Past HIV-1 Medications and the Current Status of Combined Antiretroviral Therapy Options for HIV-1 Patients
Abstract
1. Introduction
2. First AIDS Drug—AZT
3. Highly Active Antiretroviral Therapy—HAART
4. Combined Antiretroviral Therapy—cART
5. gp120-Binding Proteins Inhibit HIV-1 Infection
6. Entry Inhibitors
7. Integrase Strand Transfer Inhibitor (INSTI), Dolutegravir (DTG), and Nucleoside Reverse Transcriptase Inhibitor, Lamivudine
8. Available HIV-1 Treatment Options
8.1. Anti-CD4 Monoclonal Antibodies
8.2. Nucleoside Reverse Transcriptase Translocation Inhibitors (NRTTI)
8.3. Capsid Inhibitors
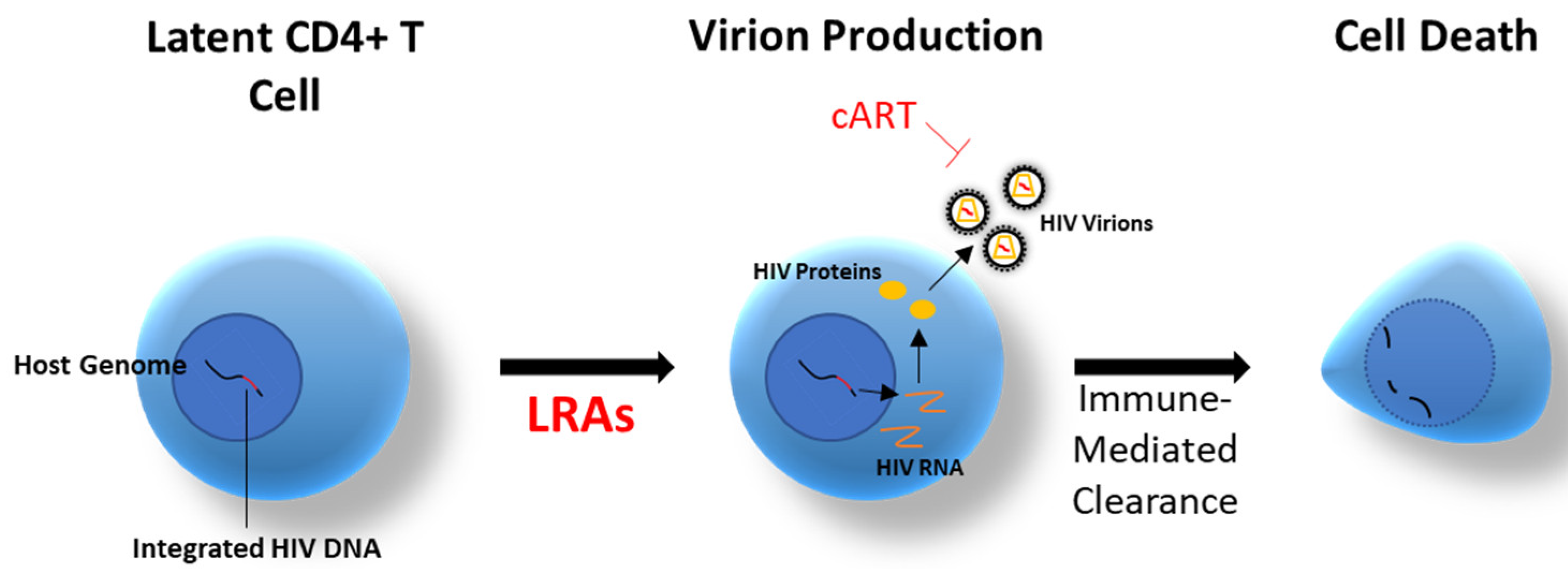
8.4. Latency-Reversing Agents (LRAs)
9. Remaining Obstacles
9.1. Viral Persistence
9.2. Side Effects
9.3. Poor Drug Penetration
10. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.-R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- Bosque, A.; Famiglietti, M.; Weyrich, A.S.; Goulston, C.; Planelles, V. Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells. PLoS Pathog. 2011, 7, e1002288. [Google Scholar] [CrossRef]
- Katlama, C.; Deeks, S.G.; Autran, B.; Martinez-Picado, J.; van Lunzen, J.; Rouzioux, C.; Miller, M.; Vella, S.; Schmitz, J.E.; Ahlers, J.; et al. Barriers to a Cure: New Concepts in targeting and eradicating HIV-1 reservoirs. Lancet 2013, 381, 9883. [Google Scholar] [CrossRef]
- Natarajan, V.; Bosche, M.; Metcalf, J.A.; Ward, D.J.; Lane, H.C.; Kovacs, J.A. HIV-1 replication in patients with undetectable plasma virus receiving HAART. Lancet 1999, 353, 119–120. [Google Scholar] [CrossRef]
- Martinez-Picado, J.; Deeks, S. Persistent HIV-1 replication during antiretroviral therapy. Curr. Opin. HIV AIDS 2016, 11, 417–423. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-Competent Noninduced Proviruses in the Latent Reservoir Increase Barrier to HIV-1 Cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef]
