Status Quo and Trends of Intra-Arterial Therapy for Brain Tumors: A Bibliometric and Clinical Trials Analysis
Abstract
:1. Introduction
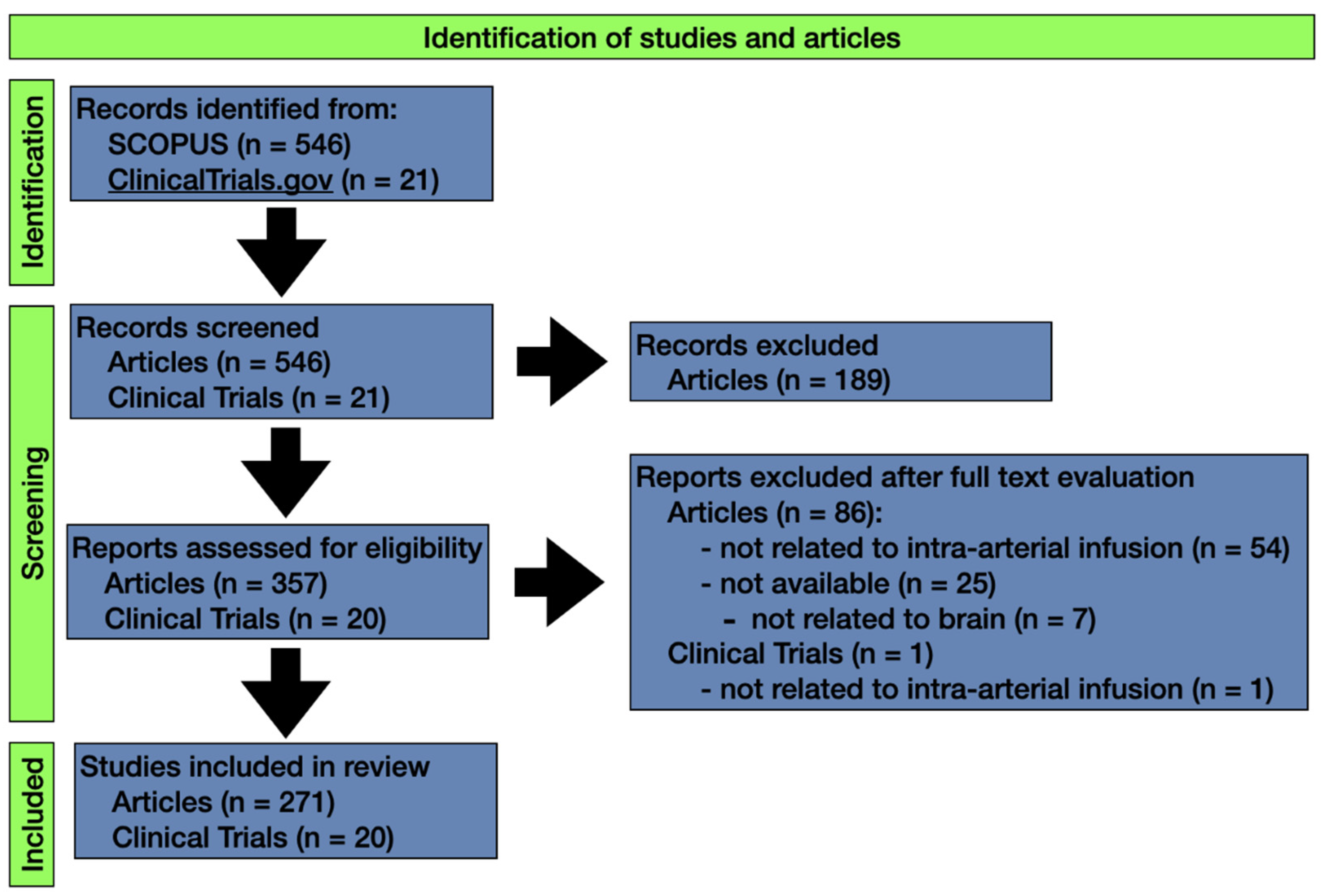
2. Methodology
3. Results
3.1. Article Characteristics
3.2. Clinical Trial Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Improving drug delivery to primary and metastatic brain tumors: Strategies to overcome the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Tzika, A.A.; Astrakas, L.G.; Zarifi, M.K.; Zurakowski, D.; Poussaint, T.Y.; Goumnerova, L.; Tarbell, N.J.; Black, P.M. Spectroscopic and perfusion magnetic resonance imaging predictors of progression in pediatric brain tumors. Cancer 2004, 100, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Arvold, N.D.; Lee, E.Q.; Mehta, M.P.; Margolin, K.; Alexander, B.M.; Lin, N.U.; Anders, C.K.; Soffietti, R.; Camidge, D.R.; Vogelbaum, M.A.; et al. Updates in the management of brain metastases. Neuro Oncol. 2016, 18, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pitz, M.W.; Desai, A.; Grossman, S.A.; Blakeley, J.O. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J. Neuro-Oncol. 2011, 104, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Sun, T.; Wan, W.; Wu, Z.; Zhang, J.; Zhang, L. Clinical outcomes and natural history of pediatric brainstem tumors: With 33 cases follow-ups. Neurosurg. Rev. 2013, 36, 311–319; discussion 319–320. [Google Scholar] [CrossRef]
- Cohen, K.J.; Jabado, N.; Grill, J. Diffuse intrinsic pontine gliomas-current management and new biologic insights. Is there a glimmer of hope? Neuro Oncol. 2017, 19, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Frazier, J.L.; Lee, J.; Thomale, U.W.; Noggle, J.C.; Cohen, K.J.; Jallo, G.I. Treatment of diffuse intrinsic brainstem gliomas: Failed approaches and future strategies. J. Neurosurg. Pediatr. 2009, 3, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Vanan, M.I.; Eisenstat, D.D. DIPG in Children—What Can We Learn from the Past? Front. Oncol. 2015, 5, 237. [Google Scholar] [CrossRef] [Green Version]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.Y.; Jones, D.T.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.A.; Tönjes, M.; et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Wu, G.; Broniscer, A.; McEachron, T.A.; Lu, C.; Paugh, B.S.; Becksfort, J.; Qu, C.; Ding, L.; Huether, R.; Parker, M.; et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012, 44, 251–253. [Google Scholar] [CrossRef] [Green Version]
- Zorzan, M.; Giordan, E.; Redaelli, M.; Caretta, A.; Mucignat-Caretta, C. Molecular targets in glioblastoma. Future Oncol. 2015, 11, 1407–1420. [Google Scholar] [CrossRef]
- Wei, W.; Shin, Y.S.; Xue, M.; Matsutani, T.; Masui, K.; Yang, H.; Ikegami, S.; Gu, Y.; Herrmann, K.; Johnson, D.; et al. Single-Cell Phosphoproteomics Resolves Adaptive Signaling Dynamics and Informs Targeted Combination Therapy in Glioblastoma. Cancer Cell 2016, 29, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood-brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef] [PubMed]
- Kumar Yadav, S.; Kumar Srivastava, A.; Dev, A.; Kaundal, B.; Roy Choudhury, S.; Karmakar, S. Nanomelatonin triggers superior anticancer functionality in a human malignant glioblastoma cell line. Nanotechnology 2017, 28, 365102. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv Rev. 2020, 165–166, 77–95. [Google Scholar] [CrossRef]
- Gutenberg, A.; Bock, H.C.; Brück, W.; Doerner, L.; Mehdorn, H.M.; Roggendorf, W.; Westphal, M.; Felsberg, J.; Reifenberger, G.; Giese, A. MGMT promoter methylation status and prognosis of patients with primary or recurrent glioblastoma treated with carmustine wafers. Br. J. Neurosurg. 2013, 27, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Householder, K.T.; Dharmaraj, S.; Sandberg, D.I.; Wechsler-Reya, R.J.; Sirianni, R.W. Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice. Sci. Rep. 2019, 9, 12587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroll, R.A.; Neuwelt, E.A. Outwitting the blood-brain barrier for therapeutic purposes: Osmotic opening and other means. Neurosurgery 1998, 42, 1083–1099; discussion 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, F.; Corti, A.; Ferreri, A.J.M. Breaching the Blood-Brain Tumor Barrier for Tumor Therapy. Cancers 2021, 13, 2391. [Google Scholar] [CrossRef] [PubMed]
- Niwińska, A.; Rudnicka, H.; Murawska, M. Breast cancer leptomeningeal metastasis: The results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin. Breast Cancer 2015, 15, 66–72. [Google Scholar] [CrossRef]
- Wait, S.D.; Prabhu, R.S.; Burri, S.H.; Atkins, T.G.; Asher, A.L. Polymeric drug delivery for the treatment of glioblastoma. Neuro Oncol. 2015, 17 (Suppl. 2), ii9–ii23. [Google Scholar] [CrossRef] [Green Version]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [Green Version]
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Kunigelis, K.E.; Vogelbaum, M.A. Therapeutic Delivery to Central Nervous System. Neurosurg. Clin. N. Am. 2021, 32, 291–303. [Google Scholar] [CrossRef]
