanocarriers (d–e), and inorganic NPs (g–i). (a) polymeric micelle, (b) polymeric NP, (c) dendrimer, (d) liposome, (e) lipid emulsion, (f) lipid NPs, (g) Au-NPs, (h) silica NPs, and (i) magnetic NPs. Hydrophobic, hydrophilic, as well as amphiphilic drugs can be embedded in corresponding regions. Meanwhile, it is possible to conjugate immune checkpoint inhibitors and/or antibodies to the surface of nanocarriers for therapeutic and/or targeting purposes. Usually, hybrid NPs are developed to obtain multiple function or improved properties for delivering the drugs.
The physiochemical properties of polymers, such as charge, hydrophobicity/hydrophilicity, as well as the features of the polymeric NPs, include size, shape, and rigidity, can be tuned for encapsulating specific immune-responsive drugs and be designed to improve the endocytic uptake, biodistribution, and body clearance properties as well [68,69,70]. Methods for preparing nano-sized polymeric particles include polymeric micelles prepared by self-assembling of co-polymer consisting of hydrophobic and hydrophilic sections, polymeric NPs prepared by solvent evaporation, emulsification/solvent diffusion, nanoprecipitation or emulsification/reverse salting-out, as well as dendrimers prepared by repetitive addition of monomers initiated from a polyfunctional center [71,72,73]. Poly(lactic-co-glycolic acid) (PLGA) and polylactic acid (PLA) are biocompatible and biodegradable polymers approved by FDA in drug-delivery systems [74,75]. Dendrimers are composed of a hydrophobic core, which favors the encapsulation of hydrophobic molecules, and the outer surface that provides sites for functionalization.
Lipids are amphiphilic molecules composed of hydrophilic headgroup and hydrophobic tail(s). Lipid-based nanocarriers for drug delivery include solid lipid nanoparticles, vesicular liposomes, nano-emulsions/nano-micelles, as well as non-spherical ones, such as nanotubes. The configuration of the nanocarriers strongly depends on the packing parameter of the lipids [76]. Phospholipids, being the major components of cell membranes, have attracted particular attention as drug-carrier materials due to their good biocompatible, low toxicity and higher permeation. Liposomes prepared from aggregated synthetic phospholipid(s) or even directly extracted from tumor cells are preferred as drug-delivery carriers [77,78]. Similar to polymeric micelles, liposomes, lipid emulsions as well as lipid NPs are composed of both hydrophobic and hydrophilic regions, which can be used to encapsulate hydrophobic and hydrophilic drugs, respectively. Amphiphilic drugs may also be encapsulated at the hydrophilic-hydrophobic interface.
Inorganic NPs are robust frameworks allowing encapsulation and incorporation of one or more drugs or therapeutic molecules. Several inorganic NPs, including gold NPs (Au-NPs), mesoporous silica NPs, magnetic NPs, and carbon nanotubes, have been investigated as nanocarriers for drug delivery [79]. In addition to the loaded therapeutic agents, some NPs exert specific functions by themselves. For example, Au-NPs can induce cell death [80], and can also adsorb light in the NIR region and dissipate heat to the surroundings. As for magnetic nanoparticles (MNPs), they not only generate nonuniform magnetic fields which affect the morphology, differentiation and function of cells by generating magnetically induced mechanical forces [81], but also activate anti-cancer immune responses through their own immunomodulatory effects [82]. Inorganic NPs coated with polymers or lipid bilayers, forming core-shell structures that enable surface modification and show enhanced biocompatibility, have also been widely studied [83].
2.2. Nanocarriers for the Delivery of Immune Checkpoint Blockade Molecules
IC blockade is the primary strategy for TNBC immunotherapy, but the effect of monotherapy is modest. Increasing the dose or choosing a combination of multiple ICIs is commonly used to overcome this problem. However, the increase in drug toxicity has led to the suspension of many clinical trials. For instance, in the randomized phase II trial NCT02519322, grade III adverse events occurred in 8% of patients treated with nivolumab monotherapy and in as high as 73% of patients treated with both nivolumab and ipilimumab [26].
