Disposition of Cefquinome in Turkeys (Meleagris gallopavo) Following Intravenous and Intramuscular Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
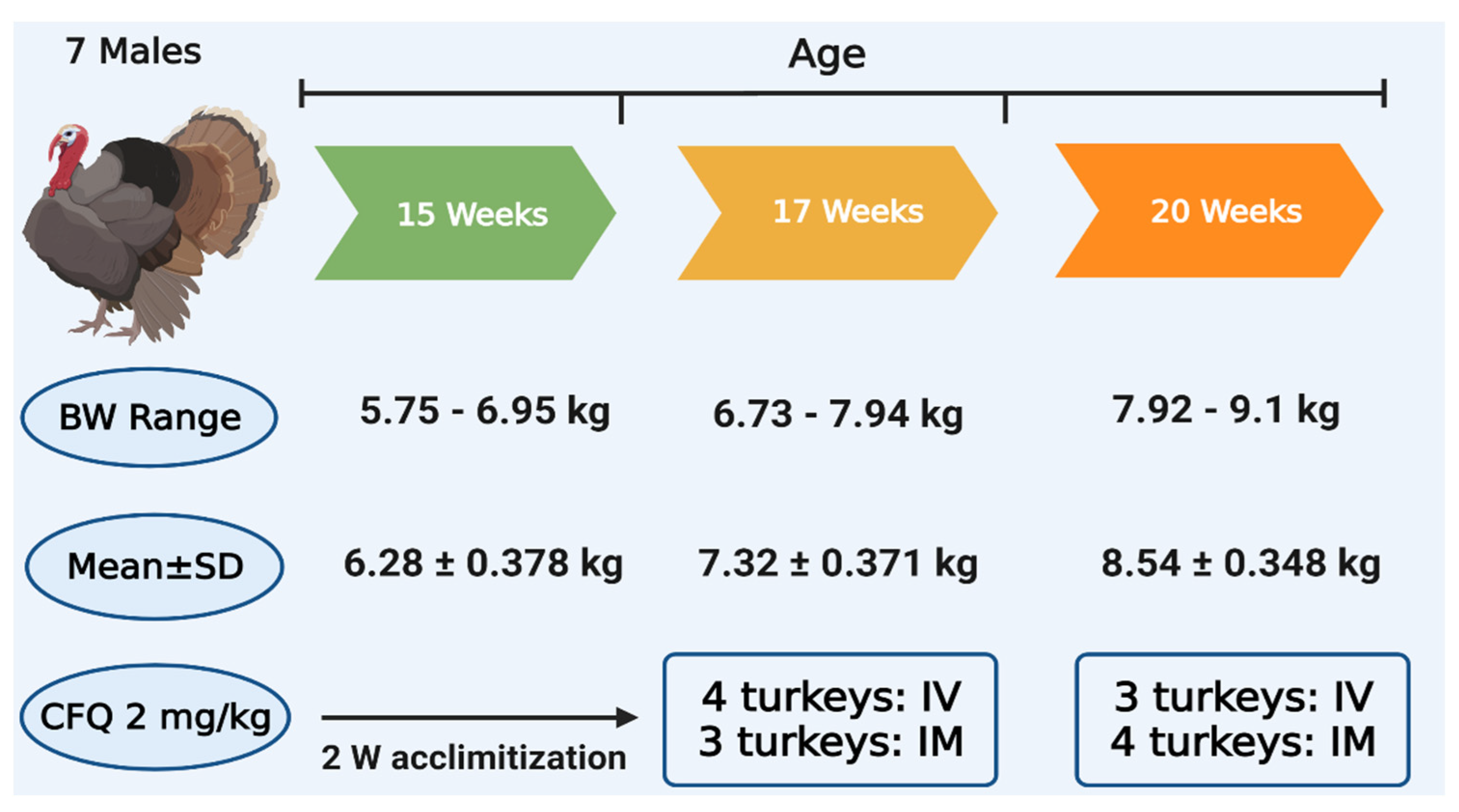
2.2. Experimental Birds
2.3. Experimental Design
2.4. Analytical Method
2.5. Method Validation
2.6. Plasma Protein Binding Extent of CFQ
2.7. Pharmacokinetic Analysis
3. Results
4. Discussion
| Species | Chickens | Ducks | Black Swans | Ducklings | Gosling | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | 2 | 5 | 2 | 2 | 2 | |||||
| Route | IV | IM | IV | IM | IV | IM | IV | IM | IV | IM |
| β (1/h) | 0.54 ± 0.04 | 0.53 ± 0.08 | 0.44 ± 0.02 | 0.39 ± 0.03 | 42.09 ± 0.09 | 0.43 ± 0.03 | - | - | - | - |
| t1/2α (h) | 0.43 ± 0.19 | 0.58 ± 0.27 | 0.19 ± 0.05 | 0.46 ± 0.30 | 0.31 ± 0.03 | - | 0.019 | 0.343 | 0.446 | 0.483 |
| t1/2β (h) | 1.29 ± 0.10 | 1.35 ± 0.20 | 1.57 ± 0.06 | 1.79 ± 0.11 | 1.69 ± 0.85 | 1.62 ± 0.11 | 0.972 | 1.717 | 1.737 | 1.403 |
| AUC0–∞ (μg·h/mL) | 5.33 ± 0.55 | 5.13 ± 1.06 | 25.12 ± 2.31 | 23.78 ± 3.87 | 16.5 ± 4.92 | 12.17 ± 4.32 | 6.248 | 4.220 | 4.396 | 5.008 |
| Vdss (L/kg) | 0.49 ± 0.05 | - | 0.41 ± 0.0 | - | 0.32 ± 0.17 | - | 0.042 | - | 0.432 | - |
| Cltot (L/kg/h) | 0.35 ± 0.04 | - | 0.22 ± 0.02 | 0.13 ± 0.04 | 0.320 | - | 0.455 | - | ||
| t1/2ab (h) | - | 0.07 ± 0.02 | - | 0.12 ± 0.02 | - | 0.12 ± 0.04 | - | - | - | |
| Cmax (µg/mL) | - | 3.04 ± 0.71 | - | 9.38 ± 1.61 | - | 5.71 ± 1.43 | - | 4.010 | - | 3.400 |
| Tmax (h) | - | 0.25 ± 0.06 | - | 0.38 ± 0.06 | - | 0.39 ± 0.19 | - | 0.163 | - | 0.203 |
| F (%) | - | 95.81 ± 5.81 | - | 93.28 ± 13.89 | - | 74.2 ± 26.3 | - | 67.5 | - | 113.9 |
| Reference | [38] | [9] | [11] | [39] | [39] | |||||
| Species | Pigs | Rabbits | Sheep | Dogs | Calves | |||||
| Dose (mg/kg) | 2 | 2 | 2 | 2 | 2 | |||||
| Route | IV | IM | IV | IM | IV | IM | IV | IM | IV | IM |
| β (1/h) | - | - | 0.76 ± 0.11 | 0.69 ± 0.13 | - | - | 0.72 ± 0.11 | 0.84 ± 0.13 | 0.40 ± 0.11 | 0.15 ± 0.02 |
| t1/2α (h) | 0.30 ± 0.08 | 1.33 ± 0.42 | - | - | 0.06 ± 0.04 | 0.31 ± 0.05 | 0.12 ± 0.05 | - | - | - |
| t1/2β (h) | 2.32 ± 0.47 | 4.92 ± 1.14 | 0.93 ± 0.14 | 1.04 ± 0.22 | 0.78 ± 0.19 | 1.88 ± 0.40 | 0.98 ± 0.14 | 0.85 ± 0.15 | 1.85 ± 0.44 | 4.47 ± 0.69 |
| AUC0–∞ (μg·h/mL) | 18.35 ± 5.32 | 17.22 ± 4.11 | 11.08 ± 4.06 | 10.40 ± 1.23 | 5.83 ± 0.45 | 5.19 ± 0.25 | 8.51 ± 1.27 | 8.24 ± 0.80 | 15.74 ± 3.57 | 22.75 ± 6.18 |
| Vdss (l/kg) | - | - | 0.21 ± 0.03 | - | 0.36 ± 0.06 | - | 0.30 ± 0.03 | - | 0.37 ± 0.10 | - |
| Cltot (L/kg/h) | 0.12 ± 0.03 | - | 0.18 ± 0.05 | - | 0.34 ± 0.03 | - | 0.24 ± 0.03 | - | 0.13 ± 0.03 | - |
| t1/2ab (h) | - | 0.24 ± 0.05 | - | - | - | 0.31 ± 0.05 | - | 0.14 ± 0.05 | - | - |
| Cmax (µg/mL) | - | 3.36 ± 0.54 | - | 8.87 ± 2.07 | - | 2.60 ± 0.14 | - | 4.83 ± 0.79 | - | 4.56 ± 0.75 |
| Tmax (h) | - | 0.83 ± 0.28 | - | 0.25 ± 0.12 | - | 0.50 ± 0.00 | - | 0.43 ± 0.11 | - | 1.00 ± 0.00 |