- Huang, R.; Boltze, J.; Li, S. Strategies for Improved Intra-arterial Treatments Targeting Brain Tumors: A Systematic Review. Front. Oncol. 2020, 10, 1443. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol 2008, 34, 1093–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavance, M.A.; Blanchette, M.; Fortin, D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008, 10, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Doolittle, N.D.; Miner, M.E.; Hall, W.A.; Siegal, T.; Jerome, E.; Osztie, E.; McAllister, L.D.; Bubalo, J.S.; Kraemer, D.F.; Fortin, D.; et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 2000, 88, 637–647. [Google Scholar] [CrossRef]
- Boockvar, J.A.; Tsiouris, A.J.; Hofstetter, C.P.; Kovanlikaya, I.; Fralin, S.; Kesavabhotla, K.; Seedial, S.M.; Pannullo, S.C.; Schwartz, T.H.; Stieg, P.; et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J. Neurosurg. 2011, 114, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Riina, H.A.; Knopman, J.; Greenfield, J.P.; Fralin, S.; Gobin, Y.P.; Tsiouris, A.J.; Souweidane, M.M.; Boockvar, J.A. Balloon-assisted superselective intra-arterial cerebral infusion of bevacizumab for malignant brainstem glioma. A technical note. Interv. Neuroradiol. 2010, 16, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Filippi, C.G.; Wong, T.; Ray, A.; Fralin, S.; Tsiouris, A.J.; Praminick, B.; Demopoulos, A.; McCrea, H.J.; Bodhinayake, I.; et al. Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: Phase I study. J. Neurooncol. 2016, 128, 405–415. [Google Scholar] [CrossRef]
- Hochberg, F.H.; Pruitt, A.A.; Beck, D.O.; DeBrun, G.; Davis, K. The rationale and methodology for intra-arterial chemotherapy with BCNU as treatment for glioblastoma. J. Neurosurg. 1985, 63, 876–880. [Google Scholar] [CrossRef] [Green Version]
- Ashby, L.S.; Shapiro, W.R. Intra-arterial cisplatin plus oral etoposide for the treatment of recurrent malignant glioma: A phase II study. J. Neurooncol. 2001, 51, 67–86. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Crowley, J.; Eyre, H.; Weiden, P.; Eltringham, J.; Stuckey, W.J. A phase II randomized study comparing sequential and combined intraarterial cisplatin and radiation therapy in primary brain tumors. A Southwest Oncology Group study. Cancer 1992, 69, 1220–1223. [Google Scholar] [CrossRef]
- Imbesi, F.; Marchioni, E.; Benericetti, E.; Zappoli, F.; Galli, A.; Corato, M.; Ceroni, M. A randomized phase III study: Comparison between intravenous and intraarterial ACNU administration in newly diagnosed primary glioblastomas. Anticancer Res. 2006, 26, 553–558. [Google Scholar] [PubMed]
- Stewart, D.J.; Belanger, J.M.; Grahovac, Z.; Curuvija, S.; Gionet, L.R.; Aitken, S.E.; Hugenholtz, H.; Benoit, B.G.; DaSilva, V.F. Phase I study of intracarotid administration of carboplatin. Neurosurgery 1992, 30, 512–516; discussion 516–517. [Google Scholar] [CrossRef]
- Theodotou, C.; Shah, A.H.; Hayes, S.; Bregy, A.; Johnson, J.N.; Aziz-Sultan, M.A.; Komotar, R.J. The role of intra-arterial chemotherapy as an adjuvant treatment for glioblastoma. Br. J. Neurosurg. 2014, 28, 438–446. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Pfiffner, P.B.; Oh, J.; Miller, T.A.; Mandl, K.D. ClinicalTrials.gov as a data source for semi-automated point-of-care trial eligibility screening. PLoS ONE 2014, 9, e111055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huser, V.; Cimino, J.J. Linking ClinicalTrials.gov and PubMed to track results of interventional human clinical trials. PLoS ONE 2013, 8, e68409. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Yoon, D.Y.; Yun, E.J.; Seo, Y.L.; Baek, S.; Gu, D.H.; Yoon, S.J.; Han, A.; Ku, Y.J.; Kim, S.S. Characteristics and trends of radiology research: A survey of original articles published in AJR and Radiology between 2001 and 2010. Radiology 2012, 264, 796–802. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; Volume 445, pp. 56–61. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, F.H.; Miller, D.C. Primary central nervous system lymphoma. J. Neurosurg. 1988, 68, 835–853. [Google Scholar] [CrossRef]
- Matsukado, K.; Inamura, T.; Nakano, S.; Fukui, M.; Bartus, R.T.; Black, K.L. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery 1996, 39, 125–133; discussion 133–124. [Google Scholar] [CrossRef]
- Angelov, L.; Doolittle, N.D.; Kraemer, D.F.; Siegal, T.; Barnett, G.H.; Peereboom, D.M.; Stevens, G.; McGregor, J.; Jahnke, K.; Lacy, C.A.; et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: A multi-institutional experience. J. Clin. Oncol. 2009, 27, 3503–3509. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.K.; Jakowatz, J.G.; Pollack, R.B.; Ceraldi, C.; Yamamoto, R.; Dett, C.; Lopez, F.; Camacho, C.; Carson, W.E.; Sentovich, S.M.; et al. Effects of intracarotid and intravenous infusion of human TNF and LT on established intracerebral rat gliomas. Lymphokine Cytokine Res. 1991, 10, 189–194. [Google Scholar]
- Mao, J.; Cao, M.; Zhang, F.; Zhang, J.; Duan, X.; Lu, L.; Yang, Z.; Zhang, X.; Zhu, W.; Zhang, Q.; et al. Peritumoral administration of IFNβ upregulated mesenchymal stem cells inhibits tumor growth in an orthotopic, immunocompetent rat glioma model. J. Immunother Cancer 2020, 8, e000164. [Google Scholar] [CrossRef] [Green Version]
- Aoki, S.; Terada, H.; Kosuda, S.; Shitara, N.; Fujii, H.; Suzuki, K.; Kutsukake, Y.; Tanaka, J.; Sasaki, Y.; Okubo, T.; et al. Supraophthalmic chemotherapy with long tapered catheter: Distribution evaluated with intraarterial and intravenous Tc-99m HMPAO. Radiology 1993, 188, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Dahlborg, S.A.; Petrillo, A.; Crossen, J.R.; Roman-Goldstein, S.; Doolittle, N.D.; Fuller, K.H.; Neuwelt, E.A. The potential for complete and durable response in nonglial primary brain tumors in children and young adults with enhanced chemotherapy delivery. Cancer J. Sci. Am. 1998, 4, 110–124. [Google Scholar] [PubMed]
