Signaling Pathway Inhibitors, miRNA, and Nanocarrier-Based Pharmacotherapeutics for the Treatment of Lung Cancer: A Review
Abstract
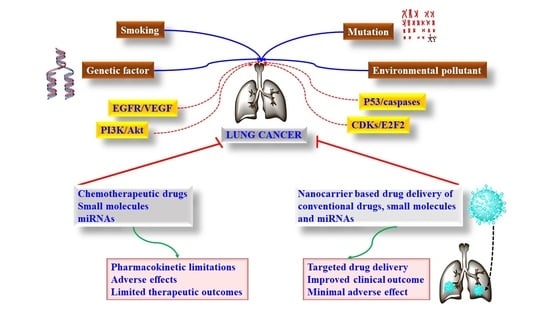
1. Introduction
2. Signaling Pathways and Targeted Therapy in LC
2.1. Epidermal Growth Factor (EGFR) Receptor Deregulation and EGFR Inhibitors in LC
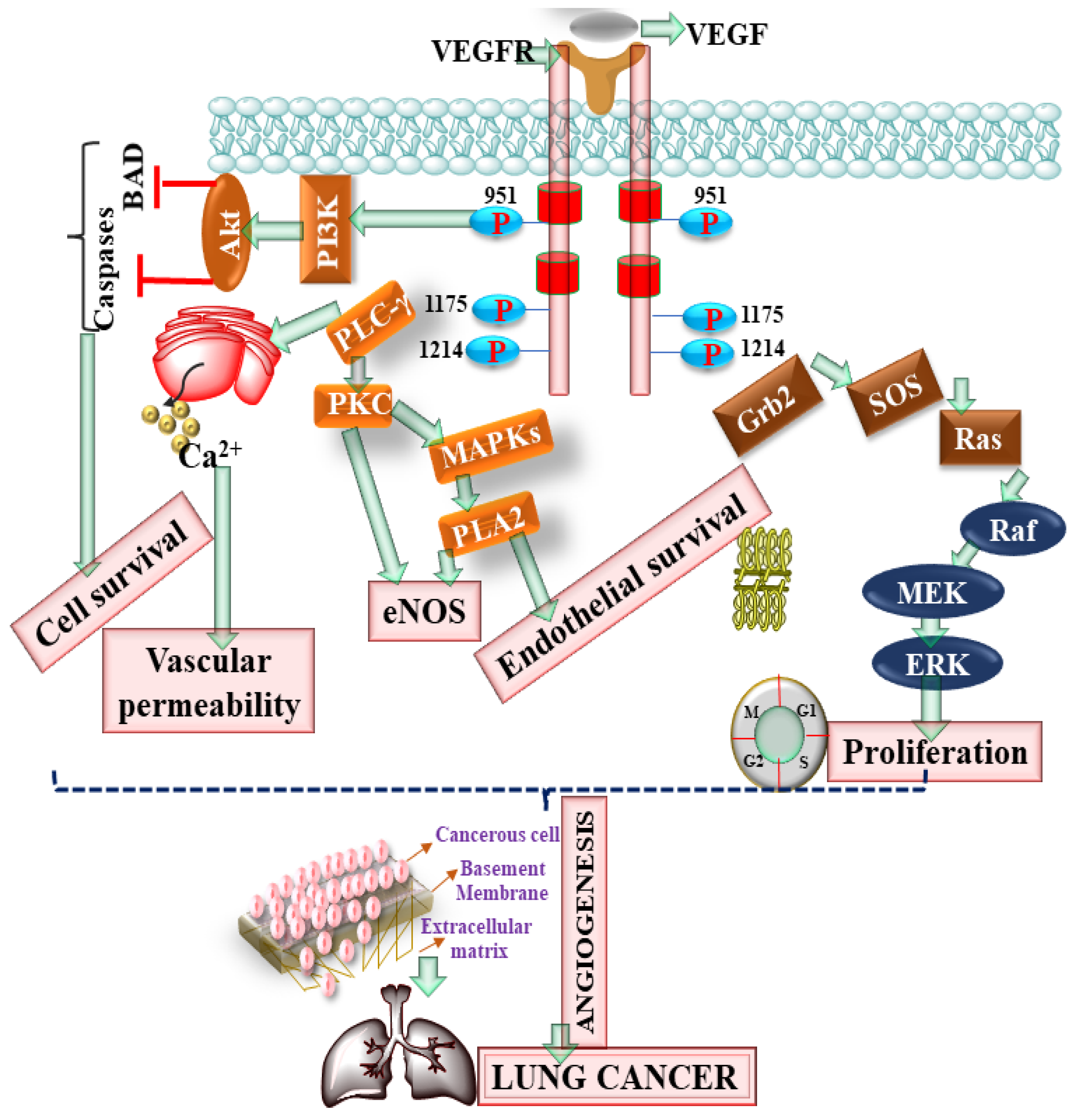
2.2. Vascular Endothelial Growth Factor (VEGF) Receptor Deregulation and VEGF Inhibitors in LC
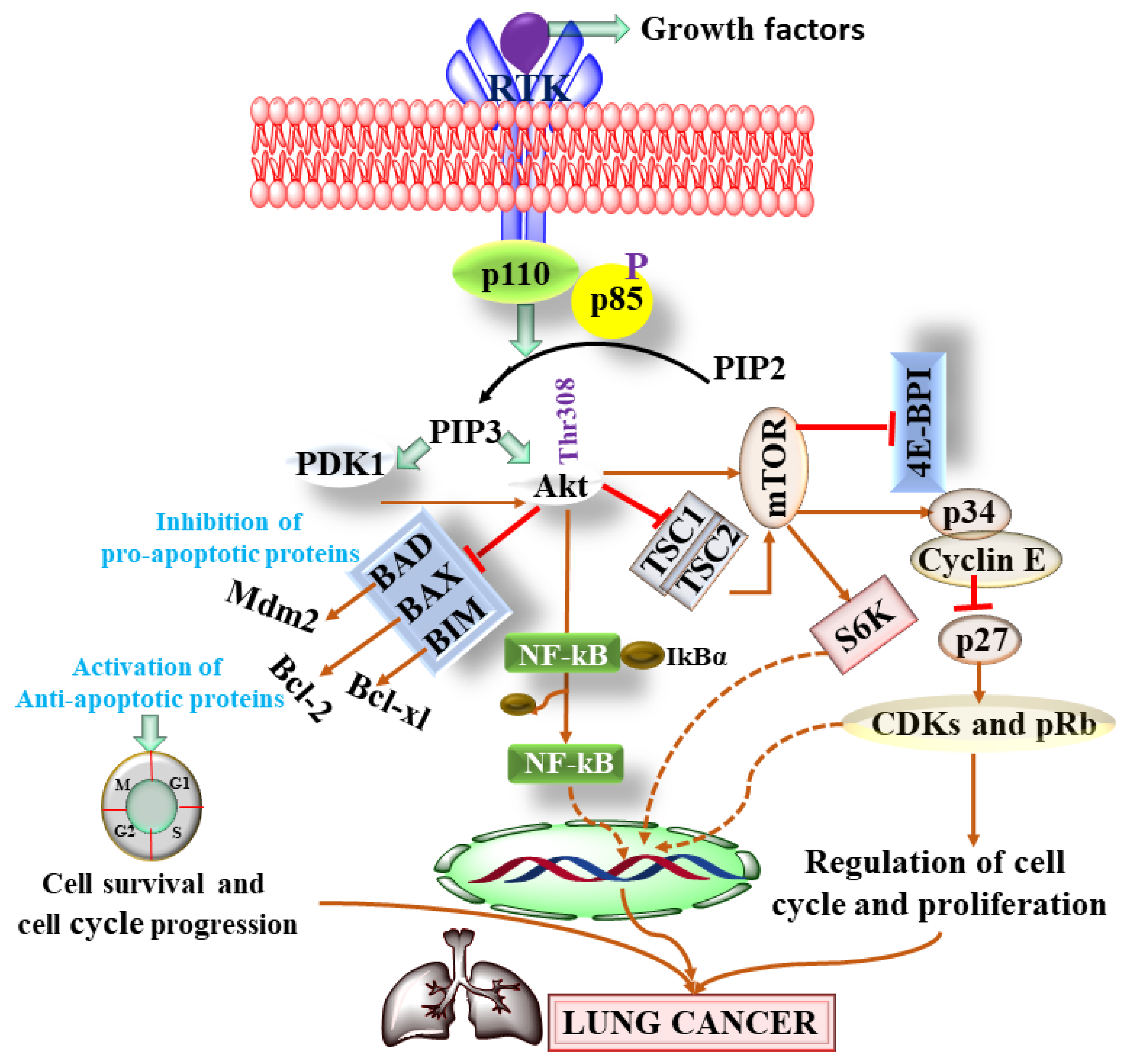
2.3. PI3K/AKT/mTOR Signaling Pathway and PI3K/AKT/mTOR Inhibitors in LC
2.4. p53, Bax/Bcl-2, Fas, and p16INK4/Cyclin D1/Rb Pathway Dysfunction and Their Inhibitors in LC
3. Limitations of the Approved and Pipeline Drugs of Lungs Cancer
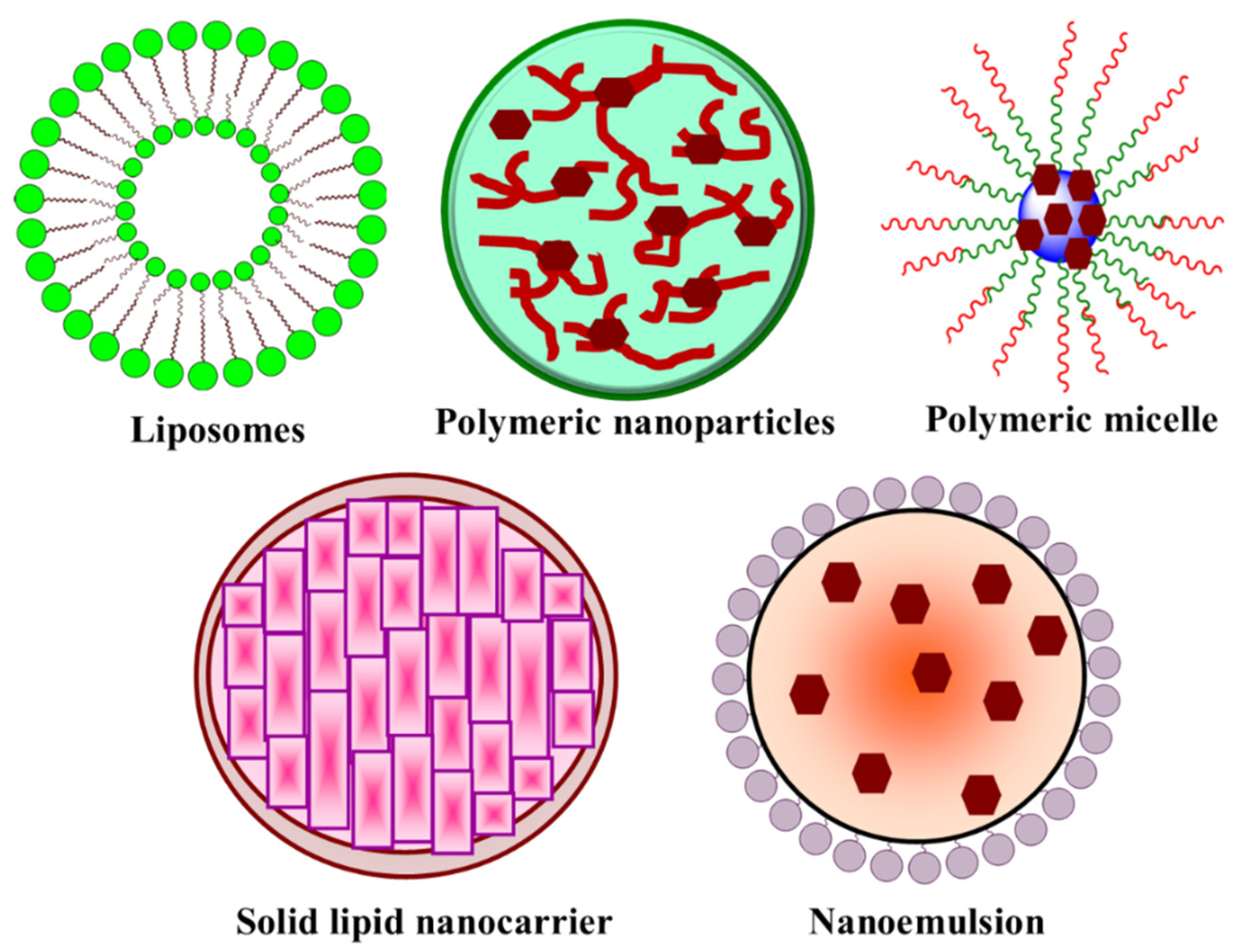
4. Nanocarrier-Based Targeted Drug Delivery in LC
4.1. Polymeric Nanoparticles
4.2. Liposome
4.3. Nanoemulsion
4.4. Polymeric Micelle
5. The Limitations of Nanocarrier Drug Delivery Systems and miRNA as Emerging Tools against Lung Cancer
6. miRNAs and Lung Cancer
6.1. Mechanism of miRNA Deregulation in Lung Cancer
6.2. Preclinical Based Evidence of miRNA in Lung Cancer
6.3. Translatory and Clinical-Based Evidence of miRNA in Lung Cancer
7. Challenges in Developing miRNA-Based Therapeutics
Nanocarrier-Based miRNA Delivery in Lung Cancer
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Jha, A.; Dr, M.; Mishra, B. Targeted drug nanocrystals for pulmonary delivery: A potential strategy for lung cancer therapy. Expert Opin. Drug Deliv. 2020, 17, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A., III; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef]
- Merewether, E.R.A.; Price, C. Report on Effects of Asbestos Dust on the Lungs and Dust Suppression in the Asbestos Industry. Part I. Occurrence of Pulmonary Pibrosis and Other Pulmonary Affections in Asbestos Workers; H.M.S.O.: London, UK, 1930.
- Cooke, W.E. Pulmonary asbestosis. Br. Med. J. 1927, 2, 1024. [Google Scholar] [CrossRef]
- Wood, W.B.; Gloyne, S.R. Pulmonary Asbestosis. Lancet 1930, 445–448. [Google Scholar] [CrossRef]
- Suraya, A.; Nowak, D.; Sulistomo, A.W.; Ghanie Icksan, A.; Syahruddin, E.; Berger, U.; Bose-O’Reilly, S. Asbestos-related lung cancer: A hospital-based case-control study in Indonesia. Int. J. Environ. Res. Public Health 2020, 17, 591. [Google Scholar] [CrossRef]
- Kwak, K.; Kang, D.; Paek, D. Environmental exposure to asbestos and the risk of lung cancer: A systematic review and meta-analysis. Occup. Environ. Med. 2021. [Google Scholar] [CrossRef]
- Cooper, W.A.; Lam, D.C.; O’Toole, S.A.; Minna, J.D. Molecular biology of lung cancer. J. Thorac. Dis. 2013, 5, S479. [Google Scholar]
- Su, D.; Ma, S.; Liu, P.; Jiang, Z.; Lv, W.; Zhang, Y.; Deng, Q.; Smith, S.; Yu, H. Genetic polymorphisms and treatment response in advanced non-small cell lung cancer. Lung Cancer 2007, 56, 281–288. [Google Scholar] [CrossRef]
- Swanton, C.; Govindan, R. Clinical implications of genomic discoveries in lung cancer. N. Engl. J. Med. 2016, 374, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Bauer, T.M.; Felip, E.; Besse, B.; James, L.P.; Clancy, J.S.; Klamerus, K.J.; Martini, J.-F.; Abbattista, A.; Shaw, A.T. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2016, 34, 9009. [Google Scholar] [CrossRef]
- Rosell, R.; Karachaliou, N.; Arrieta, O. Novel molecular targets for the treatment of lung cancer. Curr. Opin. Oncol. 2020, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Alexander, R.E.; MacLennan, G.T.; Cummings, O.W.; Montironi, R.; Lopez-Beltran, A.; Cramer, H.M.; Davidson, D.D.; Zhang, S. Molecular pathology of lung cancer: Key to personalized medicine. Mod. Pathol. 2012, 25, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Gazdar, A. Pathogenesis of lung cancer signalling pathways: Roadmap for therapies. Eur. Respir. J. 2009, 33, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Tumbrink, H.L.; Heimsoeth, A.; Sos, M.L. The next tier of EGFR resistance mutations in lung cancer. Oncogene 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Gupta, R.; Dastane, A.M.; Forozan, F.; Riley-Portuguez, A.; Chung, F.; Lopategui, J.; Marchevsky, A.M. Evaluation of EGFR abnormalities in patients with pulmonary adenocarcinoma: The need to test neoplasms with more than one method. Mod. Pathol. 2009, 22, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Robinet, G.; Szczesna, A.; Ramlau, R.; Constenla, M.; Mennecier, B.; Pfeifer, W.; O’Byrne, K.J.; Welte, T.; Kolb, R. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann. Oncol. 2008, 19, 362–369. [Google Scholar] [CrossRef]
- Langer, C.J. Emerging role of epidermal growth factor receptor inhibition in therapy for advanced malignancy: Focus on NSCLC. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 991–1002. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Gridelli, C.; Ciardiello, F.; Gallo, C.; Feld, R.; Butts, C.; Gebbia, V.; Maione, P.; Morgillo, F.; Genestreti, G.; Favaretto, A. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: The TORCH randomized trial. J. Clin. Oncol. 2012, 30, 3002–3011. [Google Scholar] [CrossRef]
- Ciuleanu, T.; Stelmakh, L.; Cicenas, S.; Miliauskas, S.; Grigorescu, A.C.; Hillenbach, C.; Johannsdottir, H.K.; Klughammer, B.; Gonzalez, E.E. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): A randomised multicentre, open-label, phase 3 study. Lancet Oncol. 2012, 13, 300–308. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, S.; Song, X.; Han, B.; Cheng, Y.; Huang, C.; Yang, S.; Liu, X.; Liu, Y.; Lu, S. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): A multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012, 13, 466–475. [Google Scholar] [CrossRef]
