Nano-Assembly of Quisinostat and Biodegradable Macromolecular Carrier Results in Supramolecular Complexes with Slow-Release Capabilities
Abstract
:1. Introduction
2. Materials and Methods
3. Results
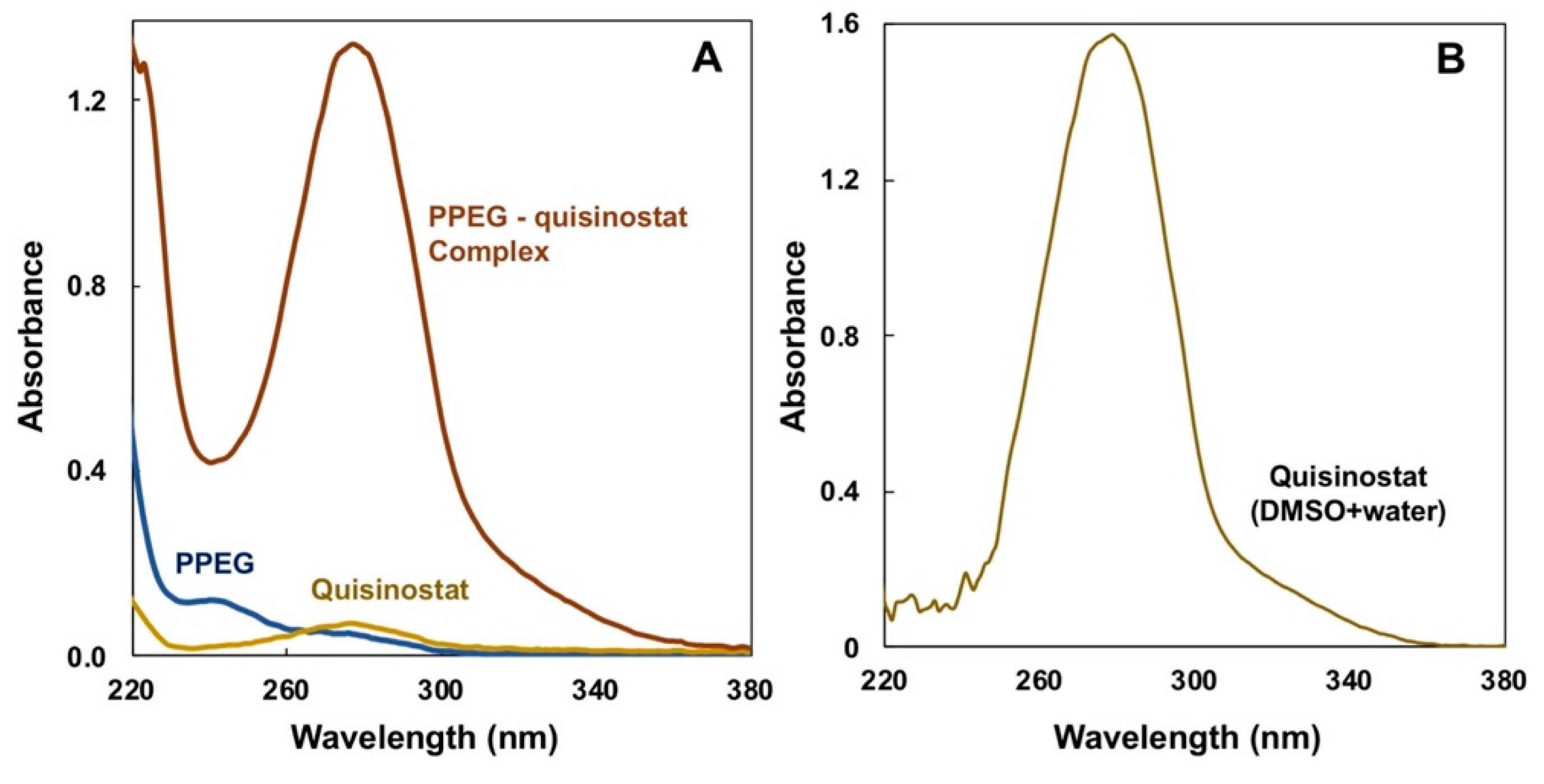
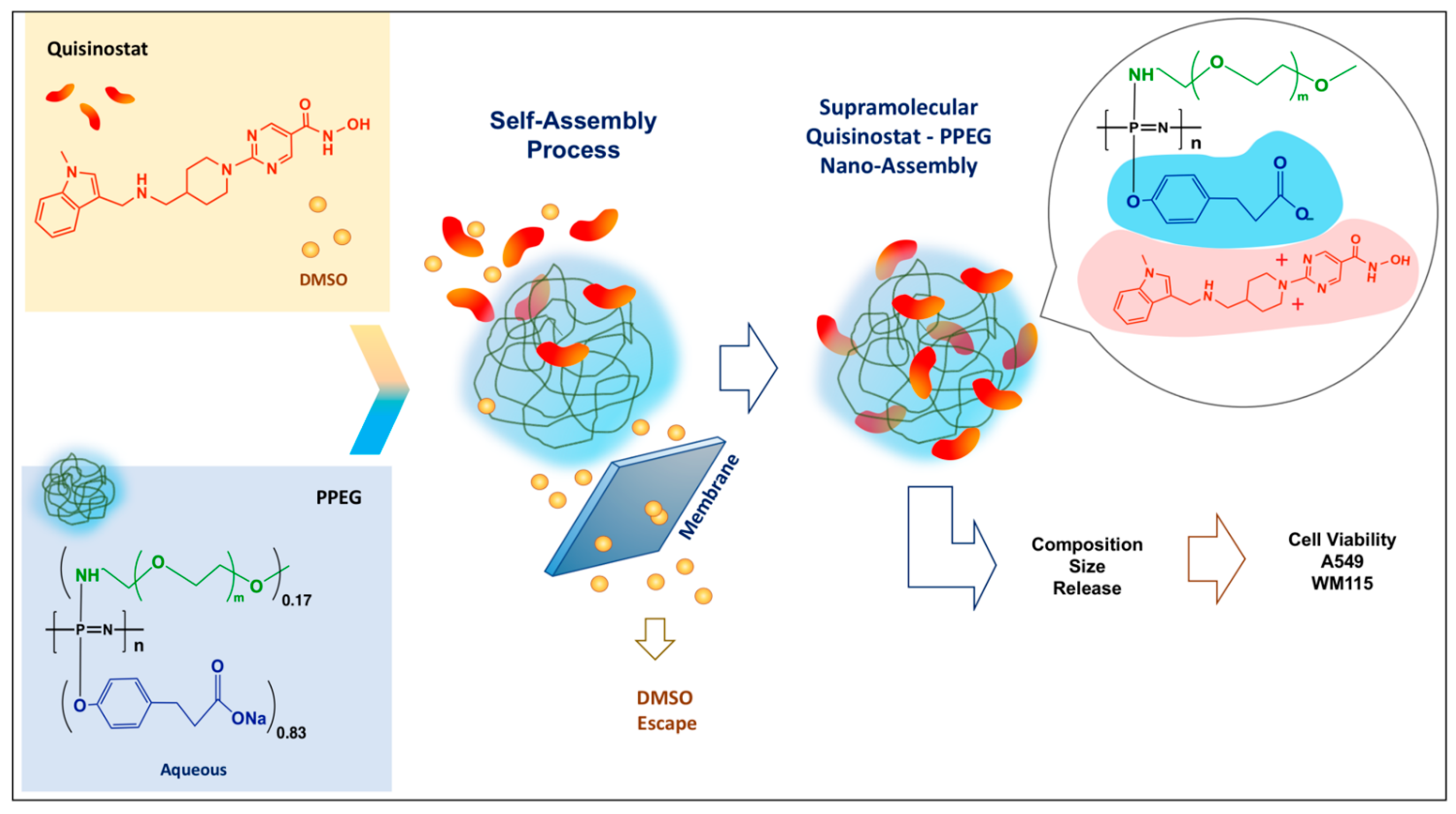
3.1. Solvent Gradient Driven Nano-Assembly of Quisinostat—Polyphosphazene Complexes
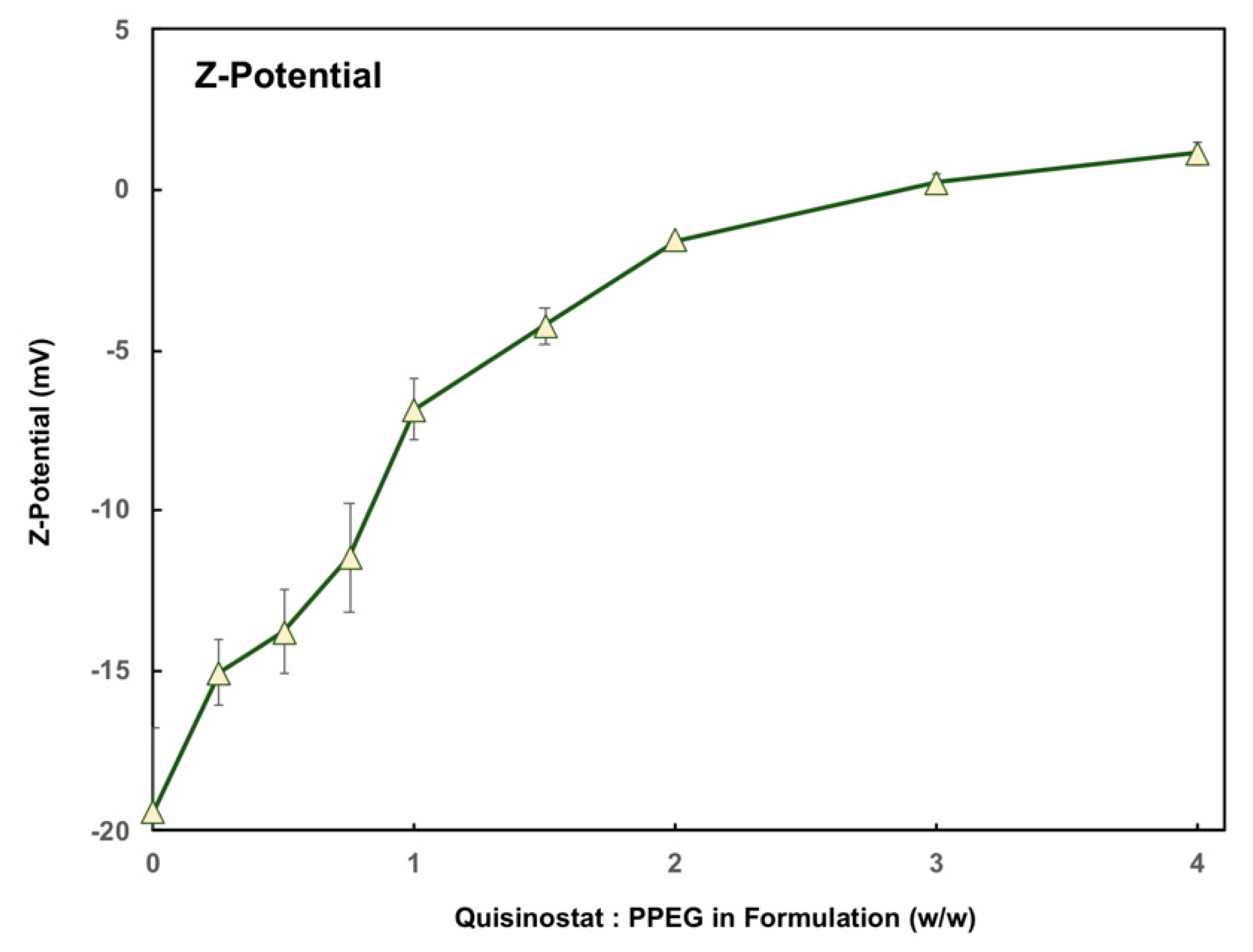
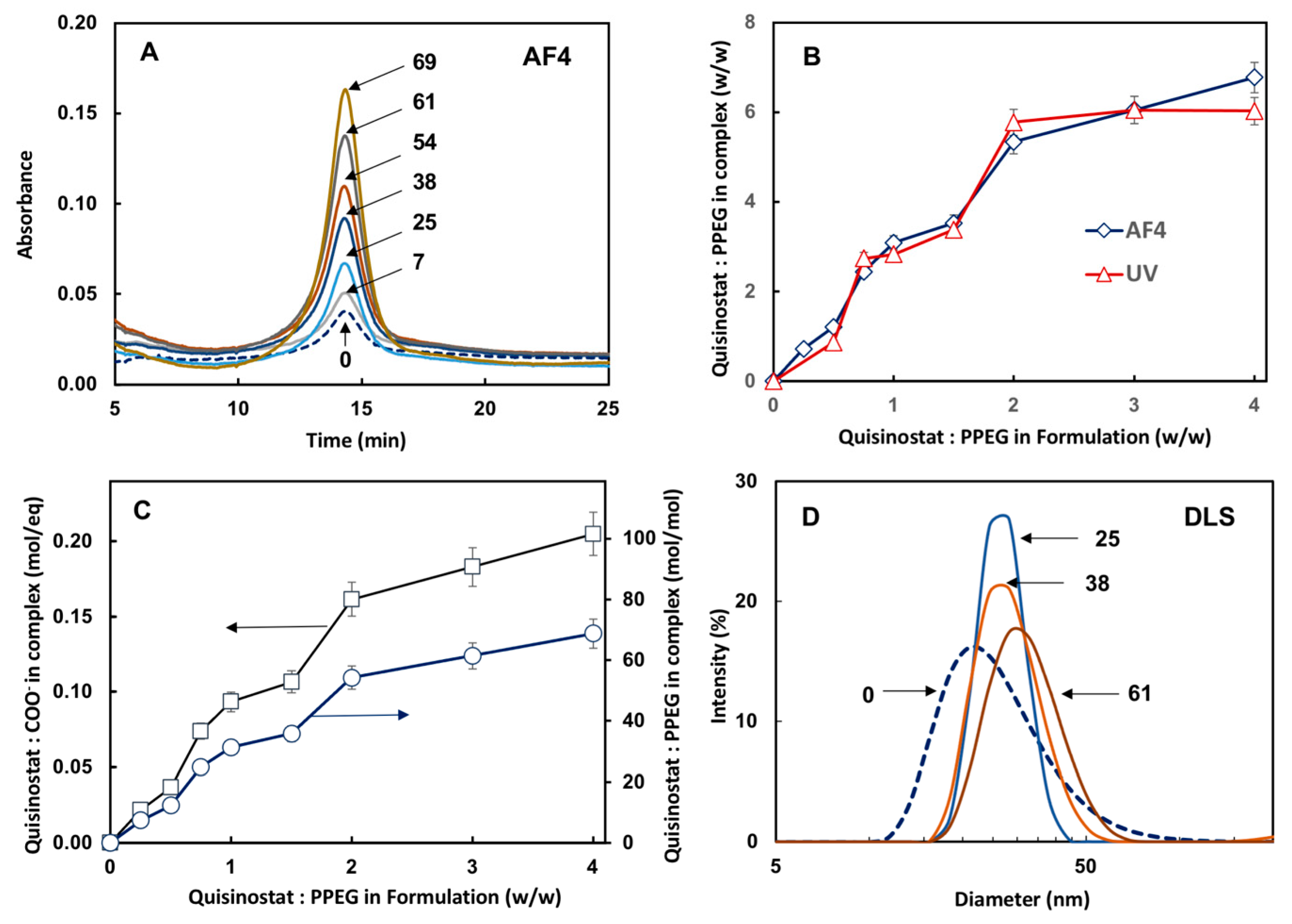
3.2. Quisinostat Associates with PPEG in a Dose Dependent Manner
3.3. Drug Release Characteristics and Hemocompatibility of Complexes
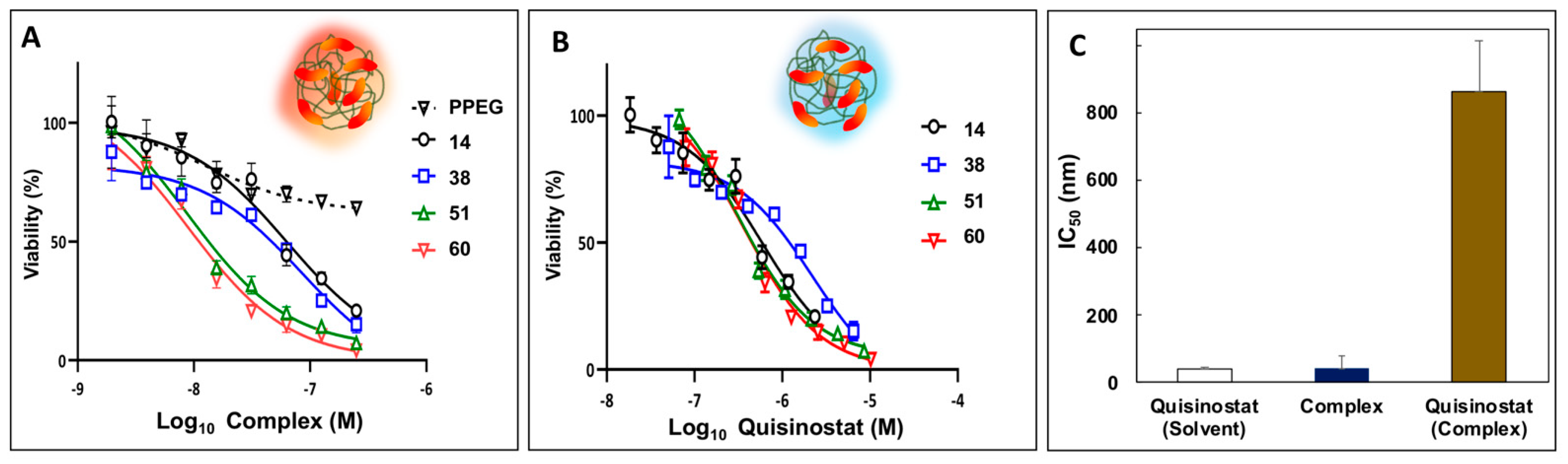
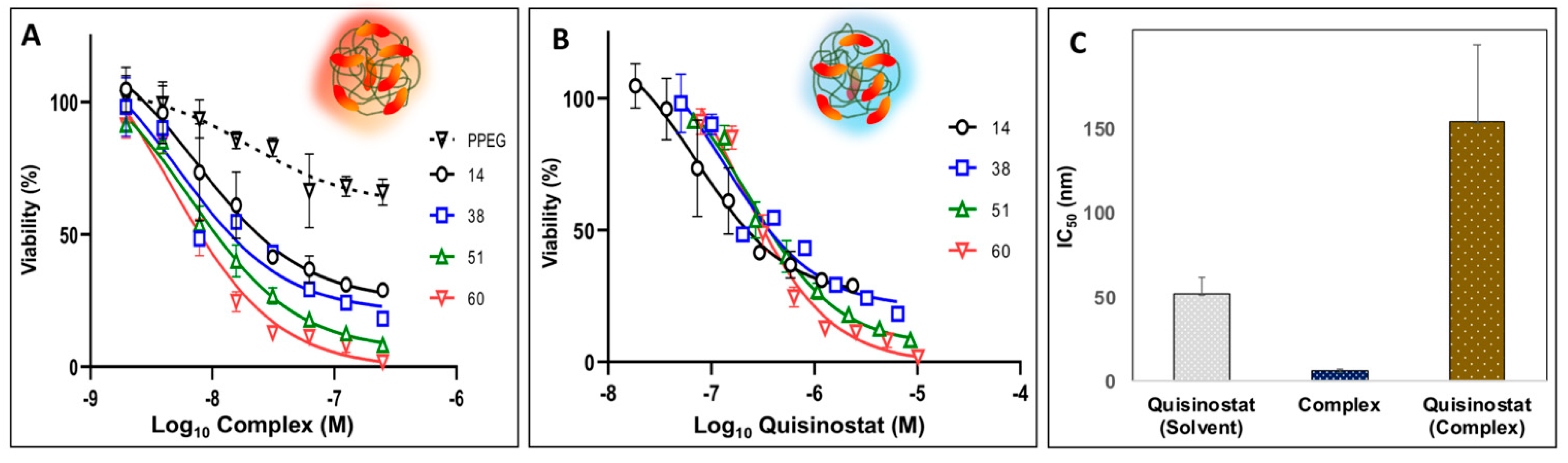
3.4. Potency of Quisinostat-PPEG Complexes in Cell-Based Assays
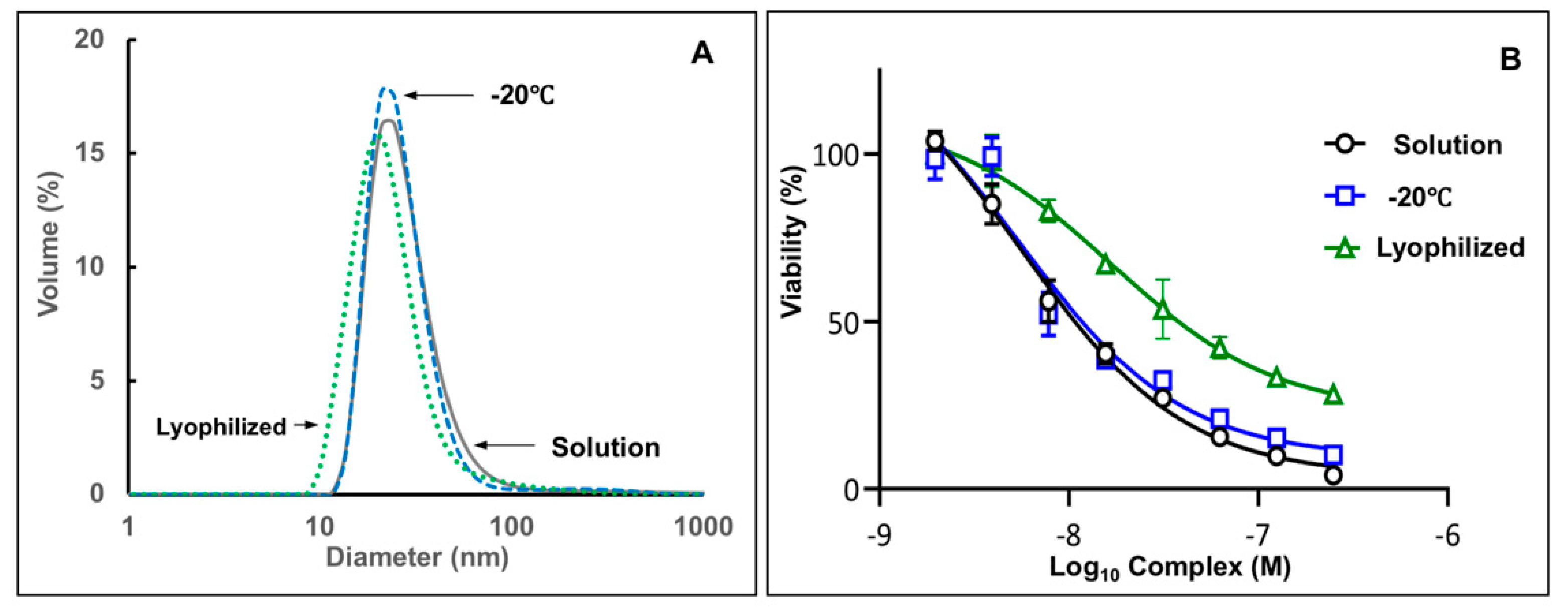
3.5. Complexes Demonstrate Stability in Freeze-Thaw and Lyophilization Stability Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Values | PPEG | Complexes (Molar Quisinostat-to-PPEG Ratios) | |||
|---|---|---|---|---|---|
| 14 | 38 | 51 | 60 | ||
| IC50 (nM) | - | 63 | 84 | 9 | 9 |
| 95% CI (nM) | - | 36–113 | 46–162 | 6–12 | 6–12 |
| R2 | Non fit | 0.94 | 0.94 | 0.98 | 0.97 |
| Values | Complexes (Molar Quisinostat-to-PPEG Ratios) | |||
|---|---|---|---|---|
| 14 | 38 | 51 | 60 | |
| IC50 (nM) | 591 | 2198 | 301 | 364 |
| 95% CI (nM range) | 340–1067 | 1211–4220 | 210–424 | 255–516 |
| R2 | 0.94 | 0.94 | 0.98 | 0.97 |
| Values | PPEG | Complexes (Molar Quisinostat-to-PPEG Ratios) | |||
|---|---|---|---|---|---|
| 14 | 38 | 51 | 60 | ||
| IC50 (nM) | - | 7.6 | 5.25 | 6.67 | 4.54 |
| 95% CI (nM) | - | 3.8–14 | 2.16–11.52 | 4.67–9.36 | 2.8–6.8 |
| R2 | Non fit | 0.91 | 0.91 | 0.98 | 0.97 |
| Values | Complexes (Molar Quisinostat-to-PPEG Ratios) | |||
|---|---|---|---|---|
| 14 | 38 | 51 | 60 | |
| IC50 (nM) | 72 | 136 | 228 | 184 |
| 95% CI (nM) | 36–135 | 56–299 | 160–321 | 117–278 |
| R2 | 0.92 | 0.92 | 0.98 | 0.97 |

References