- Roman-Goldstein, S.; Mitchell, P.; Crossen, J.R.; Williams, P.C.; Tindall, A.; Neuwelt, E.A. MR and cognitive testing of patients undergoing osmotic blood-brain barrier disruption with intraarterial chemotherapy. Am. J. Neuroradiol. 1995, 16, 543–553. [Google Scholar]
- Uluc, K.; Siler, D.A.; Lopez, R.; Varallyay, C.; Netto, J.P.; Firkins, J.; Lacy, C.; Huddleston, A.; Ambady, P.; Neuwelt, E.A. Long-Term Outcomes of Intra-Arterial Chemotherapy for Progressive or Unresectable Pilocytic Astrocytomas: Case Studies. Neurosurgery 2021, 88, E336–E342. [Google Scholar] [CrossRef]
- Muldoon, L.L.; Pagel, M.A.; Netto, J.P.; Neuwelt, E.A. Intra-arterial administration improves temozolomide delivery and efficacy in a model of intracerebral metastasis, but has unexpected brain toxicity. J. Neurooncol. 2016, 126, 447–454. [Google Scholar] [CrossRef]
- Osztie, E.; Várallyay, P.; Doolittle, N.D.; Lacy, C.; Jones, G.; Nickolson, H.S.; Neuwelt, E.A. Combined intraarterial carboplatin, intraarterial etoposide phosphate, and IV Cytoxan chemotherapy for progressive optic-hypothalamic gliomas in young children. Am. J. Neuroradiol. 2001, 22, 818–823. [Google Scholar] [PubMed]
- Doolittle, N.D.; Muldoon, L.L.; Brummett, R.E.; Tyson, R.M.; Lacy, C.; Bubalo, J.S.; Kraemer, D.F.; Heinrich, M.C.; Henry, J.A.; Neuwelt, E.A. Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin. Cancer Res. 2001, 7, 493–500. [Google Scholar]
- Kulason, K.O.; Schneider, J.R.; Chakraborty, S.; Filippi, C.G.; Pramanik, B.; Wong, T.; Fralin, S.; Tan, K.; Ray, A.; Alter, R.A.; et al. Superselective intraarterial cerebral infusion of cetuximab with blood brain barrier disruption combined with Stupp Protocol for newly diagnosed glioblastoma. J. Exp. Ther. Oncol. 2018, 12, 223–229. [Google Scholar]
- Shin, B.J.; Burkhardt, J.K.; Riina, H.A.; Boockvar, J.A. Superselective intra-arterial cerebral infusion of novel agents after blood-brain disruption for the treatment of recurrent glioblastoma multiforme: A technical case series. Neurosurg. Clin. N. Am. 2012, 23, 323–329. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Khatri, D.; Reichman, N.; Patel, N.V.; Wong, T.; Fralin, S.R.; Li, M.; Ellis, J.A.; Ortiz, R.; Langer, D.J.; et al. Super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood-brain barrier disruption: Where are we now, and where we are going. J. Neurooncol. 2020, 147, 261–278. [Google Scholar] [CrossRef]
- Riina, H.A.; Fraser, J.F.; Fralin, S.; Knopman, J.; Scheff, R.J.; Boockvar, J.A. Superselective intraarterial cerebral infusion of bevacizumab: A revival of interventional neuro-oncology for malignant glioma. J. Exp. Ther. Oncol. 2009, 8, 145–150. [Google Scholar]
- Faltings, L.; Kulason, K.O.; Patel, N.V.; Wong, T.; Fralin, S.; Li, M.; Schneider, J.R.; Filippi, C.G.; Langer, D.J.; Ortiz, R.; et al. Rechallenging Recurrent Glioblastoma with Intra-Arterial Bevacizumab with Blood Brain-Barrier Disruption Results in Radiographic Response. World Neurosurg. 2019, 131, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.K.; Riina, H.A.; Shin, B.J.; Moliterno, J.A.; Hofstetter, C.P.; Boockvar, J.A. Intra-arterial chemotherapy for malignant gliomas: A critical analysis. Interv. Neuroradiol. 2011, 17, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Huashan Hospital. Cerebral Blood Perfusion Changes after General Anesthesia for Craniotomy. Available online: https://ClinicalTrials.gov/show/NCT01642147 (accessed on 3 August 2021).
- OHSU Knight Cancer Institute; Oregon Health and Science University. Combination Chemotherapy With or Without Sodium Thiosulfate in Preventing Low Platelet Count While Treating Patients With Malignant Brain Tumors. Available online: https://ClinicalTrials.gov/show/NCT00075387 (accessed on 3 August 2021).
- Ohio State University Comprehensive Cancer Center. Carboplatin and Temozolomide (Temodar) for Recurrent and Symptomatic Residual Brain Metastases. Available online: https://ClinicalTrials.gov/show/NCT00362817 (accessed on 3 August 2021).
- Northwell Health; Feinstein Institute for Medical Research; Hofstra North Shore. Super-Selective Intraarterial Cerebral Infusion of Bevacizumab (Avastin) for Treatment of Vestibular Schwannoma. Available online: https://ClinicalTrials.gov/show/NCT01083966 (accessed on 3 August 2021).
- Northwell Health; Feinstein Institute for Medical Research. Repeated Super-Selective Intraarterial Cerebral Infusion of Bevacizumab (Avastin) for Treatment of Newly Diagnosed GBM. Available online: https://ClinicalTrials.gov/show/NCT01811498 (accessed on 3 August 2021).
- Northwell Health. Super-Selective Intra-arterial Repeated Infusion of Cetuximab for the Treatment of Newly Diagnosed Glioblastoma. Available online: https://ClinicalTrials.gov/show/NCT02861898 (accessed on 3 August 2021).
- Northwell Health. Super Selective Intra-Arterial Repeated Infusion of Cetuximab (Erbitux) with Reirradiation for Treatment of Relapsed/Refractory GBM, AA, and AOA. Available online: https://ClinicalTrials.gov/show/NCT02800486 (accessed on 3 August 2021).