- Pirker, R.; Pereira, J.R.; Von Pawel, J.; Krzakowski, M.; Ramlau, R.; Park, K.; De Marinis, F.; Eberhardt, W.E.; Paz-Ares, L.; Störkel, S. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: Analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012, 13, 33–42. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J. Clin. Med. 2020, 9, 84. [Google Scholar] [CrossRef]
- Altorki, N.; Lane, M.E.; Bauer, T.; Lee, P.C.; Guarino, M.J.; Pass, H.; Felip, E.; Peylan-Ramu, N.; Gurpide, A.; Grannis, F.W. Phase II proof-of-concept study of pazopanib monotherapy in treatment-naive patients with stage I/II resectable non–small-cell lung cancer. J. Clin. Oncol. 2010, 28, 3131–3137. [Google Scholar] [CrossRef]
- Lee, J.S.; Hirsh, V.; Park, K.; Qin, S.; Blajman, C.R.; Perng, R.-P.; Chen, Y.-M.; Emerson, L.; Langmuir, P.; Manegold, C. Vandetanib versus placebo in patients with advanced non–small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: A randomized, double-blind phase III trial (ZEPHYR). J. Clin. Oncol. 2012, 30, 1114–1121. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.-P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Dahlberg, S.; Sandler, A.; Brahmer, J.; Schiller, J.; Johnson, D. Clinical course of advanced non-small cell lung cancer (NSCLC) patients (pts) experiencing hypertension (HTN) during treatment (TX) with bevacizumab (B) in combination with carboplatin (C) and paclitaxel (P) on E4599. J. Clin. Oncol. 2009, 27, 8042. [Google Scholar] [CrossRef]
- Herbst, R.S.; Ansari, R.; Bustin, F.; Flynn, P.; Hart, L.; Otterson, G.A.; Vlahovic, G.; Soh, C.-H.; O’Connor, P.; Hainsworth, J. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. Lancet 2011, 377, 1846–1854. [Google Scholar] [CrossRef]
- Reck, M.; von Pawel, J.; Zatloukal, P.; Ramlau, R.; Gorbounova, V.; Hirsh, V.; Leighl, N.; Mezger, J.; Archer, V.; Moore, N. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer: AVAiL. J. Clin. Oncol. 2009, 27, 1227–1234. [Google Scholar] [CrossRef]
- Ray, M.R.; Jablons, D.; He, B. Lung cancer therapeutics that target signaling pathways: An update. Expert Rev. Respir. Med. 2010, 4, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non–small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1315–1326. [Google Scholar] [CrossRef]
- Tsurutani, J.; Fukuoka, J.; Tsurutani, H.; Shih, J.H.; Hewitt, S.M.; Travis, W.D.; Jen, J.; Dennis, P.A. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non–small-cell lung cancer tumors. J. Clin. Oncol. 2006, 24, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Shcherba, M.; Pendurti, G.; Liang, Y.; Piperdi, B.; Perez-Soler, R. Targeting the PI3K/AKT/mTOR pathway: Potential for lung cancer treatment. Lung Cancer Manag. 2014, 3, 67–75. [Google Scholar] [CrossRef]
- Kim, L.C.; Cook, R.S.; Chen, J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 2017, 36, 2191–2201. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Wozniak, A. Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non–small-cell lung cancer. Clin. Lung Cancer 2013, 14, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Brambilla, C. p53 and lung cancer. Pathologie-Biologie 1997, 45, 852–863. [Google Scholar]
- Zhao, H.; Yang, B.; Xu, J.; Chen, D.-m.; Xiao, C.-l. PM2. 5-induced alterations of cell cycle associated gene expression in lung cancer cells and rat lung tissues. Environ. Toxicol. Pharmacol. 2017, 52, 77–82. [Google Scholar] [CrossRef]
- Rasheduzzaman, M.; Jeong, J.-K.; Park, S.-Y. Resveratrol sensitizes lung cancer cell to TRAIL by p53 independent and suppression of Akt/NF-κB signaling. Life Sci. 2018, 208, 208–220. [Google Scholar] [CrossRef]
- Giacomini, A.; Taranto, S.; Rezzola, S.; Matarazzo, S.; Grillo, E.; Bugatti, M.; Scotuzzi, A.; Guerra, J.; Di Trani, M.; Presta, M. Inhibition of the FGF/FGFR system induces apoptosis in lung cancer cells via c-Myc downregulation and oxidative stress. Int. J. Mol. Sci. 2020, 21, 9376. [Google Scholar] [CrossRef] [PubMed]
- Shivapurkar, N.; Reddy, J.; Chaudhary, P.M.; Gazdar, A.F. Apoptosis and lung cancer: A review. J. Cell. Biochem. 2003, 88, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, M.; Amini, R.; Amri, J.; Karami, H. Gene Therapy with MiRNA-Mediated Targeting of Mcl-1 Promotes the Sensitivity of Non-Small Cell Lung Cancer Cells to Treatment with ABT-737. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 675. [Google Scholar] [CrossRef]
- Cooper, S.; Spiro, S.G. Small cell lung cancer: Treatment review. Respirology 2006, 11, 241–248. [Google Scholar] [CrossRef]
- Amararathna, M.; Goralski, K.; Hoskin, D.W.; Rupasinghe, H.V. Pulmonary nano-drug delivery systems for lung cancer: Current knowledge and prospects. J. Lung Health Dis. 2019, 3, 11–28. [Google Scholar] [CrossRef]
- Biswas, B.; Ghadyalpatil, N.; Krishna, M.; Deshmukh, J. A review on adverse event profiles of epidermal growth factor receptor-tyrosine kinase inhibitors in nonsmall cell lung cancer patients. Indian J. Cancer 2017, 54, 55. [Google Scholar] [CrossRef]