- Johnstone, R.W. Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002, 1, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R. Safety and Tolerability of Histone Deacetylase (HDAC) inhibitors in oncology. Drug Saf. 2019, 42, 235–245. [Google Scholar] [CrossRef] [PubMed]
- San José-Enériz, E.; Gimenez-Camino, N.; Agirre, X.; Prosper, F. HDAC inhibitors in acute myeloid leukemia. Cancers 2019, 11, 1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, A.A.; Chabner, B.A. Histone deacetylase inhibitors in cancer therapy. J. Clin. Oncol. 2009, 27, 5459–5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, W.K.; O’Connor, O.A.; Krug, L.M.; Chiao, J.H.; Heaney, M.; Curley, T.; MacGregore-Cortelli, B.; Tong, W.; Secrist, J.P.; Schwartz, L.; et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J. Clin. Oncol. 2005, 23, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.C.; Min, Y.; Palm, R.C.; Fiordalisi, J.J.; Wagner, K.T.; Hyder, N.; Cox, A.D.; Caster, J.M.; Tian, X.; Wang, A.Z. Nanoparticle formulations of histone deacetylase inhibitors for effective chemoradiotherapy in solid tumors. Biomaterials 2015, 51, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, P.; Facon, T.; Touzeau, C.; Benboubker, L.; Delain, M.; Badamo-Dotzis, J.; Phelps, C.; Doty, C.; Smit, H.; Fourneau, N.; et al. Quisinostat, bortezomib, and dexamethasone combination therapy for relapsed multiple myeloma. Leuk. Lymphoma 2016, 57, 1546–1559. [Google Scholar] [CrossRef]
- Stühmer, T.; Arts, J.; Chatterjee, M.; Borawski, J.; Wolff, A.; King, P.; Einsele, H.; Leo, E.; Bargou, R.C. Preclinical anti-myeloma activity of the novel HDAC-inhibitor JNJ-26481585. Br. J. Haematol. 2010, 149, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Diao, H.; Dong, N.; Su, X.; Wang, B.; Mo, Q.; Yu, H.; Wang, X.; Chen, C. Histone deacetylase inhibitor induces cell apoptosis and cycle arrest in lung cancer cells via mitochondrial injury and p53 up-acetylation. Cell Biol. Toxicol. 