Combining more than one ICIs can exert more effects than using one ICI. Considering there are more expressed CD155 and PD-L1 in TNBC than in other types of breast cancers, Chen et al. [84] designed mPEG-PLGA-PLL (PEAL) NPs loaded with CD155 siRNA (siCD155) by a double-emulsion method followed by coated with a PD-L1 blockade, termed as P/PEALsiCD155, for immunotherapy of TNBC. In CD8+ TIL cells, CD155 can bind to different receptors, such as DNAM-1, TIGIT, and CD96 [85,86]. Based on the asynchronous expression of the above-mentioned receptors, this study shows that siCD155-mediated knockdown of CD155 by P/PEALsiCD155 can achieve spatiotemporal targeting of surface receptors and intracellular mRNA, making the antitumor effect take place in favorable periods, i.e., promoted CD155-mediated immune surveillance in the early stage and inhibited CD155-mediated immune evasion in the later stage. In the 4T1 TNBC tumor model, P/PEALsiCD155 showed surprising biocompatibility, specific targeting, along with efficient inhibition of TNBC tumor progression and metastasis [84].
Discovering more efficient molecules is another option overcoming the problems along with monotherapy. NPs made of 100% BMS-200, a small-molecular inhibitor of PD-1/PD-L1 interaction, has also been developed as potential alternatives to anti-PD-L1 monoclonal antibody (α-PD-L1) [87]. Compared with α-PD-L1, BMS-202 NPs show equivalent immunotherapy effect by inhibiting >90% primary and distant tumors, and possible superior tumor penetration. The approach of using NPs for the delivery of immune checkpoint blockade molecules may provide an alternative nanomedicine for treating metastatic or advanced TNBC.
2.3. Nanoparticles for the Delivery of Combination Therapy Agents
The monotherapies of TNBC, including chemotherapy, radiotherapy, and immunotherapy based on ICs, all demonstrate limited therapeutic effect. Based on the low pH levels, endogenous H2O2, overexpressed enzymes, and other specific factors in the TME of TNBCs, combination therapy using immunotherapy with other specific treatment, such as chemotherapy, photothermal therapy (PTT), photodynamic therapy (PDT), and sonodynamic therapy (SDT), to overcome the insufficient efficacy by monotherapy and improve the therapeutic efficacy to TNBCs [88,89]. Combination therapy may also generate synergistic effect to the treatment of TNBC [90].
2.3.1. Immunotherapy Combined with PTT
PTT is a process that converts light energy into heat to induce thermal ablation of the tumor. Near-infrared region (700–1350 nm) is the most commonly used wavelength, and recent studies have shown that light in the second near-infrared region (1000–1700 nm) has deeper tissue penetration and tolerance [91]. The conversion of electromagnetic energy into heat is achieved by photothermal agents (PAT). An excellent PAT is characterized by strong light absorption, strong photothermal conversion ability, stability, good biocompatibility, and the ability to turn off PTT in the non-treatment stage [92]. Near-infrared PAT include polyaniline [93], copper sulfides (CuS) NPs [94], as well as inorganic NPs, such as gold-based NPs [95]. Inorganic NPs show high photothermal conversion efficiency but relatively poor biocompatibility and toxicity [95], while liposome-based material and organic-conjugated polymers exhibit relatively better biocompatibility [96].
As PTT cannot directly kill tumor cells but acts as an adjuvant at about 45 °C, Huang et al. [97] developed a symbiotic mild photothermal-sensitized immunotherapy (SMPAI) and put forward a hypothesis of a synergistic effect by combining mild PTT with immunotherapy. They encapsulated a photothermal and photodynamic therapy agent (IR820) and an anti-PD-L1 antibody into a lipid gel composed of soybean phosphatidylcholine (SPC) and glycerol dioleate (GDO), which undergoes a reversible gel-to-sol transition for the controllable release of aPD-L1 and enhanced infiltration of T cells into tumor under manually controlled NIR irradiation. Overall, mild PTT can activate the systemic immune response, increase the number of TILs, and increase the expression of PD-L1 in tumor cells.
To specifically target CD44, Yasothamani et al. [93] designed a conjugation of hyaluronan (HA)−polyaniline (PANi)−imiquimod (R837), denoted as HA-PANi/R837. The high photothermal conversion efficiency of PANi means that it has a significant thermal ablation effect in solid tumor. HA is a targeting ligand activating CD44 with high biosafety [98], which compensates for the poor targeting and low cellular uptake of PANi. R837 is a toll-like receptor 7 agonist, acting as an immunomodulator. In the TNBC model, HA-PANi/R837 directly killed a part of cancer cells through thermal ablation, induced the production of tumor-associated antigens, and activated immune response. Ultimately, the combination of HA-PANi/R837 and anti-CTLA-4 antibody shows enhanced immunotherapy effect and played a synergistic antitumor effect. For research on PTT, it is clear that it has a certain tumor-killing effect, immunostimulatory effect, and NPs-based PTT can produce NPs-mediated antigen capture [99].
