Biomaterials and Meniscal Lesions: Current Concepts and Future Perspective
Abstract
:1. Introduction
2. Current Concepts on Meniscus Anatomy, Structure, Lesions and Their Treatment
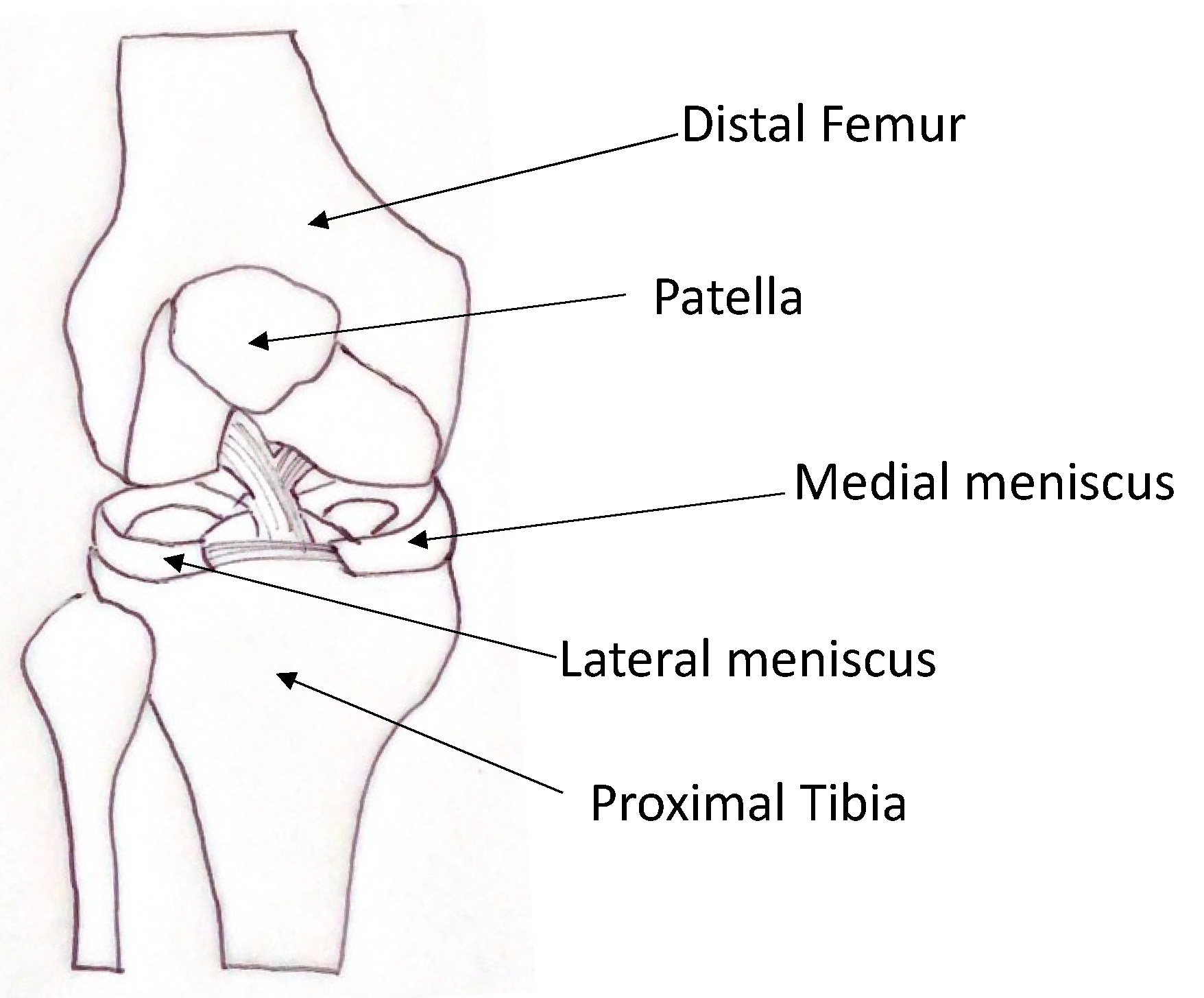
2.1. Meniscal Anatomy and Function
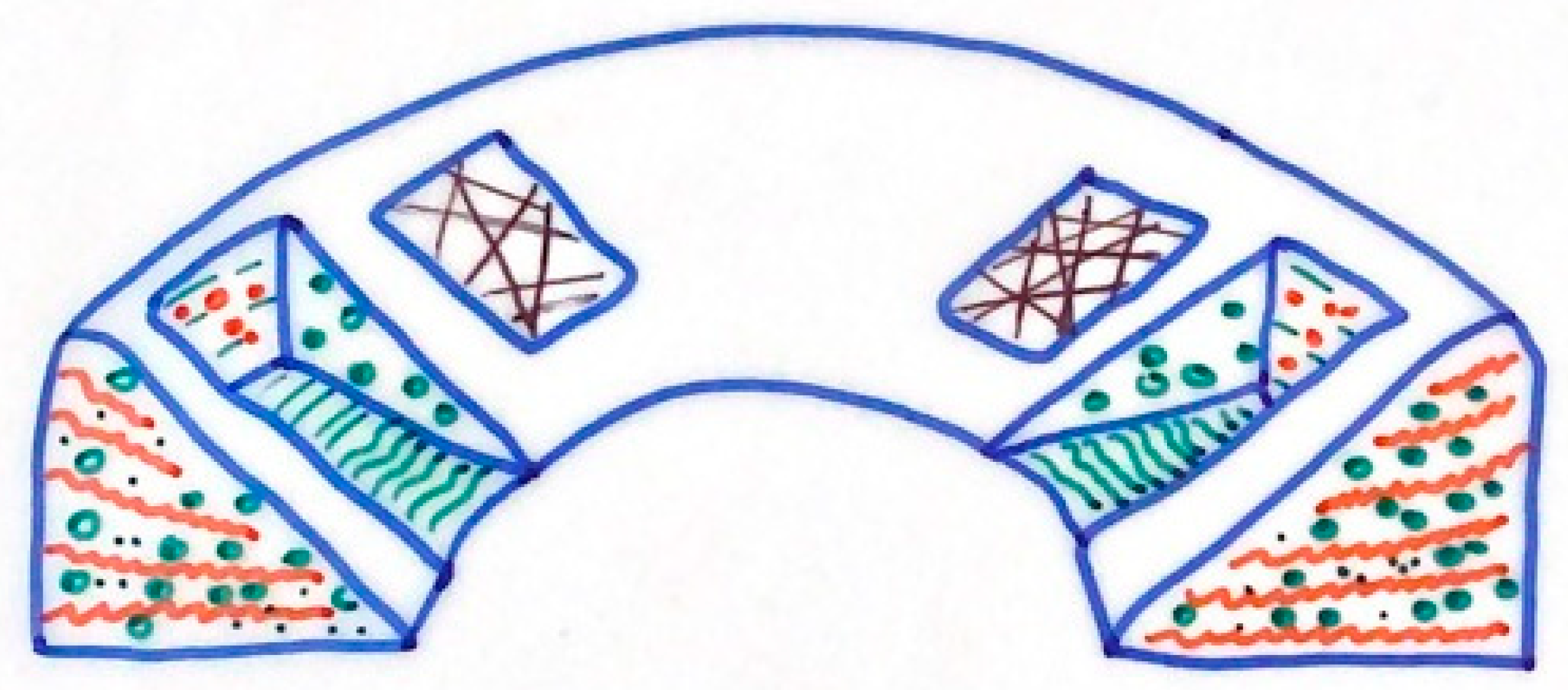
2.2. Meniscal Structure
2.3. Meniscal Lesions and Treatment
2.4. Clinical Evidence of Needing New Regenerative Strategies
3. Scaffolds
- −
- It should promote cell migration;
- −
- It should be biomimetic, mimicking the architecture, tribology and mechanical characteristics of the native meniscus;
- −
- It should resist the forces that are applied and transmitted by the knee once the repopulating cells have produced an extracellular matrix;
- −
- It must be biocompatible, in order to avoid a reaction from a foreign body;
- −
- It must be slowly biodegraded, allowing for a progressive replacement from the host tissue;
- −
- It should possess a porosity that allows for the diffusion of nutrients and substances produced by cellular catabolism;
- −
- It must be easy to handle and easy to implant.
3.1. Synthetic Polymers
3.2. Biological Scaffolds
3.3. Hydrogel Scaffolds
3.4. Decellularized Meniscal Scaffolds
3.5. Hybrid Meniscal Scaffold
| Material | Advantages | Limitation | References |
|---|---|---|---|
| Synthetic | |||
| Polilactic acid (PGA) | Excellent mechanical properties Bioresorbability | Potential adverse tissue reaction for polymer fragments | T. Murakami et al. [47]; |
| Poly-(l)-lactic acid (PLLA) | High mechanical strength Thermal stability Tunable properties | Acidic products Autocatalytic degradation | A. P. Testa Pezzin et al. [48] |
| Poly-(lactic-co-glycolitic acid) (PLGA) | Tunable degradability Biocompatibility | Acidic byproducts | Y. Gu et al. [49] |
| Polyurethane (PU) | Good mechanical properties and cytocompatibility Thermoplasticity | Low reabsorption rate | T. De Coninck et al. [50] |
| Polyester carbon | Good mechanical properties | Cytotoxicity | J. Gopinathan et al. [51] |
| poly(ε-caprolactone) | Biocompatibility Biodegradability | Hydrophobicity Host cell low interaction | Z. Abpeikar et al. [52] R. T. C. Welsing et al. [53] |
| Polietilene + PCL | Increased secretion of glycosaminoglycans | Reduced cell adhesion | U. Koller et al. [55] |
| PU + PCL | Good integration, good clinical results | Possible implant extrusion | A.Leroy et al. [60] C.Baynat et al. [61] E. Bulgheroni et al. [62] |
| Biological | |||
| Perichondrium | Good biocompatibility | Cartilage differentiation when in articular environment | C J Walsh et al. [63] |
| Small intestine submucosa (SIS) | Partial meniscal regeneration | Progression of joint damage | M P Bradley et al. [65] |
| Acellular porcine meniscal tissue | Excellent immunocompatibility | Absence of mechanical assessment | T. W. Stapleton et al. [67] |
| Bacterial cellulosa | Inexpensive, moldable and promotes cell migration | Poor mechanical properties | A.Bodin et al. [68] |
| Silk fibrous protein | Flexible processability Biocompatibility Capable of chemical modification Thermal stability Good mechanical strength | Immunogenicity Poor cell adhesion | A.Bandyopadhyay et al. [69] R. Yan et al. [70] S. E. C. Stein et al. [71] |
| Collagen | Cytocompatibility, capable of clinical use | Immunogenicity Weak mechanical strength | K. R. Stone et al. [75] W. G. Rodkey et al. [76] |
| Hydrogel | |||
| Polyvvinil alcohol (PVA) | Excellent visco-elastic and biocompatible properties | Hard fixation method, tolerance of PVA-H | M. Kobayashi et al. [82] M. Kobayashi et al. [81] |
| Methacrylate genatine (GelMA) | Biocompatibility, biodegradability | Poor mechanical properties | S. P. Grogan et al. [85] |
