Synthesis, Drug Release, and Antibacterial Properties of Novel Dendritic CHX-SrCl2 and CHX-ZnCl2 Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chlorhexidine Particle Synthesis
2.3. UV-Vis Spectrometer
2.4. Inductively Coupled Plasma-Optical Emission Spectroscopy
2.5. Structural Analysis of the CHX-SrCl2 and CHX-ZnCl2 Particles
2.6. Antimicrobial Assay
2.7. Cytotoxicity Assay
3. Results and Discussion
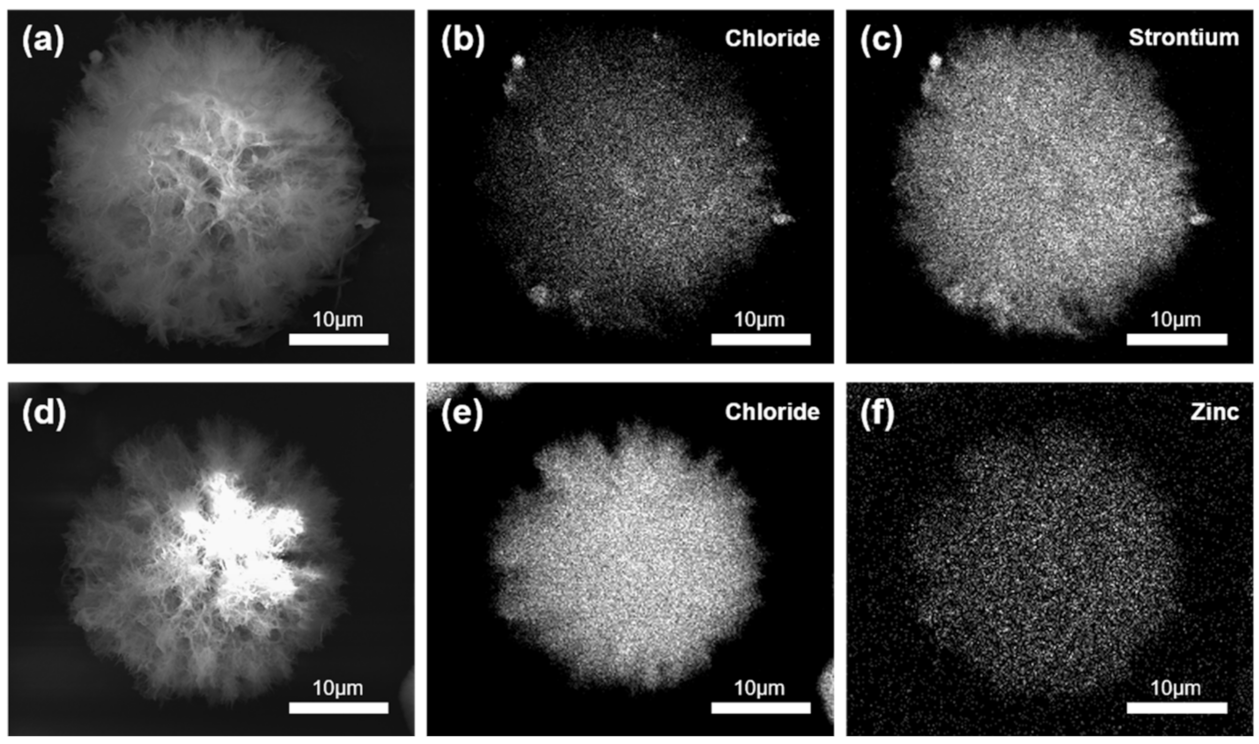
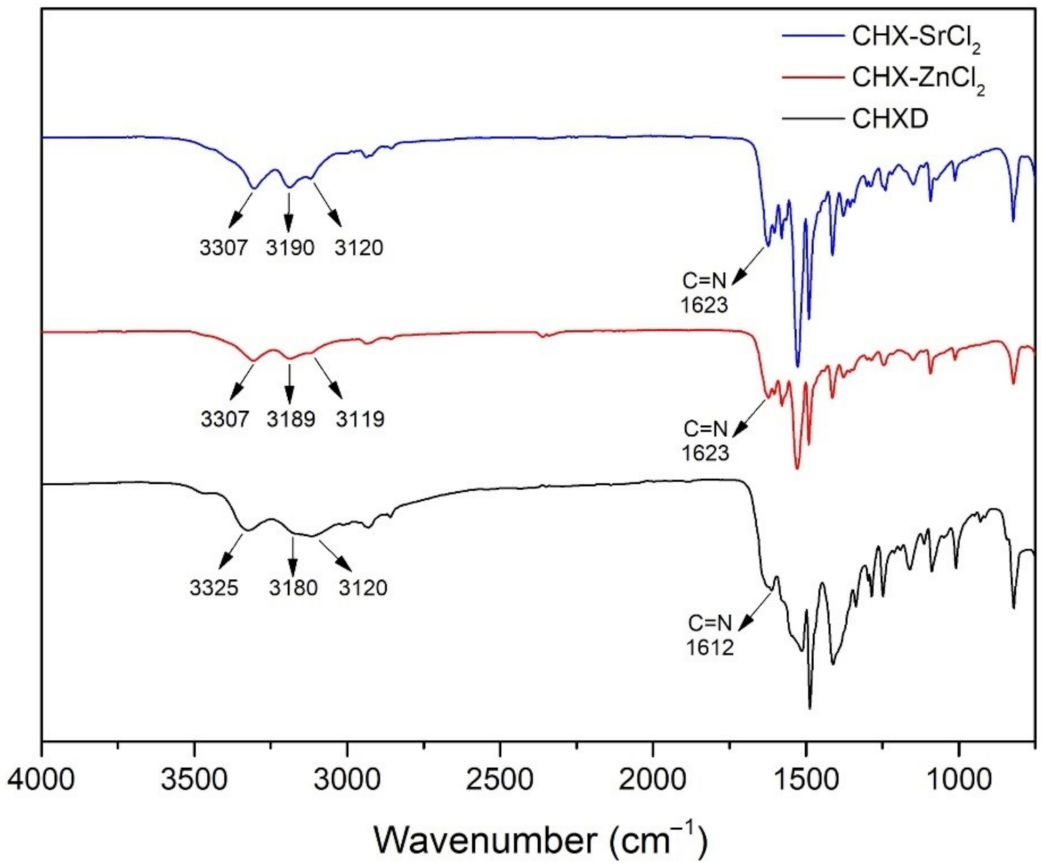
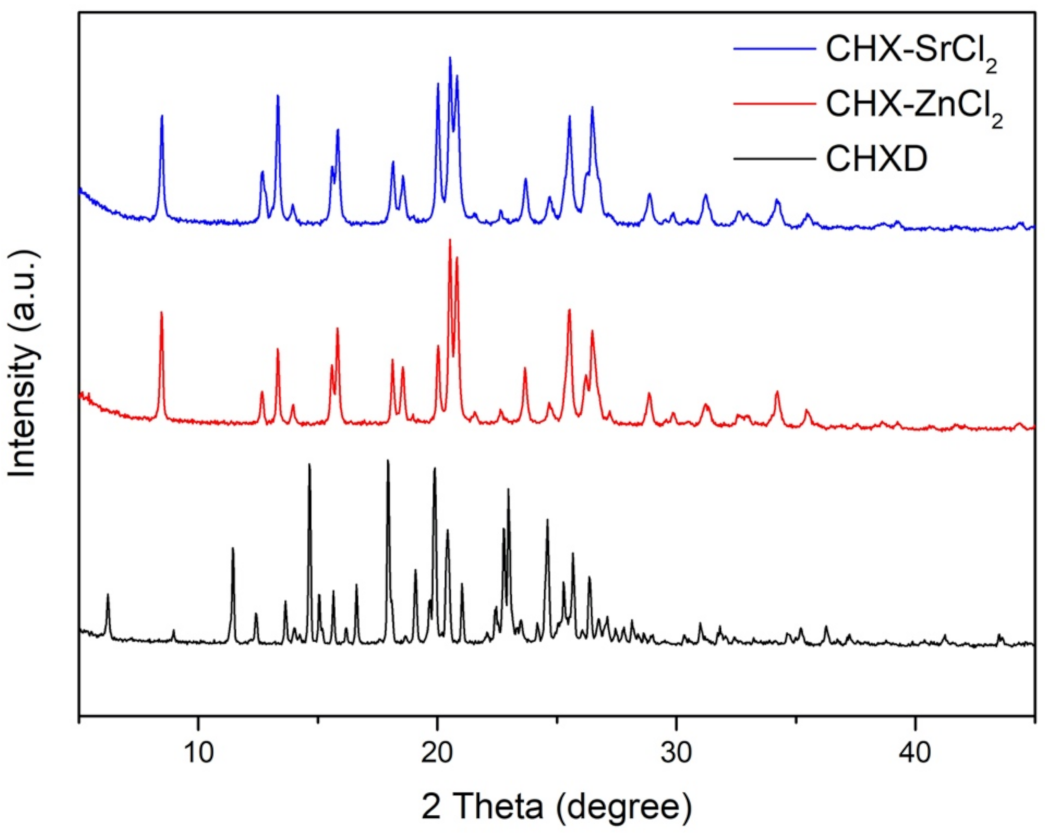
3.1. Synthesis and Characterization of CHX-SrCl2 and CHX-ZnCl2 Particles
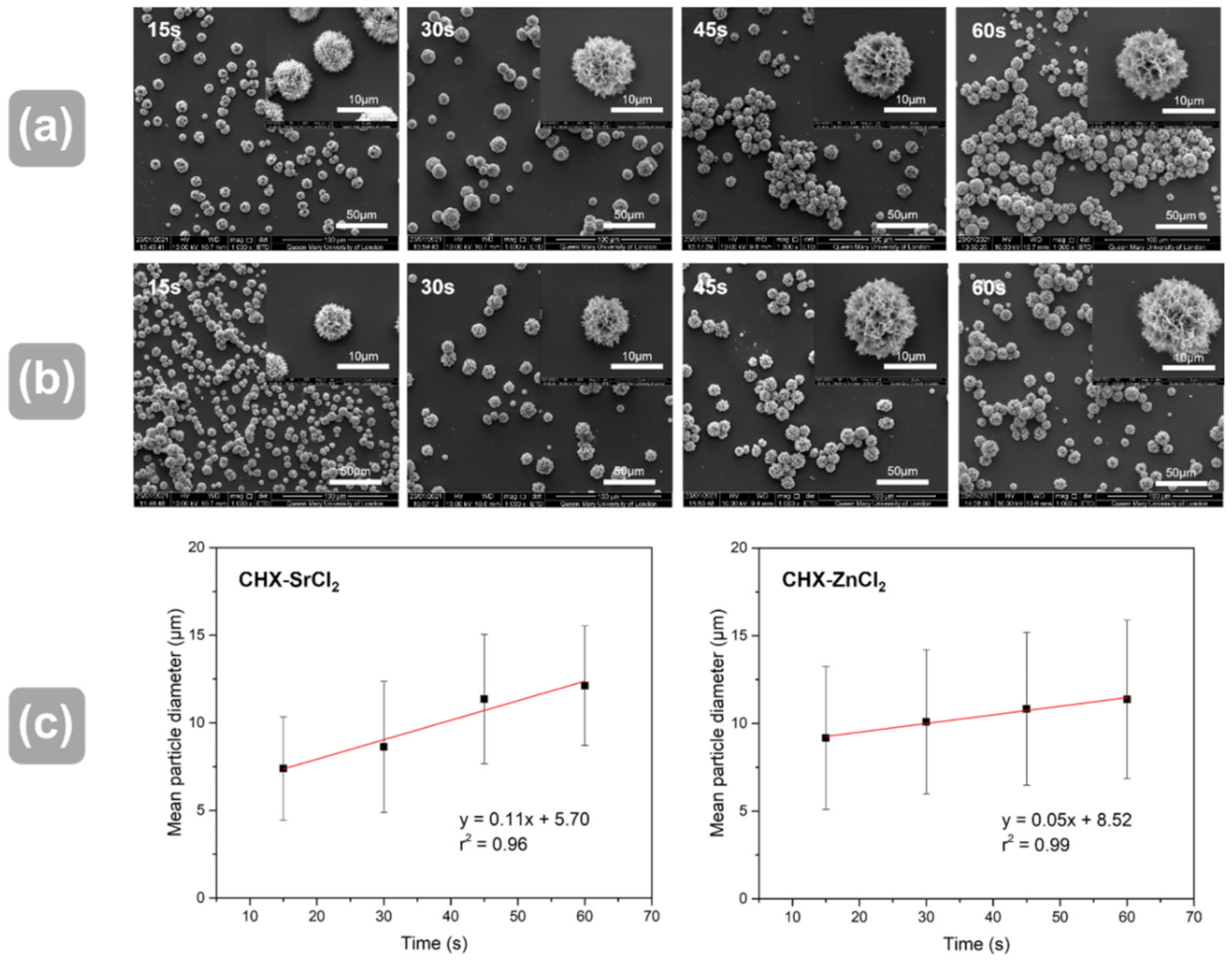
3.2. Influence of Reaction Time and Temperature on Particle Size
3.3. Antibacterial and Cytotoxicity Assay
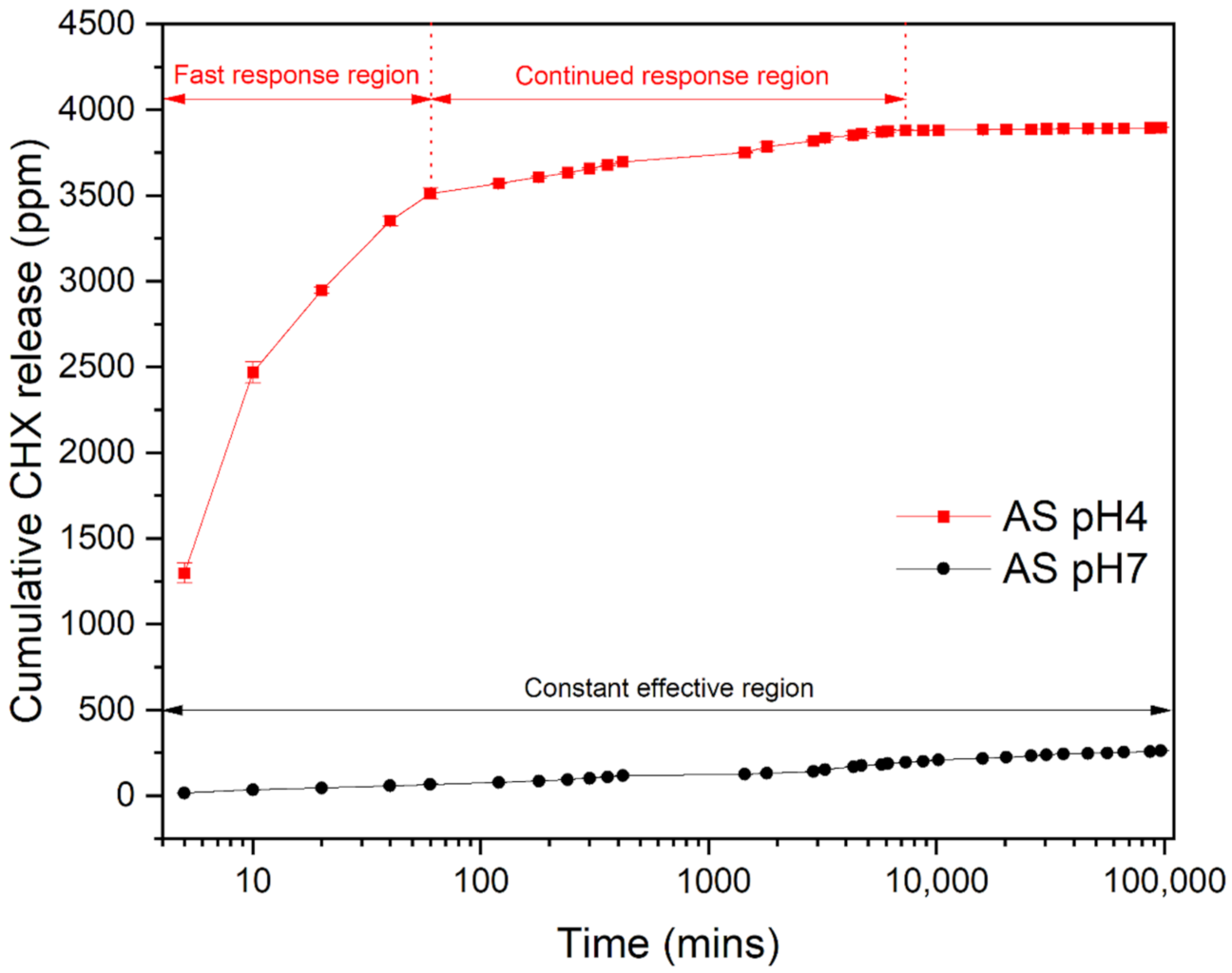
3.4. Release Kinetics of CHX Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Cao, Q.; Li, S.; Li, H.; Zhang, W. Impact of daily bathing with chlorhexidine gluconate on ventilator associated pneumonia in intensive care units: A meta-analysis. J. Thorac. Dis. 2015, 7, 746–753. [Google Scholar] [CrossRef][Green Version]
- Mullany, L.C.; Darmstadt, G.L.; Tielsch, J. Safety and Impact of Chlorhexidine Antisepsis Interventions for Improving Neonatal Health in Developing Countries. Pediatr. Infect. Dis. J. 2006, 25, 665–675. [Google Scholar] [CrossRef]
- Lawn, J.E.; Cousens, S.; Zupan, J. Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: When? Where? Why? Lancet 2005, 365, 891–900. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Rello, J.; Marshall, J.K.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Jeansonne, M.J.; White, R.R. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J. Endod. 1994, 20, 276–278. [Google Scholar] [CrossRef]
- Salim, N.; Moore, C.; Silikas, N.; Satterthwaite, J.; Rautemaa, R. Chlorhexidine is a highly effective topical broad-spectrum agent against Candida spp. Int. J. Antimicrob. Agents 2012, 41, 65–69. [Google Scholar] [CrossRef]
- Herrera, D. Chlorhexidine mouthwash reduces plaque and gingivitis. Evid. Based Dent. 2013, 14, 17–18. [Google Scholar] [CrossRef]
- Farrugia, C.; Camilleri, J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review. Dent. Mater. 2015, 31, e89–e99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.; Xu, X. Antibacterial Dental Composites with Chlorhexidine and Mesoporous Silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef]