| F (%) | - | 85.13 ± 9.93 | - | 95.23 ± 9.84 | - | 89.31 ± 6.06 | - | 97.8 ± 9.40 | - | 141.22 |
| Reference | [51] | [31] | [27] | [22] | [28] | |||||
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elbadawy, M.; Aboubakr, M.; Abugomaa, A. Pharmacokinetics of Tylvalosin in Broiler Turkeys (Meleagris gallopavo) After Single Intravenous and Oral Administration. Front. Vet. Sci. 2019, 6, 355. [Google Scholar] [CrossRef]
- Hornish, R.; Katarski, R.E.H.A.S.F. Cephalosporins in Veterinary Medicine-Ceftiofur Use in Food Animals. Curr. Top. Med. Chem. 2002, 2, 717–731. [Google Scholar] [CrossRef]
- Becker, M.; Zittlau, E.; Petz, M. Residue analysis of 15 penicillins and cephalosporins in bovine muscle, kidney and milk by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2004, 520, 19–32. [Google Scholar] [CrossRef]
- Thomas, E.; Thomas, V.; Wilhelm, C. Antibacterial activity of cefquinome against equine bacterial pathogens. Vet. Microbiol. 2006, 115, 140–147. [Google Scholar] [CrossRef]
- Broens, E.M.; Van Geijlswijk, I.M. Prudent Use of Antimicrobials in Exotic Animal Medicine. Vet. Clin. N. Am. Exot. Anim. Pr. 2018, 21, 341–353. [Google Scholar] [CrossRef]
- Chin, N.-X.; Gu, J.-W.; Fang, W.; Neu, H.C. In vitro activity of cefquinome, a new cephalosporin, compared with other cephalosporin antibiotics. Diagn. Microbiol. Infect. Dis. 1992, 15, 331–337. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, J. Activity of cefquinome against extended-spectrum β -lactamase-producing Klebsiella pneumoniae in neutropenic mouse thigh model. J. Vet. Pharmacol. Ther. 2016, 40, 392–397. [Google Scholar] [CrossRef]
- Guérin-Faublée, V.; Carret, G.; Houffschmitt, P. In vitro activity of 10 antimicrobial agents against bacteria isolated from cows with clinical mastitis. Vet. Rec. 2003, 152, 466–471. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, J.; Wang, R.; Sun, L.; Zhu, L.; Luo, X.; Fang, B.; Liu, Y. Pharmacokinetics and bioavailability of cefquinome in healthy ducks. Am. J. Vet. Res. 2011, 72, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Limbert, M.; Isert, D.; Klesel, N.; Markus, A.; Seeger, K.; Seibert, G.; Schrinner, E. Antibacterial activities in vitro and in vivo and pharmacokinetics of cefquinome (HR 111V), a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 1991, 35, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-H.; Wang, X.-F.; Wang, Q.; Li, L.-D. Pharmacokinetics, bioavailability and dose assessment of Cefquinome against Escherichia coli in black swans (Cygnus atratus). BMC Vet. Res. 2017, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Mi, K.; Li, M.; Sun, L.; Hou, Y.; Zhou, K.; Hao, H.; Pan, Y.; Liu, Z.; Xie, C.; Huang, L. Determination of Susceptibility Breakpoint for Cefquinome against Streptococcus suis in Pigs. Antibiotics 2021, 10, 958. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.S.; Amarante, A.F.; Guerra, S.T.; Latosinski, G.S.; Rossi, B.F.; Rall, V.L.M.; Pantoja, J.C.D.F. Efficacy of cefquinome and a combination of cloxacillin and ampicillin for treatment of dairy cows with Streptococcus agalactiae subclinical mastitis. PLoS ONE 2019, 14, e0216091. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Birhanu, B.T.; Lee, E.B.; Lee, S.J.; Boby, N.; Park, Y.S.; Park, S.C. Pharmacokinetic and pharmacodynamic integration for optimal dosage of cefquinome against Streptococcus equi subsp. equi in foals. Vet. Res. 2020, 51, 131. [Google Scholar] [CrossRef]
- Smiet, E.; Haritova, A.; Heil, B.A.; Fink-Gremmels, J.; Wijnberg, I.D. Comparing the pharmacokinetics of a fourth generation cephalosporin in three different age groups of New Forest ponies. Equine Vet. J. 2012, 44, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Uney, K.; Altan, F.; Altan, S.; Erol, H.; Arican, M.; Elmas, M. Plasma and synovial fluid pharmacokinetics of cefquinome following the administration of multiple doses in horses. J. Vet. Pharmacol. Ther. 2016, 40, 239–247. [Google Scholar] [CrossRef]
- Zhao, D.H.; Zhang, C.Y.; Zhang, Z.; Liu, Z.C.; Liu, B.T.; Yu, J.J.; Guo, J.P.; Deng, H.; Liu, Y.H. Population pharmacokinetics of cefquinome in pigs. J. Vet. Pharmacol. Ther. 2013, 36, 313–319. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, X.; Gu, X.; Li, X.; Gu, M.; Zhang, N.; Shen, X.; Ding, H. Pharmacokinetics and ex vivo pharmacodynamics of cefquinome in porcine serum and tissue cage fluids. Vet. J. 2014, 199, 399–405. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, X.; Huang, Z.; Zhang, N.; Wu, Y.; Cai, Q.; Shen, X.; Ding, H. Pharmacokinetic/pharmacodynamic assessment of cefquinome against Actinobacillus Pleuropneumoniae in a piglet tissue cage infection model. Vet. Microbiol. 2018, 219, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.; Huang, Z.; Kang, Z.; Chen, Y.; Shen, X.; Cai, Q.; Ding, H. Pharmacokinetic/pharmacodynamic integration of cefquinome against Pasteurella Multocida in a piglet tissue cage model. J. Vet. Pharmacol. Ther. 2018, 42, 60–66. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, X.; Li, X.; Gu, M.; Zhang, N.; Shen, X.; Li, Y.; Ding, H. Pharmacokinetics and ex-vivo pharmacodynamics of cefquinome against Klebsiella pneumonia in healthy dogs. J. Vet. Pharmacol. Ther. 2014, 37, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-F.; Zhao, D.H.; Yu, Y.; Yang, X.; Shi, W.; Peng, Y.B.; Liu, Y.H. Pharmacokinetics, bioavailability and PK/PD relationship of cefquinome for Escherichia coli in Beagle dogs. J. Vet. Pharmacol. Ther. 2015, 38, 543–548. [Google Scholar] [CrossRef]
