The Anti-Nociceptive Potential of Tulathromycin against Chemically and Thermally Induced Pain in Mice
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Equipment
2.2. Experimental Animals
2.3. Experimental Design
2.3.1. Hot-Plate Test
2.3.2. Tail-Flick Test
2.3.3. Acetic Acid-Induced Writhing Response Test
2.3.4. Formalin-Induced Paw Licking Test
2.4. Statistical Analysis
3. Results
3.1. Analgesic Effect of Tulathromycin against Thermal Stimuli-Induced Pain
3.2. Analgesic Effect of Tulathromycin against Chemical Stimuli-Induced Pain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debono, D.J.; Hoeksema, L.J.; Hobbs, R.D. Caring for Patients With Chronic Pain: Pearls and Pitfalls. J. Am. Osteopath. Assoc. 2013, 113, 620–627. [Google Scholar] [CrossRef]
- Turk, D.C.; Dworkin, R.H. What should be the core outcomes in chronic pain clinical trials? Arthritis Res. Ther. 2004, 6, 151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Merskey, H.; Bogduk, N. IASP Task Force on Taxonomy. In Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definition of Pain Terms, 2nd ed.; Merskey, H., Bogduk, N., Eds.; IASP Press: Seattle, WA, USA, 1994; pp. 209–214. [Google Scholar]
- Russell, P.; Michael, B. Chronic Pain Management. In Handbook of Neurorehabilitation; David, G.J.C.R., Ed.; Informa Healthcare: London, UK, 1994. [Google Scholar]
- Voscopoulos, C.; Lema, M. When does acute pain become chronic? BJA Br. J. Anaesth. 2010, 105 (Suppl. 1), i69–i85. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Tambaro, S.; Reali, R.; Volonterio, A.; Zanda, M.; Olimpieri, F.; Pinna, G.A.; Lazzari, P. NESS002ie: A new fluorinated thiol endopeptidase inhibitor with antinociceptive activity in an animal model of persistent pain. Pharmacol. Biochem. Behav. 2013, 110, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K. Opioid analgesics and antagonists. In Essentials of Medical Pharmacology, 7th ed.; Tripathi, K., Ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2013; p. 975. [Google Scholar]
- WHO. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2005 (including the 14th Model List of Essential Medicines); World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Ocaña, M.; Baeyens, J. Analgesic effects of centrally administered aminoglycoside antibiotics in mice. Neurosci. Lett. 1991, 126, 67–70. [Google Scholar] [CrossRef]
- Suaudeau, C.; Chait, A.; Cimetiere, C.; de Beaurepaire, R. Analgesic effects of antibiotics in rats. Pharmacol. Biochem. Behav. 1993, 46, 361–364. [Google Scholar] [CrossRef]
- Elbadawy, M. Some Pharmacodynamic Effects of Cefepime. Master’s Thesis, Faculty of Veterinary Medicine, Benha University, Benha, Egypt, 2007. [Google Scholar]
- Letavic, M.A.; Bronk, B.S.; Bertsche, C.D.; Casavant, J.M.; Cheng, H.; Daniel, K.L.; George, D.M.; Hayashi, S.F.; Kamicker, B.J.; Kolosko, N.L.; et al. Synthesis and activity of a novel class of tribasic macrocyclic antibiotics: The triamilides. Bioorg. Med. Chem. Lett. 2002, 12, 2771–2774. [Google Scholar] [CrossRef]