- Swan, J.T.; Ashton, C.M.; Bui, L.N.; Pham, V.P.; Shirkey, B.A.; Blackshear, J.E.; Bersamin, J.B.; Pomer, R.M.L.; Johnson, M.L.; Magtoto, A.D. Effect of chlorhexidine bathing every other day on prevention of hospital-acquired infections in the surgical ICU: A single-center, randomized controlled trial. Crit. Care Med. 2016, 44, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Biggar, R.J. Vaginal Cleansing and the Gold Standard. J. Women’s Health 2005, 14, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Bonesvoll, P.; Gjermo, P. A comparison between chlorhexidine and some quaternary ammonium compounds with regard to retention, salivary concentration and plaque-inhibiting effect in the human mouth after mouth rinses. Arch. Oral Biol. 1978, 23, 289–294. [Google Scholar] [CrossRef]
- Lang, N.; Brecx, M.C. Chlorhexidine digluconate-an agent for chemical plaque control and prevention of gingival inflammation. J. Periodontal Res. 1986, 21, 74–89. [Google Scholar] [CrossRef]
- Plüss, E.M.; Engelberger, P.R.; Rateitschak, K.H.; Engelberglr, P.R.; Ratehcschak, K.H. Effect of chlorhexidine on dental plaque formation under periodontal pack. J. Clin. Periodontol. 1975, 2, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Baehni, P.C.; Takeuchi, Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Dis. 2003, 9, 23–29. [Google Scholar] [CrossRef]
- Kamolnarumeth, K.; Thussananutiyakul, J.; Lertchwalitanon, P.; Rungtanakiat, P.; Mathurasai, W.; Sooampon, S.; Arunyanak, S.P. Effect of mixed chlorhexidine and hydrogen peroxide mouthrinses on developing plaque and stain in gingivitis patients: A randomized clinical trial. Clin. Oral Investig. 2020, 25, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 2001, 14, 227. [Google Scholar] [CrossRef]
- Hidalgo, E.; Dominguez, C. Mechanisms underlying chlorhexidine-induced cytotoxicity. Toxicol. In Vitro 2001, 15, 271–276. [Google Scholar] [CrossRef]
- Edmiston, C.E.; Bruden, B.; Rucinski, M.C.; Henen, C.; Graham, M.B.; Lewis, B.L. Reducing the risk of surgical site infections: Does chlorhexidine gluconate provide a risk reduction benefit? Am. J. Infect. Control 2013, 41, S49–S55. [Google Scholar] [CrossRef]
- Lang, N.; Hase, J.; Grassi, M.; Hämmerle, C.; Weigel, C.; Kelty, E.; Frutig, F. Plaque formation and gingivitis after supervised mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Dis. 2008, 4, 105–113. [Google Scholar] [CrossRef]