- Venkatachalam, D.; Dumka, V.K.; Ranjan, B. Pharmacokinetics of a single intramuscular injection of cefquinome in buffalo calves. J. Vet. Pharmacol. Ther. 2017, 41, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Corum, O.; Yildiz, R.; Ider, M.; Altan, F.; Ok, M.; Uney, K. Pharmacokinetics and bioavailability of cefquinome and ceftriaxone in premature calves. J. Vet. Pharmacol. Ther. 2019, 42, 632–639. [Google Scholar] [CrossRef]
- Shan, Q.; Yang, F.; Wang, J.; Ding, H.; He, L.; Zeng, Z. Pharmacokinetic/pharmacodynamic relationship of cefquinome against Pasteurella multocida in a tissue-cage model in yellow cattle. J. Vet. Pharmacol. Ther. 2014, 37, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Hao, H.; Huang, L.; Sanders, P.; Wang, X.; Chen, D.; Tao, Y.; Xie, S.; Xiuhua, K.; Li, J.; et al. Integration of PK/PD for dose optimization of Cefquinome against Staphylococcus aureus causing septicemia in cattle. Front. Microbiol. 2015, 6, 588. [Google Scholar] [CrossRef][Green Version]
- Uney, K.; Altan, F.; Elmas, M. Development and Validation of a High-Performance Liquid Chromatography Method for Determination of Cefquinome Concentrations in Sheep Plasma and Its Application to Pharmacokinetic Studies. Antimicrob. Agents Chemother. 2010, 55, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Corum, O.; Corum, D.D.; Er, A.; Uney, K. Pharmacokinetics of cefquinome after single and repeated subcutaneous administrations in sheep. J. Vet. Pharmacol. Ther. 2019, 42, 647–653. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, S.A.; Amer, A.M.M.; Kamel, G.M.; Eldeib, K.M.; Constable, P.D. Pharmacokinetics and pharmacodynamics of intramammary cefquinome in lactating goats with and without experimentally induced Staphylococcus aureus mastitis. J. Vet. Pharmacol. Ther. 2019, 42, 452–460. [Google Scholar] [CrossRef]
- Litterio, N.J.; Lorenzutti, A.M.; Zarazaga, M.D.P.; Himelfarb, M.A.; Andrés-Larrea, M.I.S.; Serrano-Rodríguez, J.M. Comparative pharmacokinetics and pharmacokinetic/pharmacodynamic analysis by nonlinear mixed-effects modeling of cefquinome in nonpregnant, pregnant, and lactating goats after intravenous and intramuscular administration. J. Vet. Pharmacol. Ther. 2021, 44, 68–78. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Song, I.B.; Lee, H.K.; Kim, T.W.; Kim, M.S.; Lim, J.H.; Park, B.K.; Yun, H.I. Pharmacokinetics and bioavailability of cefquinome in rabbits following intravenous and intramuscular administration. J. Vet. Pharmacol. Ther. 2011, 34, 618–620. [Google Scholar] [CrossRef]
- ElAzab, S.T.; Schrunk, D.E.; Griffith, R.W.; Ensley, S.M.; Dell’Anna, G.; Mullin, K.; Elsayed, M.G.; Amer, M.S.; El-Nabtity, S.M.; Hsu, W.H. Pharmacokinetics of cefquinome in healthy and Pasteurella multocida-infected rabbits. J. Vet. Pharmacol. Ther. 2018, 41, 374–377. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, L.; Kang, Z.; Huang, Z.; Gu, X.; Shen, X.; Ding, H. Microdialysis Determination of Cefquinome Pharmacokinetics in Murine Thigh From Healthy, Neutropenic, and Actinobacillus pleuropneumoniae-Infected Mice. Front. Pharmacol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Z.; Gu, X.; Huang, S.; Shen, X.; Ding, H. Murine Thigh Microdialysis to Evaluate the Pharmacokinetic/Pharmacodynamic Integration of Cefquinome Against Actinobacillus pleuropneumoniae. Front. Vet. Sci. 2020, 7, 448. [Google Scholar] [CrossRef]