- Northwell Health. Super-Selective Intraarterial Cerebral Infusion of Cetuximab (Erbitux) for Treatment of Relapsed/Refractory GBM and AA. Available online: https://ClinicalTrials.gov/show/NCT01238237 (accessed on 3 August 2021).
- Northwell Health. Super-Selective Intra-Arterial Cerebral Infusion of Trastuzumab for the Treatment of Cerebral Metastases of HER2/Neu Positive Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT02571530 (accessed on 3 August 2021).
- Northwell Health. Super-Selective Intraarterial Intracranial Infusion of Avastin (Bevacizumab). Available online: https://ClinicalTrials.gov/show/NCT00968240 (accessed on 3 August 2021).
- Northwell Health; Feinstein Institute for Medical Research; Hofstra North Shore. Super-Selective Intraarterial Cerebral Infusion Of Temozolomide (Temodar) For Treatment Of Newly Diagnosed GBM And AA. Available online: https://ClinicalTrials.gov/show/NCT01180816 (accessed on 3 August 2021).
- Northwell Health; Feinstein Institute for Medical Research. Repeated Super-Selective Intraarterial Cerebral Infusion of Bevacizumab (Avastin) for Treatment of Relapsed GBM and AA. Available online: https://ClinicalTrials.gov/show/NCT01269853 (accessed on 3 August 2021).
- Northwell Health. Repeated Super-Selective Intraarterial Cerebral Infusion of Bevacizumab Plus Carboplatin For Treatment Of Relapsed/Refractory GBM And Anaplastic Astrocytoma. Available online: https://ClinicalTrials.gov/show/NCT01386710 (accessed on 3 August 2021).
- OHSU Knight Cancer Institute; National Cancer Institute (NCI). Melphalan with BBBD in Treating Patients with Brain Malignancies. Available online: https://ClinicalTrials.gov/show/NCT00253721 (accessed on 3 August 2021).
- OHSU Knight Cancer Institute; National Institute of Neurological Disorders and Stroke (NINDS); Oregon Health and Science University. Melphalan, Carboplatin, Mannitol, and Sodium Thiosulfate in Treating Patients With Recurrent or Progressive CNS Embryonal or Germ Cell Tumors. Available online: https://ClinicalTrials.gov/show/NCT00983398 (accessed on 3 August 2021).
- Huazhong University of Science and Technology; Beijing Tiantan Hospital; Beijing Chao Yang Hospital; Beijing Friendship Hospital. ADV-TK Improves Outcome of Recurrent High-Grade Glioma. Available online: https://ClinicalTrials.gov/show/NCT00870181 (accessed on 3 August 2021).
- Université de Sherbrooke. IA Carboplatin + Radiotherapy in Relapsing GBM. Available online: https://ClinicalTrials.gov/show/NCT03672721 (accessed on 3 August 2021).
- Srinivasan, V.M.; Lang, F.F.; Chen, S.R.; Chen, M.M.; Gumin, J.; Johnson, J.; Burkhardt, J.K.; Kan, P. Advances in endovascular neuro-oncology: Endovascular selective intra-arterial (ESIA) infusion of targeted biologic therapy for brain tumors. J. Neurointerv. Surg. 2020, 12, 197–203. [Google Scholar] [CrossRef]
- Klopp, C.T.; Alford, T.C.; Bateman, J.; Berry, G.N.; Winship, T. Fractionated intra-arterial cancer; chemotherapy with methyl bis amine hydrochloride; a preliminary report. Ann. Surg 1950, 132, 811–832. [Google Scholar] [CrossRef] [PubMed]
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; Solving Kids’ Cancer. Intra-Arterial Chemotherapy for the Treatment of Progressive Diffuse Intrinsic Pontine Gliomas (DIPG). Available online: https://ClinicalTrials.gov/show/NCT01688401 (accessed on 3 August 2021).
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Sharma, P.; Debinski, W. Receptor-Targeted Glial Brain Tumor Therapies. Int J. Mol. Sci. 2018, 19, 3326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Ellis, J.A.; Ornstein, E.; Bruce, J.N. Intraarterial drug delivery for glioblastoma mutiforme: Will the phoenix rise again? J. Neurooncol. 2015, 124, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Chen, Y.F.; Chen, F.D.; Hwang, J.J.; Chen, J.C.; Liu, R.S.; Kai, J.J.; Chang, C.W.; Wang, H.E. Evaluation of pharmacokinetics of 4-borono-2-(18)F-fluoro-L-phenylalanine for boron neutron capture therapy in a glioma-bearing rat model with hyperosmolar blood-brain barrier disruption. J. Nucl. Med. 2005, 46, 1858–1865. [Google Scholar] [PubMed]
- Yuan, F.; Salehi, H.A.; Boucher, Y.; Vasthare, U.S.; Tuma, R.F.; Jain, R.K. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994, 54, 4564–4568. [Google Scholar]
- Neuwelt, E.A.; Barnett, P.A.; McCormick, C.I.; Remsen, L.G.; Kroll, R.A.; Sexton, G. Differential permeability of a human brain tumor xenograft in the nude rat: Impact of tumor size and method of administration on optimizing delivery of biologically diverse agents. Clin. Cancer Res. 1998, 4, 1549–1555. [Google Scholar]
- Neuwelt, E.A.; Pagel, M.A.; Hasler, B.P.; Deloughery, T.G.; Muldoon, L.L. Therapeutic efficacy of aortic administration of N-acetylcysteine as a chemoprotectant against bone marrow toxicity after intracarotid administration of alkylators, with or without glutathione depletion in a rat model. Cancer Res. 2001, 61, 7868–7874. [Google Scholar]
- Neuwelt, E.A.; Hill, S.A.; Frenkel, E.P.; Diehl, J.T.; Maravilla, K.R.; Vu, L.H.; Clark, W.K.; Rapoport, S.I.; Barnett, P.A.; Lewis, S.E.; et al. Osmotic blood-brain barrier disruption: Pharmacodynamic studies in dogs and a clinical phase I trial in patients with malignant brain tumors. Cancer Treat. Rep. 1981, 65 (Suppl. 2), 39–43. [Google Scholar]
- Rajappa, P.; Krass, J.; Riina, H.A.; Boockvar, J.A.; Greenfield, J.P. Super-selective basilar artery infusion of bevacizumab and cetuximab for multiply recurrent pediatric ependymoma. Interv. Neuroradiol. 2011, 17, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Perese, D.M.; Day, C.E.; Chardach, W.M. Chemotherapy of brain tumors by intra-arterial infusion. J. Neurosurg. 1962, 19, 215–219. [Google Scholar] [CrossRef]
- Campanella, R.; Guarnaccia, L.; Caroli, M.; Zarino, B.; Carrabba, G.; La Verde, N.; Gaudino, C.; Rampini, A.; Luzzi, S.; Riboni, L.; et al. Personalized and translational approach for malignant brain tumors in the era of precision medicine: The strategic contribution of an experienced neurosurgery laboratory in a modern neurosurgery and neuro-oncology department. J. Neurol. Sci. 2020, 417, 117083. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Parney, I. Journal of Neuro Oncology: Immunotherapy for brain tumors. J. Neurooncol. 2021, 151, 1. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Trabatti, C.; Mosconi, M.; Savasta, S.; Foiadelli, T. The impact of stem cells in neuro-oncology: Applications, evidence, limitations and challenges. Acta Biomed. 2020, 91, 51–60. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Bartus, R.T.; Elliott, P.; Hayward, N.; Dean, R.; McEwen, E.L.; Fisher, S.K. Permeability of the blood brain barrier by the bradykinin agonist, RMP-7: Evidence for a sensitive, auto-regulated, receptor-mediated system. Immunopharmacology 1996, 33, 270–278. [Google Scholar] [CrossRef]
- Englander, Z.K.; Wei, H.J.; Pouliopoulos, A.N.; Bendau, E.; Upadhyayula, P.; Jan, C.I.; Spinazzi, E.F.; Yoh, N.; Tazhibi, M.; McQuillan, N.M.; et al. Focused ultrasound mediated blood-brain barrier opening is safe and feasible in a murine pontine glioma model. Sci. Rep. 2021, 11, 6521. [Google Scholar] [CrossRef]
- Columbia University; Focused Ultrasound Foundation. Non-Invasive Focused Ultrasound (FUS) with Oral Panobinostat in Children with Progressive Diffuse Midline Glioma (DMG). Available online: https://clinicaltrials.gov/ct2/show/NCT04804709 (accessed on 30 October 2021).