- Ahrens, F. Pharmacology; Williams and Wilkens: Baltimore, MD, USA, 1996. [Google Scholar]
- Evans, N.A. Tulathromycin: An overview of a new triamilide antibiotic for livestock respiratory disease. Vet. Ther. 2005, 6, 83–95. [Google Scholar]
- Nowakowski, M.A.; Inskeep, P.B.; Risk, J.E.; Skogerboe, T.L.; Benchaoui, H.A.; Meinert, T.R.; Sherington, J.; Sunderland, S.J. Pharmacokinetics and lung tissue concentrations of tulathromycin, a new triamilide antibiotic, in cattle. Vet. Ther. 2004, 5, 60–74. [Google Scholar] [PubMed]
- Galer, D.; Hessong, S.; Beato, B.; Risk, J.; Inskeep, P.; Weerasinghe, C.; Schneider, R.P.; Langer, C.; LaPerle, J.; Renouf, D.; et al. An analytical method for the analysis of tulathromycin, an equilibrating triamilide, in bovine and porcine plasma and lung. J. Agric. Food Chem. 2004, 52, 2179–2191. [Google Scholar] [CrossRef]
- Nutsch, R.G.; Hart, F.J.; Rooney, K.A.; Weigel, D.J.; Kilgore, W.R.; Skogerboe, T.L. Efficacy of tulathromycin injectable solution for the treatment of naturally occurring Swine respiratory disease. Vet. Ther. 2005, 6, 214–224. [Google Scholar]
- Rooney, K.A.; Nutsch, R.G.; Skogerboe, T.L.; Weigel, D.J.; Gajewski, K.; Kilgore, W.R. Efficacy of tulathromycin compared with tilmicosin and florfenicol for the control of respiratory disease in cattle at high risk of developing bovine respiratory disease. Vet. Ther. 2005, 6, 154–166. [Google Scholar]
- Villarino, N.; Brown, S.A.; Martín-Jiménez, T. The role of the macrolide tulathromycin in veterinary medicine. Vet. J. 2013, 198, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Villarino, N.; Brown, S.A.; Martín-Jiménez, T. Understanding the pharmacokinetics of tulathromycin: A pulmonary perspective. J. Vet. Pharmacol. Ther. 2014, 37, 211–221. [Google Scholar] [CrossRef]
- Fischer, C.D.; Beatty, J.K.; Zvaigzne, C.G.; Morck, D.W.; Lucas, M.J.; Buret, A.G. Anti-Inflammatory benefits of antibiotic-induced neutrophil apoptosis: Tulathromycin induces caspase-3-dependent neutrophil programmed cell death and inhibits NF-kappaB signaling and CXCL8 transcription. Antimicrob. Agents Chemother. 2011, 55, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.D.; Beatty, J.K.; Duquette, S.C.; Morck, D.W.; Lucas, M.J.; Buret, A.G. Direct and indirect anti-inflammatory effects of tulathromycin in bovine macrophages: Inhibition of CXCL-8 secretion, induction of apoptosis, and promotion of efferocytosis. Antimicrob. Agents Chemother. 2013, 57, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.D.; Duquette, S.C.; Renaux, B.S.; Feener, T.D.; Morck, D.W.; Hollenberg, M.D.; Lucas, M.J.; Buret, A.G. Tulathromycin exerts proresolving effects in bovine neutrophils by inhibiting phospholipases and altering leukotriene B4, prostaglandin E2, and lipoxin A4 production. Antimicrob. Agents Chemother. 2014, 58, 4298–4307. [Google Scholar] [CrossRef]