- Guan, M.; Zhou, Y.-P.; Sun, J.-L.; Chen, S.-C. Adverse events of monoclonal antibodies used for cancer therapy. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Pandey, M.; Gorain, B.; Chatterjee, B.; Madheswaran, T.; Md, S.; Mak, K.-K.; Tambuwala, M.; Chourasia, M.K.; Kesharwani, P. Nanoemulsions as effective carriers for the treatment of lung cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–247. [Google Scholar]
- Sharma, P.; Mehta, M.; Dhanjal, D.S.; Kaur, S.; Gupta, G.; Singh, H.; Thangavelu, L.; Rajeshkumar, S.; Tambuwala, M.; Bakshi, H.A. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem.-Biol. Interact. 2019, 309, 108720. [Google Scholar] [CrossRef]
- Iqubal, A.; Syed, M.A.; Najmi, A.K.; Azam, F.; Barreto, G.E.; Iqubal, M.K.; Ali, J.; Haque, S.E. Nano-engineered nerolidol loaded lipid carrier delivery system attenuates cyclophosphamide neurotoxicity–Probable role of NLRP3 inflammasome and caspase-1. Exp. Neurol. 2020, 334, 113464. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, S.-J.; Chung, H.K.; Kang, H.-W.; Lee, S.-W.; Seo, M.H.; Park, H.J.; Song, S.Y.; Jeong, S.-Y.; Choi, E.K. Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e77–e83. [Google Scholar] [CrossRef] [PubMed]
- Ray, L. Polymeric Nanoparticle-Based Drug/Gene Delivery for Lung Cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–93. [Google Scholar]
- Kim, D.-W.; Kim, S.-Y.; Kim, H.-K.; Kim, S.-W.; Shin, S.; Kim, J.; Park, K.; Lee, M.; Heo, D.S. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2007, 18, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Jung, M.; Sym, S.J.; Shin, D.B.; Kang, S.M.; Kyung, S.Y.; Park, J.-W.; Jeong, S.H.; Cho, E.K. A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 2014, 74, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.; Nagarwal, R.C.; Pandit, J.K. Lomustine loaded chitosan nanoparticles: Characterization and in-vitro cytotoxicity on human lung cancer cell line L132. Chem. Pharm. Bull. 2011, 59, 315–320. [Google Scholar] [CrossRef]
- Nafee, N.; Schneider, M.; Friebel, K.; Dong, M.; Schaefer, U.; Mürdter, T.; Lehr, C.-M. Treatment of lung cancer via telomerase inhibition: Self-assembled nanoplexes versus polymeric nanoparticles as vectors for 2′-O-Methyl-RNA. Eur. J. Pharm. Biopharm. 2012, 80, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Al-Hallak, K.M.; Azarmi, S.; Anwar-Mohamed, A.; Roa, W.H.; Löbenberg, R. Secondary cytotoxicity mediated by alveolar macrophages: A contribution to the total efficacy of nanoparticles in lung cancer therapy? Eur. J. Pharm. Biopharm. 2010, 76, 112–119. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Bravo-Caparrós, I.; Cabeza, L.; Nieto, F.R.; Ortiz, R.; Perazzoli, G.; Fernández-Segura, E.; Cañizares, F.J.; Baeyens, J.M.; Melguizo, C. Paclitaxel antitumor effect improvement in lung cancer and prevention of the painful neuropathy using large pegylated cationic liposomes. Biomed. Pharmacother. 2021, 133, 111059. [Google Scholar] [CrossRef]
- Hussain, S. Nanomedicine for treatment of lung cancer. Lung Cancer Pers. Med.: Novel Ther. Clin. Manag. 2016, 890, 137–147. [Google Scholar]
- Garbuzenko, O.B.; Saad, M.; Pozharov, V.P.; Reuhl, K.R.; Mainelis, G.; Minko, T. Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc. Natl. Acad. Sci. USA 2010, 107, 10737–10742. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-Q.; Chen, M.-S.; Zhou, X.; Guo, L.-M.; Zhu, J.-J.; Wang, R.; Zhang, X.-X.; Gan, Y. Chitosan oligosaccharide modified liposomes enhance lung cancer delivery of paclitaxel. Acta Pharmacol. Sin. 2021, 42, 1714–1722. [Google Scholar] [CrossRef]
- Miyoshi, T.; Misumi, N.; Hiraike, M.; Mihara, Y.; Nishino, T.; Tsuruta, M.; Kawamata, Y.; Hiraki, Y.; Kozono, A.; Ichiki, M. Risk factors associated with cisplatin-induced nephrotoxicity in patients with advanced lung cancer. Biol. Pharm. Bull. 2016, 39, 2009–2014. [Google Scholar] [CrossRef]
- Choradiya, B.R.; Patil, S.B. A Comprehensive Review on Nanoemulsion as an Ophthalmic Drug Delivery System. J. Mol. Liq. 2021, 339, 116751. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Câmara, A.L.; Nagel, G.; Tschiche, H.R.; Cardador, C.M.; Muehlmann, L.A.; de Oliveira, D.M.; Alvim, P.Q.; Azevedo, R.B.; Calderón, M.; Figueiro Longo, J.P. Acid-sensitive lipidated doxorubicin prodrug entrapped in nanoemulsion impairs lung tumor metastasis in a breast cancer model. Nanomedicine 2017, 12, 1751–1765. [Google Scholar] [CrossRef]
- Kim, J.-E.; Park, Y.-J. Improved antitumor efficacy of hyaluronic acid-complexed paclitaxel nanoemulsions in treating non-small cell lung cancer. Biomol. Ther. 2017, 25, 411. [Google Scholar] [CrossRef]
- Li, X.; Du, L.; Wang, C.; Liu, Y.; Mei, X.; Jin, Y. Highly efficient and lowly toxic docetaxel nanoemulsions for intravenous injection to animals. Die Pharm. 2011, 66, 479–483. [Google Scholar]
- Wan, K.; Sun, L.; Hu, X.; Yan, Z.; Zhang, Y.; Zhang, X.; Zhang, J. Novel nanoemulsion based lipid nanosystems for favorable in vitro and in vivo characteristics of curcumin. Int. J. Pharm. 2016, 504, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lev-Ari, S.; Starr, A.; Katzburg, S.; Berkovich, L.; Rimmon, A.; Ben-Yosef, R.; Vexler, A.; Ron, I.; Earon, G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J. Nutr. Biochem. 2014, 25, 843–850. [Google Scholar] [CrossRef]