- Richman, D.D.; Margolis, D.M.; Delaney, M.; Greene, W.C.; Hazuda, D.; Pomerantz, R.J. The Challenge of Finding a Cure for HIV Infection. Science 2009, 323, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.M. How Might We Cure HIV? Curr. Infect. Dis. Rep. 2014, 16, 392. [Google Scholar] [CrossRef]
- Price, R.W.; Brew, B.; Sidtis, J.; Rosenblum, M.; Scheck, A.C.; Cleary, P. The Brain in AIDS: Central Nervous System HIV-1 Infection and AIDS Dementia Complex. Science 1988, 239, 586–592. [Google Scholar] [CrossRef]
- Gorry, P.R.; Howard, J.L.; Churchill, M.J.; Anderson, J.L.; Cunningham, A.; Adrian, D.; McPhee, D.A.; Purcell, D.F.J. Diminished Production of Human Immunodeficiency Virus Type 1 in Astrocytes Results from Inefficient Translation of gag, env, and nef mRNAs despite Efficient Expression of Tat and Rev. J. Virol. 1999, 73, 352–361. [Google Scholar] [CrossRef]
- Honeycutt, J.B.; Wahl, A.; Baker, C.; Spagnuolo, R.A.; Foster, J.; Zakharova, O.; Wietgrefe, S.; Caro-Vegas, C.; Madden, V.; Sharpe, G.; et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Investig. 2016, 126, 1353–1366. [Google Scholar] [CrossRef]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Initiating Antiretroviral Therapy in Treatment-Naive Patients Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. Available online: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf (accessed on 17 May 2021).
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids#:~:text=Since%202016%2C%20WHO%20has%20recommended,status%20or%20CD4%20cell%20count (accessed on 1 September 2021).
- Burgess, M.; Kasten, M.J.; Zeuli, J.D. Management of HIV/AIDS in older patients–drug/drug interactions and adherence to antiretroviral therapy. HIV/AIDS-Res. Palliat. Care 2015, 7, 251–264. [Google Scholar] [CrossRef]
- Vella, S.; Schwartländer, B.; Sow, S.P.; Eholie, S.P.; Murphy, R. The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS 2012, 26, 1231–1241. [Google Scholar] [CrossRef]
- GlaxoSmithKline. Package Insert-Retrovir (Zidovudine). Updated September 2018. Available online: https://gskpro.com/content/dam/global/hcpportal/en_NA/PI/Retrovir-GDS31.pdf (accessed on 17 May 2021).
