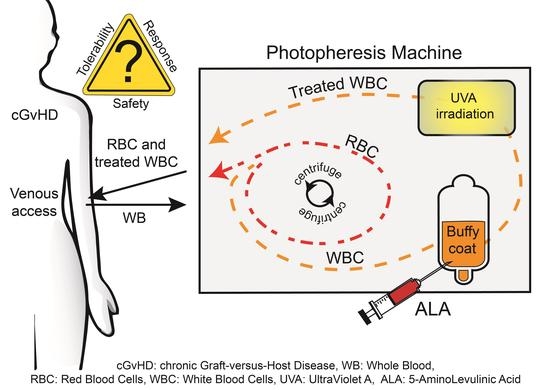
Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Patients with Chronic Graft-versus-Host Disease: A First-in-Human Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Design and Procedures
2.3. Safety and Tolerability
2.4. Organ, Performance, and Quality of Life Assessments
2.5. Statistical Methods
3. Results
3.1. Patients and Treatments
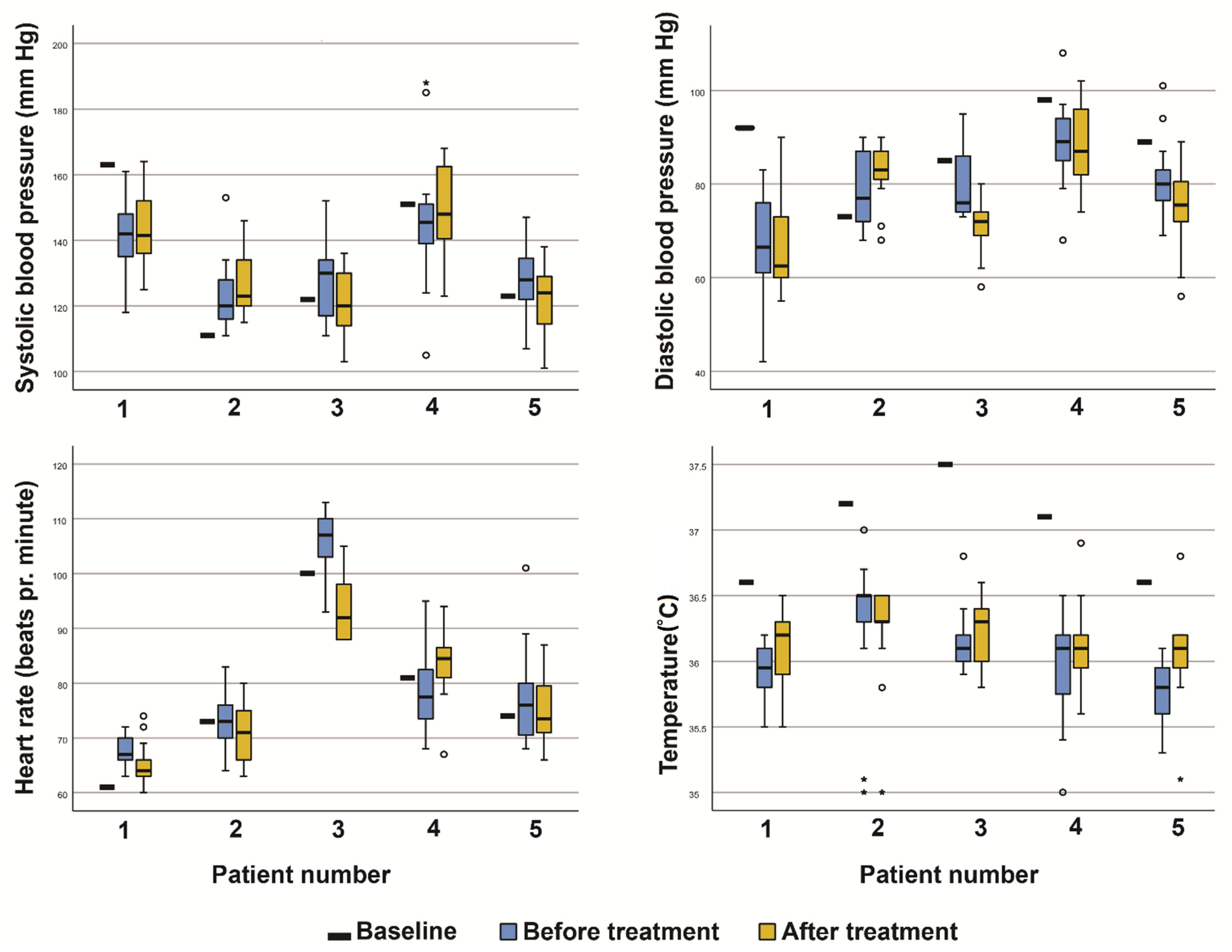
3.2. Safety and Tolerability
3.3. Organ, Performance, and Quality of Life Assessments
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edelson, R.; Berger, C.; Gasparro, F.; Jegasothy, B.; Heald, P.; Wintroub, B.; Vonderheid, E.; Knobler, R.; Wolff, K.; Plewig, G.; et al. Treatment of Cutaneous T-Cell Lymphoma by Extracorporeal Photochemotherapy. N. Engl. J. Med. 1987, 316, 297–303. [Google Scholar] [CrossRef]
- Knobler, R.; Arenberger, P.; Arun, A.; Assaf, C.; Bagot, M.; Berlin, G.; Bohbot, A.; Calzavara-Pinton, P.; Child, F.; Cho, A.; et al. European dermatology forum—Updated guidelines on the use of extracorporeal photopheresis 2020—Part 1. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2693–2716. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, M.; Wichert, S.; Berlin, G.; Toss, F. Extracorporeal photopheresis for graft-vs-host disease: A literature review and treatment guidelines proposed by the Nordic ECP Quality Group. Eur. J. Haematol. 2020, 104, 361–375. [Google Scholar] [CrossRef]
- Saidu, N.E.B.; Bonini, C.; Dickinson, A.; Grce, M.; Inngjerdingen, M.; Koehl, U.; Toubert, A.; Zeiser, R.; Galimberti, S. New Approaches for the Treatment of Chronic Graft-Versus-Host Disease: Current Status and Future Directions. Front. Immunol. 2020, 11, 578314. [Google Scholar] [CrossRef] [PubMed]
- Shulman, H.M.; Cardona, D.; Greenson, J.K.; Hingorani, S.; Horn, T.; Huber, E.; Kreft, A.; Longerich, T.; Morton, T.; Myerson, D.; et al. NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. The 2014 Pathology Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 589–603. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Tvedt, T.H.A.; Paulsen, P.Q.; Ahmed, A.B.; Gedde-Dahl, T.; Tjønnfjord, G.E.; Slåstad, H.; Heldal, D.; Reikvam, H. Extracorporeal photopheresis (photochemotherapy) in the treatment of acute and chronic graft versus host disease: Immuno-logical mechanisms and the results from clinical studies. Cancer Immunol. Immunother. 2014, 63, 757–777. [Google Scholar] [CrossRef]