- Drapeau, A.; Fortin, D. Chemotherapy Delivery Strategies to the Central Nervous System: Neither Optional nor Superfluous. Curr. Cancer Drug Targets 2015, 15, 752–768. [Google Scholar] [CrossRef]
- Charest, G.; Sanche, L.; Fortin, D.; Mathieu, D.; Paquette, B. Glioblastoma treatment: Bypassing the toxicity of platinum compounds by using liposomal formulation and increasing treatment efficiency with concomitant radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; David, A.; Chertok, B.; Cole, A.; Lee, K.; Zhang, J.; Wang, J.; Huang, Y.; Yang, V.C. Magnetic nanoparticles for tumor imaging and therapy: A so-called theranostic system. Pharm. Res. 2013, 30, 2445–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Singh-Moon, R.P.; Ellis, J.A.; Chaudhuri, D.B.; Wang, M.; Reif, R.; Bruce, J.N.; Bigio, I.J.; Straubinger, R.M. Cerebral hypoperfusion-assisted intra-arterial deposition of liposomes in normal and glioma-bearing rats. Neurosurgery 2015, 76, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Cooke, J.R.; Chan, D.K.; Ellis, J.A.; Hossain, S.S.; Singh-Moon, R.P.; Wang, M.; Bigio, I.J.; Bruce, J.N.; Straubinger, R.M. Liposome size and charge optimization for intraarterial delivery to gliomas. Drug Deliv. Transl. Res. 2016, 6, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, J.; Cooke, J.R.N.; Ellis, J.A.; Deci, M.; Emala, C.W.; Bruce, J.N.; Bigio, I.J.; Straubinger, R.M.; Joshi, S. Cationizable lipid micelles as vehicles for intraarterial glioma treatment. J. Neurooncol. 2016, 128, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, J.; Hossain, S.S.; Cooke, J.R.N.; Ellis, J.A.; Deci, M.B.; Emala, C.W.; Bruce, J.N.; Bigio, I.J.; Straubinger, R.M.; Joshi, S. Flow arrest intra-arterial delivery of small TAT-decorated and neutral micelles to gliomas. J. Neurooncol. 2017, 133, 77–85. [Google Scholar] [CrossRef]
- Rainov, N.G.; Zimmer, C.; Chase, M.; Kramm, C.M.; Chiocca, E.A.; Weissleder, R.; Breakefield, X.O. Selective uptake of viral and monocrystalline particles delivered intra-arterially to experimental brain neoplasms. Hum. Gene Ther. 1995, 6, 1543–1552. [Google Scholar] [CrossRef]
- McCrorie, P.; Vasey, C.E.; Smith, S.J.; Marlow, M.; Alexander, C.; Rahman, R. Biomedical engineering approaches to enhance therapeutic delivery for malignant glioma. J. Control. Release 2020, 328, 917–931. [Google Scholar] [CrossRef] [PubMed]
- A Power, E.; Rechberger, J.S.; Lu, V.M.; Daniels, D.J. The emerging role of nanotechnology in pursuit of successful drug delivery to H3K27M diffuse midline gliomas. Nanomedicine 2021, 16. [Google Scholar] [CrossRef]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredlau, A.L.; Dixit, S.; Chen, C.; Broome, A.M. Nanotechnology Applications for Diffuse Intrinsic Pontine Glioma. Curr Neuropharmacol 2017, 15, 104–115. [Google Scholar] [CrossRef] [Green Version]
- El-Khouly, F.E.; van Vuurden, D.G.; Stroink, T.; Hulleman, E.; Kaspers, G.J.L.; Hendrikse, N.H.; Veldhuijzen van Zanten, S.E.M. Effective Drug Delivery in Diffuse Intrinsic Pontine Glioma: A Theoretical Model to Identify Potential Candidates. Front. Oncol. 2017, 7, 254. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- Donovan, L.K.; Delaidelli, A.; Joseph, S.K.; Bielamowicz, K.; Fousek, K.; Holgado, B.L.; Manno, A.; Srikanthan, D.; Gad, A.Z.; Van Ommeren, R.; et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 2020, 26, 720–731. [Google Scholar] [CrossRef]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Theruvath, J.; Sotillo, E.; Mount, C.W.; Graef, C.M.; Delaidelli, A.; Heitzeneder, S.; Labanieh, L.; Dhingra, S.; Leruste, A.; Majzner, R.G.; et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med. 2020, 26, 712–719. [Google Scholar] [CrossRef]
- Vora, P.; Venugopal, C.; Salim, S.K.; Tatari, N.; Bakhshinyan, D.; Singh, M.; Seyfrid, M.; Upreti, D.; Rentas, S.; Wong, N.; et al. The Rational Development of CD133-Targeting Immunotherapies for Glioblastoma. Cell Stem Cell 2020, 26, 832–844.e836. [Google Scholar] [CrossRef] [PubMed]
- Ingegnere, T.; Mariotti, F.R.; Pelosi, A.; Quintarelli, C.; De Angelis, B.; Tumino, N.; Besi, F.; Cantoni, C.; Locatelli, F.; Vacca, P.; et al. Human CAR NK Cells: A New Non-viral Method Allowing High Efficient Transfection and Strong Tumor Cell Killing. Front. Immunol. 2019, 10, 957. [Google Scholar] [CrossRef] [Green Version]
- Kennis, B.A.; Michel, K.A.; Brugmann, W.B.; Laureano, A.; Tao, R.H.; Somanchi, S.S.; Einstein, S.A.; Bravo-Alegria, J.B.; Maegawa, S.; Wahba, A.; et al. Monitoring of intracerebellarly-administered natural killer cells with fluorine-19 MRI. J. Neurooncol. 2019, 142, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, J.H.; Kwack, K.; Choi, S.W. Natural Killer Cell Therapy: A New Treatment Paradigm for Solid Tumors. Cancers 2019, 11, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- City of Hope Medical Center; National Cancer Institute (NCI); Food and Drug Administration (FDA). Genetically Modified T-cells in Treating Patients with Recurrent or Refractory Malignant Glioma. Available online: https://ClinicalTrials.gov/show/NCT02208362 (accessed on 30 October 2021).
