Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of Paroxetine Nanosuspension
2.3. Assessment of Particle Size, Poly Dispersity Index, and Zeta Potential
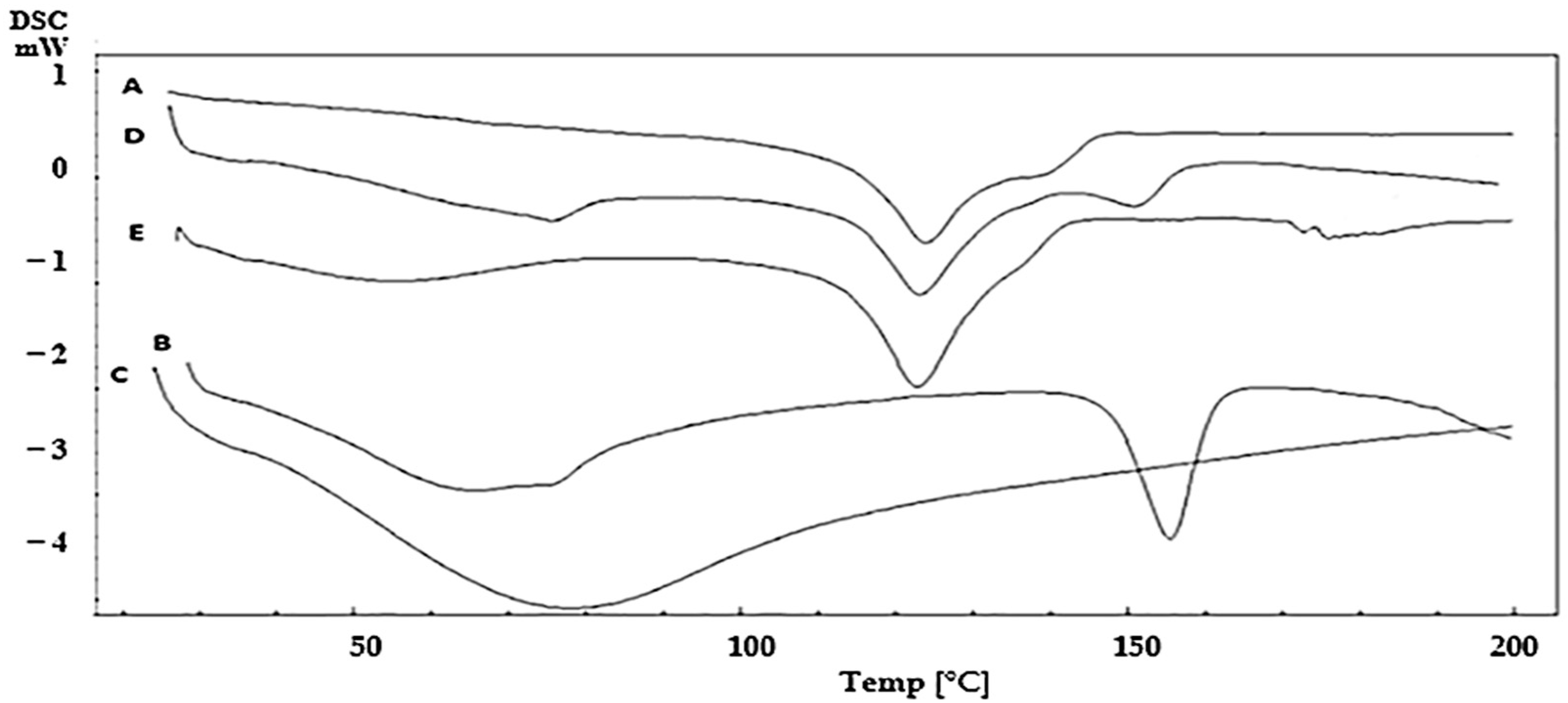
2.4. Differential Scanning Calorimetry (DSC)
2.5. Oral Fast Dissolving Films (OFDFs) Loaded with PX Nanosuspension Preparations
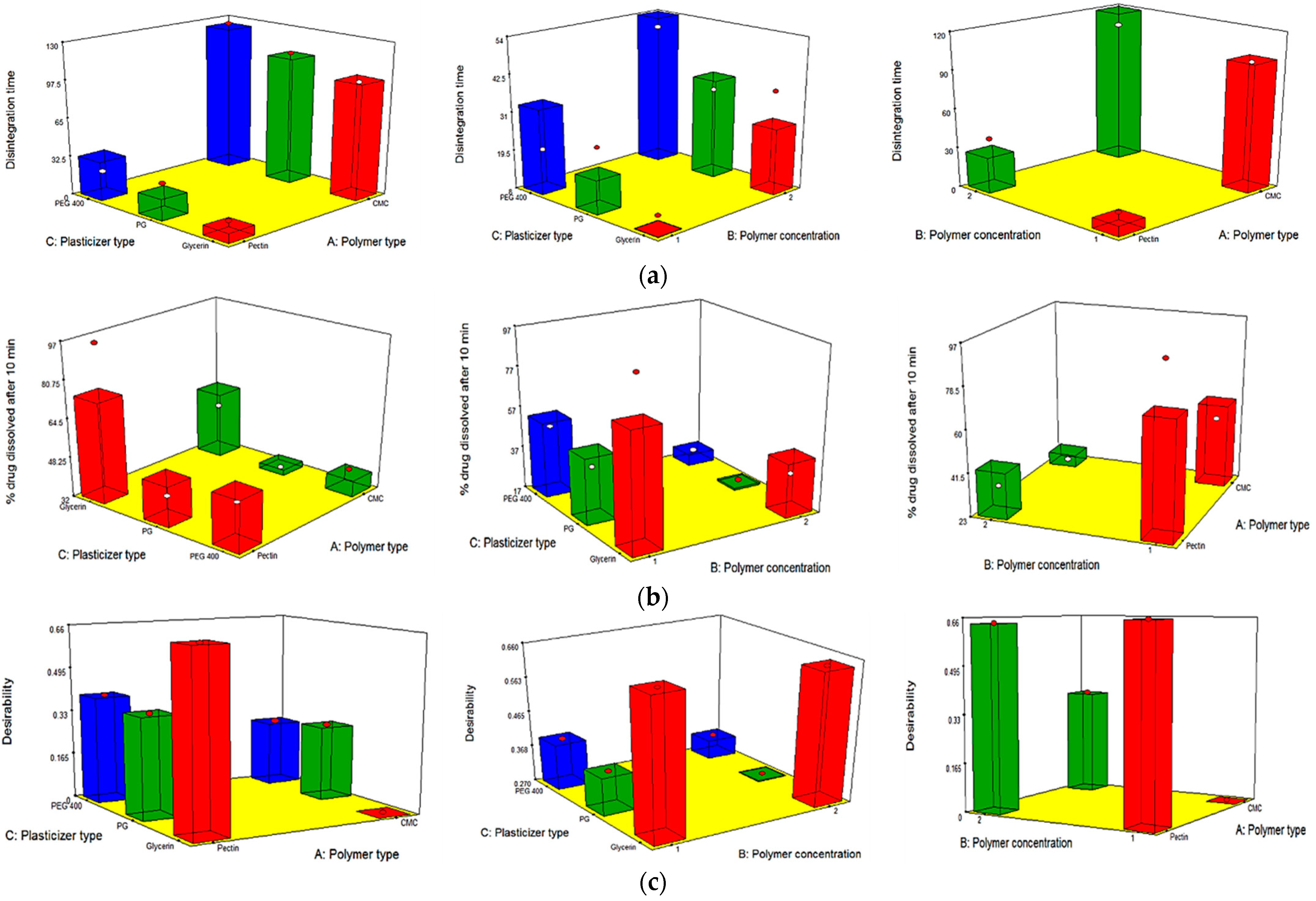
2.6. Full Factorial Statistical Design
2.7. Characterization of Paroxetine OFDFs
2.7.1. Average Weight
2.7.2. Film Thickness
2.7.3. Folding Endurance
2.7.4. Content Uniformity
2.7.5. Surface pH
2.7.6. Moisture Content %
Moisture Loss %
Moisture Absorption %
2.7.7. Mechanical Characteristics of PX OFDFs
Tensile Strength
Percent Elongation
Young’s Modulus
2.7.8. In Vitro Disintegration Time
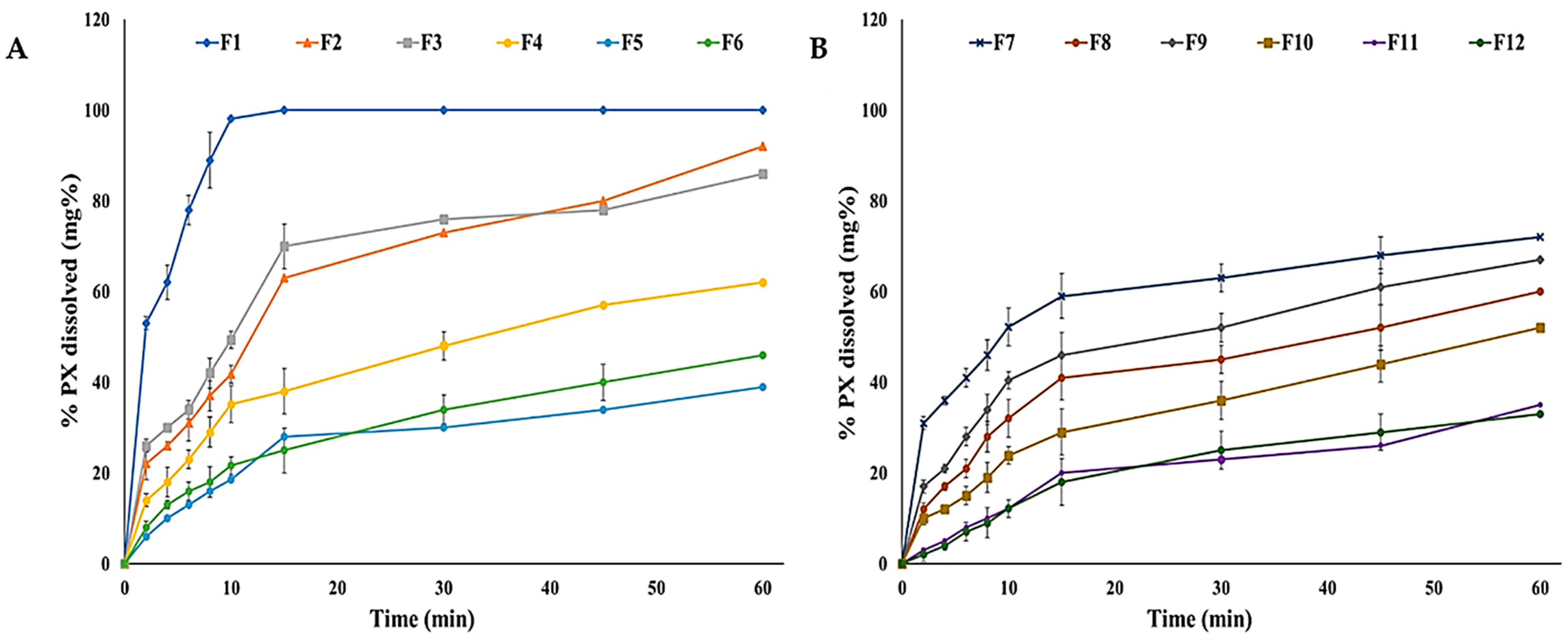
2.7.9. In Vitro Dissolution Study
2.8. Characterization of the Optimized OFDF Loaded with PX Nanosuspension
2.8.1. Re-Dispersion of PX Nanoparticles from the Optimized OFDF
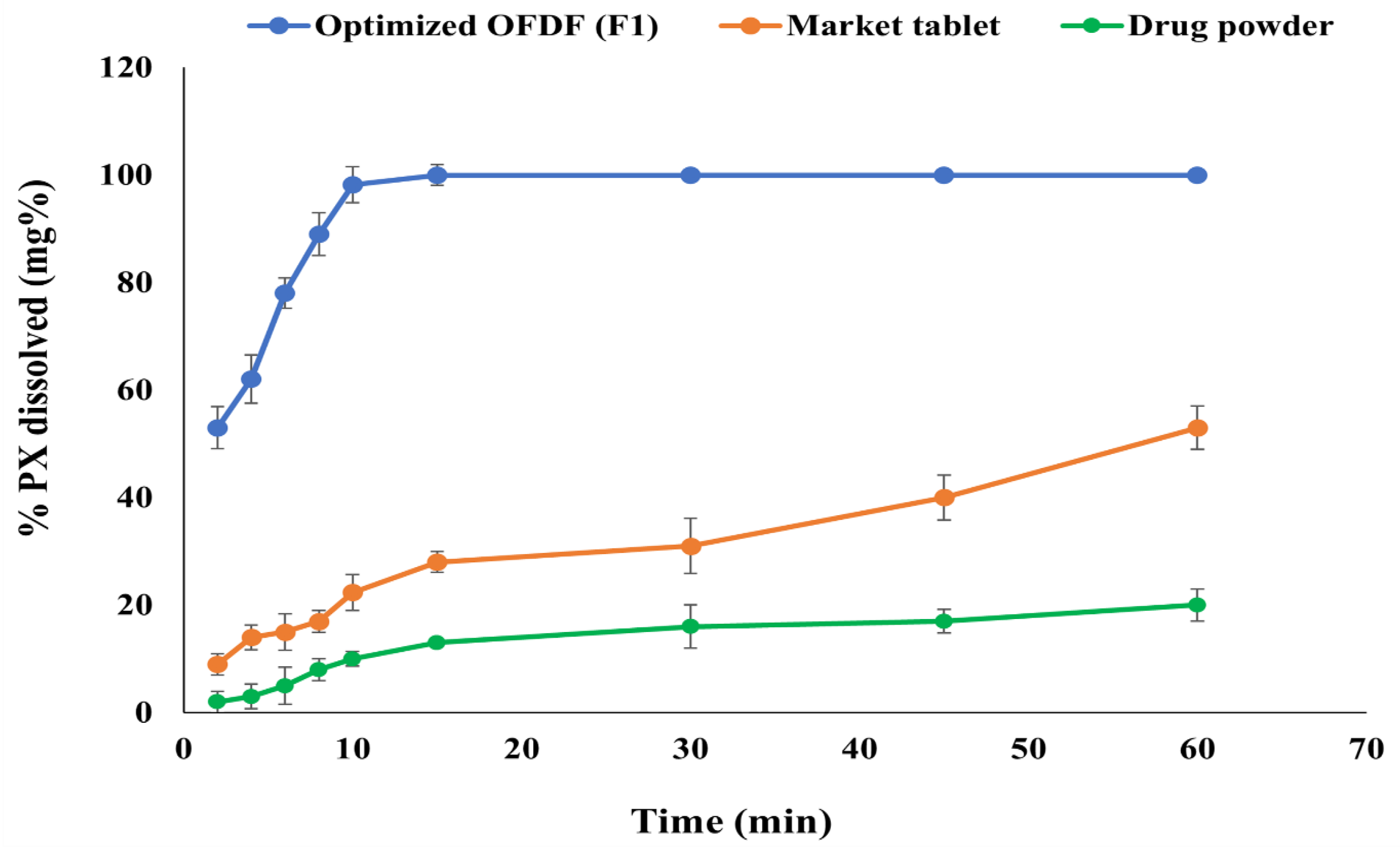
2.8.2. Comparative Dissolution Study of Optimized PX OFDF, Drug Powder, and the Market Tablet
2.8.3. Ex Vivo Permeation
Tissue Preparation
Ex Vivo Permeation Testing
Permeation Parameter Calculation
Statistical Analysis
2.8.4. Stability Study
2.9. In Vivo Clinical Studies
2.9.1. In Vivo Disintegration Time and Palatability Studies
2.9.2. Pharmacokinetic Evaluation in Healthy Human Volunteers
Study Design and Subjects
Drug Administration and Sample Collection
Sample Preparation
Chromatographic Conditions
Pharmacokinetic and Statistical Analysis
3. Results and Discussion
3.1. Particle size, Poly Dispersity Index, and Zeta Potential
3.2. Differential Scanning Calorimetry (DSC)
3.3. Preparation of OFDFs Loaded with PX Nanosuspension
3.4. Full Factorial Design Statistical Analysis
3.5. Characterization of the Prepared PX OFDFs
3.5.1. Average Weight
3.5.2. Films Thickness
3.5.3. Folding Endurance
3.5.4. Content Uniformity
3.5.5. Surface pH
3.5.6. Moisture content %
Moisture Loss %
Moisture Absorption %
3.5.7. Mechanical Characteristics of the OFDFs
Tensile Strength
Percentage Elongation
Young’s Modulus
3.5.8. In Vitro Disintegration Time