2016, 32, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Arts, J.; King, P.; Mariën, A.; Floren, W.; Beliën, A.; Janssen, L.; Pilatte, I.; Roux, B.; Decrane, L.; Gilissen, R.; et al. JNJ-26481585, a novel “second-generation” oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin. Cancer Res. 2009, 15, 6841–6851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, W.-G.; Wei, Y.; Stevenson, W.; Kuang, S.-Q.; Fang, Z.; Zhang, M.; Arts, J.; Garcia-Manero, G. Preclinical antileukemia activity of JNJ-26481585, a potent second-generation histone deacetylase inhibitor. Leuk. Res. 2010, 34, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Carol, H.; Gorlick, R.; Kolb, E.A.; Morton, C.L.; Manesh, D.M.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Wozniak, A.; et al. Initial testing (stage 1) of the histone deacetylase inhibitor, quisinostat (JNJ-26481585), by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2014, 61, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, B.; Baird, R.; Kristeleit, R.S.; Plummer, R.; Cowan, R.; Stewart, A.; Fourneau, N.; Hellemans, P.; Elsayed, Y.; Mcclue, S.; et al. A phase I study of Quisinostat (JNJ-26481585), an oral hydroxamate histone deacetylase inhibitor with evidence of target modulation and antitumor activity, in patients with advanced solid tumors. Clin. Cancer Res. 2013, 19, 4262–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouché, M.; Dong, Y.C.; Sheikh, S.; Taing, K.; Saxena, D.; Hsu, J.C.; Chen, M.H.; Salinas, R.D.; Song, H.; Burdick, J.A.; et al. Novel treatment for glioblastoma delivered by a radiation responsive and radiopaque hydrogel. ACS Biomater. Sci. Eng. 2021, 7, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Householder, K.T.; DiPerna, D.M.; Chung, E.P.; Luning, A.R.; Nguyen, D.T.; Stabenfeldt, S.E.; Mehta, S.; Sirianni, R.W. pH driven precipitation of quisinostat onto PLA-PEG nanoparticles enables