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Occhiogrosso, G.; Edgar, M.A.; Sandberg, D.I.; Souweidane, M.M. Prolonged convection-enhanced delivery into the rat brainstem. Neurosurgery 2003, 52, 388–393; discussion 393–384. [Google Scholar] [CrossRef]
- Rechberger, J.S.; Power, E.A.; Lu, V.M.; Zhang, L.; Sarkaria, J.N.; Daniels, D.J. Evaluating infusate parameters for direct drug delivery to the brainstem: A comparative study of convection-enhanced delivery versus osmotic pump delivery. Neurosurg. Focus 2020, 48, E2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therapeutics, Y.-M.; Labcorp Drug Development; Invicro. 131I-Omburtamab Delivered by Convection-Enhanced Delivery in Patients with Diffuse Intrinsic Pontine Glioma. Available online: https://ClinicalTrials.gov/show/NCT05063357 (accessed on 30 October 2021).
- OncoSynergy, Inc.; Infuseon Therapeutics, Inc. Convection-Enhanced Delivery of OS2966 for Patients with High-grade Glioma Undergoing a Surgical Resection. Available online: https://ClinicalTrials.gov/show/NCT04608812 (accessed on 30 October 2021).
- Istari Oncology, Inc. EAP for the Treatment of Glioblastoma with PVSRIPO. Available online: https://ClinicalTrials.gov/show/NCT04599647 (accessed on 30 October 2021).
- Bigner, D.; Rockefeller University. Phase 1 Trial of D2C7-IT in Combination with 2141-V11 for Recurrent Malignant Glioma. Available online: https://ClinicalTrials.gov/show/NCT04547777 (accessed on 30 October 2021).
- Istari Oncology, I. LUMINOS-101: PVSRIPO and Pembrolizumab in Patients with Recurrent Glioblastoma. Available online: https://ClinicalTrials.gov/show/NCT04479241 (accessed on 30 October 2021).
- Zacharoulis, S.; Columbia University. CED of MTX110 Newly Diagnosed Diffuse Midline Gliomas. Available online: https://ClinicalTrials.gov/show/NCT04264143 (accessed on 30 October 2021).
- Bigner, D.; Istari Oncology, Inc.; National Cancer Institute (NCI); Genentech, Inc. D2C7-IT with Atezolizumab for Recurrent Gliomas. Available online: https://ClinicalTrials.gov/show/NCT04160494 (accessed on 30 October 2021).
- Therapeutics, P.; National Cancer Institute (NCI). Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma (ReSPECT). Available online: https://ClinicalTrials.gov/show/NCT01906385 (accessed on 30 October 2021).
- Therapeutics, Y.-M.; Memorial Sloan Kettering Cancer Center. Convection-Enhanced Delivery of 124I-Omburtamab for Patients with Non-Progressive Diffuse Pontine Gliomas Previously Treated with External Beam Radiation Therapy. Available online: https://ClinicalTrials.gov/show/NCT01502917 (accessed on 30 October 2021).