4. New Technology and Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bland-Sutton, J. Ligaments; Their Nature and Morphology, 2nd ed.; H. K. Lewis: London, UK, 1897; p. 114. [Google Scholar]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The basic science of human knee menisci: Structure, composition, and function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, M.R.; Johnson, D.L. Meniscal injuries in the young, athletically active patient. Physician Sportsmed. 2011, 39, 123–130. [Google Scholar] [CrossRef]
- McDermott, I.D.; Amis, A.A. The consequences of meniscectomy. J. Bone Jt. Surg. Br. 2006, 88, 1549–1556. [Google Scholar] [CrossRef] [Green Version]
- King, D. The function of semilunar cartilages. JBJS 1936, 18, 1069–1076. [Google Scholar]
- Fox, A.J.S.; Wanivenhaus, F.; Burge, A.J.; Warren, R.F.; Rodeo, S.A. The human meniscus: A review of anatomy, function, injury, and advances in treatment. Clin. Anat. 2015, 28, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.C. Neural and vascular anatomy of the menisci of the human knee. J. Orthop. Sports Phys. Ther. 1999, 29, 23–30. [Google Scholar] [CrossRef]
- Hamrock, B.J.; Schmid, S.R.; Jacobson, B.O. Fundamentals of Fluid Film Lubrication; CRC press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Aagaard, H.; Verdonk, R. Function of the normal meniscus and consequences of meniscal resection. Scand. J. Med. Sci. Sports 1999, 9, 134–140. [Google Scholar] [CrossRef]
- McDevitt, C.A.; Webber, R.J. The ultrastructure and biochemistry of meniscal cartilage. Clin. Orthop. Relat. Res. 1990, 252, 8–18. [Google Scholar] [CrossRef]
- Ibarra, C.; Koski, J.A.; Warren, R.F. Tissue engineering meniscus: Cells and matrix. Orthop. Clin. N. Am. 2000, 31, 411–418. [Google Scholar] [CrossRef]
- Jones, R.S.; Keene, G.; Learmonth, D.; Bickerstaff, D.; Nawana, N.; Costi, J.; Pearcy, M. Direct measurement of hoop strains in the intact and torn human medial meniscus. Clin. Biomech. 1996, 11, 295–300. [Google Scholar] [CrossRef]
- Paton, R. Basic Orthopaedic Sciences: The Stanmore Guide. Ann. R. Coll. Surg. Engl. 2008, 90, 358. [Google Scholar]
- Bryceland, J.K.; Powell, A.J.; Nunn, T. Knee Menisci: Structure, function, and management of pathology. Cartilage 2017, 8, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.M.; Burke, D.L. In-vitro measurement of static pressure distribution in synovial joints—Part I: Tibial surface of the knee. J. Biomech. Eng. 1983, 105, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.; Allum, R.L. Relevance of history of injury to the diagnosis of meniscal tears. Ann. R. Coll. Surg. Engl. 1993, 75, 229–230. [Google Scholar] [PubMed]
- McDermott, I. Meniscal tears, repairs and replacement: Their relevance to osteoarthritis of the knee. Br. J. Sports Med. 2011, 45, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.R.; Morrison, W.B.; Carrino, J.A. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? Am. J. Roentgenol. 2004, 183, 17–23. [Google Scholar] [CrossRef]
- Englund, M. The role of the meniscus in osteoarthritis genesis. Med. Clin. N. Am. 2009, 93, 37–43. [Google Scholar] [CrossRef]
- McGinity, R.; Geuss, J.B.; Marvin, L.F. Partial or total meniscectomy: A comparative analysis. J. Bone Jt. Surg. 1977, 59, 763–766. [Google Scholar] [CrossRef]
- Beamer, B.S.; Walley, K.; Okajima, S.; Manoukian, O.S.; Perez-Viloria, M.; DeAngelis, J.P.; Ramappa, A.J.; Nazarian, A. Changes in Contact Area in Meniscus Horizontal Cleavage Tears Subjected to Repair and Resection. Arthroscopy 2017, 33, 617–624. [Google Scholar] [CrossRef]
- Goyal, K.S.; Pan, T.J.; Tran, D.; Dumpe, S.C.; Zhang, X.; Harner, C.D. Vertical Tears of the Lateral Meniscus: Effects on In Vitro Tibiofemoral Joint Mechanics. Orthop. J. Sport. Med. 2014, 2, 2325967114541237. [Google Scholar] [CrossRef]
- Seedhom, B.B.; Hargreaves, D.J. Transmission of the Load in the Knee Joint with Special Reference to the Role of the Menisci. Eng. Med. 1979, 8, 220–228. [Google Scholar] [CrossRef]