- Turner, J.; Badireddy, M. Anemia; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Abers, M.S.; Shandera, W.X.; Kass, J.S. Neurological and Psychiatric Adverse Effects of Antiretroviral Drugs. CNS Drugs 2013, 28, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, E.R.; Naylor, A.J.D. Mechanisms of Zidovudine-Induced Mitochondrial Toxicity and Myopathy. Pharmacology 2008, 82, 83–88. [Google Scholar] [CrossRef]
- Kravcik, S.; Gallicano, K.; Roth, V.; Cassol, S.; Hawley-Foss, N.; Badley, A.; Cameron, D.W. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and Saquinavir. J. Acquir. Immune Defic. Syndr. 1999, 21, 371–375. [Google Scholar] [CrossRef]
- Dragsted, U.B.; Gerstoft, J.; Pedersen, C.; Peters, B.; Duran, A.; Obel, N.; Castagna, A.; Cahn, P.; Clumeck, N.; Bruun, J.N.; et al. Randomized Trial to Evaluate Indinavir/Ritonavir versus Saquinavir/Ritonavir in Human Immunodeficiency Virus Type 1—Infected Patients: The MaxCmin1 Trial. J. Infect. Dis. 2003, 188, 635–642. [Google Scholar] [CrossRef]
- Whitesid, A.; Winsbury, R. Vancouver AIDS conference: Special report. The role of the military: To protect society and themselves. AIDS Anal. Africa. 1996, 6, 4. [Google Scholar] [PubMed]
- Da-Yong, L.; Hong-Ying, W.; Yarla, N.S.; Xu, B.; Ding, J.; Lu, T.-R. HAART in HIV/AIDS Treatments: Future Trends. Infect. Discord Drug Targets 2018, 18, 15–22. [Google Scholar]
- El-Sadr, W.M.; Holmes, C.B.; Mugyenyi, P.; Thirumurthy, H.; Ellerbrock, T.; Ferris, R.; Sanne, I.; Asiimwe, A.; Hirnschall, G.; Nkambule, N.R.; et al. Scale-up of HIV treatment through PEPFAR: A historic public health achievement. J. Acquir. Immune Defic. Syndr. 2012, 60 (Suppl. 3), S96–S104. [Google Scholar] [CrossRef]
- Chow, D. Epidemiological evidence of increasing blood pressure in HIV-1-infected individuals in the era of HAART. Antivir. Ther. 2000, 5, 13. [Google Scholar]
- Maggiolo, F.; Arici, C.; Airoldi, M.; Ripamonti, D.; Quinzan, G.; Gregis, G.; Ravasio, V.; Bombana, E.; Suter, F. Reasons for discontinuation of nevirapine-containing HAART: Results from an unselected population of a large clinical cohort. J. Antimicrob. Chemother. 2007, 59, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Max, B.; Sherer, R. Management of the Adverse Effects of Antiretroviral Therapy and Medication Adherence. Clin. Infect. Dis. 2000, 30, S96–S116. [Google Scholar] [CrossRef]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A Review of the Toxicity of HIV Medications. J. Med. Toxicol. 2013, 10, 26–39. [Google Scholar] [CrossRef]
- The INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.L.; Chao, C.R.; Leyden, W.A.; Xu, L.; Quesenberry, C.P.; Klein, D.B.; Towner, W.J.; Horberg, M.A.; Silverberg, M.J. Narrowing the Gap in Life Expectancy Between HIV-Infected and HIV-Uninfected Individuals With Access to Care. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 73, 39–46. [Google Scholar] [CrossRef]
- Abel, S.; Back, D.J.; Vourvahis, M. Maraviroc: Pharmacokinetics and drug interactions. Antivir. Ther. 2009, 14, 607–618. [Google Scholar]
- Latinovic, O.S.; Le, N.; Reitz, M.; Pal, R.; DeVico, A.; Foulke, J.S.; Redfield, R.R.; Heredia, A. Synergistic Inhibition of CCR5-tropic HIV-1 by Maraviroc and CCR5 Antibody HGS004 in Primary Cells: Potential Implications for Treatment and Prevention. AIDS 2011, 25, 1232–1235. [Google Scholar] [CrossRef]
- Heredia, A.; Latinovic, O.S.; Gallo, R.C.; Melikian, G.B.; Reitz, M.; Le, N.; Redfield, R.R. Low doses of Rapamycin enhance the antiviral activity of CCR5 antagonist VCV against wild-type and drug resistant R5 HIV-1 (co-first authorship). Proc. Natl. Acad. Sci. USA 2008, 105, 20476–20481. [Google Scholar] [CrossRef]