3.5.9. In Vitro Dissolution Studies
3.6. Selection of the Optimized OFDF Loaded with PX Nanosuspension
3.7. Characterization of the Optimized OFDF Loaded with PX Nanosuspension
3.7.1. Re-Dispersion of PX Nanoparticles from the Optimized OFDF
3.7.2. Comparative Dissolution Study of the Optimized OFDF (F1), Pure Drug, and the Market Tablet
3.7.3. Ex Vivo Permeation Studies
3.7.4. Stability Study
3.8. In Vivo Clinical Studies
3.8.1. In Situ Disintegration Time and Palatability Studies
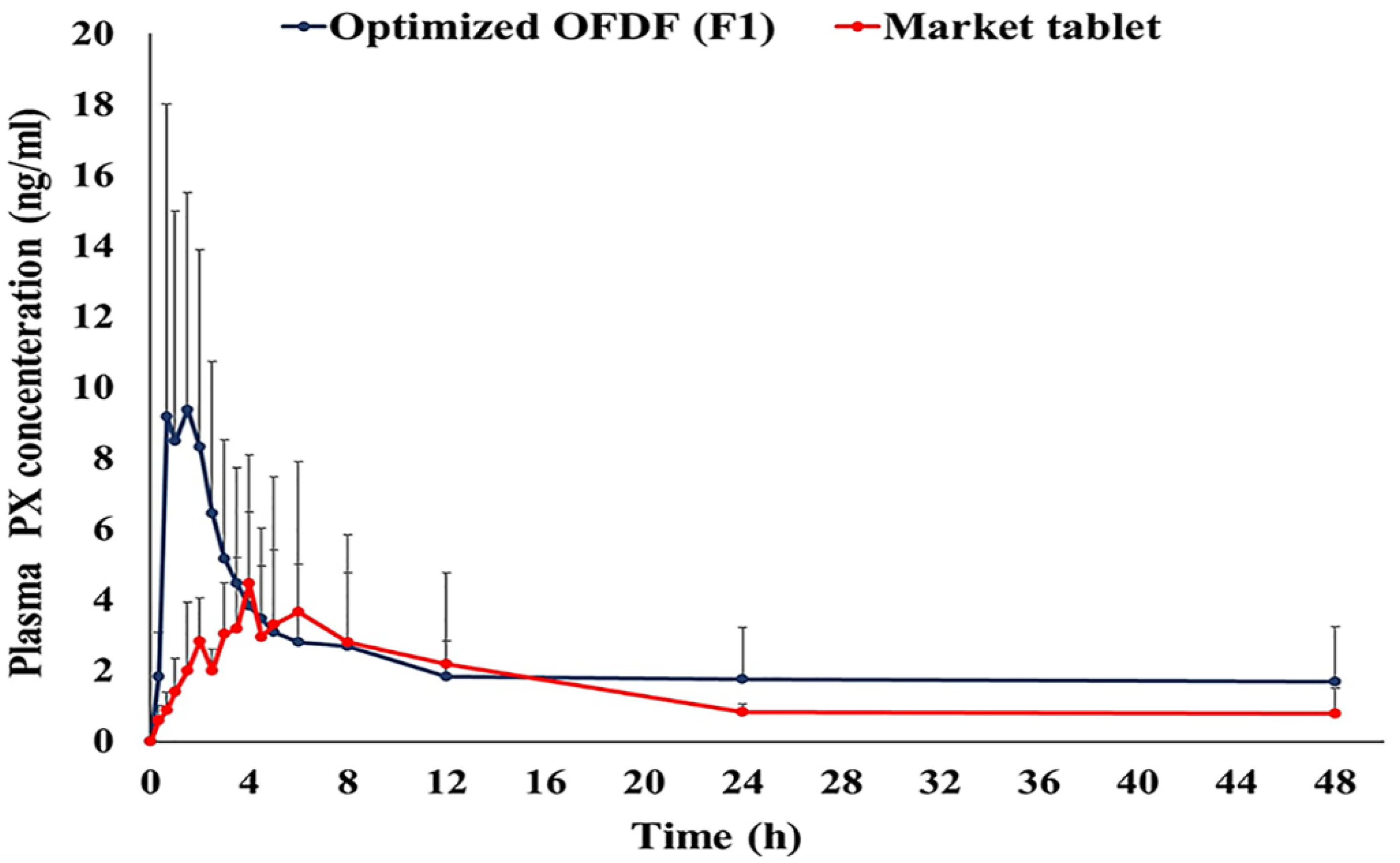
3.8.2. Pharmacokinetic Parameters of PX in Healthy Human Volunteer s
LC-MS/MS Method for Detection of Paroxetine in Human Plasma
Estimation of Bioequivalence
Statistical Analysis of Paroxetine Pharmacokinetic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jin, S.J.; Yoo, Y.H.; Kim, M.S.; Kim, J.S.; Park, J.S.; Hwang, S.J. Paroxetine hydrochloride-controlled release POLYOX® matrix tablets: Screening of formulation variables using Plackett–Burman screening design. Arch. Pharm. Res. 2008, 31, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Pandey, Y.R.; Kumar, S.; Gupta, B.K.; Ali, J.; Baboota, S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: Formulation, behavioural and biochemical estimation. Nanotechnology 2016, 27, 25102. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Bendas, E.R.; Tag, R.; El Rehem, A.; Abary, M.Y.S. Transdermal drug delivery of paroxetine through lipid-vesicular formulation to augment its bioavailability. Int. J. Pharm. 2013, 443, 307–317. [Google Scholar] [CrossRef]
- Gudas, G.K.; Battacharjee, C. Design and evaluation of buccoadhesive bi-layer tablet of paroxetine hydrochloride. Indian J. Pathol. Res. Pract. 2011, 2, 167–169. [Google Scholar]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Lian, R.; Zheng, S.; Yin, Z.; Lu, Y.; Wu, W. Enhanced dissolution and oral bioavailability of aripiprazole nanosuspensions prepared by nanoprecipitation/homogenization based on acid-base neutralization. Int. J. Pharm. 2012, 438, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Muller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef]