- Shan, Q.; Zhu, X.; Liu, S.; Bai, Y.; Ma, L.; Yin, Y.; Zheng, G. Pharmacokinetics of cefquinome in tilapia (Oreochromis niloticus) after a single intramuscular or intraperitoneal administration. J. Vet. Pharmacol. Ther. 2015, 38, 601–605. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, J.; Yang, F.; Ma, L.; Yin, Y.; Liu, S.; Li, L.; Zheng, G. Pharmacokinetics of cefquinome in crucian carp (Carassius auratus gibelio) after oral, intramuscular, intraperitoneal, and bath administration. J. Vet. Pharmacol. Ther. 2018, 41, 734–738. [Google Scholar] [CrossRef]
- Martín, B.N.S.; Bataglia, J.; Hernandez, P.; Quiroz, A.; Cañon, H. Absorption and Excretion of Cefquinome in Coho Salmon (Oncorhynchus kisutch) in Freshwater at 10 °C. J. Vet. Med. Ser. A 1998, 45, 615–623. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, X.; Wang, T.; Du, S. Pharmacokinetic analysis of cefquinome in healthy chickens. Br. Poult. Sci. 2013, 54, 81–86. [Google Scholar] [CrossRef]
- Cheng, P.; Feng, T.; Zhang, Y.; Li, X.; Tian, L.; Wu, J.; Cheng, F.; Zeng, Y.; Chen, H.; He, X.; et al. Comparative pharmacokinetics of intravenous and intramuscular cefquinome sulfate administration in ducklings and goslings. Am. J. Vet. Res. 2020, 81, 837–877. [Google Scholar] [CrossRef]
- Saleem, R. Pharmacokinetics of Cefquinome in Layer Birds Following Intramuscular and Intravenous Administration. Pak. Vet. J. 2019, 39, 493–498. [Google Scholar] [CrossRef]
- Lashev, L.D.; Haritova, A. Allometric analysis of antibacterial drugs in avian species. Bulg. J. Vet. Med. 2012, 15, 93–109. [Google Scholar]
- Poźniak, B.; Tikhomirov, M.; Motykiewicz-Pers, K.; Bobrek, K.; Świtała, M. The influence of age and body weight gain on enrofloxacin pharmacokinetics in turkeys—Allometric approach to dose optimization. J. Vet. Pharmacol. Ther. 2019, 43, 67–78. [Google Scholar] [CrossRef]
- Sibbald, B.; Roberts, C. Understanding controlled trials: Crossover trials. BMJ 1998, 316, 1719–1720. [Google Scholar] [CrossRef]
- Craig, W.; Suh, B. Protein binding and the antimicrobial effects: Methods for determination of protein binding. In Antibiotics in Laboratory Medicine, 3rd ed.; Lorian, V., Ed.; Williams and Wilkins: Baltimore, MD, USA, 1991; pp. 367–402. [Google Scholar]
- Gibaldi, M.; Perrier, D. Noncompartmental analysis based on statistical moment theory. In Pharmacokinetics, 2nd ed.; Gibaldi, M., Perrier, D., Eds.; Informa Healthcare: New York, NY, USA, 1982; pp. 409–417. [Google Scholar]