- Majewski, M.; Stoll, R.; Widmer, H.; Müller, W.; Friederich, N.F. Midterm and long-term results after arthroscopic suture repair of isolated, longitudinal, vertical meniscal tears in stable knees. Am. J. Sports Med. 2006, 34, 1072–1076. [Google Scholar] [CrossRef]
- Steenbrugge, F.; Verdonk, R.; Hürel, C.; Verstraete, K. Arthroscopic meniscus repair: Inside-out technique vs. Biofix meniscus arrow. Knee Surg. Sports Traumatol. Arthrosc. 2004, 12, 43–49. [Google Scholar] [CrossRef]
- Hoffelner, T.; Resch, H.; Forstner, R.; Michael, M.; Minnich, B.; Tauber, M. Arthroscopic all-inside meniscal repair—Does the meniscus heal? A clinical and radiological follow-up examination to verify meniscal healing using a 3-T MRI. Skelet. Radiol. 2011, 40, 181–187. [Google Scholar] [CrossRef]
- Beaufils, P.; Pujol, N. Meniscal repair: Technique. Orthop. Traumatol. Surg. Res. 2018, 104, S137–S145. [Google Scholar] [CrossRef]
- Jacobi, M.; Jakob, R.P. Meniscal repair: Enhancement of healing process. In The Meniscus; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Scordino, L.E.; Deberardino, T.M. Biologic enhancement of meniscus repair. Clin. Sports Med. 2012, 31, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Campi, S.; Romeo, G.; Spiezia, F.; Maffulli, N.; Denaro, V. Biological strategies to enhance healing of the avascular area of the meniscus. Stem Cells Int. 2012, 2012, 528359. [Google Scholar] [CrossRef]
- Hirschmann, M.; Friederich, N. Meniscal lesions in children: Classifications. In The Meniscus; Springer: Berlin/Heidelberg, Germany, 2009; pp. 241–246. [Google Scholar]
- Arnold, M.P.; Hirschmann, M.T.; Verdonk, P.C.M. See the whole picture: Knee preserving therapy needs more than surface repair. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 195–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, D.W.; Whelan, J.; Simon, T.M. Cell survival after transplantation of fresh meniscal allografts. DNA probe analysis in a goat model. Am. J. Sports Med. 1993, 21, 540–550. [Google Scholar] [CrossRef]
- Cavendish, P.A.; DiBartola, A.C.; Everhart, J.S.; Kuzma, S.; Kim, W.J.; Flanigan, D.C. Meniscal allograft transplantation: A review of indications, techniques, and outcomes. Sport. Traumatol. Arthrosc. 2020, 28, 3539–3550. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, D.; Mercurio, M.; Gasparini, G.; Galasso, O. Predictors of Meniscal Allograft Transplantation Outcome: A Systematic Review. J. Knee Surg. 2021, 34, 303–321. [Google Scholar] [CrossRef]
- Figueroa, F.; Figueroa, D.; Calvo, R.; Vaisman, A.; Espregueira-Mendes, J. Meniscus allograft transplantation: Indications, techniques and outcomes. EFORT Open Rev. 2019, 4, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, P.C.M.; Demurie, A.; Almqvist, K.F.; Veys, E.M.; Verbruggen, G.; Verdonk, R. Transplantation of viable meniscal allograft. Survivorship analysis and clinical outcome of one hundred cases. J. Bone Jt. Surg. Am. 2005, 87, 715–724. [Google Scholar] [CrossRef]
- Yow, B.G.; Donohue, M.; Tennent, D.J. Meniscal Allograft Transplantation. Sports Med. Arthrosc. 2021, 29, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Elattar, M.; Dhollander, A.; Verdonk, R.; Almqvist, K.F.; Verdonk, P. Twenty-six years of meniscal allograft transplantation: Is it still experimental? A meta-analysis of 44 trials. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kazi, H.A.; Abdelrahman, W.; Brady, P.A.; Cameron, J.C. Meniscal allograft with or without osteotomy: A 15-year follow-up study. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 303–309. [Google Scholar] [CrossRef]
- Wirth, C.J.; Peters, G.; Milachowski, K.A.; Weismeier, K.G.; Kohn, D. Long-term results of meniscal allograft transplantation. Am. J. Sports Med. 2002, 30, 174–181. [Google Scholar] [CrossRef]