To suppress recurrence and metastasis of TNBC, Cheng et al. [94] designed a biomimetic nanoplatform AM@DLMSN@CuS/R848, based on dendritic large-pore mesoporous silica nanoparticles (DLMSNs) loaded with CuS NPs, immune adjuvant R848 (resiquimod), TNBC cell membrane, and AUNP-12 (a PD-1/PD-L1 peptide inhibitor). AUNP-12 conjugated to the outer TNBC cell membrane by benzoic-imide bond readily released from the nanoparticle in the weakly acidic pH of tumor microenvironment. This nanocarrier exhibits targeted TNBC delivery, high photothermal efficiency of CuS NPs, photothermal-triggered release of R848, and pH-responsive release of AUNP-12, which together lead to strong antitumor efficacy and enhanced therapy against metastatic TNBC.
2.3.2. Immunotherapy Combined with PDT
PDT induces chemical cytotoxic effects to tumor cells on the generation of reactive oxygen species (ROS) [100]. Light, photosensitizer, and molecular oxygen are three indispensable elements for PDT. Light wavelengths in visible (400–700 nm) and near-infrared ranges (700–1350 nm) are commonly used. The photosensitizer absorbs photons and transforms from ground singlet state to excited singlet state, which afterwards generates excited triplet state and relaxation by undergoing intersystem crossing, accompanied by energy emitted as fluorescence, heat, and/or other photophysical energy [92]. The tetrapyrrole structure is extremely common in photosensitizer, and it is related to the photon absorption ability and the ability to convert the relatively low active triplet ground-state molecular oxygen (3O2) into more active singlet oxygen (1O2) [101]. Because oxygen content in TME is low (as TME is in a hypoxic state), it is challenging to provide enough molecular oxygen at tumor site for oxygen-dependent PDT.
Combining PDT with immunotherapy could play a synergistic effect. The release of ROS or PDT-induced exposure and/or release of damage-associated molecular patterns (DAMPs) would stimulate the body’s immune system. The increase in immunogenicity induces the maturation of DCs and activation of cytotoxic T lymphocytes to increase the number of TILs [102]. In addition to the immune response caused by PDT itself, the combination of PDT with ICB also plays a synergistic effect in preclinical research. Zhang et al. [87] proved that BMS-202 NPs in combination with Ce6 NPs exhibited a better antitumor and antimetastatic effects. Chung et al. [103] designed smart multifunctional nanoparticle cluster, FM@VP, which combined co-assembly of a nanocomplex formed by a functional polysaccharide fucoidan and a bioreducible polyamidoamine dendrimer, and MnO2 NPs encapsulated with a photosensitizer verteporfin. It was able to target P-selectin-overexpressed TNBC. Moreover, due to the high concentration of glutathione, FM@VP clusters would rapidly disintegrate in tumor. The released verteporfin from the clusters enhanced PDT and inhibited yes-associated protein (involved in tumor development), which weakened tumor-mediated immunosuppression. Meanwhile, MnO2 NPs could efficiently convert H2O2 into oxygen in TME, reducing the adverse impression caused by low-oxygen environments [104]. This discovery provided a powerful strategy for synergistic tumor targeting, PDT and immunotherapy.
2.3.3. Immunotherapy Combined with SDT
SDT is a non-invasive therapeutic modality based on ultrasound, which is more acceptable to patients than chemotherapy and radiotherapy. Low-intensity focused ultrasound is commonly used to activate sonosensitizers [105]. Compared with PTT and PDT, the tissue penetration ability of SDT is extremely powerful, thus therapeutic effect for tumors in deep positions is more significant [106,107]. Meanwhile, SDT generates many types of ROS, not only 1O2, but also hydroxyl (•OH) and superoxide radicals (•O2−) [108]. Its functions include direct tumor cells killing, and indirect tumor-specific immunity activation through damaging blood vessels or inhibiting the regeneration of tumor tissues, et al. Sonosensitizers, including organic molecules (such as porphyrin derivatives), inorganic nanomaterials (such as TiO2, ZnO) as well as the hybrid ones, have been developed for SDT [109]. To better exert its immunomodulatory effect, the sonosensitizer was delivered through NPs drug-delivery system (DDS) when combined with immune adjuvants in some research.