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neurooncol. 2021, 151, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Rechberger, J.S.; Lu, V.M.; Zhang, L.; Power, E.A.; Daniels, D.J. Clinical trials for diffuse intrinsic pontine glioma: The current state of affairs. Childs Nerv. Syst. 2020, 36, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Singleton, W.G.B.; Bienemann, A.S.; Woolley, M.; Johnson, D.; Lewis, O.; Wyatt, M.J.; Damment, S.J.P.; Boulter, L.J.; Killick-Cole, C.L.; Asby, D.J.; et al. The distribution, clearance, and brainstem toxicity of panobinostat administered by convection-enhanced delivery. J. Neurosurg. Pediatr. 2018, 22, 288–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameter | Outcome (n = 271 Publications) * |
|---|---|
| Publication Type | |
| Original articles | 227 (84%) |
| Review articles | 44 (16%) |
| Clinical articles | 201 (74%) |
| Basic science articles | 70 (26%) |
| Open access | 54 (20%) |
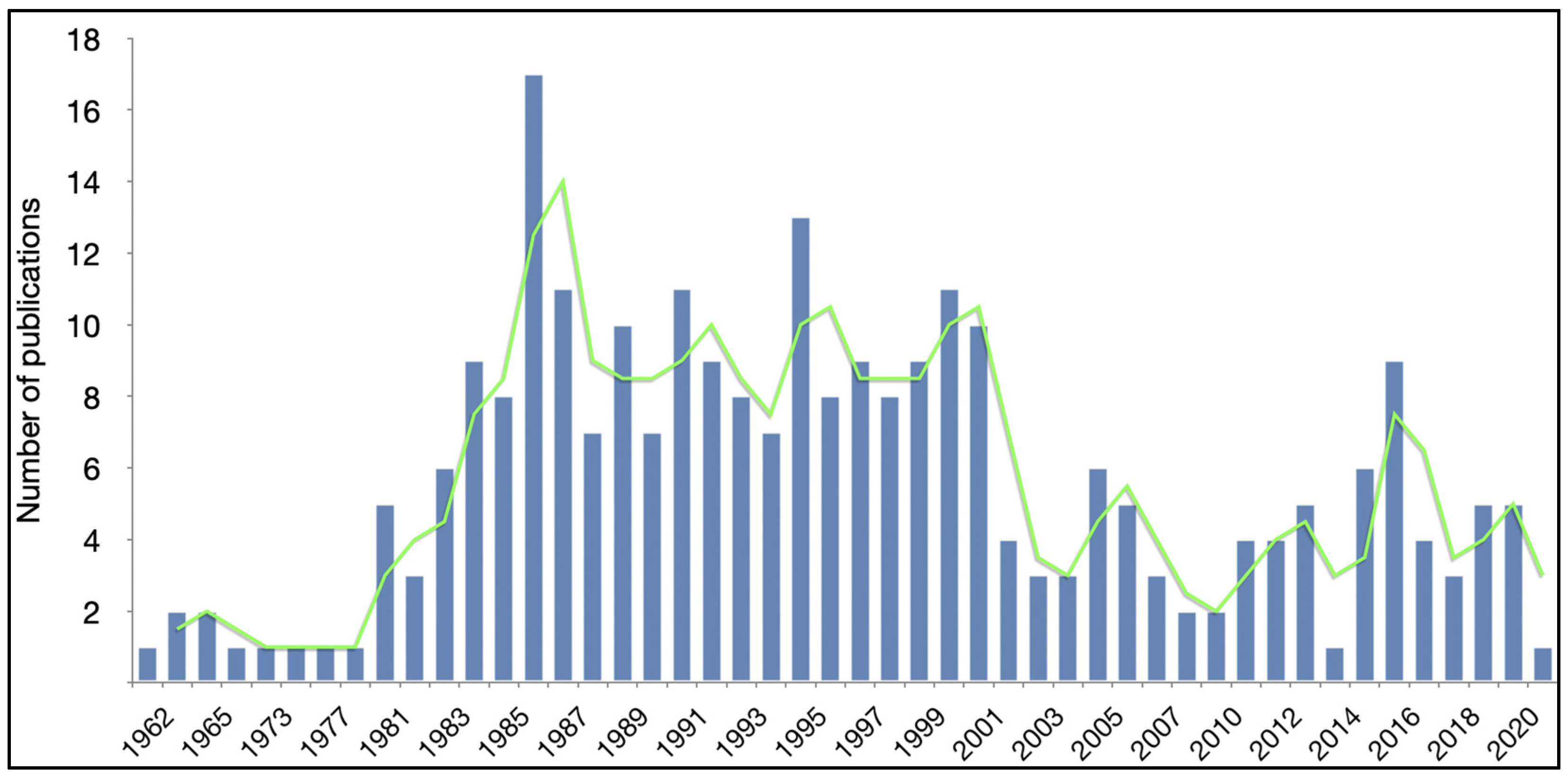
| Year of publication | |
| Range in years | 1962–2021 |
| Peak year | 1986 |
| Number of publications in peak year | 17 |
| Median publications per year | 5 |
| Citations | |
| Median | 15 |
| Most cited publication (n) | Primary central nervous system lymphoma (607) |
| Most cited original article (n) | Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood–brain barrier for the treatment of patients with malignant brain tumors (300) |
| Most cited review article (n) | Primary central nervous system lymphoma (607) |
| Authors | |
| Median number of authors per publication | 5 |
| Most authored publications (n) | Neuwelt E.A. (14) |
| Most first authored publications (n) | Nakagawa H. (7) |
| Most senior authored publications (n) | Neuwelt E.A. (8), Boockvar J.A. (8) |
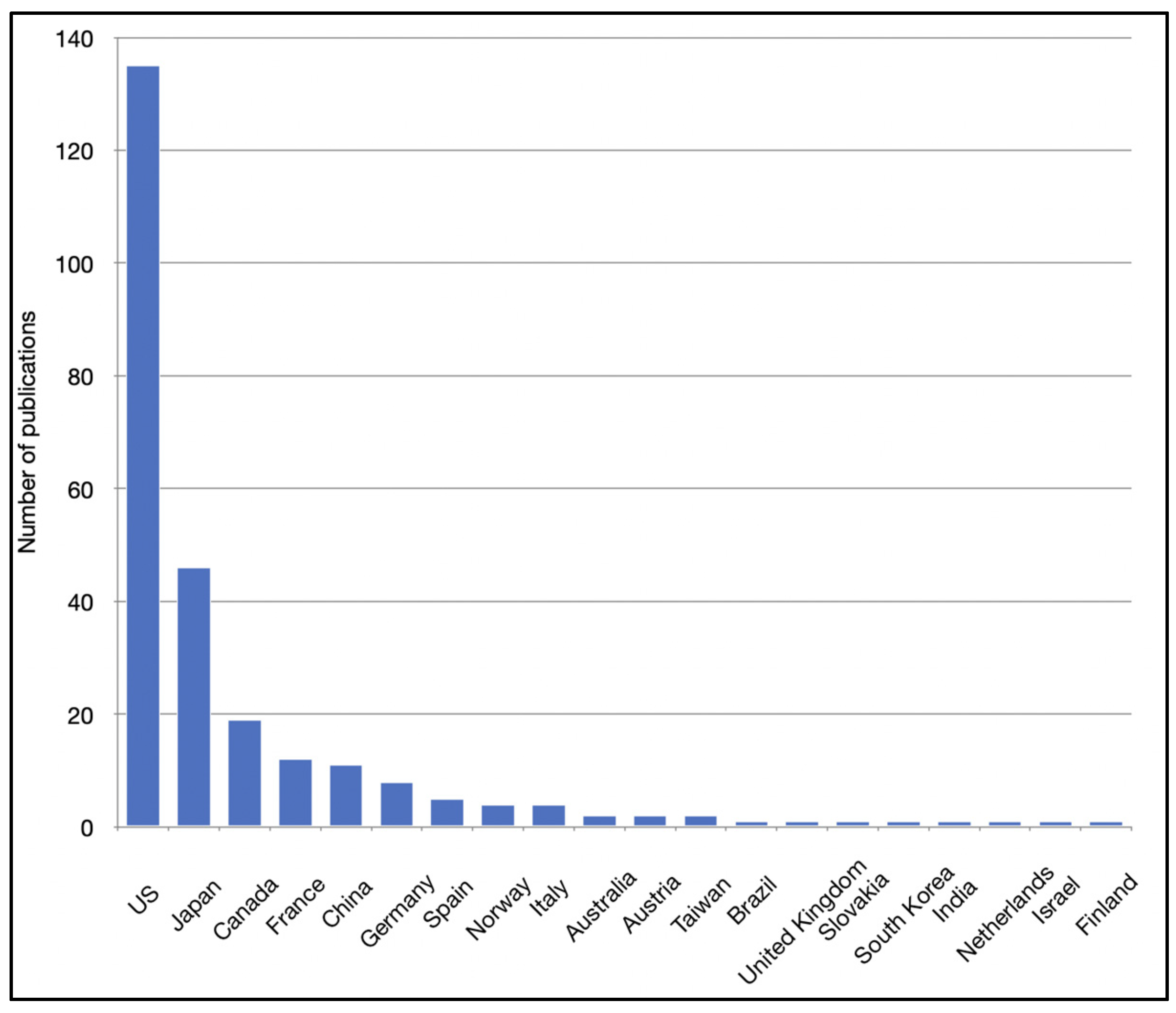
| Country of correspondence | |
| Total countries involved | 20 |
| Countries with most publications | |
| US | 135 (50%) |
| Japan | 46 (17%) |
| Canada | 19 (7%) |
| Contributing journals | |
| Total number of journals involved | 120 |
| Journals with most publications | |
| Journal of Neuro-Oncology | 48 (18%) |
| Japanese Journal of Cancer and Chemotherapy | 14 (5%) |
| Neurosurgery | 13 (5%) |
| Tumor type # | |
| Most common | |
| Glioma (combined) | 184 (68%) |
| Multiple (>1 tumor type) | 66 (24%) |
| Therapies # | |
| Chemotherapy | 215 (79%) |
| Targeted Therapy | 40 (15%) |
| Immunotherapy | 13 (5%) |
| Radiosensitizing/neutron capture therapy | 17 (6%) |
| Stem cell therapy | 5 (2%) |
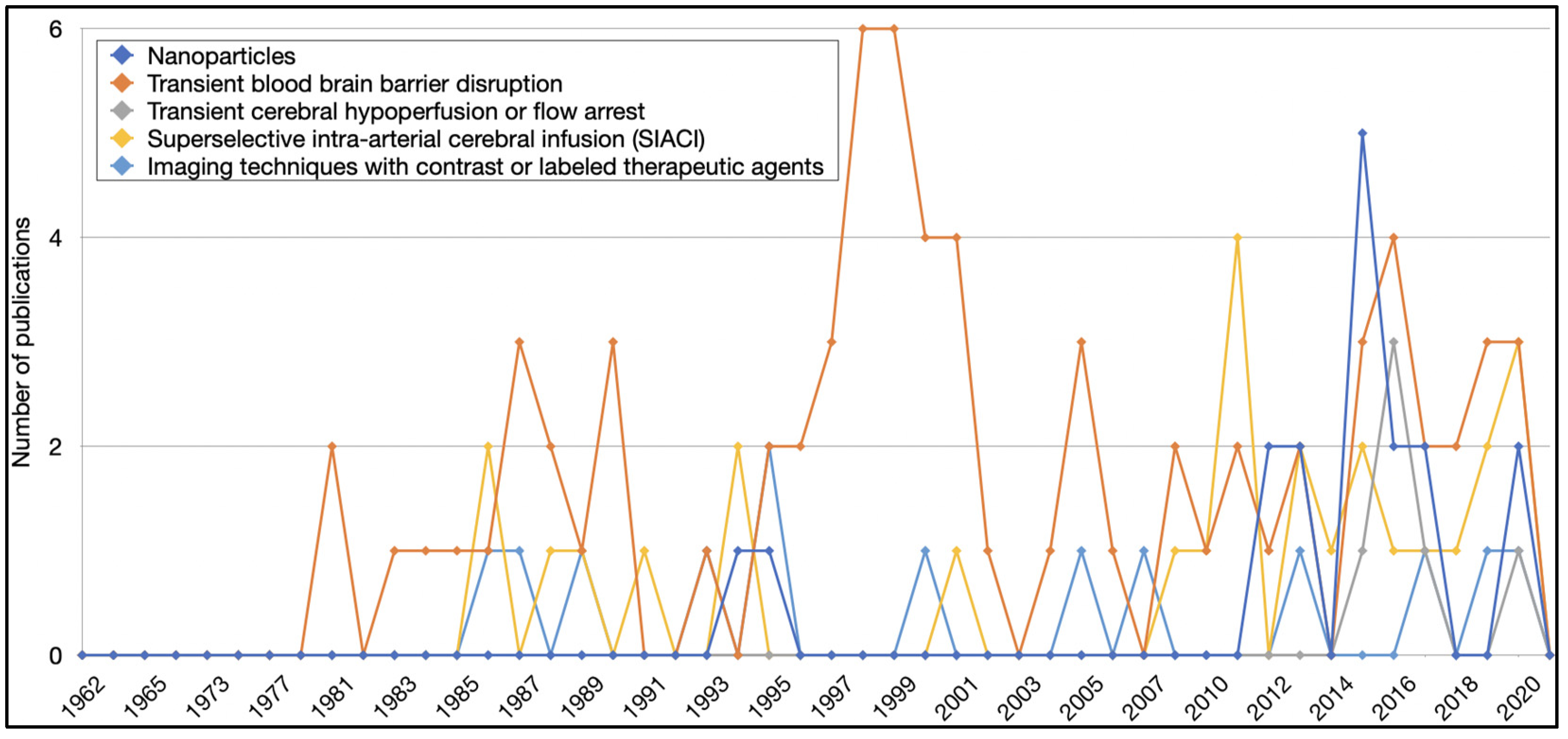
| Treatment strategies # | |
| Number of publications using: | |
| Nanoparticles | 17 (6%) |
| Transient blood–brain barrier disruption | 74 (27%) |
| Transient cerebral hypoperfusion or flow arrest | 6 (2%) |
| Superselective intra-arterial cerebral infusion | 27 (10%) |
| Imaging techniques with contrast or labelled therapeutic agents | 13 (5%) |
| Parameter | Outcome (n = 20 Trials) * |
|---|---|
| Time (expected) | |
| Start year | 2011 (1998–2019) |
| Completion year | 2022 (2008–2025) |
| Results first posted | 2015 ^ |
| Last updated | 2020 (2013–2021) |
| Status | |
| Current status as of August 2021 | |
| Recruiting | 8 (40%) |
| Completed | 6 (30%) |
| Suspended | 2 (10%) |
| Active, not recruiting | 2 (10%) |