- Chan, D.C.; Kim, P.S. HIV Entry and Its Inhibition. Cell 1998, 93, 681–684. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, X.; Zhu, Y.; Chong, H.; Cui, S.; He, J.; Wang, X.; He, Y. Structural and functional characterization of HIV-1 cell fusion inhibitor T20. AIDS 2019, 33, 1–11. [Google Scholar] [CrossRef]
- Latinovic, O.; Kuruppu, J.; Davis, C.; Le, N.; Heredia, A. Pharmacotherapy of HIV-1 Infection: Focus on CCR5 Antagonist Maraviroc. Clin. Med. 2009, 1, 1497–1510. [Google Scholar] [CrossRef]
- Hyland, R.; Dickins, M.; Collins, C.; Jones, H.; Jones, B. Maraviroc: In vitro assessment of drug-drug interaction potential. Br. J. Clin. Pharmacol. 2008, 66, 498–507. [Google Scholar] [CrossRef]
- Gardner, M.R.; Farzan, M. Engineering antibody-like inhibitors to prevent and treat HIV-1 infection. Curr. Opin. HIV AIDS 2017, 12, 294–301. [Google Scholar] [CrossRef]
- Deen, K.C.; McDouga, J.S.; Inacker, R.; Folena-Wasserman, G.; Arthos, J.; Rosenberg, J.; Maddon, P.J.; Axel, R.; Sweet, R.W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature 1988, 331, 82–84. [Google Scholar] [CrossRef]
- Gardner, M.R.; Kattenhorn, L.M.; Kondur, H.R.; von Schaewen, M.; Dorfman, T.; Chiang, J.J.; Haworth, K.G.; Decker, J.M.; Alpert, M.D.; Bailey, C.C.; et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 2015, 519, 87–91. [Google Scholar] [CrossRef]
- Huang, J.; Kang, B.H.; Ishida, E.; Zhou, T.; Griesman, T.; Sheng, Z.; Wu, F.; Doria-Rose, N.A.; Zhang, B.; McKee, K.; et al. Identification of a CD4-Binding-Site Antibody to HIV that Evolved Near-Pan Neutralization Breadth. Immunity 2016, 45, 1108–1121. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, Z.; Feng, Y.; Dimitrov, D.S. Human domain antibodies to conserved sterically restricted regions on gp120 as exceptionally potent cross-reactive HIV-1 neutralizers. Proc. Natl. Acad. Sci. USA 2008, 105, 17121–17126. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.; Fiume, G.; Caivano, A.; De Laurentiis, A.; Falcone, C.; Masci, F.F.; Iaccino, E.; Mimmi, S.; Palmieri, C.; Pisano, A.; et al. Design and Characterization of a Peptide Mimotope of the HIV-1 gp120 Bridging Sheet. Int. J. Mol. Sci. 2012, 13, 5674–5699. [Google Scholar] [CrossRef]
- Wang, X.; Cao, M.; Wu, Y.; Xu, W.; Wang, Q.; Ying, T.; Lu, L.; Jiang, S. Synergistic Effect by Combining a gp120-Binding Protein and a gp41-Binding Antibody to Inactivate HIV-1 Virions and Inhibit HIV-1 Infection. Molecules 2021, 26, 1964. [Google Scholar] [CrossRef]
- Andreatta, K.; Willkom, M.; Martin, R.; Chang, S.; Wei, L.; Liu, H.; Liu, Y.-P.; Graham, H.; Quirk, E.; Martin, H.; et al. Erratum to: Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J. Antimicrob. Chemother. 2019, 74, 3646–3647. [Google Scholar] [CrossRef]
- Brehm, T.T.; Franz, M.; Hüfner, A.; Hertling, S.; Schmiedel, S.; Degen, O.; Kreuels, B.; Schulze Zur Weisch, J. Safety and efficacy of elvitegravir, dolutegravir, and raltegravir in a real-world cohort of treatment-naïve and -experienced patients. Medicine 2019, 98, e16721. [Google Scholar] [CrossRef]
- Cahn, P.; Pozniak, A.L.; Mingrone, H.; Shuldyakov, A.; Brites, C.; Andrade-Villanueva, J.F.; Richmond, G.; Buendia, C.B.; Fourie, J.; Ramgopal, M.; et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013, 382, 700–708. [Google Scholar] [CrossRef]
- McAllister, J.W.; Towns, J.M.; McNulty, A.; Pierce, A.B.; Foster, R.; Richardson, R.; Carr, A. Dolutegravir with tenofovir disoproxil fumarate–emtricitabine as HIV postexposure prophylaxis in gay and bisexual men. AIDS 2017, 31, 1291–1295. [Google Scholar] [CrossRef]