- Urso, R.; Blardi, P.; Giorgi, G. A short introduction to pharmacokinetics. Eur. Rev. Med. Pharmacol. Sci. 2003, 6, 33–44. [Google Scholar]
- Tekeli, I.O.; Turk, E.; Corum, D.D.; Corum, O.; Kirgiz, F.C.; Sakin, F.; Uney, K. Effect of ketoprofen co-administration on pharmacokinetics of cefquinome following repeated administration in goats. J. Vet. Pharmacol. Ther. 2020, 43, 440–447. [Google Scholar] [CrossRef]
- Toutain, P.L.; Lees, P. Integration and modelling of pharmacokinetic and pharmacodynamic data to optimize dosage regimens in veterinary medicine. J. Vet. Pharmacol. Ther. 2004, 27, 467–477. [Google Scholar] [CrossRef]
- Committee for Veterinary Medicinal Products (CVMP). Cefquinome Summary Report; EMEA/MRL/005/95; European Medicines Agency: London, UK, 1995; Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500011877.pdf (accessed on 27 July 2021).
- Li, X.B.; Wu, W.X.; Su, D.; Wang, Z.J.; Jiang, H.Y.; Shen, J.Z. Pharmacokinetics and bioavailability of cefquinome in healthy piglets. J. Vet. Pharmacol. Ther. 2008, 31, 523–527. [Google Scholar] [CrossRef]
- LU, G.F.; Yang, H.F.; LI, Y.J.; Jiang, C.M. Pharmacokinetics of cefquinome sulfate suspension in pigs. J. Yangzhou Univ. 2007, 28, 18–20. (In Chinese) [Google Scholar]
- Zhang, S.; Dai, W.; Lu, Z.; Lei, Z.; Yang, B.; He, B.; Zhou, H.; Cao, J. Preparation and evaluation of cefquinome-loaded gelatin microspheres and the pharmacokinetics in pigs. J. Vet. Pharmacol. Ther. 2017, 41, 117–124. [Google Scholar] [CrossRef]
- Venkatachalam, D.; Dumka, V.K. Pharmacokinetic profile of cefquinome after oral subchronic flubendiamide exposure and in vitro plasma protein binding in buffalo calves. Environ. Toxicol. Pharmacol. 2015, 39, 321–326. [Google Scholar] [CrossRef]
- Toutain, P.-L.; Bousquet-Melou, A. Plasma terminal half-life. J. Vet. Pharmacol. Ther. 2004, 27, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.A.; Remsberg, C.M.; Sayre, C.L.; Forrest, M.L.; Davies, N.M. Flip-flop pharmacokinetics–delivering a reversal of disposition: Challenges and opportunities during drug development. Ther. Deliv. 2011, 2, 643–672. [Google Scholar] [CrossRef]
- Mckellar, Q.A.; Bogan, J.A.; von Fellenberg, R.-L.; Ludwig, B.; Cawley, G.D. Pharmacokinetic, biochemical and tolerance studies on carprofen in the horse. Equine Vet. J. 1991, 23, 280–284. [Google Scholar] [CrossRef]
- Holz, P.; Barker, I.K.; Burger, J.P.; Crawshaw, G.J.; Conlon, P.D. The effect of the renal portal system on pharmacokinetic parameters in the red-eared slider (Trachemys scripta elegans). J. Zoo Wildl. Med. 1997, 28, 386–393. [Google Scholar]
- Toutain, P.-L.; Ferran, A.A.; Bousquet-Melou, A. Species Differences in Pharmacokinetics and Pharmacodynamics. Comp. Vet. Pharmacol. 2010, 199, 19–48. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, X.; Li, Y.; Li, X.; Gu, M.; Zhang, N.; Shen, X.; Ding, H. In vivo evaluation of mutant selection window of cefquinome against Escherichia coliin piglet tissue-cage model. BMC Vet. Res. 2014, 10, 448. [Google Scholar] [CrossRef]