- El-Feky, Y.A.; Mostafa, D.A.; Al-Sawahli, M.M.; El-Telbany, R.F.; Zakaria, S.; Fayez, A.M.; Ahmed, K.A.; Alolayan, E.M.; El-Telbany, D.F. Reduction of intraocular pressure using timolol orally dissolving strips in the treatment of induced primary open-angle glaucoma in rabbits. J. Pharm. Pharmacol. 2020, 72, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.N.; Garg, G.; Sharma, P.K. A short review on-a novel approach in oral fast dissolving drug delivery system and their patents. Adv. Biol. Res. 2011, 5, 291–303. [Google Scholar]
- Liu, C.; Chang, D.; Zhang, X.; Sui, H.; Kong, Y.; Zhu, R.; Wang, W. Oral fast-dissolving films containing lutein nanocrystals for improved bioavailability: Formulation development, in vitro and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2957–2964. [Google Scholar] [CrossRef]
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.W.; Chow, S.F.; Lam, T.N.; Lee, W.Y.T. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B 2018, 163, 9–18. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Ranjan, S.; Dasgupta, N.; Mishra, R.K.; Tomas, S. (Eds.) Nanocarriers for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Garsuch, V.; Breitkreutz, J. Comparative investigations on different polymers for the preparation of fast-dissolving oral films. J. Pharm. Pharmacol. 2010, 62, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. 2015, 24, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakarani, M.; Misra, A.K.; Patel, J.K.; Vaghani, S.S. Itraconazole nanosuspension for oral delivery: Formulation, characterization and in vitro comparison with marketed formulation. Daru 2010, 18, 84–90. [Google Scholar] [PubMed]
- Londhe, V.; Shirsat, R. Formulation and characterization of fast-dissolving sublingual film of iloperidone using Box–Behnken design for enhancement of oral bioavailability. AAPS PharmSciTech 2018, 19, 1392–1400. [Google Scholar] [CrossRef]
- Rai, V.; Tan, H.S.; Michniak-Kohn, B. Effect of surfactants and pH on naltrexone (NTX) permeation across buccal mucosa. Inter. J. Pharm. 2011, 411, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Shariare, M.; Sharmin, S.; Jahan, I.; Reza, H.; Mohsin, K. The impact of process parameters on carrier free paracetamol nanosuspension prepared using different stabilizers by antisolvent precipitation method. J. Drug Deliv. Sci. Technol. 2018, 43, 122–128. [Google Scholar] [CrossRef]