- Raffi, F.; Jaeger, H.; Quiros-Roldan, E.; Albrecht, H.; Belonosova, E.; Gatell, J.M.; Baril, J.-G.; Domingo, P.; Brennan, C.; Almond, S.; et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2013, 13, 927–935. [Google Scholar] [CrossRef]
- Powderly, W.G. Integrase inhibitors in the treatment of HIV-1 infection. J. Antimicrob. Chemother. 2010, 65, 2485–2488. [Google Scholar] [CrossRef]
- Venter, E.D.F.; Moorhouse, S.; Sokhela, L.; Fairlie, L.; Mashabane, N.; Masenya, M.; Serenata, C.; Akpomiemie, G.; Qavi, A.; Chandiwana, N.; et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N. Engl. J. Med. 2019, 381, 803–815. [Google Scholar] [CrossRef]
- Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf (accessed on 3 June 2021).
- Wohl, D.A.; Yazdanpanah, Y.; Baumgarten, A.; Clarke, A.; Thompson, M.A.; Brinson, C.; Hagins, D.; Ramgopal, M.N.; Antinori, A.; Wei, X.; et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: Week 96 results from a randomized, double-blind, multicenter, phase3, non-inferiority trial. Lancet 2019, 6, e355–e363. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves First Two-Drug Regimen for Certain Patients with HIV. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-two-drug-regimen-certain-patients-hiv (accessed on 17 May 2021).
- U.S. Food and Drug Administration. FDA Approves First Two-Drug Complete Regimen for HIV-Infected Patients Who Have Never Received Antiretroviral Treatment. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-two-drug-complete-regimen-hiv-infected-patients-who-have-never-received (accessed on 17 May 2021).
- U.S. Preventive Services Task Force. Final Draft Recommendation: Human Immunodeficiency Virus (HIV) Infection: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/human-immunodeficiency-virus-hiv-infection-screening (accessed on 17 May 2021).
- U.S. Food and Drug Administration. Drug Approval Package. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202895_prezista_toc.cfm (accessed on 7 September 2021).
- U.S. Food and Drug Administration. FDA Approves Cabenuva and Vocabria for the Treatment of HIV-1 Infection. Available online: https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/1890 (accessed on 27 January 2021).
- Pace, C.S.; Fordyce, M.W.; Franco, D.; Kao, C.-Y.; Seaman, M.S.; Ho, D.D. Anti-CD4 Monoclonal Antibody Ibalizumab Exhibits Breadth and Potency Against HIV-1, With Natural Resistance Mediated by the Loss of a V5 Glycan in Envelope. JAIDS J. Acquir. Immune Defic. Syndr. 2013, 62, 1–9. [Google Scholar] [CrossRef]
- Emu, B.; Fessel, J.; Schrader, S.; Kumar, P.; Richmond, G.; Win, S.; Weinheimer, S.; Marsolais, C.; Lewis, S. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. N. Engl. J. Med. 2018, 379, 645–654. [Google Scholar] [CrossRef]
- Grobler, J.A.; Huang, Q.; Hazuda, D.J.; Lai, M.-T. Efficacy of MK-8591 against diverse HIV-1 subtypes and NRTI-resistant clinical isolates. J. Int. AIDS Soc. 2018, 2, O343. [Google Scholar]
- Grobler, J.; Friedman, E.; Barrett, S.E.; Wood, S.L.; Ankrom, W.; Fillgrove, K.L.; Lai, M.-T.; Gindy, M.; Iwamoto, M.; Hazuda, D.J. Long-acting oral and parenteral dosing of MK-8591 for HIV treatment or prophylaxis. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, USA, 22–25 February 2016. [Google Scholar]
- Matthews, R.P.; Schurmann, D.; Rudd, D.J.; Levine, V.; Fox-Bosetti, S.; Zhang, S.; Robberechts, M.; Huser, A.; Hazuda, D.J.; Iwamoto, M.; et al. Single doses as low as 0.5 mg of the novel NRTTI MK-8591 suppress HIV for at least seven days. In Proceedings of the International AIDS Society Conference, Paris, France, 23–26 July 2017. [Google Scholar]
- CROI 2021 Presentation 88. Available online: https://www.croiconference.org/ (accessed on 3 June 2021).