- Pilloni, A.; Zeza, B.; Mongardini, C.; Dominici, F.; Cassini, M.; Polimeni, A. A preliminary comparison of the effect of 0.3% versus 0.2% chlorhexidine mouth rinse onde novoplaque formation: A monocentre randomized double-blind crossover trial. Int. J. Dent. Hyg. 2013, 11, 198–202. [Google Scholar] [CrossRef]
- Heasman, P.A.; Heasman, L.; Stacey, F.; McCracken, G.I. Local delivery of chlorhexidine gluconate (PerioChipTM) in periodontal maintenance patients. J. Clin. Periodontol. 2008, 28, 90–95. [Google Scholar] [CrossRef]
- Hiraishi, N.; Yiu, C.K.Y.; King, N.M.; Tay, F.R. Chlorhexidine release and antibacterial properties of chlorhexidine-incorporated polymethyl methacrylate-based resin cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 134–140. [Google Scholar] [CrossRef]
- Socransky, S.; Haffajee, A.; Cugini, M.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Mai, H.N.; Kim, D.Y.; Hyun, D.C.; Park, J.H.; Lee, S.M.; Lee, D.H. A New Antibacterial Agent-Releasing Polydimethylsiloxane Coating for Polymethyl Methacrylate Dental Restorations. J. Clin. Med. 2019, 8, 1831. [Google Scholar] [CrossRef]
- Luo, D.; Shahid, S.; Wilson, R.M.; Cattell, M.J.; Sukhorukov, G.B. Novel Formulation of Chlorhexidine Spheres and Sustained Release with Multilayered Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 12652–12660. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.D.; Balu, R.; Kumar, T.S. Strontium-Substituted Calcium Deficient Hydroxyapatite Nanoparticles: Synthesis, Characterization, and Antibacterial Properties. J. Am. Ceram. Soc. 2012, 95, 2700–2708. [Google Scholar] [CrossRef]
- Badea, M.; Olar, R.; Iliş, M.; Georgescu, R.; Călinescu, M. Synthesis, characterization, and thermal decomposition of new copper (II) complex compounds with chlorhexidine. J. Therm. Anal. Calorim. 2012, 111, 1763–1770. [Google Scholar] [CrossRef]
- Călinescu, M.; Negreanu-Pîrjol, T.; Georgescu, R.; Călinescu, O. Synthesis and characterization of new copper(II) complex compounds with chlorhexidine. Part I. Open Chem. 2010, 8, 543–549. [Google Scholar] [CrossRef][Green Version]
- Burlet, N.; Reginster, J.-Y. Strontium Ranelate. Clin. Orthop. Relat. Res. 2006, 443, 55–60. [Google Scholar] [CrossRef]
- Dahl, S.; Allain, P.; Marie, P.; Mauras, Y.; Boivin, G.; Ammann, P.; Tsouderos, Y.; Delmas, P.; Christiansen, C. Incorporation and distribution of strontium in bone. Bone 2001, 28, 446–453. [Google Scholar] [CrossRef]
- Canalis, E. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 1996, 18, 517–523. [Google Scholar] [CrossRef]