- Chandra, A.; Chondkar, A.D.; Shirodkar, R.; Lewis, S.A. Rapidly dissolving lacidipine nanoparticle strips for transbuccal administration. J. Drug Deliv. Sci. Technol. 2018, 47, 259–267. [Google Scholar] [CrossRef]
- Shen, C.; Shen, B.; Xu, H.; Bai, J.; Dai, L.; Lv, Q.; Han, J.; Yuan, H. Formulation and optimization of a novel oral fast dissolving film containing drug nanoparticles by Box–Behnken design response surface methodology. Drug Dev. Ind. Pharm. 2014, 40, 649–656. [Google Scholar] [CrossRef]
- Chavan, D.U.; Marques, S.M.; Bhide, P.J.; Kumar, L.; Shirodkar, R.K. Rapidly dissolving Felodipine nanoparticle strips—Formulation using design of experiment and characterisation. J. Drug Deliv. Sci. Technol. 2020, 60, 102053. [Google Scholar] [CrossRef]
- Elsayed, I.; El-Dahmy, R.M.; Elshafeey, A.H.; Abd El Gawad, N.A.; El Gazayerly, O.N. Tripling the bioavailability of rosuvastatin calcium through development and optimization of an in situ forming nanovesicular system. Pharmaceutics 2019, 11, 275. [Google Scholar] [CrossRef] [Green Version]
- Shivhare, U.D.; Bodkhe, P.D.; Bhusari, K.P.; Mathur, V.B. Formulation and evaluation of buccoadhesive films of losartan potassium. Pharm. Lett. 2010, 2, 251–260. [Google Scholar]
- Smriti, T. Mouth dissolving films: A review. Int. J. Pharma Bio. Sci. 2013, 4, 899–908. [Google Scholar]
- Satyanarayana, D.A.; Keshavarao, K.P. Fast disintegrating films containing anastrazole as a dosage form for dysphagia patients. Arch. Pharm. Res. 2012, 35, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.B.; Ball, R.L.; Matthew, H.W.T. Chitosan films with improved tensile strength and toughness from N-acetyl-cysteine mediated disulfide bonds. Carbohydr Polym. 2016, 139, 1–9. [Google Scholar] [CrossRef]
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432. [Google Scholar] [CrossRef]