- Safety and Pharmacokinetics of Oral Islatravir (MK-8591) Once Monthly in Participants at Low Risk of Human Immunodeficiency Virus 1 (HIV-1) Infection (MK-8591-016). Available online: https://clinicaltrials.gov/ct2/show/NCT04003103 (accessed on 17 May 2021).
- Matthews, R. First-in-Human Trial of MK-8591-Eluting Implants Demonstrates Concentrations Suitable for HIV Prophylaxis for at Least One Year. In Proceedings of the International AIDS Society Conference, Mexico City, Mexico, 21–24 July 2019. [Google Scholar]
- Tse, W.L.J.O.; Mulato, A.; Niedziela-Majka, A.; Rowe, W.; Somoza, J.R.; Villasenor, A.G.; Yant, S.R.; Zhang, J.R.; Zheng, J. Discovery of Novel Potent HIV Capsid Inhibitors with Long-Acting Potential. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, DC, USA, 13–16 February 2017. [Google Scholar]
- Carnes, S.K.; Sheehan, J.H.; Aiken, C. Inhibitors of the HIV-1 capsid, a target of opportunity. Curr. Opin. HIV AIDS 2018, 13, 359–365. [Google Scholar] [CrossRef]
- Safety, Pharmacokinetics, and Antiviral Activity of GS-6207 Administered Subcutaneously in HIV-1 Infected Adults. Available online: https://ClinicalTrials.gov/show/NCT03739866 (accessed on 17 May 2021).
- Mascolini, M. Sharp Drops in HIV Load after 10 Days of Capsid Inhibitor Monotherapy; International AIDS Society: Mexico City, Mexico, 2019. [Google Scholar]
- Hamer, D.H. Can HIV be Cured? Mechanisms of HIV Persistence and Strategies to Combat It. Curr. HIV Res. 2004, 2, 99–111. [Google Scholar] [CrossRef]
- Timmons, A.; Fray, E.; Kumar, M.; Wu, F.; Dai, W.; Bullen, C.K.; Kim, P.; Hetzel, C.; Yang, C.; Beg, S.; et al. HSF1 inhibition attenuates HIV-1 latency reversal mediated by several candidate LRAs In Vitro and Ex Vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 15763–15771. [Google Scholar] [CrossRef]
- Abner, E.; Jordan, A. HIV “shock and kill” therapy: In need of revision. Antivir. Res. 2019, 166, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ammar, A.; Kula, A.; Darcis, G.; Verdikt, R.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Rohr, O.; Van Lint, C. Current Status of Latency Reversing Agents Facing the Heterogeneity of HIV-1 Cellular and Tissue Reservoirs. Front. Microbiol. 2020, 10, 3060. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Siliciano, R.F. Targeting the Latent Reservoir for HIV-1. Immunity 2018, 48, 872–895. [Google Scholar] [CrossRef]
- Vanhamel, J.; Bruggemans, A.; Debyser, Z. Establishment of latent HIV-1 reservoirs: What do we really know? J. Virus Erad. 2019, 5, 3–9. [Google Scholar] [CrossRef]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “kill” into “shock and kill”: Strategies to eliminate latent HIV. Cell Host Microbe. 2018, 23, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Archin, N.M.; Kirchherr, J.L.; Sung, J.A.; Clutton, G.; Sholtis, K.; Xu, Y.; Allard, B.; Stuelke, E.; Kashuba, A.D.; Kuruc, J.D.; et al. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J. Clin. Investig. 2017, 127, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Archin, N.M.; Liberty, A.L.; Kashuba, A.D.; Choudhary, S.K.; Kuruc, J.D.; Crooks, A.M.; Parker, D.C.; Anderson, E.M.; Kearney, M.F.; Strain, M.C.; et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012, 487, 482–485. [Google Scholar] [CrossRef]