- Braux, J.; Velard, F.; Guillaume, C.; Bouthors, S.; Jallot, E.; Nedelec, J.-M.; Laurent-Maquin, D.; Laquerrière, P. A new insight into the dissociating effect of strontium on bone resorption and formation. Acta Biomater. 2011, 7, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Billington, R.W.; Pearson, G.J. Kinetics of fluoride release from glass components of glass ionomers. J. Dent. 2009, 37, 495–501. [Google Scholar] [CrossRef]
- Lippert, F.; Hara, A. Strontium and Caries: A Long and Complicated Relationship. Caries Res. 2012, 47, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Mouriño, V.; Boccaccini, A.R. Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci. 2012, 1, 254–256. [Google Scholar] [CrossRef]
- Hameed, A.S.H.; Karthikeyan, C.; Sasikumar, S.; Kumar, V.S.; Kumaresan, S.; Ravi, G. Impact of alkaline metal ions Mg2+, Ca2+, Sr2+ and Ba2+ on the structural, optical, thermal and antibacterial properties of ZnO nanoparticles prepared by the co-precipitation method. J. Mater. Chem. B 2013, 1, 5950–5962. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.J. Zinc in the mouth, its interactions with dental enamel and possible effects on caries: A review of the literature. Int. Dent. J. 2011, 61, 46–54. [Google Scholar] [CrossRef]
- Chen, X.; Brauer, D.; Karpukhina, N.; Waite, R.; Barry, M.; McKay, I.; Hill, R. ‘Smart’ acid-degradable zinc-releasing silicate glasses. Mater. Lett. 2014, 126, 278–280. [Google Scholar] [CrossRef]
- Lin, S.-C.; Huang, C.-F.; Shen, L.-J.; Wang, H.-J.; Lin, C.-Y.; Wu, F.-L.L. Formulation and stability of an extemporaneous 0.02% chlorhexidine digluconate ophthalmic solution. J. Formos. Med. Assoc. 2015, 114, 1162–1169. [Google Scholar] [CrossRef]
- Boaro, L.C.C.; Campos, L.M.; Varca, G.H.C.; dos Santos, T.M.R.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Müller, K.; Brandt, W.C.; Braga, R.R.; et al. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Rajabnejadkeleshteri, A.; Kamyar, A.; Khakbiz, M.; Bakalani, Z.L.; Basiri, H. Synthesis and characterization of strontium fluor-hydroxyapatite nanoparticles for dental applications. Microchem. J. 2019, 153, 104485. [Google Scholar] [CrossRef]
- Garley, A.; Hoff, S.E.; Saikia, N.; Jamadagni, S.N.; Baig, A.; Heinz, H. Adsorption and Substitution of Metal Ions on Hydroxyapatite as a Function of Crystal Facet and Electrolyte pH. J. Phys. Chem. C 2019, 123, 16982–16993. [Google Scholar] [CrossRef]