treatment of intracranial glioblastoma. Colloids Surf. B 2018, 166, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, H.; Kühne, M.; Grune, C.; Warncke, P.; Hofmann, S.; Koschella, A.; Godmann, M.; Fischer, D.; Heinzel, T.; Heinze, T. Polysaccharide nanoparticles bearing HDAC inhibitor as nontoxic nanocarrier for drug delivery. Macromol. Biosci. 2020, 20, e2000039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, S.; Fowler, M.J.; Baker, C.; Stopka, S.A.; Regan, M.S.; Sablatura, L.; Broughton, C.W.; Knight, B.E.; Stabenfeldt, S.E.; Agar, N.Y.R.; et al. β-Cyclodextrin-poly (β-Amino Ester) nanoparticles are a generalizable strategy for high loading and sustained release of HDAC inhibitors. ACS Appl. Mater. Interfaces 2021, 13, 20960–20973. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Wang, R.; Karauzum, H.; Chowdhury, A.; Agnihotri, P.; Yunus, A.S.; Mariuzza, R.A.; Fuerst, T.R. Supramolecular assembly of Toll-like receptor 7/8 agonist into multimeric water-soluble constructs enables superior immune stimulation in vitro and in vivo. ACS Appl. Bio Mater. 2020, 3, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Langer, R. Polyphosphazene immunoadjuvants: Historical perspective and recent advances. J. Control Release 2021, 329, 299–315. [Google Scholar] [CrossRef]
- Guest, J.D.; Wang, R.; Elkholy, K.H.; Chagas, A.; Chao, K.L.; Cleveland, T.E.; Kim, Y.C.; Keck, Z.-Y.; Marin, A.; Yunus, A.S.; et al. Design of a native-like secreted form of the hepatitis C virus E1E2 heterodimer. Proc. Natl. Acad. Sci. USA 2021, 118, e2015149118. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Marin, A.; Martinez, A.P.; Weidman, J.L.; Fuerst, T.R. Hydrolytically degradable PEGylated polyelectrolyte nanocomplexes for protein delivery. Biomacromolecules 2018, 19, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Qamar, B.; Solomon, M.; Marin, A.; Fuerst, T.R.; Andrianov, A.K.; Muro, S. Intracellular