- Ali, M.; Vijendar, C.; Kumar, S.; Krishnaveni, J. Formulation and evaluation of fast dissolving oral films of diazepam. Aust. J. Pharm. 2016, 4, 1–5. [Google Scholar]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Dave, R.H. Formulation and characterization of acetaminophen nanoparticles in orally disintegrating films. Drug Deliv. 2016, 23, 540–549. [Google Scholar] [CrossRef]
- Elsayed, I.; El-Dahmy, R.M.; El-Emam, S.Z.; Elshafeey, A.H.; Abd El Gawad, N.A.; El-Gazayerly, O.N. Response surface optimization of biocompatible elastic nanovesicles loaded with rosuvastatin calcium: Enhanced bioavailability and anticancer efficacy. Drug Deliv. Transl. Res. 2020, 10, 1459–1475. [Google Scholar] [CrossRef]
- El-Dahmy, R.M.; Elsayed, I.; Elshafeey, A.H.; Abd El Gawad, N.A.; El-Gazayerly, O.N. Optimization of long circulating mixed polymeric micelles containing vinpocetine using simple lattice mixture design, in vitro and in vivo characterization. Int. J. Pharm. 2014, 477, 39–46. [Google Scholar] [CrossRef]
- Wong, C.F.; Yuen, K.H.; Peh, K.K. An in vitro method for buccal adhesion studies: Importance of instrument variables. Int. J. Pharm. 1999, 180, 47–57. [Google Scholar] [CrossRef]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. An integrated buccal delivery system combining chitosan films impregnated with peptide loaded PEG-b-PLA nanoparticles. Colloids Surf. B. Biointerfaces 2013, 112, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; El Menshawe, S.F.; Abou-Taleb, H.A.; Elkarmalawy, M.H. Loratadine bioavailability via buccal transferosomal gel: Formulation, statistical optimization, in vitro/in vivo characterization, and pharmacokinetics in human volunteers. Drug Deliv. 2017, 24, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; Hassan, A.H.; Eissa, E.M.; Aboud, H.M. Response surface optimization of ultra-elastic nanovesicles loaded with deflazacort tailored for transdermal delivery: Accentuated bioavailability and anti-inflammatory efficacy. Int. J. Nanomed. 2021, 2021, 591–607. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Stability Testing of New Drug Substances and Products Q1A (R2). ICH Harmonized Tripartite Guidelines. 2003. Available online: http://www.alz.org/what-is-dementia.asp (accessed on 6 February 2021).