- Matsunaga, K.; Murata, H. Strontium Substitution in Bioactive Calcium Phosphates: A First-Principles Study. J. Phys. Chem. B 2009, 113, 3584–3589. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Wren, A.W.; Towler, M.R.; Boyd, D. The effect of ionic dissolution products of Ca–Sr–Na–Zn–Si bioactive glass on in vitro cytocompatibility. J. Mater. Sci. Mater. Electron. 2010, 21, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Woo, L.C.; Yuen, V.G.; Thompson, K.H.; McNeill, J.H.; Orvig, C. Vanadyl–biguanide complexes as potential synergistic insulin mimics. J. Inorg. Biochem. 1999, 76, 251–257. [Google Scholar] [CrossRef]
- Holešová, S.; Samlíková, M.; Pazdziora, E.; Valášková, M. Antibacterial activity of organomontmorillonites and organovermiculites prepared using chlorhexidine diacetate. Appl. Clay Sci. 2013, 83, 17–23. [Google Scholar] [CrossRef]
- Bharatam, P.V.; Patel, A.D.S.; Iqbal, P. Pharmacophoric Features of Biguanide Derivatives: An Electronic and Structural Analysis. J. Med. Chem. 2005, 48, 7615–7622. [Google Scholar] [CrossRef]
- Rao, N. Cities in Transition: Growth, Change and Governance in Six Metropolitan Areas; Routledge: London, UK, 2007; ISBN 0203391152. [Google Scholar]
- Lu, L.; Gao, X.; Zhu, M.; Wang, S.; Wu, Q.; Xing, S.; Fu, X.; Liu, Z.; Guo, M. Exploration of biguanido–oxovanadium complexes as potent and selective inhibitors of protein tyrosine phosphatases. BioMetals 2012, 25, 599–610. [Google Scholar] [CrossRef]
- Wakshlak, R.B.-K.; Pedahzur, R.; Menagen, B.; Avnir, D. An antibacterial copper composite more bioactive than metallic silver. J. Mater. Chem. B 2016, 4, 4322–4329. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Mardziah, C.; Ramesh, S.; Wahid, M.A.; Chandran, H.; Sidhu, A.; Krishnasamy, S.; Purbolaksono, J. Effect of zinc ions on the structural characteristics of hydroxyapatite bioceramics. Ceram. Int. 2020, 46, 13945–13952. [Google Scholar] [CrossRef]
- Shedam, M.; Rao, A.V. Effect of temperature on nucleation and growth of cadmium oxalate single crystals in silica gels. Mater. Chem. Phys. 1998, 52, 263–266. [Google Scholar] [CrossRef]
- Haas, I.; Shanmugam, S.; Gedanken, A. Pulsed Sonoelectrochemical Synthesis of Size-Controlled Copper Nanoparticles Stabilized by Poly(N-vinylpyrrolidone). J. Phys. Chem. B 2006, 110, 16947–16952. [Google Scholar] [CrossRef] [PubMed]
- Joop, H.; Sefcik, J. The Handbook of Continuous Crystallization; Yazdanpanah, N., Nagy, Z.K., Eds.; Royal Society of Chemistry: Cambridge, UK, 2020; ISBN 978-1-78801-214-0. [Google Scholar]