delivery of active proteins by polyphosphazene polymers. Pharmaceutics 2021, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Andrianov, A.K.; Marin, A.; Fuerst, T.R. Self-assembly of polyphosphazene immunoadjuvant with poly(ethylene oxide) enables advanced nanoscale delivery modalities and regulated pH-dependent cellular membrane activity. Heliyon 2016, 2, e00102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yessine, M.-A.; Lafleur, M.; Meier, C.; Petereit, H.-U.; Leroux, J.-C. Characterization of the membrane-destabilizing properties of different pH-sensitive methacrylic acid copolymers. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1613, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. Update on in vitro cytotoxicity assays for drug development. Expert Opin. Drug Discov. 2008, 3, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Corrales, J.Á.; Josan, J.S. Resazurin live cell assay: Setup and fine-tuning for reliable cytotoxicity results. In Proteomics for Drug Discovery: Methods and Protocols; Lazar, I.M., Kontoyianni, M., Lazar, A.C., Eds.; Springer: New York, NY, USA, 2017; pp. 207–219. [Google Scholar]
- Zhong, L.; Zhou, S.; Tong, R.; Shi, J.; Bai, L.; Zhu, Y.; Duan, X.; Liu, W.; Bao, J.; Su, L.; et al. Preclinical assessment of histone deacetylase inhibitor quisinostat as a therapeutic agent against esophageal squamous cell carcinoma. Investig. New Drugs 2019, 37, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, T.; Wang, Z.; Chen, X.; Liu, R. Histone deacetylase inhibitor quisinostat activates caspase signaling and upregulates p53 acetylation to inhibit the proliferation of HepG2 cells. Mol. Med. Rep. 2017, 16, 6094–6101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quisinostat (JNJ-26481585) 2HCl. Available online: https://www.selleckchem.com/products/JNJ-26481585.html (accessed on 29 September 2021).