In TNBC therapy, Chen et al. [110] designed a liposome-encapsulated anganese-protoporphyrin complex (MnP) and modified with folic acid (FA). In the 4T1 model, the multifunctional nanosonosensitizer FA-MnPs exhibited significant effect on both deep-state and superficial tumors. In addition to induce chemical damage to tumor cells, SDT could also exert an effect through immune regulation. For instance, M2 macrophages would develop towards the antitumor M1 phenotype. Similar to PDT, the damage-associated molecular patterns caused by SDT can also stimulate systemic immunity, including the maturation of DCs and the activation of cytotoxic T lymphocytes.
2.3.4. Others
In addition to the direct delivery of immunomodulatory agent to the tumor site through the EPR effect, there are other studies focused on TME targeting. For example, although chemotherapy exert less efficacy to the treatment of TNBC, it also shows immunomodulatory effect on TME. Xu et al. [111] designed a peptide-based, structure-transformable NPs, 2-(Nap)-FFKPt-2TPA-ADDGGGPLGVRG-WKYMVm-mPEG1000, for contemporaneous delivery of chemotherapy agent for TNBC, cisplatin and adjudin, as well as WKYMVm—an FPR-1 agonist functions as an immune adjuvant, which synergistically elicit and promote immunogenic cell death for TNBC immunotherapy. In another study, Xu et al. [112] prepared a pueratin nanoemulsion (nanoPue) surface modified with aminoethyl anisamide as targeting ligand to tumor-associated fibroblasts (TAFs). Together with improved solubility and bioavailability, the delivered puerarin significantly deactivate the stromal microenvironment, which synergistically enhances the activity of checkpoint blockade immunotherapy in a TNBC mode when combined with α-PD-L1.
3. Conclusions and Perspective
In conclusion, immunotherapy has achieved many promising results in alleviating TNBC. In addition to the classic immune checkpoints PD-1/PD-L1 and CTLA-4, new immune checkpoints are constantly being discovered. At the same time, progress has been made in new treatments, including CAR-T and tumor immunization vaccines. ICIs are commonly used in combination with other therapy, such as chemotherapy, radiotherapy, CAR-T, and tumor vaccine, to obtain synergistic effects for more effective treatment of TNBCs. Moreover, he nano-delivery system integrated with TNBC immunotherapy provides strategy that can achieve precise targeting and reduce off-target effects. Immunotherapy has indeed brought hope to the treatment of TNBC. Although there are problems need to be solved, immunotherapy for TNBC is full of potential.
Finally, we may identify several issues and problems related to the immunotherapy of TNBC. These include: (1) The fundamental understanding of tumor heterogeneity, molecular changes, immunogenomics, and treatment resistance mechanisms in TNBC should be advanced to give better treatments to the patients. (2) The narrow therapeutic window is also a potential problem that needs to be resolved. Although the indication of drugs for monotherapy is clear, it still needs be redefined in combination therapies. Most importantly, because the drug-delivery system of combination therapy is complex, it is more difficult to understand the mechanism. In particular, TME has changed tremendously when it comes to immunotherapy. Most of the research focus on DCs and TILs, but these are definitely not enough. Therefore, immune-regulatory mechanism needs to be further clarified when nanocarrier is involved in immunotherapy. (3) The discovery of new antigens specifically expressed in TNBC cells would facilitate the development of new immunotherapies. (4) For nanocarrier in immunotherapy of TNBC, the safety of nanomaterials is complicated. Particular attention should be paid to inorganic materials with poor biocompatibility and organic nanomaterials with good biocompatibility but strong immunogenicity. (5) Immunoconjugates are effective with minimal toxicity, showing promise for clinical translation. Trodelvy (Sacituzumab govitecan), an antibody-drug conjugate consisting of an antibody Sacituzumab targeting the Trop-2 protein (found in more than 90% of TNBC), and coupled to chemotherapy drugs 7-ethyl-10-hydroxycamptothecin (SN-38, a topoisomerase I inhibitor to interfere with the replication of cancer cells) with a hydrolysable linker, is a first-in-class medicine for advanced TNBC therapy.