- Fan, Z.; Duan, X.; Cai, H.; Wang, L.; Li, M.; Qu, J.; Li, W.; Wang, Y.; Wang, J. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol. Rep. 2015, 34, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wan, K.; Hu, X.; Zhang, Y.; Yan, Z.; Feng, J.; Zhang, J. Functional nanoemulsion-hybrid lipid nanocarriers enhance the bioavailability and anti-cancer activity of lipophilic diferuloylmethane. Nanotechnology 2016, 27, 085102. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Kaur, K.; Pandey, R.S.; Jain, U.K.; Chandra, R.; Madan, J. Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: In vitro and in vivo studies. J. Colloid Interface Sci. 2015, 445, 219–230. [Google Scholar] [CrossRef]
- Arbain, N.A.N.; Basri, M.; Salim, N.; Wui, W.; Rahman, M.A. Aerosolized nanoemulsion system encapsulating quercertin for lung cancer treatment. Mater. Today Proc. 2018, 5, S137–S142. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, H.; Gong, T.; Zhang, Z. Nanoemulsion loaded with lycobetaine–oleic acid ionic complex: Physicochemical characteristics, in vitro, in vivo evaluation, and antitumor activity. Int. J. Nanomed. 2013, 8, 1959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.-H.; Jiang, C.; Lü, J. Tanshinones: Sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef]
- Lee, W.Y.; Liu, K.W.; Yeung, J.H. Reactive oxygen species-mediated kinase activation by dihydrotanshinone in tanshinones-induced apoptosis in HepG2 cells. Cancer Lett. 2009, 285, 46–57. [Google Scholar] [CrossRef]
- Lee, W.; Liang, Y.; Chen, B. Effects of tanshinone nanoemulsion and extract on inhibition of lung cancer cells A549. Nanotechnology 2016, 27, 495101. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-t.; Mu, L.-Q.; Dai, W.; Wang, C.-b.; Liu, X.-Y.; Xiang, D.-X. Preparation, characterization, and evaluation of antitumor effect of Brucea javanica oil cationic nanoemulsions. Int. J. Nanomed. 2016, 11, 2515. [Google Scholar] [CrossRef]
- Bahman, F.; Elkaissi, S.; Greish, K.; Taurin, S. Polymeric Micelles in Management of Lung Cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–216. [Google Scholar]
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef]
- Maeda, H.; Ueda, M.; Morinaga, T.; Matsumoto, T. Conjugation of poly (styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: Pronounced improvements in pharmacological properties. J. Med. Chem. 1985, 28, 455–461. [Google Scholar] [CrossRef]
- Dalela, M.; Shrivastav, T.; Kharbanda, S.; Singh, H. pH-sensitive biocompatible nanoparticles of paclitaxel-conjugated poly (styrene-co-maleic acid) for anticancer drug delivery in solid tumors of syngeneic mice. ACS Appl. Mater. Interfaces 2015, 7, 26530–26548. [Google Scholar] [CrossRef]
- Yang, Z.L.; Li, X.R.; Yang, K.W.; Liu, Y. Amphotericin B-loaded poly (ethylene glycol)–poly (lactide) micelles: Preparation, freeze-drying, and in vitro release. J. Biomed. Mater. Res. Part A 2008, 85, 539–546. [Google Scholar] [CrossRef]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol–gels for drug delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent advances in PEG–PLA block copolymer nanoparticles. Int. J. Nanomed. 2010, 5, 1057. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Wu, Y.; Hu, Y.-l.; Nan, K.; Nie, G.; Chen, H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 2011, 32, 8281–8290. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Min, Y.; Rodgers, Z.; Au, K.M.; Hagan, C.T.; Zhang, M.; Roche, K.; Yang, F.; Wagner, K.; Wang, A.Z. Co-delivery of paclitaxel and cisplatin with biocompatible PLGA–PEG nanoparticles enhances chemoradiotherapy in non-small cell lung cancer models. J. Mater. Chem. B 2017, 5, 6049–6057. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Wong, S.W.; Yeo, H.L.; Zhao, Y. Nanocarriers for cancer treatment: Clinical impact and safety. NanoImpact 2020, 20, 100253. [Google Scholar] [CrossRef]
- James, N.; Coker, R.; Tomlinson, D.; Harris, J.; Gompels, M.; Pinching, A.; Stewart, J. Liposomal doxorubicin (Doxil): An effective new treatment for Kaposi’s sarcoma in AIDS. Clin. Oncol. 1994, 6, 294–296. [Google Scholar] [CrossRef]
- Uddin, A.; Chakraborty, S. Role of miRNAs in lung cancer. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef]
- Xia, H.; Wu, J.; Li, M. MiRNA Identification, Characterization and Integrated Network Analysis for Flavonoid Biosynthesis in Brassicacoraphanus. Hortic. Plant J. 2021. [Google Scholar] [CrossRef]
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in cancer. Ann. Rev. Pathol.: Mech. Dis. 2014, 9, 287–314. [Google Scholar] [CrossRef]
- Lin, P.; Yu, S.; Yang, P. MicroRNA in lung cancer. Br. J. Cancer 2010, 103, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Arora, S.; Prakasam, G.; Calin, G.A.; Syed, M.A. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Asp. Med. 2019, 70, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hsu, S.h.; Wang, X.; Kutay, H.; Bid, H.K.; Yu, J.; Ganju, R.K.; Jacob, S.T.; Yuneva, M.; Ghoshal, K. Reciprocal regulation of microRNA-122 and c-Myc in hepatocellular cancer: Role of E2F1 and transcription factor dimerization partner 2. Hepatology 2014, 59, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2009, 17, 193–199. [Google Scholar] [CrossRef]
- Suzuki, H.; Maruyama, R.; Yamamoto, E.; Kai, M. Epigenetic alteration and microRNA dysregulation in cancer. Front. Genet. 2013, 4, 258. [Google Scholar] [CrossRef]
- Fazi, F.; Racanicchi, S.; Zardo, G.; Starnes, L.M.; Mancini, M.; Travaglini, L.; Diverio, D.; Ammatuna, E.; Cimino, G.; Lo-Coco, F. Epigenetic silencing of the myelopoiesis regulator microRNA-223 by the AML1/ETO oncoprotein. Cancer Cell 2007, 12, 457–466. [Google Scholar] [CrossRef]