- Cheow, W.S.; Hadinoto, K. Self-assembled amorphous drug-polyelectrolyte nanoparticle complex with enhanced dissolution rate and saturation solubility. J. Colloid Interface Sci. 2012, 367, 518. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.S. Counterion condensation theory constructed from different models. Physics A 1996, 231, 236–253. [Google Scholar] [CrossRef]
- Jeon, J.; Dobrynin, A.V. Necklace globule and counterion condensation. Macromolecules 2007, 40, 7695–7706. [Google Scholar] [CrossRef]
- Dobrynin, A.V. Polyelectrolytes: On the doorsteps of the second century. Polymer 2020, 202, 122714. [Google Scholar] [CrossRef]
- Messaud, F.A.; Sanderson, R.D.; Runyon, J.R.; Otte, T.; Pasch, H.; Williams, S.K.R. An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers. Prog. Polym. Sci. 2009, 34, 351–368. [Google Scholar] [CrossRef]
- Lappan, U.; Geißler, U.; Oelmann, M.; Schwarz, S. Apparent dissociation constants of polycarboxylic acids in presence of polycations. Colloid Polym. Sci. 2012, 290, 1665–1670. [Google Scholar] [CrossRef]
- Facchetti, F.; Previdi, S.; Ballarini, M.; Minucci, S.; Perego, P.; Porta, C.A.M.L. Modulation of pro- and anti-apoptotic factors in human melanoma cells exposed to histone deacetylase inhibitors. Apoptosis 2004, 9, 573–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Liu, Y.; Gaber, M.W.; Bumgardner, J.D.; Haggard, W.O.; Yang, Y. Development of chitosan–ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cells. J. Biomed. Mater. Res. Part. B 2009, 90B, 145–155. [Google Scholar] [CrossRef]
- Matafora, V.; Farris, F.; Restuccia, U.; Tamburri, S.; Martano, G.; Bernardelli, C.; Sofia, A.; Pisati, F.; Casagrande, F.; Lazzari, L.; et al. Amyloid aggregates accumulate in melanoma metastasis modulating YAP activity. EMBO Rep. 2020, 21, e50446. [Google Scholar] [CrossRef] [PubMed]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Fu, J.; Schlenoff, J.B. Driving forces for oppositely charged polyion association in aqueous solutions: Enthalpic, entropic, but not electrostatic. J. Am. Chem. Soc. 2016, 138, 980–990. [Google Scholar] [CrossRef]