- Dangelmajer, S.; Familiari, F.; Simonetta, R.; Kaymakoglu, M.; Huri, A.G. Meniscal Transplants and Scaffolds: A Systematic Review of the Literature. Knee Surg. Relat. Res. 2017, 29, 3–10. [Google Scholar] [CrossRef]
- Scotti, C.; Hirschmann, M.; Antinolfi, P.; Martin, I.; Peretti, G. Meniscus repair and regeneration: Review on current methods and research potential. Eur. Cells Mater. 2013, 26, 150–170. [Google Scholar] [CrossRef]
- van Tienen, T.G.; Hannink, G.; Buma, P. Meniscus replacement using synthetic materials. Clin. Sports Med. 2009, 28, 143–156. [Google Scholar] [CrossRef]
- Klompmaker, J.; Jansen, H.W.; Veth, R.; Nielsen, H.K.; de Groot, J.H.; Pennings, A.J. Porous implants for knee joint meniscus reconstruction: A preliminary study on the role of pore sizes in ingrowth and differentiation of fibrocartilage. Clin. Mater. 1993, 14, 1–11. [Google Scholar] [CrossRef]
- Shimomura, K.; Bean, A.C.; Lin, H.; Nakamura, N.; Tuan, R.S. In Vitro Repair of Meniscal Radial Tear Using Aligned Electrospun Nanofibrous Scaffold. Tissue Eng. Part A 2015, 21, 2066–2075. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Otsuki, S.; Nakagawa, K.; Okamoto, Y.; Inoue, T.; Sakamoto, Y.; Sato, H.; Neo, M. Establishment of novel meniscal scaffold structures using polyglycolic and poly-l-lactic acids. J. Biomater. Appl. 2017, 32, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Pezzin, A.P.T.; Cardoso, T.P.; Rincón, M.D.C.A.; Zavaglia, C.A.D.C.; Duek, E.A.D.R. Bioreabsorbable polymer scaffold as temporary meniscal prosthesis. Artif. Organs 2003, 27, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhu, W.; Hao, Y.; Lu, L.; Chen, Y.; Wang, Y. Repair of meniscal defect using an induced myoblast-loaded polyglycolic acid mesh in a canine model. Exp. Ther. Med. 2012, 3, 293–298. [Google Scholar] [CrossRef] [Green Version]
- De Coninck, T.; Huysse, W.; Willemot, L.; Verdonk, R.; Verstraete, K.; Verdonk, P. Two-year follow-up study on clinical and radiological outcomes of polyurethane meniscal scaffolds. Am. J. Sports Med. 2013, 41, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Pillai, M.M.; Sahanand, K.S.; Rai, B.K.D.; Selvakumar, R.; Bhattacharyya, A. Synergistic effect of electrical conductivity and biomolecules on human meniscal cell attachment, growth, and proliferation in poly-ε-caprolactone nanocomposite scaffolds. Biomed. Mater. 2017, 12, 065001. [Google Scholar] [CrossRef] [PubMed]
- Abpeikar, Z.; Moradi, L.; Javdani, M.; Kargozar, S.; Soleimannejad, M.; Hasanzadeh, E.; Mirzaei, S.A.; Asadpour, S. Characterization of Macroporous Polycaprolactone/Silk Fibroin/Gelatin/Ascorbic Acid Composite Scaffolds and In Vivo Results in a Rabbit Model for Meniscus Cartilage Repair. Cartilage 2021, 19476035211035416. [Google Scholar]
- Welsing, R.T.; van Tienen, T.G.; Ramrattan, N.; Heijkants, R.; Schouten, A.J.; Veth, R.P.; Buma, P. Effect on tissue differentiation and articular cartilage degradation of a polymer meniscus implant: A 2-year follow-up study in dogs. Am. J. Sports Med. 2008, 36, 1978–1989. [Google Scholar] [CrossRef] [Green Version]
- Tienen, T.G.; Heijkants, R.G.; de Groot, J.H.; Pennings, A.J.; Schouten, A.J.; Veth, R.P.; Buma, P. Replacement of the knee meniscus by a porous polymer implant: A study in dogs. Am. J. Sports Med. 2006, 34, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Koller, U.; Nehrer, S.; Vavken, P.; Kapeller, B.; Windhager, R.; Chiari, C. Polyethylene terephthalate (PET) enhances chondrogenic differentiation of ovine meniscocytes in a hyaluronic acid/polycaprolactone scaffold in vitro. Int. Orthop. 2012, 36, 1953–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, B.M.; Handorf, A.M.; Ionescu, L.C.; Li, W.