Author Contributions
Writing—original draft preparation, Y.C. and C.C.; writing—review and editing, Y.C., Y.T., W.L. and P.W.; funding acquisition, P.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Technology Department of Zhejiang Province, China, Grant No. 2017C04009 and the Ministry of Science and Technology of the People’s Republic of China, Grant No. 2017YFE0130100.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [Green Version]
- Makhoul, I.; Atiq, M.; Alwbari, A.; Kieber-Emmons, T. Breast cancer immunotherapy: An update. Breast Cancer (Auckl) 2018, 12, 1–15. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Emens, L.A. Breast cancer immunotherapy: Facts and hopes. Clin. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef]
- Shihab, I.; Khalil, B.A.; Elemam, N.M.; Hachim, I.Y.; Hachim, M.Y.; Hamoudi, R.A.; Maghazachi, A.A. Understanding the role of innate immune cells and identifying genes in breast cancer microenvironment. Cancers 2020, 12, 2226. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin. Ther. Targets 2018, 22, 353–363. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Wang, S.; Xie, K.; Liu, T. Cancer immunotherapies: From efficacy to resistance mechanisms-Not only checkpoint matters. Front. Immunol. 2021, 12, 690112. [Google Scholar] [CrossRef]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Babaloo, Z.; Baradaran, B. CTLA-4: From mechanism to autoimmune therapy. Int. ImmunoPharmacol. 2020, 80, 106221. [Google Scholar] [CrossRef]
- Boussiotis, V.A.; Longo, D.L. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [Green Version]
- Gatalica, Z.; Snyder, C.; Maney, T.; Ghazalpour, A.; Holterman, D.; Nianqing, X.; Overberg, P.; Rose, I.; Basu, G.D.; Vranic, S.; et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2965–2970. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, A.H.; Freeman, G.J. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002, 2, 116–126. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. ImmunoPharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Markham, A.; Duggan, S. Cemiplimab: First global approval. Drugs 2018, 78, 1841–1846. [Google Scholar] [CrossRef]
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccin Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Shen, J.; Ivaturi, V.; Gopalakrishnan, M.; Feng, Y.; Schmidt, B.J.; Statkevich, P.; Goodman, V.; Gobburu, J.; Bello, A.; et al. Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types. Ann. Oncol. 2020, 31, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.T.; Lee, J.Y.; Lim, H.; Lee, S.H.; Moon, Y.J.; Pyo, H.J.; Ryu, S.E.; Shin, W.; Heo, Y.S. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci. Rep. 2017, 7, 5532. [Google Scholar] [CrossRef] [Green Version]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11, 728–734. [Google Scholar]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef]
- Mediratta, K.; El-Sahli, S.; D’Costa, V.; Wang, L. Current progresses and challenges of immunotherapy in triple-negative breast cancer. Cancers 2020, 12, 3529. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.Q.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 2 November 2021).
- Joller, N.; Kuchroo, V.K. Tim-3, Lag-3, and TIGIT. Curr Top. Microbiol. Immunol. 2017, 410, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Yang, M.; Turner, A.; Xu, C.; Ferris, R.L.; Huang, J.; Kane, L.P.; Lu, B. TIM-3 as a target for cancer immunotherapy and mechanisms of action. Int. J. Mol. Sci. 2017, 18, 645. [Google Scholar] [CrossRef]
- Byun, K.D.; Hwang, H.J.; Park, K.J.; Kim, M.C.; Cho, S.H.; Ju, M.H.; Lee, J.H.; Jeong, J.S. T-cell immunoglobulin mucin 3 expression on tumor infiltrating lymphocytes as a positive prognosticator in triple-negative breast cancer. J. Breast Cancer 2018, 21, 406–414. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Iain, A.; Murray, A.D.P.; Gary, H. Perdew. AH receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Brochez, L.; Chevolet, I.; Kruse, V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur. J. Cancer 2017, 76, 167–182. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, S.; Li, M.; Li, F.; Wei, F.; Liu, J.; Ren, X. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front. Immunol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Asghar, K.; Loya, A.; Rana, I.A.; Tahseen, M.; Ishaq, M.; Farooq, A.; Bakar, M.A.; Masood, I. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manag. Res. 2019, 11, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.; Wu, Y.H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef]