- Saito, Y.; Liang, G.; Egger, G.; Friedman, J.M.; Chuang, J.C.; Coetzee, G.A.; Jones, P.A. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 2006, 9, 435–443. [Google Scholar] [CrossRef]
- Lujambio, A.; Calin, G.A.; Villanueva, A.; Ropero, S.; Sánchez-Céspedes, M.; Blanco, D.; Montuenga, L.M.; Rossi, S.; Nicoloso, M.S.; Faller, W.J. A microRNA DNA methylation signature for human cancer metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13556–13561. [Google Scholar] [CrossRef]
- Wu, Y.; Crawford, M.; Mao, Y.; Lee, R.J.; Davis, I.C.; Elton, T.S.; Lee, L.J.; Nana-Sinkam, S.P. Therapeutic delivery of microRNA-29b by cationic lipoplexes for lung cancer. Mol. Ther.-Nucleic Acids 2013, 2, e84. [Google Scholar] [CrossRef]
- Ye, Z.; Yin, S.; Su, Z.; Bai, M.; Zhang, H.; Hei, Z.; Cai, S. Downregulation of miR-101 contributes to epithelial-mesenchymal transition in cisplatin resistance of NSCLC cells by targeting ROCK2. Oncotarget 2016, 7, 37524. [Google Scholar] [CrossRef]
- Jin, Z.; Guan, L.; Song, Y.; Xiang, G.; Chen, S.; Gao, B. MicroRNA-138 regulates chemoresistance in human non-small cell lung cancer via epithelial mesenchymal transition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1080–1086. [Google Scholar] [PubMed]
- Nishijima, N.; Seike, M.; Soeno, C.; Chiba, M.; Miyanaga, A.; Noro, R.; Sugano, T.; Matsumoto, M.; Kubota, K.; Gemma, A. miR-200/ZEB axis regulates sensitivity to nintedanib in non-small cell lung cancer cells. Int. J. Oncol. 2016, 48, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yang, J.; Luo, M.; Cho, W.C.; Liu, X. MicroRNA-targeted therapeutics for lung cancer treatment. Expert Opin. Drug Deliv. 2017, 12, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-T.; Xu, M.; Xu, C.-X.; Song, Z.-G.; Jin, H. Decreased expression of miR216a contributes to non–small-cell lung cancer progression. Clin. Cancer Res. 2014, 20, 4705–4716. [Google Scholar] [CrossRef]
- Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Patrawala, L.; Brown, D.; Bader, A.G. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010, 70, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.; Tzankov, A.; Flori, M.; Schmid, C.; Bader, A.; Müller, A. Systemic microRNA-34a delivery induces apoptosis and abrogates growth of diffuse large B-cell lymphoma in vivo. Leukemia 2012, 26, 2421–2424. [Google Scholar] [CrossRef]
- Quemener, A.M.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M.D. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip. Rev. RNA 2020, 11, e1594. [Google Scholar] [CrossRef]
- Shi, S.-J.; Zhong, Z.-R.; Liu, J.; Zhang, Z.-R.; Sun, X.; Gong, T. Solid lipid nanoparticles loaded with anti-microRNA oligonucleotides (AMOs) for suppression of microRNA-21 functions in human lung cancer cells. Pharm. Res. 2012, 29, 97–109. [Google Scholar] [CrossRef]
- Vester, B.; Wengel, J. LNA (locked nucleic acid): High-affinity targeting of complementary RNA and DNA. Biochemistry 2004, 43, 13233–13241. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C. MicroRNAs as therapeutic targets for lung cancer. Expert Opin. Ther. Targets 2010, 14, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Trang, P.; Medina, P.P.; Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Homer, R.; Brown, D.; Bader, A.G.; Weidhaas, J.B. Regression of murine lung tumors by the let-7 microRNA. Oncogene 2010, 29, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Nakagawa, Y.; Naoe, T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol. Pharm. Bull. 2006, 29, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Monroig-Bosque, P.d.C.; Rivera, C.A.; Calin, G.A. MicroRNAs in cancer therapeutics:“from the bench to the bedside”. Expert Opin. Biol. Ther. 2015, 15, 1381–1385. [Google Scholar] [CrossRef]
- Bansal, P.; Christopher, A.; Kaur, R.; Kaur, G.; Kaur, A.; Gupta, V. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef]
- Elmen, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.F.; Berger, U.; et al. Kauppinen S2008 LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef]
- Lindow, M.; Kauppinen, S. Discovering the first microRNA-targeted drug. J. Cell Biol. 2012, 199, 407–412. [Google Scholar] [CrossRef]
- Beg, M.S.; Brenner, A.; Sachdev, J.; Ejadi, S.; Borad, M.; Kang, Y.-K.; Lim, H.; Kim, T.; Bader, A.; Stoudemire, J. Abstract C43: Safety, tolerability, and clinical activity of MRX34, the first-in-class liposomal miR-34 mimic, in patients with advanced solid tumors. Clin. Trials 2015, 14, C43. [Google Scholar]
- Hong, D.S.; Kang, Y.-K.; Brenner, A.J.; Sachdev, J.C.; Ejadi, S.; Borad, M.J.; Kim, T.-Y.; Lim, H.Y.; Park, K.; Becerra, C. MRX34, a liposomal miR-34 mimic, in patients with advanced solid tumors: Final dose-escalation results from a first-in-human phase I trial of microRNA therapy. J. Clin. Oncol. 2016, 34, 2508. [Google Scholar] [CrossRef]
- Mollaei, H.; Safaralizadeh, R.; Rostami, Z. MicroRNA replacement therapy in cancer. J. Cell. Physiol. 2019, 234, 12369–12384. [Google Scholar] [CrossRef]
- Segal, M.; Slack, F.J. Challenges Identifying Efficacious miRNA Therapeutics for Cancer; Taylor & Francis: Abingdon, UK, 2020. [Google Scholar]
- Kumar, V.; Mundra, V.; Peng, Y.; Wang, Y.; Tan, C.; Mahato, R.I. Pharmacokinetics and biodistribution of polymeric micelles containing miRNA and small-molecule drug in orthotopic pancreatic tumor-bearing mice. Theranostics 2018, 8, 4033. [Google Scholar] [CrossRef]