-J.; Mauck, R.L. New directions in nanofibrous scaffolds for soft tissue engineering and regeneration. Expert Rev. Med. Devices 2009, 6, 515–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, M.B.; Henning, E.A.; Söegaard, N.; Bostrom, M.; Esterhai, J.L.; Mauck, R.L. Engineering meniscus structure and function via multi-layered mesenchymal stem cell-seeded nanofibrous scaffolds. J. Biomech. 2015, 48, 1412–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kon, E.; Chiari, C.; Marcacci, M.; Delcogliano, M.; Salter, D.M.; Martin, I.; Ambrosio, L.; Fini, M.; Tschon, M.; Tognana, E.; et al. Tissue engineering for total meniscal substitution: Animal study in sheep model. Tissue Eng. Part A 2008, 14, 1067–1080. [Google Scholar] [CrossRef] [Green Version]
- Chiari, C.; Koller, U.; Dorotka, R.; Eder, C.; Plasenzotti, R.; Lang, S.; Ambrosio, L.; Tognana, E.; Kon, E.; Salter, D.; et al. A tissue engineering approach to meniscus regeneration in a sheep model. Osteoarthr. Cartil. 2006, 14, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, A.; Beaufils, P.; Faivre, B.; Steltzlen, C.; Boisrenoult, P.; Pujol, N. Actifit® polyurethane meniscal scaffold: MRI and functional outcomes after a minimum follow-up of 5 years. Orthop. Traumatol. Surg. Res. 2017, 103, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Baynat, C.; Andro, C.; Vincent, J.; Schiele, P.; Buisson, P.; Dubrana, F.; Gunepin, F. Actifit synthetic meniscal substitute: Experience with 18 patients in Brest, France. Orthop. Traumatol. Surg. Res. 2014, 100 (Suppl. 8), S385–S389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgheroni, E.; Grassi, A.; Campagnolo, M.; Bulgheroni, P.; Mudhigere, A.; Gobbi, A. Comparative Study of Collagen versus Synthetic-Based Meniscal Scaffolds in Treating Meniscal Deficiency in Young Active Population. Cartilage 2016, 7, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.J.; Goodman, D.; Caplan, A.I.; Goldberg, V.M. Meniscus regeneration in a rabbit partial meniscectomy model. Tissue Eng. 1999, 5, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Bruns, J.; Kahrs, J.; Kampen, J.; Behrens, P.; Plitz, W. Autologous perichondral tissue for meniscal replacement. J. Bone Jt. Surg. Br. 1998, 80, 918–923. [Google Scholar] [CrossRef]
- Bradley, M.P.; Fadale, P.D.; Hulstyn, M.J.; Muirhead, W.R.; Lifrak, J.T. Porcine small intestine submucosa for repair of goat meniscal defects. Orthopedics 2007, 30, 650–656. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Wan, X.; Zhao, C.; Qiu, P.; Lin, X.; Zhang, J.; Huang, Y. Preparation and Characterization of an Optimized Meniscal Extracellular Matrix Scaffold for Meniscus Transplantation. Front. Bioeng. Biotechnol. 2020, 8, 779. [Google Scholar] [CrossRef]
- Stapleton, T.W.; Ingram, J.; Fisher, J.; Ingham, E. Investigation of the regenerative capacity of an acellular porcine medial meniscus for tissue engineering applications. Tissue Eng. Part A 2011, 17, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Bodin, A.; Concaro, S.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential meniscus implant. J. Tissue Eng. Regen. Med. 2007, 1, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Mandal, B.B. A three-dimensional printed silk-based biomimetic tri-layered meniscus for potential patient-specific implantation. Biofabrication 2019, 12, 015003. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Chen, Y.; Gu, Y.; Tang, C.; Huang, J.; Hu, Y.; Zheng, Z.; Ran, J.; Heng, B.; Chen, X.; et al. A collagen-coated sponge silk scaffold for functional meniscus regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 156–173. [Google Scholar] [CrossRef]