- Elliott, J.H.; McMahon, J.; Chang, C.C.; Lee, S.A.; Hartogensis, W.; Bumpus, N.; Savic, R.; Roney, J.; Hoh, R.; Solomon, A.; et al. Short-term administration of disulfiram for reversal of latent HIV infection: A phase 2 dose-escalation study. Lancet HIV 2015, 2, e520–e529. [Google Scholar] [CrossRef]
- Elliott, J.H.; Wightman, F.; Solomon, A.; Ghneim, K.; Ahlers, J.; Cameron, M.J.; Smith, M.Z.; Spelman, T.; McMahon, J.; Velayudham, P.; et al. Activation of HIV Transcription with Short-Course Vorinostat in HIV-Infected Patients on Suppressive Antiretroviral Therapy. PLoS Pathog. 2014, 10, e1004473. [Google Scholar] [CrossRef] [PubMed]
- Hütter, G.; Nowak, D.; Mossner, M.; Ganepola, S.; Müssig, A.; Allers, K.; Schneider, T.; Hofmann, J.; Kücherer, C.; Blau, O.; et al. Long-Term Control of HIV byCCR5Delta32/Delta32 Stem-Cell Transplantation. N. Engl. J. Med. 2009, 360, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Michael, N.L.; Louie, L.G.; Sheppard, H.W. CCR5-delta 32 gene deletion in HIV-1 infected patients. Lancet 1997, 350, 741–742. [Google Scholar] [CrossRef]
- Liu, R.; Paxton, W.A.; Choe, S.; Ceradini, D.; Martin, S.R.; Horuk, R.; MacDonald, M.E.; Stuhlmann, H.; Koup, R.A.; Landau, N.R. Homozygous Defect in HIV-1 Coreceptor Accounts for Resistance of Some Multiply-Exposed Individuals to HIV-1 Infection. Cell 1996, 86, 367–377. [Google Scholar] [CrossRef]
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; et al. Maraviroc (UK-427,857), a Potent, Orally Bioavailable, and Selective Small-Molecule Inhibitor of Chemokine Receptor CCR5 with Broad-Spectrum Anti-Human Immunodeficiency Virus Type 1 Activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Rosenberg, P.S.; Goedert, J.J.; Ashton, L.J.; Benfield, T.L.; Buchbinder, S.P.; Coutinho, R.A.; Eugen-Olsen, J.; Gallart, T.; Katzenstein, T.L.; et al. International Meta-Analysis of HIV Host Genetics: Effects of CCR5-Delta32, CCR2-64I, and SDF-13′A alleles on HIV-1 disease progression: An International Meta-Analysis of Individual Patient Data. Ann. Intern Med. 2001, 135, 782–795. [Google Scholar] [CrossRef]
- Feria, M.G.; Taborda, N.A.; Hernandez, J.C.; Rugeles, M.T. HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood. PLoS ONE 2018, 13, e0192845. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.R.; Roche, M.; Flynn, J.K.; Wesselingh, S.L.; Gorry, P.R.; Churchill, M.J. Is the central nervous system a reservoir of HIV-1? Curr. Opin. HIV AIDS 2014, 9, 552–558. [Google Scholar] [CrossRef]
- Walker-Sperling, V.E.; Pohlmeyer, C.W.; Tarwater, P.M.; Blankson, J.N. The Effect of Latency Reversal Agents on Primary CD8+ T Cells: Implications for Shock and Kill Strategies for Human Immunodeficiency Virus Eradication. EBioMedicine 2016, 8, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Kessing, C.F.; Nixon, C.C.; Li, C.; Tsai, P.; Takata, H.; Mousseau, G.; Ho, P.T.; Honeycutt, J.B.; Fallahi, M.; Trautmann, L.; et al. In Vivo Suppression of HIV Rebound by Didehydro-Cortistatin A, a “Block-and-Lock” Strategy for HIV-1 Treatment. Cell Rep. 2017, 21, 600–611. [Google Scholar] [CrossRef]