- Lu, L.F.; Liston, A. MicroRNA in the immune system, microRNA as an immune system. Immunology 2009, 127, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Ganju, A.; Khan, S.; Hafeez, B.B.; Behrman, S.W.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. miRNA nanotherapeutics for cancer. Drug Discov. Today 2017, 22, 424–432. [Google Scholar] [CrossRef]
- Wu, Y.; Crawford, M.; Yu, B.; Mao, Y.; Nana-Sinkam, S.P.; Lee, L.J. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol. Pharm. 2011, 8, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Uchida, S.; Nagatoishi, S.; Koji, K.; Hong, T.; Fukushima, S.; Tsumoto, K.; Ishihara, K.; Kataoka, K.; Cabral, H. Polymeric nanocarriers with controlled chain flexibility boost mRNA delivery in vivo through enhanced structural fastening. Adv. Healthc. Mater. 2020, 9, 2000538. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Weirauch, U.; Thomas, M.; Grünweller, A.; Hartmann, R.K.; Aigner, A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011, 71, 5214–5224. [Google Scholar] [CrossRef]
- Chiou, G.-Y.; Cherng, J.-Y.; Hsu, H.-S.; Wang, M.-L.; Tsai, C.-M.; Lu, K.-H.; Chien, Y.; Hung, S.-C.; Chen, Y.-W.; Wong, C.-I. Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial–mesenchymal transdifferentiation and cancer stem-like properties and in lung adenocarcinoma. J. Control. Release 2012, 159, 240–250. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, X.; He, D.; Hsieh, J.-T. A prostate cancer-targeted polyarginine-disulfide linked PEI nanocarrier for delivery of microRNA. Cancer Lett. 2015, 365, 156–165. [Google Scholar] [CrossRef]
- Gao, S.; Tian, H.; Guo, Y.; Li, Y.; Guo, Z.; Zhu, X.; Chen, X. miRNA oligonucleotide and sponge for miRNA-21 inhibition mediated by PEI-PLL in breast cancer therapy. Acta Biomater. 2015, 25, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wen, J.; Meng, Y.; Zhang, K.; Zhu, J.; Ren, Y.; Qian, X.; Yuan, X.; Lu, Y.; Kang, C. Efficient delivery of therapeutic miRNA nanocapsules for tumor suppression. Adv. Mater. 2015, 27, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Piñeiro, I.; Badiola, I.; Sanchez, A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnol. Adv. 2017, 35, 350–360. [Google Scholar] [CrossRef]
- Mittal, A.; Chitkara, D.; Behrman, S.W.; Mahato, R.I. Efficacy of gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials 2014, 35, 7077–7087. [Google Scholar] [CrossRef]
- Liu, Q.; Li, R.-T.; Qian, H.-Q.; Wei, J.; Xie, L.; Shen, J.; Yang, M.; Qian, X.-P.; Yu, L.-X.; Jiang, X.-Q. Targeted delivery of miR-200c/DOC to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles. Biomaterials 2013, 34, 7191–7203. [Google Scholar] [CrossRef]
- Cheng, C.J.; Saltzman, W.M. Polymer nanoparticle-mediated delivery of microRNA inhibition and alternative splicing. Mol. Pharm. 2012, 9, 1481–1488. [Google Scholar] [CrossRef]
- Li, F.; Wang, F.; Zhu, C.; Wei, Q.; Zhang, T.; Zhou, Y.L. miR-221 suppression through nanoparticle-based miRNA delivery system for hepatocellular carcinoma therapy and its diagnosis as a potential biomarker. Int. J. Nanomed. 2018, 13, 2295. [Google Scholar] [CrossRef]
- Devulapally, R.; Sekar, N.M.; Sekar, T.V.; Foygel, K.; Massoud, T.F.; Willmann, J.R.K.; Paulmurugan, R. Polymer nanoparticles mediated codelivery of antimiR-10b and antimiR-21 for achieving triple negative breast cancer therapy. ACS Nano 2015, 9, 2290–2302. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Wang, Y.; Chen, M. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomed.: Nanotechnol. Biol. Med. 2016, 12, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhou, X.; Mei, M.; Yuan, X.-B.; Han, L.; Wang, G.-X.; Jia, Z.-F.; Xu, P.; Pu, P.-Y.; Kang, C.-S. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer 2010, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Oliva, N.; Atilano, M.; Song, H.S.; Artzi, N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nat. Mater. 2016, 15, 353–363. [Google Scholar] [CrossRef]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Santos-Carballal, B.; Aaldering, L.; Ritzefeld, M.; Pereira, S.; Sewald, N.; Moerschbacher, B.; Götte, M.; Goycoolea, F.M. Physicochemical and biological characterization of chitosan-microRNA nanocomplexes for gene delivery to MCF-7 breast cancer cells. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, M.; Deng, X.; Xiao, X.; Yin, Z.; Hu, Q.; Zhou, Z.; Zhang, F.; Zhang, R.; Wu, Y. Degradable Hyaluronic Acid/Protamine Sulfate Interpolyelectrolyte Complexes as miRNA-Delivery Nanocapsules for Triple-Negative Breast Cancer Therapy. Adv. Healthc. Mater. 2015, 4, 281–290. [Google Scholar] [CrossRef]
- Hao, Z.; Fan, W.; Hao, J.; Wu, X.; Zeng, G.Q.; Zhang, L.J.; Nie, S.F.; Wang, X.D. Efficient delivery of micro RNA to bone-metastatic prostate tumors by using aptamer-conjugated atelocollagen in vitro and in vivo. Drug Deliv. 2016, 23, 864–871. [Google Scholar] [CrossRef]
- Kurmi, B.D.; Kayat, J.; Gajbhiye, V.; Tekade, R.K.; Jain, N.K. Micro-and nanocarrier-mediated lung targeting. Expert Opin. Drug Deliv. 2010, 7, 781–794. [Google Scholar] [CrossRef]