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.E. Third international conference on harmonization of technical requirements for registration of pharmaceuticals for human use—A toxicologist’s perspective. Toxicol. Pathol. 1996, 24, 519–528. [Google Scholar] [CrossRef]
- Auda, S.H.; Elbadry, M.; Ibrahim, M.A. Design, formulation and characterization of fast dissolving films containing dextromethorphan. Dig. J. Nanomat. Biostruc. 2014, 9, 133–141. [Google Scholar]
- Khan, S.; Kataria, P.; Nakhat, P.; Yeole, P. Taste masking of ondansetron hydrochloride by polymer carrier system and formulation of rapid-disintegrating tablets. AAPS PharmSciTech 2007, 8, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshafeey, A.H.; Kamel, A.O.; Awad, G.A.S. Ammonium methacrylate units polymer content and their effect on acyclovir colloidal nanoparticles properties and bioavailability in human volunteers. Colloids Surf. B Biointerfaces 2010, 75, 398–404. [Google Scholar] [CrossRef]
- Shah, V.P.; Midha, K.K.; Dighe, S.; McGilveray, I.J.; Skelly, J.P.; Yacobi, A.; Layloff, T.; Viswanathan, C.T.; Cook, C.E.; McDowall, R.D.; et al. Analytical methods validation: Bioavailability, bioequivalence and pharmacokinetic studies. Eur. J. Drug Metab. Pharmacokinet. 1991, 16, 249. [Google Scholar] [PubMed]
- Massaroti, P.; Cassiano, N.M.; Duarte, L.F.; Campos, D.R.; Marchioretto, M.A.M.; Bernasconi, G.; Calafatti, S.; Barros, F.A.P.; Meurer, E.C.; Pedrazzoli, J. Validation of a selective method for determination of paroxetine in human plasma by LC-MS/MS. J. Pharm. Pharm. Sci. 2005, 8, 340–347. [Google Scholar] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Vueba, M.L.; Batista, D.; Carvalho, L.A.E.; Veiga, F.; Sousa, J.J.; Pina, M.E. Influence of cellulose ether polymers on ketoprofen release from hydrophilic matrix tablets. Eur. J. Pharm. Biopharm. 2004, 58, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iijima, M.; Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Phase transition of pectin with sorbed water. Carbohydr. Polym. 2000, 41, 101–106. [Google Scholar] [CrossRef]
- Akhtar, H.M.S.; Riaz, A.; Hamed, Y.S.; Abdin, M.; Chen, G.; Wan, P.; Zeng, X. Production and characterization of CMC-based antioxidant and antimicrobial films enriched with chickpea hull polysaccharides. Int. J. Biol. Macromol. 2018, 118, 469–477. [Google Scholar] [CrossRef]
- Singh, H.; Kaur, M.; Verma, H. Optimization and evaluation of desloratadine oral strip: An innovation in paediatric medication. Sci. World J. 2013, 2013, 395681. [Google Scholar] [CrossRef]
- Panda, B.; Dey, N.; Rao, M. Development of innovative orally fast disintegrating film dosage forms: A review. Int. J. Pharm. Sci. Nanotechnol. 2012, 5, 1666–1674. [Google Scholar] [CrossRef]
- Cilurzo, F.; Cupone, I.E.; Minghetti, P.; Buratti, S.; Gennari, C.G.; Montanari, L. Diclofenac fast-dissolving film: Suppression of bitterness by a taste-sensing system. Drug Dev. Ind. Pharm. 2011, 37, 252–259. [Google Scholar] [CrossRef]
- Allam, A.; Fetih, G. Sublingual fast dissolving niosomal films for enhanced bioavailability and prolonged effect of metoprolol tartrate. Drug Des. Dev. Ther. 2016, 10, 2421–2433. [Google Scholar]
- Dinge, A.; Nagarsenker, M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS PharmSciTech. 2008, 9, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Maher, E.M.; Mahmoud, A.; Ali, A.; Farouk, H.; Abdelrahman, A.A.; Magdy, E.; Mahmoud, A.; Ali, A.; Salem, H.F.; Maher, E.M.; et al. In vitro/in vivo evaluation of an optimized fast dissolving oral film containing olanzapine co-amorphous dispersion with selected carboxylic acids. Drug Deliv. 2016, 23, 3088–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Fishman, M.L.; Coffin, D.R.; Konstance, R.P.; Onwulata, C.I. Extrusion of pectin/starch blends plasticized with glycerol. Carbohydr. Polym. 2000, 41, 317–325. [Google Scholar] [CrossRef]