- Stein, S.E.C.; Von Luebken, F.; Warnecke, D.; Gentilini, C.; Skaer, N.; Walker, R.; Kessler, O.; Ignatius, A.; Duerselen, L. The challenge of implant integration in partial meniscal replacement: An experimental study on a silk fibroin scaffold in sheep. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Warnecke, D.; Stein, S.; Haffner-Luntzer, M.; de Roy, L.; Skaer, N.; Walker, R.; Kessler, O.; Ignatius, A.; Dürselen, L. Biomechanical, structural and biological characterisation of a new silk fibroin scaffold for meniscal repair. J. Mech. Behav. Biomed. Mater. 2018, 86, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Gruchenberg, K.; Ignatius, A.; Friemert, B.; von Lübken, F.; Skaer, N.; Gellynck, K.; Kessler, O.; Dürselen, L. In vivo performance of a novel silk fibroin scaffold for partial meniscal replacement in a sheep model. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2218–2229. [Google Scholar] [CrossRef] [Green Version]
- Buma, P.; Ramrattan, N.; van Tienen, T.; Veth, R. Tissue engineering of the meniscus. Biomaterials 2004, 25, 1523–1532. [Google Scholar] [CrossRef]
- Stone, K.R.; Rodkey, W.G.; Webber, R.; McKinney, L.; Steadman, J.R. Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am. J. Sports Med. 1992, 20, 104–111. [Google Scholar] [CrossRef]
- Rodkey, W.G.; DeHaven, K.E.; Montgomery, W.H.; Baker, J.C.L.; Beck, J.C.L.; Hormel, S.E.; Steadman, J.R.; Cole, B.J.; Briggs, K. Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J. Bone Jt. Surg. Am. 2008, 90, 1413–1426. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Marcheggiani Muccioli, G.M.; Lopomo, N.; Bruni, D.; Giordano, G.; Ravazzolo, G.; Molinari, M.; Marcacci, M. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: A minimum 10-year follow-up study. Am. J. Sports Med. 2011, 39, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Ballyns, J.J.; Gleghorn, J.P.; Niebrzydowski, V.; Rawlinson, J.J.; Potter, H.G.; Maher, S.A.; Wright, T.M.; Bonassar, L.J. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng. Part A 2008, 14, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
- Ballyns, J.J.; Wright, T.M.; Bonassar, L.J. Effect of media mixing on ECM assembly and mechanical properties of anatomically-shaped tissue engineered meniscus. Biomaterials 2010, 31, 6756–6763. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Toguchida, J.; Oka, M. Preliminary study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 2003, 24, 639–647. [Google Scholar] [CrossRef]
- Kobayashi, M. A study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus in vivo. Biomed. Mater. Eng. 2004, 14, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Curley, C.; Hayes, J.C.; Rowan, N.J.; Kennedy, J.E. An evaluation of the thermal and mechanical properties of a salt-modified polyvinyl alcohol hydrogel for a knee meniscus application. J. Mech. Behav. Biomed. Mater. 2014, 40, 13–22. [Google Scholar] [CrossRef]
- Hayes, J.C.; Curley, C.; Tierney, P.; Kennedy, J.E. Biomechanical analysis of a salt-modified polyvinyl alcohol hydrogel for knee meniscus applications, including comparison with human donor samples. J. Mech. Behav. Biomed. Mater. 2016, 56, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Chung, P.H.; Soman, P.; Chen, P.; Lotz, M.K.; Chen, S.; D’Lima, D.D. Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater. 2013, 9, 7218–7226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simson, J.A.; Strehin, I.A.; Allen, B.W.; Elisseeff, J.H. Bonding and fusion of meniscus fibrocartilage using a novel chondroitin sulfate bone marrow tissue adhesive. Tissue Eng. Part A 2013, 19, 1843–1851. [Google Scholar] [CrossRef] [Green Version]
- Ishida, K.; Kuroda, R.; Miwa, M.; Tabata, Y.; Hokugo, A.; Kawamoto, T.; Sasaki, K.; Doita, M.; Kurosaka, M. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007, 13, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Minehara, H.; Urabe, K.; Naruse, K.; Mehlhorn, A.T.; Uchida, K.; Südkamp, N.P.; Itoman, M. A new technique for seeding chondrocytes onto solvent-preserved human meniscus using the chemokinetic effect of recombinant human bone morphogenetic protein-2. Cell Tissue Bank. 2011, 12, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Sandmann, G.H.; Eichhorn, S.; Vogt, S.; Adamczyk, C.; Aryee, S.; Hoberg, M.; Milz, S.; Imhoff, A.B.; Tischer, T. Generation and characterization of a human acellular meniscus scaffold for tissue engineering. J. Biomed. Mater. Res. A 2009, 91, 567–574. [Google Scholar] [CrossRef]