- Piao, L.; Zhang, M.; Datta, J.; Xie, X.; Su, T.; Li, H.; Teknos, T.N.; Pan, Q. Lipid-based nanoparticle delivery of Pre-miR-107 inhibits the tumorigenicity of head and neck squamous cell carcinoma. Mol. Ther. 2012, 20, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Decastro, M.; Saijoh, Y.; Schoenwolf, G.C. Optimized cationic lipid-based gene delivery reagents for use in developing vertebrate embryos. Dev. Dyn. 2006, 235, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Bikram, M.; Lee, M.; Chang, C.-W.; Janát-Amsbury, M.-M.; Kern, S.E.; Kim, S.W. Long-circulating DNA-complexed biodegradable multiblock copolymers for gene delivery: Degradation profiles and evidence of dysopsonization. J. Control. Release 2005, 103, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, F.; Ma, J.-l.; Sun, W.-z.; Song, L.-p. The potential clinical applications and prospects of microRNAs in lung cancer. OncoTargets Ther. 2014, 7, 901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peng, J.; Wang, R.; Sun, W.; Huang, M.; Wang, R.; Li, Y.; Wang, P.; Sun, G.; Xie, S. Delivery of miR-320a-3p by gold nanoparticles combined with photothermal therapy for directly targeting Sp1 in lung cancer. Biomater. Sci. 2021, 9, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Singh, L.C.; Shohet, J.M.; Gunaratne, P.H. A gold nanoparticle platform for the delivery of functional microRNAs into cancer cells. Biomaterials 2013, 34, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Ekin, A.; Karatas, O.F.; Culha, M.; Ozen, M. Designing a gold nanoparticle-based nanocarrier for microRNA transfection into the prostate and breast cancer cells. J. Gene Med. 2014, 16, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ni, D.; Teng, J.; Cheng, Y.; Zhuang, B.; Yang, Z. Folic Acid-Conjugated Mesoporous Silica Nanoparticles and MicroRNA-128-3p Combined with Adriamycin Alleviates the Progression of Non-Small Cell Lung Cancer. J. Biomater. Tissue Eng. 2021, 11, 1714–1721. [Google Scholar] [CrossRef]
- Tivnan, A.; Orr, W.S.; Gubala, V.; Nooney, R.; Williams, D.E.; McDonagh, C.; Prenter, S.; Harvey, H.; Domingo-Fernández, R.; Bray, I.M. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS ONE 2012, 7, e38129. [Google Scholar] [CrossRef]
- Bertucci, A.; Prasetyanto, E.A.; Septiadi, D.; Manicardi, A.; Brognara, E.; Gambari, R.; Corradini, R.; De Cola, L. Combined delivery of temozolomide and anti-miR221 PNA using mesoporous silica nanoparticles induces apoptosis in resistant glioma cells. Small 2015, 11, 5687–5695. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, B.P.; Lee, K.B. Combined magnetic nanoparticle-based microRNA and hyperthermia therapy to enhance apoptosis in brain cancer cells. Small 2014, 10, 4106–4112. [Google Scholar] [CrossRef]
- Lellouche, E.; Israel, L.; Bechor, M.; Attal, S.; Kurlander, E.; Asher, V.; Dolitzky, A.; Shaham, L.; Izraeli, S.; Lellouche, J.-P. MagRET nanoparticles: An iron oxide nanocomposite platform for gene silencing from MicroRNAs to long noncoding RNAs. Bioconjugate Chem. 2015, 26, 1692–1701. [Google Scholar] [CrossRef]
- Hossain, S.; Stanislaus, A.; Chua, M.J.; Tada, S.; Tagawa, Y.-i.; Chowdhury, E.H.; Akaike, T. Carbonate apatite-facilitated intracellularly delivered siRNA for efficient knockdown of functional genes. J. Control. Release 2010, 147, 101–108. [Google Scholar] [CrossRef]
- Inoue, A.; Mizushima, T.; Wu, X.; Okuzaki, D.; Kambara, N.; Ishikawa, S.; Wang, J.; Qian, Y.; Hirose, H.; Yokoyama, Y. A miR-29b byproduct sequence exhibits potent tumor-suppressive activities via inhibition of NF-κB signaling in KRAS-mutant colon cancer cells. Mol. Cancer Ther. 2018, 17, 977–987. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhang, C.; Zhang, C.; Wang, H. A signature of tumor immune microenvironment genes associated with the prognosis of non-small cell lung cancer. Oncol. Rep. 2020, 43, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.; Hainaut, P.; Boffetta, P. Genetics of lung-cancer susceptibility. Lancet Oncol. 2011, 12, 399–408. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, B.; Hutchinson, A.; Pesatori, A.C.; Consonni, D.; Caporaso, N.E.; Zhang, T.; Wang, D.; Shi, J.; Landi, M.T. Clinical Implications of Inter-and Intratumor Heterogeneity of Immune Cell Markers in Lung Cancer. J. Natl. Cancer Inst. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.S.; Upadhya, A.; Misra, A. Targeted drug therapy in nonsmall cell lung cancer: Clinical significance and possible solutions-part II (role of nanocarriers). Expert Opin. Drug Deliv. 2021, 18, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Mayekar, M.K.; Bivona, T.G. Current landscape of targeted therapy in lung cancer. Clin. Pharmacol. Ther. 2017, 102, 757–764. [Google Scholar] [CrossRef]
- Rosell, R.; Karachaliou, N. Optimizing lung cancer treatment approaches. Nat. Rev. Clin. Oncol. 2015, 12, 75–76. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, J.; Kim, Y.-J.; Wong, M.K.; Wang, P. Codelivery of doxorubicin and paclitaxel by cross-linked multilamellar liposome enables synergistic antitumor activity. Mol. Pharm. 2014, 11, 1651–1661. [Google Scholar] [CrossRef]
- Babu, A.; Templeton, A.K.; Munshi, A.; Ramesh, R. Nanoparticle-based drug delivery for therapy of lung cancer: Progress and challenges. J. Nanomater. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Kumar, K.; Chawla, R. Nanocarriers-mediated therapeutics as a promising approach for treatment and diagnosis of lung cancer. J. Drug Deliv. Sci. Technol. 2021, 65, 102677. [Google Scholar] [CrossRef]

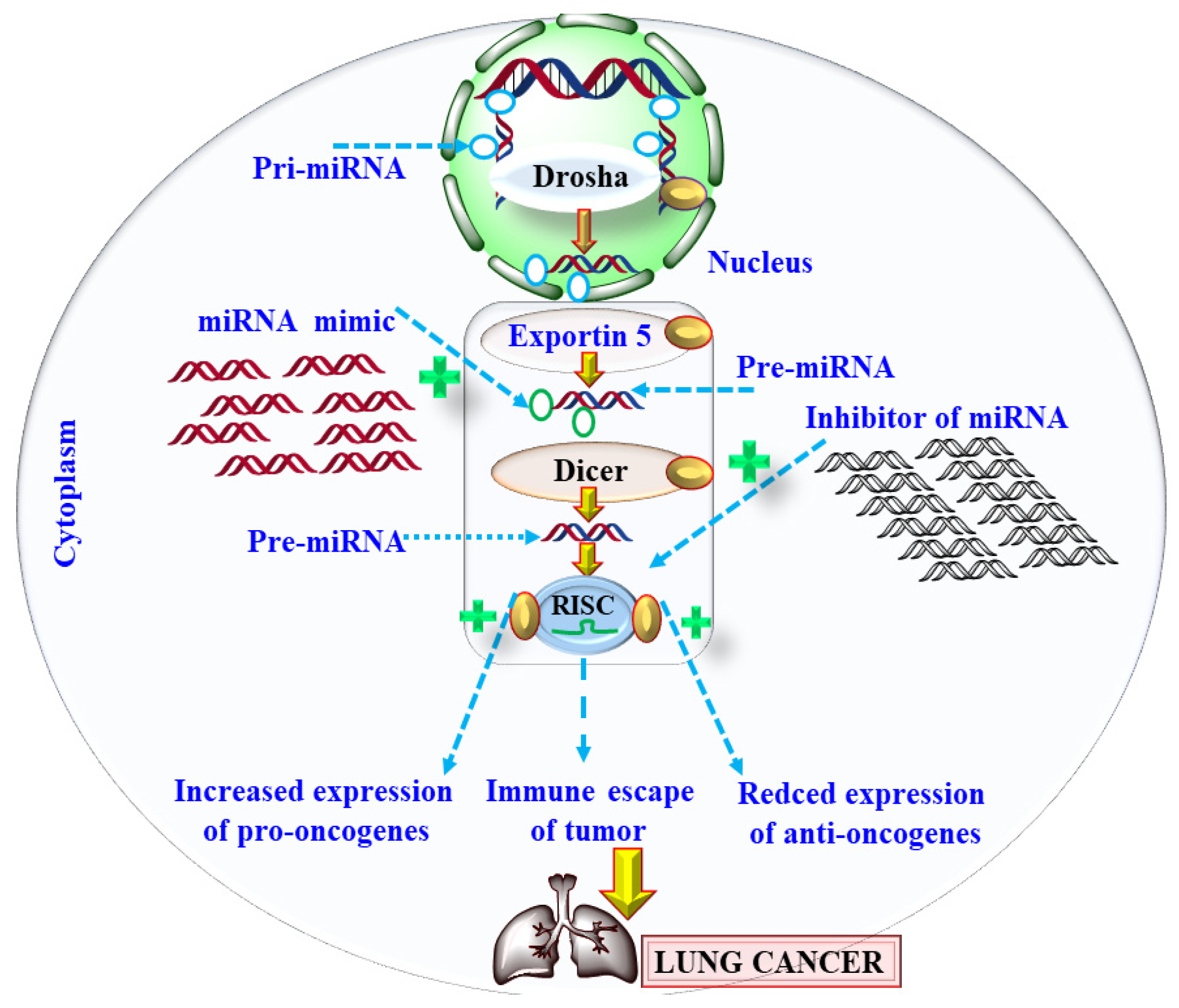
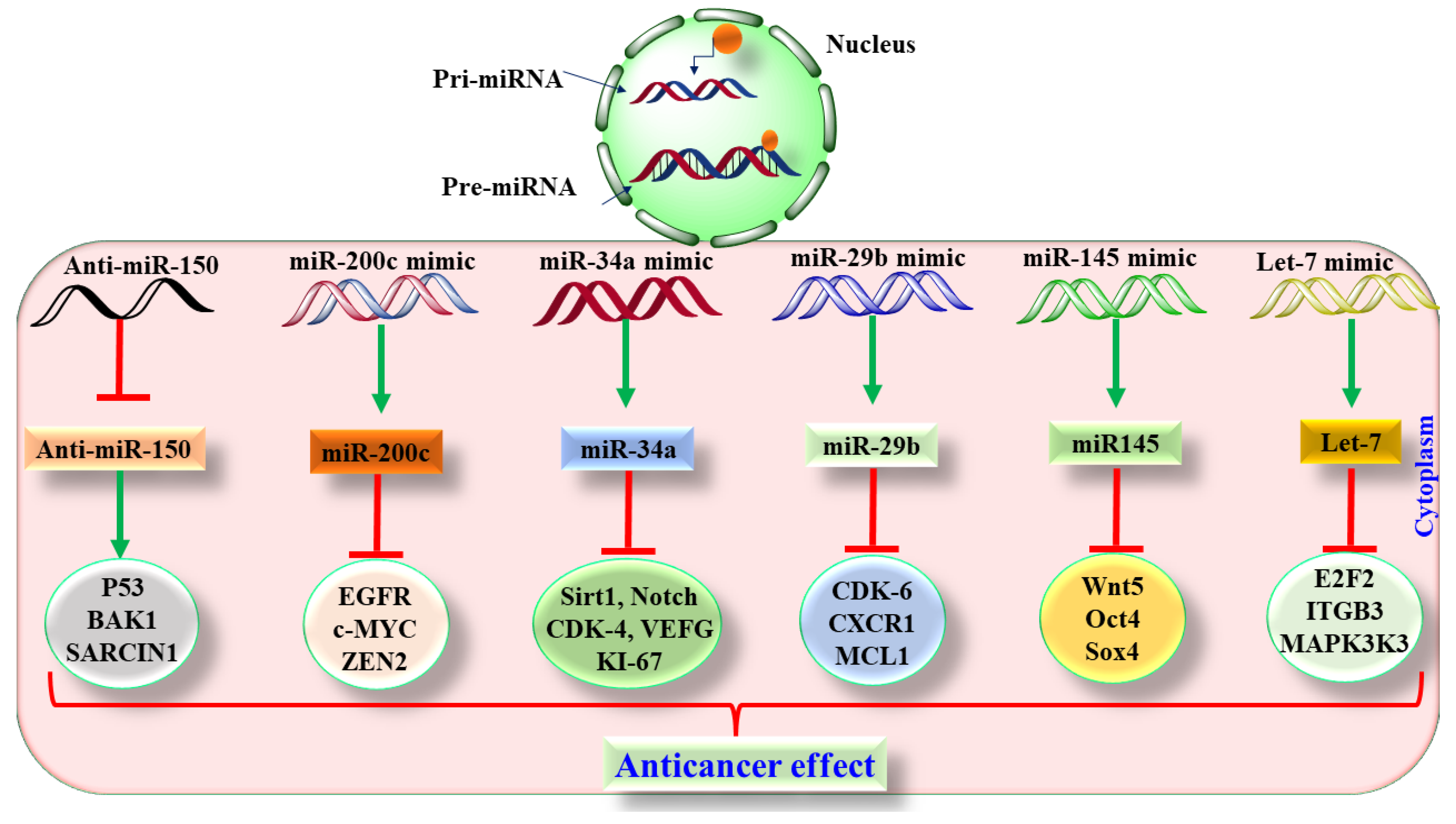
| Drugs | Year of Approval | Mechanism of Action | Adverse Effect | References |
|---|---|---|---|---|
| Afatinib | 2013 | EGFR tyrosine kinase inhibitor | Diarrhea, rash, mucositis, swelling of the lips, nail infection, and nose bleeds. | [48] |