- Kulpa, D.A.; Chomont, N. HIV persistence in the setting of antiretroviral therapy: When, where and how does HIV hide? J. Virus Erad. 2015, 1, 59–66. [Google Scholar] [CrossRef]
- Shultz, L.D.; Brehm, M.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: Progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Siliciano, J.D.; Kajdas, J.; Finzi, D.; Quinn, T.C.; Chadwick, K.; Margolick, J.B.; Kovacs, C.; Gange, S.; Siliciano, R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003, 9, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.P.; Javed, S.; Lever, A. Stable gene expression occurs from a minority of integrated HIV-1-based vectors: Transcriptional silencing is present in the majority. Gene Ther. 2007, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, L. HIV-1 immunopathogenesis in humanized mouse models. Cell. Mol. Immunol. 2012, 9, 237–244. [Google Scholar] [CrossRef]
- Descours, B.; Petitjean, G.; Lopez-Zaragoza, J.L.; Bruel, T.; Raffel, R.; Psomas, C.; Reynes, J.; Lacabaratz, C.; Levy, Y.; Schwartz, O.; et al. CD32 is a marker of a CD4 T-cell HIV reservoir harboring replication-competent proviruses. Nature 2017, 543, 564–567. [Google Scholar] [CrossRef]
| Antiretroviral Drug Group | Leading Drug Option | Phase of Current Development | FDA Approval Status | Route of Administration in HIV-1 Patients |
|---|---|---|---|---|
| Entry Inhibitors | Enfuvirtide (T-20) | IV Completed | Yes | Oral |
| Maraviroc | IV Completed | Yes | Oral | |
| Zinc-Finger Nuclease | II Completed | No | Infusion | |
| Attachment Inhibitors | Fostemsavir (Rukobia) | III Active | Yes | Oral |
| Anti-CD4 Monoclonal Antibodies | Ibalizumab | III Completed | Yes | Infusion |
| Nucleoside Reverse Transcriptase Translocation Inhibitors (NRTTI) | Islatravir | I Completed, II Active | No | Oral |
| Integrase Strand Transfer Inhibitors (INSTI) | Dolutegravir | IV Completed | Yes | Oral |
| Capsid Inhibitors | GS-6207 | Ib Completed, II/III Active | No | Oral/Subcutaneous |
| Latency-Reversing Agents (LRAs) | Romidepsin | II Completed | No | Infusion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weichseldorfer, M.; Reitz, M.; Latinovic, O.S. Past HIV-1 Medications and the Current Status of Combined Antiretroviral Therapy Options for HIV-1 Patients. Pharmaceutics 2021, 13, 1798. https://doi.org/10.3390/pharmaceutics13111798
Weichseldorfer M, Reitz M, Latinovic OS. Past HIV-1 Medications and the Current Status of Combined Antiretroviral Therapy Options for HIV-1 Patients. Pharmaceutics. 2021; 13(11):1798. https://doi.org/10.3390/pharmaceutics13111798
Chicago/Turabian StyleWeichseldorfer, Matthew, Marvin Reitz, and Olga S. Latinovic. 2021. "Past HIV-1 Medications and the Current Status of Combined Antiretroviral Therapy Options for HIV-1 Patients" Pharmaceutics 13, no. 11: 1798. https://doi.org/10.3390/pharmaceutics13111798
APA StyleWeichseldorfer, M., Reitz, M., & Latinovic, O. S. (2021). Past HIV-1 Medications and the Current Status of Combined Antiretroviral Therapy Options for HIV-1 Patients. Pharmaceutics, 13(11), 1798. https://doi.org/10.3390/pharmaceutics13111798