- Villarino, N.; Brown, S.A.; Martín-Jiménez, T. Pharmacokinetics of tulathromycin in healthy and neutropenic mice challenged intranasally with lipopolysaccharide from Escherichia coli. Antimicrob. Agents Chemother. 2012, 56, 4078–4086. [Google Scholar] [CrossRef]
- Villarino, N.; Denny, J.E.; Schmidt, N.W. Antimalarial activity of tulathromycin in a murine model of malaria. Antimicrob. Agents Chemother. 2015, 59, 3672–3674. [Google Scholar] [CrossRef]
- Wong, C.L.; Wai, M.K. Increased naloxone potency induced by pretreatment with morphine and nalbuphine in mice. Clin. Exp. Pharmacol. Physiol. 1984, 11, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L. The effect of nalbuphine on gastrointestinal transit in mice. Eur. J. Pharmacol. 1987, 135, 219–223. [Google Scholar] [CrossRef]
- Hawk, C.; Leary, S.; Morris, T. Ketoprofen. In Formulary for Laboratory Animals; Blackwell Publishing: Ames, IA, USA, 2005; p. 30. [Google Scholar]
- Plumb, D. Ketoprofen. In Plumb’s Veterinary Drug Handbook; Wiley-Blackwell: Ames, IA, USA, 2011; p. 579. [Google Scholar]
- Woolfe, G.; Macdonald, A.D. The evaluation of the analgesic action of pethidine hydrochloride (Demerol). J. Pharmacol. Exp. Ther. 1944, 80, 300. [Google Scholar]
- Harris, L.S.; Pierson, A.K. Some narcotic antagonists in the benzomorphan series. J. Pharmacol. Exp. Ther. 1964, 143, 141. [Google Scholar]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Janssen, P.A.; Niemegeers, C.J.; Dony, J.G. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung 1963, 13, 502–507. [Google Scholar]
- Koster, R.; Anderson, M.; De Beer, E. Acetic acid-induced analgesic screening. Fed. Proc. 1959, 18, 412. [Google Scholar]
- El-Mahmoudy, A.; Gheith, I. The anti-nociceptive potential of tilmicosin against chemical-induced but not thermal-induced pain in mice. Int. J. Immunopathol. Pharmacol. 2016, 29, 9–16. [Google Scholar] [CrossRef]
- Hunskaar, S.; Fasmer, O.B.; Hole, K. Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 1985, 14, 69–76. [Google Scholar] [CrossRef]
- Yongna, Z.; Wantana, R.; Pisit, B.; Zhongkun, L.; Rongping, Z. Analgesic and antipyretic activities of the aqueous extract of Urtica macrorrhiza in experimental animals. Fitoterapia 2005, 76, 91–95. [Google Scholar] [CrossRef]
- Gladue, R.P.; Bright, G.M.; Isaacson, R.E.; Newborg, M.F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: Possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 1989, 33, 277–282. [Google Scholar] [CrossRef]
- Frank, M.O.; Sullivan, G.W.; Carper, H.T.; Mandell, G.L. In vitro demonstration of transport and delivery of antibiotics by polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 1992, 36, 2584–2588. [Google Scholar] [CrossRef] [PubMed]