| Alectinib | 2017 | EGFR tyrosine kinase inhibitor | Bloody urine, joint pain or swelling, increased blood pressure, immobility, and nephrotoxicity. | [48] |
| Amivantamab-vmjw | 2021 | EGFR tyrosine kinase inhibitor | Shortness of breath, muscle and joint pain, swelling of hands. | [48] |
| Atezolizumab | 2020 | PD-1 receptor inhibitor | Bladder pain, bloating, ear congestion and dyspnea. | [47] |
| Bevacizumab | 2006 | VEGF inhibitor | Cardiotoxicity, alopecia, xeroderma, hemorrhage, proteinuria, and necrotizing fasciitis. | [49] |
| Brigatinib | 2020 | Inhibitor of AKT, ERK, and STAT3 | Headache, skin rashes, nausea, constipation and numbness. | [47] |
| Capmatinib | 2020 | MET kinase inhibitor | Loss of appetite, chest pain and bloating. | [47] |
| Cemiplimab-rwlc | 2021 | PD-1 receptor inhibitor | Blisters, immobility, gland and joint swelling and mouth ulcers. | [47] |
| Ceritinib | 2017 | ALK phosphorylation inhibitor | Reduced hemoglobin, hepatotoxicity, and nephrotoxicity. | [47] |
| Crizotinib | 2016 | RTK inhibitor | Oedema, reduced appetite, loss of taste and hepatotoxicity. | [47] |
| Dabrafenib | 2017 | BRAF and RAF kinase inhibitor | Joint pain, papilloma, alopecia, and hepatotoxicity. | [47] |
| Dacomitinib | 2018 | EGFR tyrosine kinase inhibitor | Dermatitis, acne, stomatitis, dry skin, and paronychia. | [48] |
| Docetaxel | 2005 | Microtubule depolymerization inhibition | Neutropenia, dysgeusia hypersensitivity, anemia, thrombocytopenia, anorexia, nail disorders and fluid retention. | [47] |
| Doxorubicin | 1970 | Topoisomerase II inhibitor | Cardiotoxicity, hepatotoxicity and nephrotoxicity. | [47] |
| Durvalumab | 2020 | PD-1 receptor inhibitor | Musculoskeletal pain, loss of appetite, and UTI. | [47] |
| Entrectinib | 2019 | RTK inhibitor | Peripheral edema, hepato-reno toxicity, myelotoxicity. | [47] |
| Erlotinib | 2010 | EGFR tyrosine kinase inhibitor | Fatigue, rashes, hepatotoxicity, cough, mouth ulceration, and dry skin. | [48] |
| Everolimus | 2016 | mTORC1 inhibitor | Insomnia, weight loss, and dry mouth. | [48] |
| Gefitinib | 2015 | EGFR tyrosine kinase inhibitor | Rash, diarrhea, hepatotoxicity, acne, and anorexia. | [48] |
| Gemcitabine | 2005 | DNA synthesis inhibitor | Hair loss, nausea, mouth ulcer. | [47] |
| Ipilimumab | 2020 | Inhibition of T-cell inactivation | Diarrhea, fatigue, skin rash, and pruritus. | [49] |
| Methotrexate | 1970 | Dihydrofolate reductase inhibitor | Alopecia, hepatotoxicity, and tender gums. | [47] |
| Necitumumab | 2015 | EGFR tyrosine kinase inhibitor | Weight loss, hypokalemia, mouth ulcer, acne, and chest infection. | [47] |
| Nivolumab | 2018 | PD-1 receptor inhibitor | Lymphopenia, fatigue, diarrhea, pruritus, and vitiligo. | [49] |
| Osimertinib | 2020 | EGFR tyrosine kinase inhibitor | Diarrhea, nausea, reduced appetite, dry skin, paronychia. | [48] |
| Paclitaxel protein-bound nanoparticle | 2012 | Causes cell cycle arrest | Low blood counts, alopecia, mouth ulcer, peripheral neuropathy, arthralgias, and myalgias. | [47] |
| Pembrolizumab | 2016 | PD-1 receptor inhibitor | Anemia, hypertension, hyponatremia, hypoalbuminemia, and cough. | [49] |
| Pemetrexed | 2017 | Purine and pyrimidine synthesis inhibitor | Weight loss, vomiting, fatigue, loss of appetite, and insomnia. | [49] |
| Pralsetinib | 2020 | RET kinase inhibitor | Shortness of breath, cough, bleeding gums, nosebleeds, and mental confusion. | [49] |
| Ramucirumab | 2020 | VEGF inhibitor | Cardiotoxicity, wound healing problem and skin rashes. | [49] |
| Selpercatinib | 2020 | RTK inhibitor | Dry mouth, hypertension, peripheral edema, diabetes, and hepatotoxicity. | [47] |
| Sotorasib | 2021 | KRAS G12C inhibitor | Bone/joint pain, constipation, and stomach pain. | [47] |
| Tepotinib | 2021 | Kinase inhibitor | Anxiety, tachycardia, loss of appetite, sore throat, and stomach pain. | [47] |
| Trametinib | 2015 | MEK ½ inhibitor | Losing of fingernails, eye dryness, damaged taste buds, dry skin, and canker sores. | [47] |
| Vinorelbine | 1994 | Cycle arrest via binding with microtubular spindle | Muscle or joint pain, constipation, and loss of appetite | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md, S.; Alhakamy, N.A.; Karim, S.; Gabr, G.A.; Iqubal, M.K.; Murshid, S.S.A. Signaling Pathway Inhibitors, miRNA, and Nanocarrier-Based Pharmacotherapeutics for the Treatment of Lung Cancer: A Review. Pharmaceutics 2021, 13, 2120. https://doi.org/10.3390/pharmaceutics13122120
Md S, Alhakamy NA, Karim S, Gabr GA, Iqubal MK, Murshid SSA. Signaling Pathway Inhibitors, miRNA, and Nanocarrier-Based Pharmacotherapeutics for the Treatment of Lung Cancer: A Review. Pharmaceutics. 2021; 13(12):2120. https://doi.org/10.3390/pharmaceutics13122120
Chicago/Turabian StyleMd, Shadab, Nabil A. Alhakamy, Shahid Karim, Gamal A Gabr, Mohammad Kashif Iqubal, and Samar S. A. Murshid. 2021. "Signaling Pathway Inhibitors, miRNA, and Nanocarrier-Based Pharmacotherapeutics for the Treatment of Lung Cancer: A Review" Pharmaceutics 13, no. 12: 2120. https://doi.org/10.3390/pharmaceutics13122120
APA StyleMd, S., Alhakamy, N. A., Karim, S., Gabr, G. A., Iqubal, M. K., & Murshid, S. S. A. (2021). Signaling Pathway Inhibitors, miRNA, and Nanocarrier-Based Pharmacotherapeutics for the Treatment of Lung Cancer: A Review. Pharmaceutics, 13(12), 2120. https://doi.org/10.3390/pharmaceutics13122120