- Stapleton, T.W.; Ingram, J.; Katta, J.; Knight, R.; Korossis, S.; Fisher, J.; Ingham, E. Development and characterization of an acellular porcine medial meniscus for use in tissue engineering. Tissue Eng. Part A 2008, 14, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Stabile, K.J.; Odom, D.; Smith, T.L.; Northam, C.; Whitlock, P.W.; Smith, B.P.; Van Dyke, M.E.; Ferguson, C.M. An acellular, allograft-derived meniscus scaffold in an ovine model. Arthroscopy 2010, 26, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, S.; Hao, C.; Guo, W.; Gao, S.; Wang, M.; Chen, M.; Sun, Z.; Xu, Y.; Wang, Y.; et al. AMECM/DCB scaffold prompts successful total meniscus reconstruction in a rabbit total meniscectomy model. Biomaterials 2016, 111, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Bakhshandeh, B.; Zarintaj, P.; Oftadeh, M.O.; Keramati, F.; Fouladiha, H.; Sohrabi-Jahromi, S.; Ziraksaz, Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Bilgen, B.; Jayasuriya, C.T.; Owens, B.D. Current Concepts in Meniscus Tissue Engineering and Repair. Adv. Healthc. Mater. 2018, 7, e1701407. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Cheng, J.; Sun, M.; Yang, P.; Zhao, F.; Zhang, J.; Duan, X.; Fu, X.; Zhang, J.; et al. Biomechanically, structurally and functionally meticulously tailored polycaprolactone/silk fibroin scaffold for meniscus regeneration. Theranostics 2020, 10, 5090–5106. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, P.; Yang, Z.; Gao, C.; Fu, L.; Liao, Z.; Zhao, T.; Cao, F.; Chen, W.; Peng, Y.; et al. Meniscal Regenerative Scaffolds Based on Biopolymers and Polymers: Recent Status and Applications. Front. Cell Dev. Biol. 2021, 9, 661802. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Rodeo, S.A.; Fortier, L.A.; Lu, C.; Erisken, C.; Mao, J.J. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 2014, 6, 266ra171. [Google Scholar] [CrossRef] [Green Version]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. A 3D printed PCL/hydrogel construct with zone-specific biochemical composition mimicking that of the meniscus. Biofabrication 2019, 11, 025002. [Google Scholar] [CrossRef]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural polymers-based light-induced hydrogels: Promising biomaterials for biomedical applications. Coord. Chem. Rev. 2020, 420, 213432. [Google Scholar] [CrossRef]
- Chae, S.; Lee, S.-S.; Choi, Y.-J.; Hong, D.H.; Gao, G.; Wang, J.H.; Cho, D.-W. 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink. Biomaterials 2021, 267, 120466. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar]
- Hall, B.K. Selective proliferation and accumulation of chondroprogenitor cells as the mode of action of biomechanical factors during secondary chondrogenesis. Teratology 1979, 20, 81–91. [Google Scholar] [CrossRef]
- Fang, J.; Hall, B.K. Differential expression of neural cell adhesion molecule (NCAM) during osteogenesis and secondary chondrogenesis in the embryonic chick. Int. J. Dev. Biol. 1995, 39, 519–528. [Google Scholar] [PubMed]
- Mizuno, S.; Ogawa, R. Using changes in hydrostatic and osmotic pressure to manipulate metabolic function in chondrocytes. Am. J. Physiol. Cell Physiol. 2011, 300, C1234–C1245. [Google Scholar] [CrossRef] [Green Version]
- Carroll, S.; Buckley, C.; Kelly, D. Cyclic hydrostatic pressure promotes a stable cartilage phenotype and enhances the functional development of cartilaginous grafts engineered using multipotent stromal cells isolated from bone marrow and infrapatellar fat pad. J. Biomech. 2014, 47, 2115–2121. [Google Scholar] [CrossRef]