- Junmahasathien, T.; Panraksa, P.; Protiarn, P.; Hormdee, D.; Noisombut, R.; Kantrong, N.; Jantrawut, P. Preparation and evaluation of metronidazole-loaded pectin films for potentially targeting a microbial infection associated with periodontal disease. Polymers 2018, 10, 1021. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.J.; Rowe, R.C. The Young’s modulus of pharmaceutical materials. Int. J. Pharm. 1987, 37, 15–18. [Google Scholar] [CrossRef]
- Bermudez-Oria, A.; Rodriguez- Guiterrez, G.; Voque, B.; Rubio-Senet, F.; Demandez-Bolanos, J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxytyrosol and 3,4-dihydroxyphenylglycol. Carbohyd. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef]
- Pathare, Y.S.; Hastak, V.S.; Bajaj, A.N. Polymers used for fast disintegrating oral films: A review. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 169–178. [Google Scholar]
- Opanasopit, P.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Ruktanonchai, U. Development and characterization of pectinate micro/nanoparticles for gene delivery. AAPS PharmSciTech 2008, 9, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sujja-Areevath, J.; Munday, D.L.; Cox, P.J.; Khan, K.A. Relationship between swelling, erosion and drug release in hydrophillic natural gum mini-matrix formulations. Eur. J. Pharm. Sci. 1998, 6, 207–217. [Google Scholar] [CrossRef]
- Shaikh, M.T.M.; Gore, A.A.; Salunkhe, K.S.; Chaudhari, S.R. Formulation development and evaluation of fast dissolving oral film of amlodipine besilate by solvent casting technique. Int. J. Pharm. Biol. Sci. 2013, 2, 534–544. [Google Scholar]
- Abdelbary, A.A.; Li, X.; El-Nabarawi, M.; Elassasy, A.; Jasti, B. Comparison of nanomilling and coprecipitation on the enhancement of in vitro dissolution rate of poorly water-soluble model drug aripiprazole. Pharm. Dev. Technol. 2014, 19, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar]
- Abd El Azim, H.; Nafee, N.; Ramadan, A.; Khalafallah, N. Liposomal buccal mucoadhesive film for improved delivery and permeation of water-soluble vitamins. Inter. J. Pharm. 2015, 488, 78–85. [Google Scholar] [CrossRef]


| Formulations | Factors (Independent Variables) | ||
|---|---|---|---|
| Polymer Type | Polymer Concentration (%w/v) | Plasticizer Type | |
| F1 | Pectin | 1% | Glycerol |
| F2 | Pectin | 1% | PG |
| F3 | Pectin | 1% | PEG 400 |
| F4 | Pectin | 2% | Glycerol |
| F5 | Pectin | 2% | PG |
| F6 | Pectin | 2% | PEG 400 |
| F7 | CMC | 1% | Glycerol |
| F8 | CMC | 1% | PG |
| F9 | CMC | 1% | PEG 400 |
| F10 | CMC | 2% | Glycerol |
| F11 | CMC | 2% | PG |
| F12 | CMC | 2% | PEG 400 |
| Responses | Tensile Strength (Mpa) | % Elongation | Young’s Modulus (Mpa) | Disintegration Time (s) | % PX Dissolved after 10 Minutes |
|---|---|---|---|---|---|
| Minimum | 1.04 ± 0.11 | 6.03 ± 0.45 | 8.09 ± 0.15 | 17.09 ± 1.30 | 12.14 ± 0.08 |
| Maximum | 15.5 ± 0.68 | 53.08 ± 1.28 | 383.66 ± 11.06 | 160.06 ± 4.20 | 96.02 ± 3.46 |
| F value | 17.21 | 20.73 | 15.61 | 79.52 | 11.31 |
| p-value | 0.0010 | 0.0006 | 0.0013 | < 0.0001 | 0.0036 |
| Adequate precision | 13.39 | 9.83 | 18.01 | 22.99 | 10.88 |
| Adjusted R2 | 0.855 | 0.877 | 0.842 | 0.966 | 0.789 |
| Predicted R2 | 0.729 | 0.772 | 0.704 | 0.934 | 0.686 |
| R2 | 0.908 | 0.922 | 0.899 | 0.978 | 0.896 |
| Significant factors | X1, X2 and X3 | X3 | X3 | X1, X2 and X3 | X1, X2 and X3 |
| Observed values of optimum OFDF (F1) | 3.89 | 53.08 | 8.12 | 17.09 | 96.02 |
| Predicted values of optimum OFDF (F1) | 3.46 | 50.07 | 9.90 | 20.28 | 97.14 |
| Average Weight (mg) | Film Thickness (mm) | Folding Endurance | Content Uniformity (%) | pH | Moisture Loss % | Moisture Absorption % | Tensile Strength (Mpa) | Percentage Elongation | Young’s Modulus (Mpa) | Disintegration Time (s) | % PX Dissolved after 10 Minutes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 38.02 ± 1.45 | 0.11 ± 0.02 | >300 | 96.68 ± 3.62 | 6.80 ± 0.17 | 1.08 ± 0.02 | 1.39 ± 0.11 | 3.89 ± 0.19 | 53.08 ± 1.28 | 8.12 ± 0.13 | 17.09 ± 1.30 | 96.02 ± 3.46 |