- Wang, J.; Shan, Q.; Ding, H.; Liang, C.; Zeng, Z. Pharmacodynamics of Cefquinome in a Neutropenic Mouse Thigh Model of Staphylococcus aureus Infection. Antimicrob. Agents Chemother. 2014, 58, 3008–3012. [Google Scholar] [CrossRef]
- McKellar, Q.A.; Sanchez Bruni, S.F.; Jones, D.G. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J. Vet. Pharmacol. Ther. 2004, 27, 503–514. [Google Scholar] [CrossRef]
- Barbour, A.; Scaglione, F.; Derendorf, H. Class-dependent relevance of tissue distribution in the interpretation of anti-infective pharmacokinetic/pharmacodynamic indices. Int. J. Antimicrob. Agents 2010, 35, 431–438. [Google Scholar] [CrossRef]
- Orden, J.A.; Ruiz-Santa-Quiteria, J.A.; García, S.; Cid, D.; de la Fuente, R. In Vitro Activities of Cephalosporins and Quinolones against Escherichia coli Strains Isolated from Diarrheic Dairy Calves. Antimicrob. Agents Chemother. 1999, 43, 510–513. [Google Scholar] [CrossRef]
- Wisselink, H.J.; Veldman, K.T.; Eede, C.V.D.; Salmon, S.A.; Mevius, D.J. Quantitative susceptibility of Streptococcus suis strains isolated from diseased pigs in seven European countries to antimicrobial agents licenced in veterinary medicine. Vet. Microbiol. 2006, 113, 73–82. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Hasman, H.; Veldman, K.; Mevius, D. Evaluation of Eight Different Cephalosporins for Detection of Cephalosporin Resistance in Salmonella enterica and Escherichia coli. Microb. Drug Resist. 2010, 16, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Ak, S.; Keles, O.; Bakirel, T. In vitro antibacterial activities of tilmicosin and cefquinome alone or in combination against certain poultry pathogens. Turk. J. Vet. Anim. Sci. 2001, 25, 577–580. [Google Scholar]
- Sellyei, B.; Varga, Z.; Szentesi-Samu, K.; Kaszanyitzky, É.; Magyar, T. Antimicrobial susceptibility of Pasteurella multocida isolated from swine and poultry. Acta Vet. Hung. 2009, 57, 357–367. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Ding, H.; Mei, X.; Liu, W.; Zeng, J.; Zeng, Z. In vitro susceptibility of four antimicrobials against Riemerella anatipestifer isolates: A comparison of minimum inhibitory concentrations and mutant prevention concentrations for ceftiofur, cefquinome, florfenicol, and tilmicosin. BMC Vet. Res. 2016, 12, 250. [Google Scholar] [CrossRef]
- Shan, Q.; Liang, C.; Wang, J.; Li, J.; Zeng, Z. In VivoActivity of Cefquinome against Escherichia coli in the Thighs of Neutropenic Mice. Antimicrob. Agents Chemother. 2014, 58, 5943–5946. [Google Scholar] [CrossRef][Green Version]
- Craig, W.A. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 1995, 22, 89–96. [Google Scholar] [CrossRef]