- Bosnar, M.; Kelneric, Z.; Munic, V.; Erakovic, V.; Parnham, M.J. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob. Agents Chemother. 2005, 49, 2372–2377. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, X.; Zhao, X.; Zhang, M.; Zhang, W.; Hou, S.; Yuan, W.; Zhang, H.; Shi, L.; Jia, H.; et al. Tylvalosin exhibits anti-inflammatory property and attenuates acute lung injury in different models possibly through suppression of NF-kappaB activation. Biochem. Pharmacol. 2014, 90, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Moges, R.; De Lamache, D.D.; Sajedy, S.; Renaux, B.S.; Hollenberg, M.D.; Muench, G.; Abbott, E.M.; Buret, A.G. Anti-Inflammatory Benefits of Antibiotics: Tylvalosin Induces Apoptosis of Porcine Neutrophils and Macrophages, Promotes Efferocytosis, and Inhibits Pro-Inflammatory CXCL-8, IL1α, and LTB(4) Production, While Inducing the Release of Pro-Resolving Lipoxin A(4) and Resolvin D1. Front. Vet. Sci. 2018, 5, 57. [Google Scholar] [PubMed]
- Buret, A.G. Immuno-modulation and anti-inflammatory benefits of antibiotics: The example of tilmicosin. Can. J. Vet. Res. 2010, 74, 1–10. [Google Scholar]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef]
- Elbadawy, M.; Aboubakr, M.; Abugomaa, A. Pharmacokinetics of Tylvalosin in Broiler Turkeys (Meleagris Gallopavo) After Single Intravenous and Oral Administration. Front. Vet. Sci. 2019, 6, 355. [Google Scholar] [CrossRef] [PubMed]
- Collier, H.O.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968, 32, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Pethő, G.; Reeh, P.W. Sensory and Signaling Mechanisms of Bradykinin, Eicosanoids, Platelet-Activating Factor, and Nitric Oxide in Peripheral Nociceptors. Physiol. Rev. 2012, 92, 1699–1775. [Google Scholar] [CrossRef]
- Balogh, M.; Zádori, Z.S.; Lázár, B.; Karádi, D.; László, S.; Mousa, S.A.; Hosztafi, S.; Zádor, F.; Riba, P.; Schäfer, M.; et al. The Peripheral Versus Central Antinociception of a Novel Opioid Agonist: Acute Inflammatory Pain in Rats. Neurochem. Res. 2018, 43, 1250–1257. [Google Scholar] [CrossRef]
- Al-Khrasani, M.; Spetea, M.; Friedmann, T.; Riba, P.; Király, K.; Schmidhammer, H.; Furst, S. DAMGO and 6β-glycine substituted 14-O-methyloxymorphone but not morphine show peripheral, preemptive antinociception after systemic administration in a mouse visceral pain model and high intrinsic efficacy in the isolated rat vas deferens. Brain Res. Bull. 2007, 74, 369–375. [Google Scholar] [CrossRef]
- Steiner, A.A.; Ivanov, A.I.; Serrats, J.; Hosokawa, H.; Phayre, A.N.; Robbins, J.R.; Roberts, J.L.; Kobayashi, S.; Matsumura, K.; Sawchenko, P.E.; et al. Cellular and Molecular Bases of the Initiation of Fever. PLoS Biol. 2006, 4, e284. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Takizawa, H.; Ohtoshi, T.; Takeuchi, N.; Kohyama, T.; Nakamura, H.; Kasama, T.; Kobayashi, K.; Nakahara, K.; Morita, Y.; et al. Roxithromycin inhibits cytokine production by and neutrophil attachment to human bronchial epithelial cells in vitro. Antimicrob. Agents Chemother. 1998, 42, 1499–1502. [Google Scholar] [CrossRef]