- Wang, Q.; Schlenoff, J.B. The polyelectrolyte complex/coacervate continuum. Macromolecules 2014, 47, 3108–3116. [Google Scholar] [CrossRef]
- Fu, J.; Fares, H.M.; Schlenoff, J.B. Ion-pairing strength in polyelectrolyte complexes. Macromolecules 2017, 50, 1066–1074. [Google Scholar] [CrossRef]
- Von Ferber, C.; Löwen, H. Complexes of polyelectrolytes and oppositely charged ionic surfactants. J. Chem. Phys. 2003, 118, 10774–10779. [Google Scholar] [CrossRef]
- Lei, Q.-L.; Hadinoto, K.; Ni, R. Complexation of polyelectrolytes with hydrophobic drug molecules in salt-free solution: Theory and simulations. Langmuir 2017, 33, 3900–3909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shang, Y.; Feng, J.; Peng, C.; Liu, H.; Hu, Y. Effect of hydrophilicity or hydrophobicity of polyelectrolyte on the interaction between polyelectrolyte and surfactants: Molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 5516–5526. [Google Scholar] [CrossRef]
- Brilliantov, N.V.; Kuznetsov, D.V.; Klein, R. Chain collapse and counterion condensation in dilute polyelectrolyte solutions. Phys. Rev. Lett. 1998, 81, 1433–1436. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Ling, D.; Tang, T.; Huang, Z.; Wang, M.; Ding, Y.; Liu, T.; Wei, H.; Xu, W.; Mao, F.; et al. Discovery of novel plasmodium falciparum HDAC1 inhibitors with dual-stage antimalarial potency and improved safety based on the clinical anticancer drug candidate quisinostat. J. Med. Chem. 2021, 64, 2254–2271. [Google Scholar] [CrossRef]
- Martinez, A.P.; Qamar, B.; Fuerst, T.R.; Muro, S.; Andrianov, A.K. Biodegradable “smart” polyphosphazenes with intrinsic multifunctionality as intracellular protein delivery vehicles. Biomacromolecules 2017, 18, 2000–2011. [Google Scholar] [CrossRef]