- Slater, B.T.; Han, X.; Chen, L.; Xiong, Y. Structural insight into T cell coinhibition by PD-1H (VISTA). Proc. Natl. Acad. Sci. USA 2020, 117, 1648–1657. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, J.; Shi, Z.; Liu, W.; Hu, X.; Qie, C.; Chen, W.; Wang, Y.; Wang, L.; Jiang, J.; et al. The expression pattern and clinical significance of the immune checkpoint regulator VISTA in human breast cancer. Front. Immunol. 2020, 11, 563044. [Google Scholar] [CrossRef]
- Gao, J.; Ward, J.F.; Pettaway, C.A.; Shi, L.Z.; Subudhi, S.K.; Vence, L.M.; Zhao, H.; Chen, J.; Chen, H.; Efstathiou, E.; et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017, 23, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Ren, X.; Zhou, Y.; Mao, F.; Lin, Y.; Wu, H.; Sun, Q. VISTA expression on immune cells correlates with favorable prognosis in patients with triple-negative breast cancer. Front. Oncol. 2020, 10, 583966. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Z.; Kuang, Y.; Wu, Z.; Zhao, S.; Zhang, Z.; Li, H.; Zheng, M.; Zhang, N.; Long, C.; et al. Intercellular adhesion molecule-1 as target for CAR-T-cell therapy of triple-negative breast cancer. Front. Immunol. 2020, 11, 573823. [Google Scholar] [CrossRef]
- Cummins, K.D.; Gill, S. Anti-CD123 chimeric antigen receptor T-cells (CART): An evolving treatment strategy for hematological malignancies, and a potential ace-in-the-hole against antigen-negative relapse. Leuk. Lymphoma 2018, 59, 1539–1553. [Google Scholar] [CrossRef]
- Wei, J.; Han, X.; Bo, J.; Han, W. Target selection for CAR-T therapy. J. Hematol. Oncol. 2019, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Song, D.G.; Ye, Q.; Poussin, M.; Chacon, J.A.; Figini, M.; Powell, D.J., Jr. Effective adoptive immunotherapy of triple-negative breast cancer by folate receptor-alpha redirected CAR T cells is influenced by surface antigen expression level. J. Hematol. Oncol. 2016, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yazdanifar, M.; Roy, L.D.; Whilding, L.M.; Gavrill, A.; Maher, J.; Mukherjee, P. CAR T cells targeting the tumor MUC1 glycoprotein reduce triple-negative breast cancer growth. Front. Immunol. 2019, 10, 1149. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Zheng, Z.; Liu, J.Y.; Chen, Y.J.; Ding, J.; Hu, G.S.; Hu, Y.H.; Liu, S.; Luo, W.X.; Xia, N.S.; et al. Targeting triple-negative breast cancer with combination therapy of EGFR CAR T cells and CDK7 inhibition. Cancer Immunol. Res. 2021, 9, 707–722. [Google Scholar] [CrossRef]
- Stuber, T.; Monjezi, R.; Wallstabe, L.; Kuhnemundt, J.; Nietzer, S.L.; Dandekar, G.; Wockel, A.; Einsele, H.; Wischhusen, J.; Hudecek, M. Inhibition of TGF-beta-receptor signaling augments the antitumor function of ROR1-specific CAR T-cells against triple-negative breast cancer. J. Immunother. Cancer 2020, 8, e000676. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson, B.A., 3rd; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Qin, Y.; Gao, W.; Tao, L.; Su, W.; Zhong, J. Neoantigen: A new breakthrough in tumor immunotherapy. Front. Immunol. 2021, 12, 672356. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Miao, L.; Liu, Q.; Musetti, S.; Li, J.; Huang, L. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol. Ther. 2018, 26, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Pack, C.D.; Bommireddy, R.; Munoz, L.E.; Patel, J.M.; Bozeman, E.N.; Dey, P.; Radhakrishnan, V.; Vartabedian, V.F.; Venkat, K.; Ramachandiran, S.; et al. Tumor membrane-based vaccine immunotherapy in combination with anti-CTLA-4 antibody confers protection against immune checkpoint resistant murine triple-negative breast cancer. Hum. Vaccin Immunother. 2020, 16, 3184–3193. [Google Scholar] [CrossRef]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A Phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Bernier, C.; Soliman, A.; Gravel, M.; Dankner, M.; Savage, P.; Petrecca, K.; Park, M.; Siegel, P.M.; Shore, G.C.; Roulston, A. DZ-2384 has a superior preclinical profile to taxanes for the treatment of triple-negative breast cancer and is synergistic with anti-CTLA-4 immunotherapy. Anticancer Drugs 2018, 29, 774–785. [Google Scholar] [CrossRef]