- Cervin, A. The anti-inflammatory effect of erythromycin and its derivatives, with special reference to nasal polyposis and chronic sinusitis. Acta Otolaryngol. 2001, 121, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sassa, K.; Mizushima, Y.; Kobayashi, M. Differential modulatory effects of clarithromycin on the production of cytokines by a tumor. Antimicrob. Agents Chemother. 1999, 43, 2787–2789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schultz, M.J.; Speelman, P.; Zaat, S.; van Deventer, S.J.; van der Poll, T. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob. Agents Chemother. 1998, 42, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Dong, M.; Shen, J.Z.; Wu, B.B.; Wu, C.M.; Du, X.D.; Wang, Z.; Qi, Y.T.; Li, B.Y. Tilmicosin and tylosin have anti-inflammatory properties via modulation of COX-2 and iNOS gene expression and production of cytokines in LPS-induced macrophages and monocytes. Int. J. Antimicrob. Agents 2006, 27, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G. Tilmicosin Phosphate. In Saunders Handbook of Veterinary Drugs, 4th ed.; Papich, M.G., Ed.; W.B. Saunders: St. Louis, MO, USA, 2016; pp. 789–791. [Google Scholar]
- Mercado, F.; Almanza, A.; Simón-Arceo, K.; López, O.; Vega, R.; Coffeen, U.; Contreras, B.; Soto, E.; Pellicer, F. Inhibition of peripheral nociceptors by aminoglycosides produces analgesia in inflammatory pain models in the rat. Inflammation 2015, 38, 649–657. [Google Scholar] [CrossRef]
- Garza, A.; López-Ramírez, O.; Vega, R.; Soto, E. The aminoglycosides modulate the acid-sensing ionic channel currents in dorsal root ganglion neurons from the rat. J. Pharmacol. Exp. Ther. 2010, 332, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Bian, J.; Li, Q.; Zhang, Y. The preemptive analgesia of pre-electroacupuncture in rats with formalin-induced acute inflammatory pain. Mol. Pain 2019, 15, 1744806919866529. [Google Scholar] [CrossRef] [PubMed]
- Besserer, F.; Chuang, R.; Mink, M.; Massey, L.; Cload, B. Tilmicosin toxicity: A case of accidental human tilmicosin injection managed with calcium, high-dose insulin and intravenous lipid emulsion therapy. Clin. Toxicol. 2016, 54, 812–813. [Google Scholar] [CrossRef]
- Er, A.; Altan, F.; Cetin, G.; Dik, B.; Elmas, M.; Yazar, E. Assessment of the cardiotoxicity of tulathromycin in rabbits. Acta Vet. Hung 2011, 59, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki-Zahn, E.M.; Segelcke, D.; Schug, S.A. Postoperative pain-from mechanisms to treatment. Pain Rep. 2017, 2, e588. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, E.; Schmidtko, A.; Gao, W.; Kühlein, H.; Ehnert, C.; Geisslinger, G. Impaired acute and inflammatory nociception in mice lacking the p50 subunit of NF-kappaB. Eur. J. Pharmacol. 2007, 559, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Verleden, S.E.; Vandooren, J.; Vos, R.; Willems, S.; Dupont, L.J.; Verleden, G.M.; van Raemdonck, D.E.; Opdenakker, G.; Vanaudenaerde, B.M. Azithromycin decreases MMP-9 expression in the airways of lung transplant recipients. Transpl. Immunol. 2011, 25, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [PubMed]


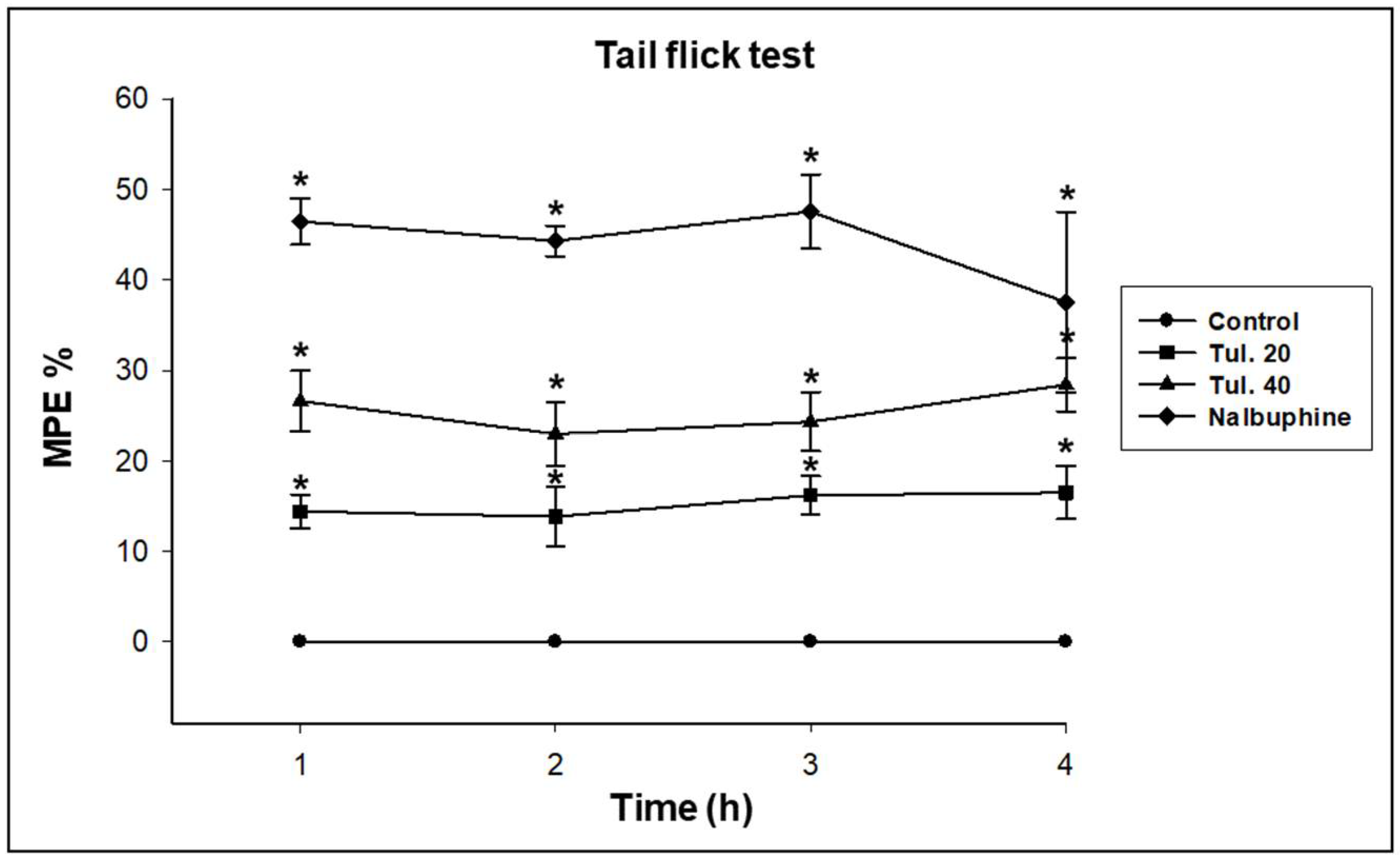


| Group One | Dose, s.c. (mg/kg BW) | Latency of Nociceptive Response (s) | |||
|---|---|---|---|---|---|
| After 1 h | After 2 h | After 3 h | After 4 h | ||