| Terminated | 1 (5%) |
| Unknown status | 1 (5%) |
| Study results available | 1 (5%) |
| Cohort | |
| Minimum age of enrollment (years) | 18 (0–18) |
| Maximum age of enrollment (years) | 99 (17–120) |
| Design | |
| Interventional studies | 20 (100%) |
| Phase | |
| Phase 1 | 9 (45%) |
| Phase 1 + Phase 2 | 8 (40%) |
| Phase 2 | 3 (15%) |
| Outcomes # | |
| Primary | |
| Safety and toxicity | 11 (55%) |
| PFS | 6 (35%) |
| OS | 5 (25%) |
| Secondary | |
| PFS | 11 (55%) |
| Safety and toxicity | 9 (45%) |
| OS | 8 (40%) |
| QOL | 5 (25%) |
| Location and funding | |
| Two most common corresponding institutes | |
| Northwell Health | 10 (50%) |
| OHSU Knight Cancer Institute | 3 (15%) |
| Trials per Country | |
| US | 18 (90%) |
| China, Canada | 1 (5%) each |
| Number of sites involved | 1 (1–2) |
| Therapies # | |
| Number of research studies using: | |
| Targeted Therapy | 13 (65%) |
| Chemotherapy | 8 (40%) |
| Treatment mechanism # | |
| Number of research studies using: | |
| Superselective intra-arterial cerebral Infusion 1 | 13 (65%) |
| Transient blood–brain barrier disruption using mannitol 2 | 12 (60%) |
| Three most common conditions # | |
| Glioblastoma | 13 (65%) |
| Anaplastic Astrocytoma | 8 (40%) |
| Brain Metastasis | 3 (15%) |
| Parameter | Outcome |
|---|---|
| NCT number | NCT00362817 |
| Title | Carboplatin and Temozolomide (Temodar) for Recurrent and Symptomatic Residual Brain Metastases |
| Location | Ohio State University, Columbus, Ohio, United States |
| Start date | October 2004 |
| Finish date | January 2008 |
| Results first posted | May 2015 |
| Enrolment size | 17 |
| Age range (years) | 18 and older |
| Primary outcome | |
| Response rate | 42.8% |
| Secondary outcome | |
| Overall survival in weeks | 25.2 |
| Time to progression in weeks (mean) | 22.6 |
| Incidence of CNS toxicities | 0 |
| Cause of death | CNS tumor = 0, systemic disease progression = 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rechberger, J.S.; Thiele, F.; Daniels, D.J. Status Quo and Trends of Intra-Arterial Therapy for Brain Tumors: A Bibliometric and Clinical Trials Analysis. Pharmaceutics 2021, 13, 1885. https://doi.org/10.3390/pharmaceutics13111885
Rechberger JS, Thiele F, Daniels DJ. Status Quo and Trends of Intra-Arterial Therapy for Brain Tumors: A Bibliometric and Clinical Trials Analysis. Pharmaceutics. 2021; 13(11):1885. https://doi.org/10.3390/pharmaceutics13111885
Chicago/Turabian StyleRechberger, Julian S., Frederic Thiele, and David J. Daniels. 2021. "Status Quo and Trends of Intra-Arterial Therapy for Brain Tumors: A Bibliometric and Clinical Trials Analysis" Pharmaceutics 13, no. 11: 1885. https://doi.org/10.3390/pharmaceutics13111885
APA StyleRechberger, J. S., Thiele, F., & Daniels, D. J. (2021). Status Quo and Trends of Intra-Arterial Therapy for Brain Tumors: A Bibliometric and Clinical Trials Analysis. Pharmaceutics, 13(11), 1885. https://doi.org/10.3390/pharmaceutics13111885