- Li, M.; Xing, S.; Zhang, H.; Shang, S.; Li, X.; Ren, B.; Li, G.; Chang, X.; Li, Y.; Li, W. A matrix metalloproteinase inhibitor enhances anti-cytotoxic T lymphocyte antigen-4 antibody immunotherapy in breast cancer by reprogramming the tumor microenvironment. Oncol. Rep. 2016, 35, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liu, X.; Pan, W.; Li, N.; Tang, B. Designing and engineering of nanocarriers for bioapplication in cancer immunotherapy. ACS Appl. Bio Mater. 2020, 3, 8321–8337. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Gu, Z.; Da Silva, C.G.; Van der Maaden, K.; Ossendorp, F.; Cruz, L.J. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics 2020, 12, 1054. [Google Scholar] [CrossRef]
- Johnson, R.; Sabnis, N.; McConathy, W.J.; Lacko, A.G. The potential role of nanotechnology in therapeutic approaches for triple negative breast cancer. Pharmaceutics 2013, 5, 353–370. [Google Scholar] [CrossRef] [Green Version]
- Jain, V.; Kumar, H.; Anod, H.V.; Chand, P.; Gupta, N.V.; Dey, S.; Kesharwani, S.S. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J. Control. Release 2020, 326, 628–647. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Jones, M.-C.; Leroux, J.-C. Polymeric micelles–A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002, 4, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold nanoparticles-induced cytotoxicity in triple negative breast cancer involves different epigenetic alterations depending upon the surface charge. Sci. Rep. 2018, 8, 12295. [Google Scholar] [CrossRef]
- Wosik, J.; Chen, W.; Qin, K.; Ghobrial, R.M.; Kubiak, J.Z.; Kloc, M. Magnetic field changes macrophage phenotype. Biophys. J. 2018, 114, 2001–2013. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.W.; Tsao, H.Y.; Chiang, C.S.; Chen, S.Y. Advances in magnetic nanoparticle-mediated cancer immune-theranostics. Adv. Healthc. Mater. 2021, 10, e2001451. [Google Scholar] [CrossRef]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic nanoparticles for cancer therapy: A transition from lab to clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Q.; Fu, H.; Yu, J.; Wang, L.; Sun, Y.; Zhang, J.; Duan, Y. Asynchronous blockade of PD-L1 and CD155 by polymeric nanoparticles inhibits triple-negative breast cancer progression and metastasis. Biomaterials 2021, 275, 120988. [Google Scholar] [CrossRef]
- Manieri, N.A.; Chiang, E.Y.; Grogan, J.L. TIGIT: A key inhibitor of the cancer immunity cycle. Trends Immunol. 2017, 38, 20–28. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Madore, J.; Li, X.Y.; Smyth, M.J. Tumor intrinsic and extrinsic immune functions of CD155. Semin. Cancer Biol. 2020, 65, 189–196. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Z.; Lv, H.; Li, F.; Sun, S.; Li, J.; Lee, C.S. Immune checkpoint blockade mediated by a small-molecule nanoinhibitor targeting the PD-1/PD-L1 pathway synergizes with photodynamic therapy to elicit antitumor immunity and antimetastatic effects on breast cancer. Small 2019, 15, e1903881. [Google Scholar] [CrossRef]
- Sau, S.; Alsaab, H.O.; Bhise, K.; Alzhrani, R.; Nabil, G.; Iyer, A.K. Multifunctional nanoparticles for cancer immunotherapy: A groundbreaking approach for reprogramming malfunctioned tumor environment. J. Control. Release 2018, 274, 24–34. [Google Scholar] [CrossRef]