- Jiang, L.; Sun, G.; Zhou, Z.; Sun, S.; Wang, Q.; Yan, S.; Li, H.; Tian, J.; Guo, J.; Zhou, B.; et al. Size-Controllable Synthesis of Monodispersed SnO2 Nanoparticles and Application in Electrocatalysts. J. Phys. Chem. B 2005, 109, 8774–8778. [Google Scholar] [CrossRef]
- Manzoor, U.; Zahra, F.T.; Rafique, S.; Moin, M.T.; Mujahid, M. Effect of Synthesis Temperature, Nucleation Time, and Postsynthesis Heat Treatment of ZnO Nanoparticles and Its Sensing Properties. J. Nanomater. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- De Geest, B.; Dejugnat, C.; Verhoeven, E.; Sukhorukov, G.; Jonas, A.; Plain, J.; Demeester, J.; De Smedt, S. Layer-by-layer coating of degradable microgels for pulsed drug delivery. J. Control. Release 2006, 116, 159–169. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, R.; DeSouza-Edwards, A.O.; Frueh, J.; Sukhorukov, G.B. Microchamber arrays made of biodegradable polymers for enzymatic release of small hydrophilic cargos. Soft Matter 2020, 16, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Cline, N.V.; Layman, D.L. The Effects of Chlorhexidine on the Attachment and Growth of Cultured Human Periodontal Cells. J. Periodontol. 1992, 63, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Pucher, J.J.; Daniel, C. The Effects of Chlorhexidine Digluconate on Human Fibroblasts In Vitro. J. Periodontol. 1992, 63, 526–532. [Google Scholar] [CrossRef]
- Giannelli, M.; Chellini, F.; Margheri, M.; Tonelli, P.; Tani, A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol. In Vitro 2008, 22, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Wyganowska-Swiatkowska, M.; Kotwicka, M.; Urbaniak, P.; Nowak, A.N.-T.; Skrzypczak-Jankun, E.; Jankun, J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int. J. Mol. Med. 2016, 37, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Faria, G.; Celes, M.R.; De Rossi, A.; Silva, L.; Silva, J.S.; Rossi, M.A. Evaluation of Chlorhexidine Toxicity Injected in the Paw of Mice and Added to Cultured L929 Fibroblasts. J. Endod. 2007, 33, 715–722. [Google Scholar] [CrossRef]
- Tsourounakis, I.; Palaiologou-Gallis, A.A.; Stoute, D.; Maney, P.; Lallier, T.E. Effect of Essential Oil and Chlorhexidine Mouthwashes on Gingival Fibroblast Survival and Migration. J. Periodontol. 2013, 84, 1211–1220. [Google Scholar] [CrossRef]
- Abdel-Sayed, P.; Tornay, D.; Hirt-Burri, N.; Roessingh, A.D.B.; Raffoul, W.; Applegate, L.A. Implications of chlorhexidine use in burn units for wound healing. Burns 2020, 46, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Edmiston, C.E.; Okoli, O.; Graham, M.B.; Sinski, S.; Seabrook, G.R. Evidence for Using Chlorhexidine Gluconate Preoperative Cleansing to Reduce the Risk of Surgical Site Infection. AORN J. 2010, 92, 509–518. [Google Scholar] [CrossRef]