| F2 | 40.17 ± 0.98 | 0.13 ± 0.04 | >300 | 93.14 ± 4.24 | 6.92 ± 0.11 | 1.24 ± 0.03 | 1.06 ± 0.08 | 9.68 ± 0.12 | 11.31 ± 1.06 | 306.41 ± 12.73 | 26.14 ± 3.06 | 41.74 ± 3.08 |
| F3 | 39.29 ± 2.37 | 0.13 ± 0.01 | >300 | 92.35 ± 1.28 | 6.64 ± 0.25 | 0.93 ± 0.02 | 1.21 ± 0.10 | 6.24 ± 0.26 | 11.06 ± 1.00 | 383.66 ± 9.06 | 20.27 ± 2.00 | 49.36 ± 2.63 |
| F4 | 52.03 ± 1.88 | 0.15 ± 0.03 | >300 | 91.50 ± 4.68 | 7.00 ± 0.16 | 1.16 ± 0.04 | 3.04 ± 0.27 | 6.63 ± 0.34 | 46.07 ± 2.46 | 13.98 ± 1.41 | 38.04 ± 1.38 | 35.15 ± 1.70 |
| F5 | 52.61 ± 2.67 | 0.20 ± 0.01 | 203 ± 8.40 | 91.27 ± 2.89 | 6.62 ± 0.32 | 1.04 ± 0.02 | 3.62 ± 0.30 | 15.5 ± 0.68 | 18.1 ± 1.88 | 378.95 ± 16.86 | 34.25 ± 2.01 | 18.5 ± 1.55 |
| F6 | 50.12 ± 1.48 | 0.18 ± 0.03 | 187 ± 5.71 | 94.04 ± 5.94 | 6.83 ± 0.24 | 1.17 ± 0.05 | 2.98 ± 0.12 | 6.71 ± 0.15 | 9.69 ± 2.08 | 337.75 ± 11.28 | 50.18 ± 1.33 | 21.62 ± 2.91 |
| F7 | 41.07 ± 0.17 | 0.16 ± 0.06 | >300 | 90.66 ± 3.36 | 7.01 ± 0.40 | 1.20 ± 0.04 | 5.66 ± 0.20 | 1.04 ± 0.11 | 49.82 ± 1.36 | 9.44 ± 0.27 | 97.23 ± 5.40 | 52.14 ± 4.07 |
| F8 | 36.86 ± 3.96 | 0.15 ± 0.02 | 237 ± 10.00 | 93.78 ± 2.06 | 6.57 ±0.24 | 1.14 ± 0.03 | 6.35 ± 0.41 | 6.33 ± 0.32 | 6.03 ± 1.05 | 497.75 ± 22.47 | 110.79 ± 4.51 | 32.03 ± 1.08 |
| F9 | 40.33 ± 2.07 | 0.13 ± 0.06 | >300 | 92.04 ± 2.31 | 6.90 ± 0.14 | 0.96 ± 0.01 | 6.09 ± 0.26 | 3.68 ± 0.18 | 23.84 ± 2.04 | 211.5 ± 13.20 | 127.04 ± 4.38 | 40.44 ± 2.59 |
| F10 | 49.20 ± 1.43 | 0.22 ± 0.01 | >300 | 90.13 ± 4.63 | 6.84 ± 0.36 | 1.00 ± 0.04 | 8.73 ± 0.38 | 1.83 ± 0.10 | 46.93 ± 3.02 | 8.09 ± 0.15 | 107.23 ± 2.46 | 23.87 ± 1.16 |
| F11 | 52.20 ± 3.75 | 0.22 ± 0.04 | 198 ± 6.70 | 89.48 ± 1.09 | 6.78 ± 0.12 | 1.18 ± 0.06 | 7.22 ± 0.19 | 8.95 ± 0.28 | 19.46 ± 1.80 | 361.87 ± 20.89 | 123.51 ± 3.87 | 12.19 ± 1.64 |
| F12 | 50.49 ± 2.03 | 0.23 ± 0.03 | 240 ± 12.00 | 90.29 ± 2.43 | 6.61 ± 0.38 | 0.99 ± 0.05 | 8.64 ± 0.42 | 6.19 ± 0.27 | 20.13 ± 1.07 | 296.52 ± 14.04 | 160.06 ± 4.20 | 12.14 ± 0.08 |
| Optimized OFDF (F1) | Content Uniformity (%) | Tensile Strength (Mpa) | % Elongation | Young’s Modulus (Mpa) | Disintegration Time (s) | % PX Dissolved after 10 min |
|---|---|---|---|---|---|---|
| Freshly prepared | 96.68 ± 3.62 | 3.89 ± 0.19 | 53.08 ±1.28 | 8.12 ± 0.13 | 17.09 ± 1.30 | 96.02 ± 3.46 |
| After 3 months | 95.70 ± 3.14 | 4.02 ± 0.25 | 48.34 ± 0.03 | 8.06 ± 0.32 | 15.24 ± 0.87 | 96.50 ± 1.78 |
| After 6 months | 93.89 ± 4.08 | 3.93 ± 0.12 | 48.29 ± 0.16 | 7.99 ± 0.30 | 20.33 ± 1.01 | 95.63 ± 2.44 |
| Pharmacokinetics Parameter | Treatment (Mean ± SD) | |
|---|---|---|
| Optimized OFDF (F1) | Market Tablet | |
| Cmax (ng/mL) a | 11.18 ± 7.86 | 6.44 ± 3.77 |
| AUC0–48 (ng.h/mL) a | 108.92 ± 81.31 | 69.79 ± 52.92 |
| AUC0-∞ (ng.h/mL) a | 165.07 ± 135.10 | 92.51 ± 67.35 |
| tmax (h) a | 0.94 ± 0.54 | 3.08 ± 1.88 |
| t1/2 (h) a | 22.54 ± 4.11 | 22.32 ± 4.81 |
| K (l/h) a | 0.030 ± 0.01 | 0.030 ± 0.01 |
| MRT a | 37.90 ± 7.29 | 34.06 ± 8.24 |
| % Relative bioavailability (%RB) | 178.43 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshafeey, A.H.; El-Dahmy, R.M. Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers. Pharmaceutics 2021, 13, 1869. https://doi.org/10.3390/pharmaceutics13111869
Elshafeey AH, El-Dahmy RM. Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers. Pharmaceutics. 2021; 13(11):1869. https://doi.org/10.3390/pharmaceutics13111869
Chicago/Turabian StyleElshafeey, Ahmed Hassen, and Rania Moataz El-Dahmy. 2021. "Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers" Pharmaceutics 13, no. 11: 1869. https://doi.org/10.3390/pharmaceutics13111869
APA StyleElshafeey, A. H., & El-Dahmy, R. M. (2021). Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers. Pharmaceutics, 13(11), 1869. https://doi.org/10.3390/pharmaceutics13111869