- Martinez, A.P.; Qamar, B.; Marin, A.; Fuerst, T.R.; Muro, S.; Andrianov, A.K. Biodegradable “Scaffold” polyphosphazenes for non-covalent PEGylation of proteins. In Polyphosphazenes in Biomedicine, Engineering, and Pioneering Synthesis; Andrianov, A., Allcock, H.R., Eds.; American Chemical Society: Washington, DC, USA, 2018; Volume 1298, pp. 121–141. [Google Scholar]
- Authelin, J.-R.; Rodrigues, M.A.; Tchessalov, S.; Singh, S.K.; McCoy, T.; Wang, S.; Shalaev, E. Freezing of biologicals revisited: Scale, stability, excipients, and degradation stresses. J. Pharm. Sci. 2020, 109, 44–61. [Google Scholar] [CrossRef]
- Rayfield, W.J.; Kandula, S.; Khan, H.; Tugcu, N. Impact of freeze/thaw process on drug substance storage of therapeutics. J. Pharm. Sci. 2017, 106, 1944–1951. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, A.; Marin, A.; Weber, D.J.; Andrianov, A.K. Nano-Assembly of Quisinostat and Biodegradable Macromolecular Carrier Results in Supramolecular Complexes with Slow-Release Capabilities. Pharmaceutics 2021, 13, 1834. https://doi.org/10.3390/pharmaceutics13111834
Chowdhury A, Marin A, Weber DJ, Andrianov AK. Nano-Assembly of Quisinostat and Biodegradable Macromolecular Carrier Results in Supramolecular Complexes with Slow-Release Capabilities. Pharmaceutics. 2021; 13(11):1834. https://doi.org/10.3390/pharmaceutics13111834
Chicago/Turabian StyleChowdhury, Ananda, Alexander Marin, David J. Weber, and Alexander K. Andrianov. 2021. "Nano-Assembly of Quisinostat and Biodegradable Macromolecular Carrier Results in Supramolecular Complexes with Slow-Release Capabilities" Pharmaceutics 13, no. 11: 1834. https://doi.org/10.3390/pharmaceutics13111834
APA StyleChowdhury, A., Marin, A., Weber, D. J., & Andrianov, A. K. (2021). Nano-Assembly of Quisinostat and Biodegradable Macromolecular Carrier Results in Supramolecular Complexes with Slow-Release Capabilities. Pharmaceutics, 13(11), 1834. https://doi.org/10.3390/pharmaceutics13111834