- Lee, A.; Harlan, R.; Breaud, A.R.; Speck, K.; Perl, T.M.; Clarke, W.; Milstone, A.M. Blood Concentrations of Chlorhexidine in Hospitalized Children Undergoing Daily Chlorhexidine Bathing. Infect. Control. Hosp. Epidemiol. 2011, 32, 395–397. [Google Scholar] [CrossRef]
- Shapur, N.K.; Duvdevani, M.; Friedman, M.; Zaks, B.; Gati, I.; Lavy, E.; Katz, R.; Landau, E.H.; Pode, D.; Gofrit, O.N.; et al. Second Prize: Sustained Release Varnish Containing Chlorhexidine for Prevention of Biofilm Formation on Urinary Catheter Surface: In Vitro Study. J. Endourol. 2012, 26, 26–31. [Google Scholar] [CrossRef]
- Tomás, I.; Cousido, M.; García-Caballero, L.; Rubido, S.; Limeres, J.; Diz, P. Substantivity of a single chlorhexidine mouthwash on salivary flora: Influence of intrinsic and extrinsic factors. J. Dent. 2010, 38, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Friedman, M. Development of sustained-release devices for modulation of dental plaque biofilm and treatment of oral infectious diseases. Drug Dev. Res. 2000, 50, 555–565. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Mitali, K.; Lu, T.B.; Handral, H.K.; Dubey, N.; Fawzy, A.S. PLGA nanoparticles as chlorhexidine-delivery carrier to resin-dentin adhesive interface. Dent. Mater. 2017, 33, 830–846. [Google Scholar] [CrossRef]
- Guida, A.; Towler, M.R.; Wall, J.G.; Hill, R.; Eramo, S. Preliminary work on the antibacterial effect of strontium in glass ionomer cements. J. Mater. Sci. Lett. 2003, 22, 1401–1403. [Google Scholar] [CrossRef]
- Steen, K.H.; Steen, A.E.; Reeh, P. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J. Neurosci. 1995, 15, 3982–3989. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.-L.; Liewehr, F.R. Relationships between Caries Bacteria, Host Responses, and Clinical Signs and Symptoms of Pulpitis. J. Endod. 2007, 33, 213–219. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Lee, K.-H.; Hwang, J.-K. Activity of panduratin A isolated from Kaempferia pandurata Roxb. against multi-species oral biofilms in vitro. J. Oral Sci. 2009, 51, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, G.; Korachi, M. Inhibition and Disruption Properties of Chlorhexidine Gluconate on Single and Multispecies Oral Biofilms. Jundishapur J. Microbiol. 2012, 6, 61–66. [Google Scholar] [CrossRef]