- Correia, C.; Pereira, A.L.; Duarte, A.; Frias, A.M.; Pedro, A.; Oliveira, J.T.; Sousa, R.; Reis, R.L. Dynamic culturing of cartilage tissue: The significance of hydrostatic pressure. Tissue Eng. Part A 2012, 18, 1979–1991. [Google Scholar] [CrossRef] [Green Version]
- Elder, S.H.; Shim, J.W.; Borazjani, A.; Robertson, H.M.; E Smith, K.; Warnock, J.N. Influence of hydrostatic and distortional stress on chondroinduction. Biorheology 2008, 45, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Elder, B.D.; Athanasiou, K.A. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J. Orthop. Res. 2008, 26, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Huwe, L.W.; Paschos, N.; Aryaei, A.; Gegg, C.A.; Hu, J.C.; Athanasiou, K.A. Tension stimulation drives tissue formation in scaffold-free systems. Nat. Mater. 2017, 16, 864–873. [Google Scholar] [CrossRef] [Green Version]
- Luis, E.; Pan, H.M.; Sing, S.L.; Bajpai, R.; Song, J.; Yeong, W.Y. 3D Direct Printing of Silicone Meniscus Implant Using a Novel Heat-Cured Extrusion-Based Printer. Polymers 2020, 12, 1031. [Google Scholar] [CrossRef]
- Luis, E.; Pan, H.M.; Bastola, A.K.; Bajpai, R.; Sing, S.L.; Song, J.; Yeong, W.Y. 3D Printed Silicone Meniscus Implants: Influence of the 3D Printing Process on Properties of Silicone Implants. Polymers 2020, 12, 2136. [Google Scholar] [CrossRef]
- Hu, J.C.; Athanasiou, K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006, 12, 969–979. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [Green Version]
- Huey, D.J.; Athanasiou, K.A. Alteration of the fibrocartilaginous nature of scaffoldless constructs formed from leporine meniscus cells and chondrocytes through manipulation of culture and processing conditions. Cells Tissues Organs 2013, 197, 360–371. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, H.; Yuan, Z.; Fu, L.; Jiang, S.; Gao, C.; Wang, F.; Zha, K.; Tian, G.; Sun, Z.; et al. Endogenous cell recruitment strategy for articular cartilage regeneration. Acta Biomater. 2020, 114, 31–52. [Google Scholar] [CrossRef]
- Patel, J.M.; Saleh, K.S.; Burdick, J.A.; Mauck, R.L. Bioactive factors for cartilage repair and regeneration: Improving delivery, retention, and activity. Acta Biomater. 2019, 93, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Z.; Jiang, D.; Ding, J.-X.; Wang, S.-J.; Zhang, L.; Zhang, J.-Y.; Qi, Y.-S.; Chen, X.-S.; Yu, J.-K. Role of scaffold mean pore size in meniscus regeneration. Acta Biomater. 2016, 43, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Gulko, J.; Kim, D.; Sim, K.H.; Gutman, S.; Yang, J.; Cook, J.; Lee, C.H. Effect of dose and release rate of CTGF and TGFβ3 on avascular meniscus healing. J. Orthop. Res. 2019, 37, 1555–1562. [Google Scholar] [CrossRef]
- Baek, J.; Lee, E.; Lotz, M.K.; D’Lima, D.D. Bioactive proteins delivery through core-shell nanofibers for meniscal tissue regeneration. Nanomedicine 2020, 23, 102090. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, M.D.M.; Mangiavini, L.; Peretti, G.M. Biomaterials and Meniscal Lesions: Current Concepts and Future Perspective. Pharmaceutics 2021, 13, 1886. https://doi.org/10.3390/pharmaceutics13111886
Lombardo MDM, Mangiavini L, Peretti GM. Biomaterials and Meniscal Lesions: Current Concepts and Future Perspective. Pharmaceutics. 2021; 13(11):1886. https://doi.org/10.3390/pharmaceutics13111886
Chicago/Turabian StyleLombardo, Michele D. M., Laura Mangiavini, and Giuseppe M. Peretti. 2021. "Biomaterials and Meniscal Lesions: Current Concepts and Future Perspective" Pharmaceutics 13, no. 11: 1886. https://doi.org/10.3390/pharmaceutics13111886
APA StyleLombardo, M. D. M., Mangiavini, L., & Peretti, G. M. (2021). Biomaterials and Meniscal Lesions: Current Concepts and Future Perspective. Pharmaceutics, 13(11), 1886. https://doi.org/10.3390/pharmaceutics13111886