| Control | NS | 6.20 ± 0.63 | 5.20 ± 0.57 | 5.64 ± 0.67 | 5.74 ± 0.25 |
| Nalbuphine HCl | 2.2 | 13.9 ± 0.55 * | 18.0 ± 1.23 * | 16.8 ± 0.44 * | 15.3 ± 0.49 * |
| Tulathromycin | 20 | 11.3 ± 2.96 * | 13.2 ± 1.30 * | 14.6 ± 0.60 * | 13.4 ± 0.55 * |
| Tulathromycin | 40 | 14.1 ± 2.8 * | 11.1 ± 1.02 * | 11.8 ± 0.51 * | 10.8 ± 0.84 * |
| Group Two | Dose, s.c. (mg/kg BW) | Latency of Nociceptive Response | |||
|---|---|---|---|---|---|
| After 1 h | After 2 h | After 3 h | After 4 h | ||
| Control | NS | 2.30 ± 0.61 | 2.36 ± 0.42 | 2.32 ± 0.44 | 2.22 ± 0.23 |
| Nalbuphine HCl | 2.2 | 7.44 ± 0.44 * | 8.34 v 0.42 * | 9.30 ± 0.42 * | 7.98 ± 0.15 * |
| Tulathromycin | 20 | 4.01 ± 0.45 * | 4.06 ± 0.40 * | 4.72 ± 0.83 * | 4.50 ± 0.55 * |
| Tulathromycin | 40 | 5.05 ± 0.89 * | 5.88 ± 0.30 * | 6.06 ± 0.32 * | 5.76 ± 0.27 * |
| Group Three | Dose, s.c. (mg/kg BW) | Nociceptive Response | |
|---|---|---|---|
| Number of Writhes | Inhibition % | ||
| Control | NS | 65 ± 5.24 | 0.00 |
| Ketoprofen HCl | 5 | 14.4 ± 3.44 * | 77.9 ± 4.62 * |
| Tulathromycin | 20 | 48.8 ± 3.77 * | 24.7 ± 6.0 * |
| Tulathromycin | 40 | 34.8 ± 4.15 * | 46.6 ± 3.13 * |
| Group Four | Dose, s.c. (mg/kg BW) | Total Paw Licking Time (s) | |||
|---|---|---|---|---|---|
| Early Phase (0–5 min) | Inhibition % | Late Phase (20–30 min) | Inhibition % | ||
| Control | NS | 81.4 ± 2.19 | 0.00 | 91.6 ± 3.21 | 0.00 |
| Ketoprofen HCl | 5 | 60.2 ± 3.96 * | 26.0 ± 4.79 * | 28.2 ± 2.05 * | 69.2 ± 2.82 * |
| Tulathromycin | 20 | 77.4 ± 2.51 | 4.88 ± 3.21 | 65.0 ± 3.08 * | 29.0 ± 3.92 * |
| Tulathromycin | 40 | 72.2 ± 4.66 * | 11.2 ± 7.30 * | 55.0 ± 3.61 * | 39.9 ± 4.23 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbadawy, M.; Abugomaa, A.; El-Husseiny, H.M.; Mandour, A.S.; Abdel-Daim, M.M.; Aboelenin, S.M.; Soliman, M.M.; El-Mleeh, A. The Anti-Nociceptive Potential of Tulathromycin against Chemically and Thermally Induced Pain in Mice. Pharmaceutics 2021, 13, 1247. https://doi.org/10.3390/pharmaceutics13081247
Elbadawy M, Abugomaa A, El-Husseiny HM, Mandour AS, Abdel-Daim MM, Aboelenin SM, Soliman MM, El-Mleeh A. The Anti-Nociceptive Potential of Tulathromycin against Chemically and Thermally Induced Pain in Mice. Pharmaceutics. 2021; 13(8):1247. https://doi.org/10.3390/pharmaceutics13081247
Chicago/Turabian StyleElbadawy, Mohamed, Amira Abugomaa, Hussein M. El-Husseiny, Ahmed S. Mandour, Mohamed M. Abdel-Daim, Salama Mostafa Aboelenin, Mohamed Mohamed Soliman, and Amany El-Mleeh. 2021. "The Anti-Nociceptive Potential of Tulathromycin against Chemically and Thermally Induced Pain in Mice" Pharmaceutics 13, no. 8: 1247. https://doi.org/10.3390/pharmaceutics13081247
APA StyleElbadawy, M., Abugomaa, A., El-Husseiny, H. M., Mandour, A. S., Abdel-Daim, M. M., Aboelenin, S. M., Soliman, M. M., & El-Mleeh, A. (2021). The Anti-Nociceptive Potential of Tulathromycin against Chemically and Thermally Induced Pain in Mice. Pharmaceutics, 13(8), 1247. https://doi.org/10.3390/pharmaceutics13081247