- Bonesvoll, P.; Lökken, P.; Rölla, G. Influence of concentration, time, temperature and pH on the retention of chlorhexidine in the human oral cavity after mouth rinses. Arch. Oral Biol. 1974, 19, 1025–1029. [Google Scholar] [CrossRef]
- Zeng, P.; Rao, A.; Wiedmann, T.S.; Bowles, W. Solubility Properties of Chlorhexidine Salts. Drug Dev. Ind. Pharm. 2009, 35, 172–176. [Google Scholar] [CrossRef]
- Hiller, H.; Reimert, R.; Marschner, F.; Renner, H.-J.; Boll, W.; Supp, E.; Brejc, M.; Liebner, W.; Schaub, G.; Hochgesand, G.; et al. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2000; ISBN 9783527303854. [Google Scholar]
- Senanayake, G. Gold leaching by copper(II) in ammoniacal thiosulphate solutions in the presence of additives. Part I: A review of the effect of hard–soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115, 1–20. [Google Scholar] [CrossRef]
- Sampson, V.; Kamona, N.; Sampson, A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent. J. 2020, 228, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef] [PubMed]



| Concentration of CHX-SrCl2, CHX-ZnCl2 or CHXD | 24 h | 48 h |
|---|---|---|
| 0.00025% | MIC | MBC |
| 0.0005% | MBC | MBC |
| 0.001% | MBC | MBC |
| Concentration of CHX-SrCl2, CHX-ZnCl2, or CHXD | 24 h | 48 h |
|---|---|---|
| 0.0005% | MIC | MIC |
| 0.001% | MBC | MBC |
| 0.002% | MBC | MBC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Zhang, J.; Whiley, R.A.; Sukhorukov, G.B.; Cattell, M.J. Synthesis, Drug Release, and Antibacterial Properties of Novel Dendritic CHX-SrCl2 and CHX-ZnCl2 Particles. Pharmaceutics 2021, 13, 1799. https://doi.org/10.3390/pharmaceutics13111799
Sun R, Zhang J, Whiley RA, Sukhorukov GB, Cattell MJ. Synthesis, Drug Release, and Antibacterial Properties of Novel Dendritic CHX-SrCl2 and CHX-ZnCl2 Particles. Pharmaceutics. 2021; 13(11):1799. https://doi.org/10.3390/pharmaceutics13111799
Chicago/Turabian StyleSun, Rui, Jiaxin Zhang, Robert A. Whiley, Gleb B. Sukhorukov, and Michael J. Cattell. 2021. "Synthesis, Drug Release, and Antibacterial Properties of Novel Dendritic CHX-SrCl2 and CHX-ZnCl2 Particles" Pharmaceutics 13, no. 11: 1799. https://doi.org/10.3390/pharmaceutics13111799
APA StyleSun, R., Zhang, J., Whiley, R. A., Sukhorukov, G. B., & Cattell, M. J. (2021). Synthesis, Drug Release, and Antibacterial Properties of Novel Dendritic CHX-SrCl2 and CHX-ZnCl2 Particles. Pharmaceutics, 13(11), 1799. https://doi.org/10.3390/pharmaceutics13111799