- Hou, X.; Tao, Y.; Pang, Y.; Li, X.; Jiang, G.; Liu, Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int. J. Cancer 2018, 143, 3050–3060. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Niu, Z.; Hou, B.; Zhou, L.; Li, Y.; Yu, H. Enhancing triple negative breast cancer immunotherapy by ICG-templated self-assembly of Paclitaxel nanoparticles. Adv. Funct. Mater. 2019, 30, 1906605. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Zhang, Z.; Ji, L.; Zhang, J.; Wang, Q.; Guo, T.; Ni, S.; Cai, R.; Mu, X.; et al. Recent progress on NIR-II photothermal therapy. Front. Chem. 2021, 9, 728066. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Yasothamani, V.; Karthikeyan, L.; Shyamsivappan, S.; Haldorai, Y.; Seetha, D.; Vivek, R. Synergistic effect of photothermally targeted NIR-responsive nanomedicine-induced immunogenic cell death for effective triple negative breast cancer therapy. Biomacromolecules 2021, 22, 2472–2490. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Q.; Guo, Z.; Li, M.; Yang, X.; Wan, G.; Chen, H.; Zhang, Q.; Wang, Y. An intelligent biomimetic nanoplatform for holistic treatment of metastatic triple-negative breast cancer via photothermal ablation and immune remodeling. ACS Nano 2020, 14, 15161–15181. [Google Scholar] [CrossRef]
- Khafaji, M.; Zamani, M.; Golizadeh, M.; Bavi, O. Inorganic nanomaterials for chemo/photothermal therapy: A promising horizon on effective cancer treatment. Biophys. Rev. 2019, 11, 335–352. [Google Scholar] [CrossRef]
- Lv, S.; Miao, Y.; Liu, D.; Song, F. Recent development of photothermal agents (PTAs) based on small organic molecular dyes. ChemBioChem 2020, 21, 2098–2110. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Du, Y.; Zhang, Y.; Wang, X.; Ding, Y.; Yang, X.; Meng, F.; Tu, J.; Luo, L.; et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat. Commun. 2019, 10, 4871. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Sweeney, E.E.; Ramanujam, A.S.; Fernandes, R. Photothermal therapies to improve immune checkpoint blockade for cancer. Int. J. Hyperth. 2020, 37, 34–49. [Google Scholar] [CrossRef]
- Luby, B.M.; Walsh, C.D.; Zheng, G. Advanced photosensitizer activation strategies for smarter photodynamic therapy beacons. Angew. Chem. Int. Ed. Engl. 2019, 58, 2558–2569. [Google Scholar] [CrossRef]
- Ming, L.; Cheng, K.; Chen, Y.; Yang, R.; Chen, D. Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Med. 2021, 10, 257–268. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, F.; Deng, H.; Lin, L.; Wang, S.; Kang, F.; Yu, G.; Lau, J.; Tian, R.; Zhang, M.; et al. Smart nanovesicle-mediated immunogenic cell death through tumor microenvironment modulation for effective photodynamic immunotherapy. ACS Nano 2020, 14, 620–631. [Google Scholar] [CrossRef]
- Chung, C.-H.; Lu, K.-Y.; Lee, W.-C.; Hsu, W.-J.; Lee, W.-F.; Dai, J.-Z.; Shueng, P.-W.; Lin, C.-W.; Mi, F.-L. Fucoidan-based, tumor-activated nanoplatform for overcoming hypoxia and enhancing photodynamic therapy and antitumor immunity. Biomaterials 2020, 257, 120227. [Google Scholar] [CrossRef]
- Shamay, Y.; Elkabets, M.; Li, H.; Shah, J.; Brook, S.; Wang, F.; Adler, K.; Baut, E.; Scaltriti, M.; Jena, P.V. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci. Transl. Med. 2016, 8, 345ra87. [Google Scholar] [CrossRef] [Green Version]
- Lafond, M.; Yoshizawa, S.; Umemura, S.I. Sonodynamic therapy: Advances and challenges in clinical translation. J. Ultrasound. Med. 2019, 38, 567–580. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef]
- Wan, G.Y.; Liu, Y.; Chen, B.W.; Liu, Y.Y.; Wang, Y.S.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Bai, L.; Wang, H.; Wu, Q.; Wang, H.; Liu, S.; Xu, B.; Shi, X.; Liu, H. Metal-organic-framework-derived carbon nanostructure augmented sonodynamic cancer therapy. Adv. Mater. 2018, 30, e1800180. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Ma, A.; Yin, T.; Chen, Z.; Liang, R.; Qiu, Y.; Zheng, M.; Cai, L. Noninvasively immunogenic sonodynamic therapy with manganese protoporphyrin liposomes against triple-negative breast cancer. Biomaterials 2021, 269, 120639. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Y.; Sun, Y.; Kong, L.; Yang, C.; Hu, M.; Yang, T.; Zhang, J.; Hu, Q.; Zhang, Z. Transformable nanoparticle-enabled synergistic elicitation and promotion of immunogenic cell death for triple-negative breast cancer immunotherapy. Adv. Funct. Mater. 2019, 29, 1905213. [Google Scholar] [CrossRef]
- Xu, H.; Hu, M.; Liu, M.; An, S.; Guan, K.; Wang, M.; Li, L.; Zhang, J.; Li, J.; Huang, L. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials 2020, 235, 119769. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).