- Craig, W.A. State-of-the-Art Clinical Article: Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef]
- Nie, H.; Feng, X.; Peng, J.; Liang, L.; Lu, C.; Tiwari, R.V.; Tang, S.; He, J. Comparative pharmacokinetics of ceftiofur hydrochloride and ceftiofur sodium after administration to water buffalo (Bubalus bubalis). Am. J. Vet. Res. 2016, 77, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Poźniak, B.; Pasławska, U.; Motykiewicz-Pers, K.; Switala, M. The influence of growth and E. coli endotoxaemia on amoxicillin pharmacokinetics in turkeys. Br. Poult. Sci. 2017, 58, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Świtała, M.; Poźniak, B.; Pasławska, U.; Grabowski, T.; Motykiewicz-Pers, K.; Bobrek, K. Metronidazole pharmacokinetics during rapid growth in turkeys-relation to changes in haemodynamics and drug metabolism. J. Vet. Pharmacol. Ther. 2016, 39, 373–380. [Google Scholar] [CrossRef]

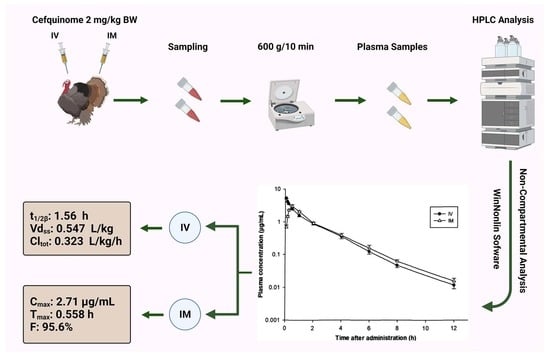
| Parameters | Unit | IV | IM |
|---|---|---|---|
| C0 | µg/mL | 6.63 ± 0.302 | — |
| t1/2ab | h | — | 0.253 ± 0.0580 |
| t1/2ʎz | h | 1.56 ± 0.0631 | 1.71 ± 0.076 |
| AUC0–∞ | μg·h/mL | 6.22 ± 0.428 | 5.94 ± 0.443 |
| AUMC0–∞ | μg·h/mL | 10.6 ± 1.23 | 12.3 ± 1.52 |
| MRT | h | 1.70 ± 0.082 | 2.07 ± 0.117 |
| MAT | h | — | 0.374 ± 0.062 |
| Vdss | L/kg | 0.547 ± 0.0133 | — |
| Cltot | L/kg/h | 0.323 ± 0.0255 | — |
| Cmax | µg/mL | — | 2.71 ± 0.161 |
| Tmax | h | — | 0.558 ± 0.018 |
| F | % | — | 95.6 ± 1.78 |
| Fortified CFQ Concentrations (µg/mL) in Blank Plasma | Protein Binding % | SD |
|---|---|---|
| 0.01 | 13.01 | 1.78 |
| 0.1 | 11.9 | 0.853 |
| 1 | 11.3 | 1.48 |
| 5 | 10.1 | 1.12 |
| Mean | 11.5 | 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbadawy, M.; Soliman, A.; Abugomaa, A.; Alkhedaide, A.; Soliman, M.M.; Aboubakr, M. Disposition of Cefquinome in Turkeys (Meleagris gallopavo) Following Intravenous and Intramuscular Administration. Pharmaceutics 2021, 13, 1804. https://doi.org/10.3390/pharmaceutics13111804
Elbadawy M, Soliman A, Abugomaa A, Alkhedaide A, Soliman MM, Aboubakr M. Disposition of Cefquinome in Turkeys (Meleagris gallopavo) Following Intravenous and Intramuscular Administration. Pharmaceutics. 2021; 13(11):1804. https://doi.org/10.3390/pharmaceutics13111804
Chicago/Turabian StyleElbadawy, Mohamed, Ahmed Soliman, Amira Abugomaa, Adel Alkhedaide, Mohamed Mohamed Soliman, and Mohamed Aboubakr. 2021. "Disposition of Cefquinome in Turkeys (Meleagris gallopavo) Following Intravenous and Intramuscular Administration" Pharmaceutics 13, no. 11: 1804. https://doi.org/10.3390/pharmaceutics13111804
APA StyleElbadawy, M., Soliman, A., Abugomaa, A., Alkhedaide, A., Soliman, M. M., & Aboubakr, M. (2021). Disposition of Cefquinome in Turkeys (Meleagris gallopavo) Following Intravenous and Intramuscular Administration. Pharmaceutics, 13(11), 1804. https://doi.org/10.3390/pharmaceutics13111804