- Szodoray, P.; Papp, G.; Nakken, B.; Harangi, M.; Zeher, M. The molecular and clinical rationale of extracorporeal pho-tochemotherapy in autoimmune diseases, malignancies and transplantation. Autoimmun. Rev. 2010, 9, 459–464. [Google Scholar] [CrossRef]
- Christensen, E.; Warloe, T.; Kroon, S.; Funk, J.; Helsing, P.; Soler, A.M.; Stang, H.J.; Vatne, O.; Mørk, C. Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 505–512. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Dunn, J.; Lovat, L. Photodynamic therapy using 5-aminolaevulinic acid for the treatment of dysplasia in Barrett’s oesophagus. Expert Opin. Pharmacother. 2008, 9, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Warloe, T.; Berg, K.; Moan, J.; Kongshaug, M.; Giercksky, K.E.; Nesland, J.M. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 1997, 79, 2282–2308. [Google Scholar] [CrossRef]
- Darvekar, S.; Juzenas, P.; Oksvold, M.; Kleinauskas, A.; Holien, T.; Christensen, E.; Stokke, T.; Sioud, M.; Peng, Q. Selective Killing of Activated T Cells by 5-Aminolevulinic Acid Mediated Photodynamic Effect: Potential Improvement of Extracorporeal Photopheresis. Cancers 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Noodt, B.B.; Berg, K.S.; Stokke, T.; Peng, Q.; Nesland, J.M. Apoptosis and necrosis induced with light and 5-aminolaevulinic acid-derived protoporphyrin IX. Br. J. Cancer 1996, 74, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Holien, T.; Gederaas, O.A.; Darvekar, S.R.; Christensen, E.; Peng, Q. Comparison between 8-methoxypsoralen and 5-aminolevulinic acid in killing T cells of photopheresis patients ex vivo. Lasers Surg. Med. 2018, 50, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Čunderlíková, B.; Vasovič, V.; Randeberg, L.; Christensen, E.; Warloe, T.; Nesland, J.; Peng, Q. Modification of extracorporeal photopheresis technology with porphyrin precursors. Comparison between 8-methoxypsoralen and hexaminolevulinate in killing human T-cell lymphoma cell lines in vitro. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Moan, J.; Warloe, T.; Nesland, J.M.; Rimington, C. Distribution and photosensitizing efficiency of porphyrins induced by application of exogenous 5-aminolevulinic acid in mice bearing mammary carcinoma. Int. J. Cancer 1992, 52, 433–443. [Google Scholar] [CrossRef]
- Gomer, C.J.; Jester, J.V.; Razum, N.J.; Szirth, B.C.; Murphree, A.L. Photodynamic therapy of intraocular tumors: Examination of hematoporphyrin derivative distribution and long-term damage in rabbit ocular tissue. Cancer Res. 1985, 45, 3718–3725. [Google Scholar] [PubMed]
- Karnofsky, D.A. The clinical evaluation of chemotherapeutic agents in cancer. In Evaluation of Chemotherapeutic Agents; MacLeod, C., Ed.; Columbia University Press: New York, NY, USA, 1949; pp. 191–205. [Google Scholar]
- Chren, M.-M. The Skindex Instruments to Measure the Effects of Skin Disease on Quality of Life. Dermatol. Clin. 2012, 30, 231–236. [Google Scholar] [CrossRef]
- Kopp, M.; Schweigkofler, H.; Holzner, B.; Nachbaur, D.; Niederwieser, D.; Fleischhacker, W.W.; Kemmler, G.; Sperner-Unterweger, B. EORTC QLQ-C30 and FACT-BMT for the measurement of quality of life in bone marrow transplant recipients: A comparison. Eur. J. Haematol. 2000, 65, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Luckett, T.; King, M.T.; Butow, P.N.; Oguchi, M.; Rankin, N.; Price, M.A.; Hackl, N.A.; Heading, G. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: Issues, evidence and recommendations. Ann. Oncol. 2011, 22, 2179–2190. [Google Scholar] [CrossRef]
- Stallvik, M.; Nordstrand, B.; Kristensen, Ø.; Bathen, J.; Skogvoll, E.; Spigset, O. Corrected QT interval during treatment with methadone and buprenorphine—Relation to doses and serum concentrations. Drug Alcohol Depend. 2013, 129, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Berg, K.; Moan, J.; Kongshaug, M.; Nesland, J.M. 5-Aminolevulinic Acid-Based Photodynamic Therapy: Principles and Experimental Research. Photochem. Photobiol. 1997, 65, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Čunderlíková, B.; Vasovič, V.; Sieber, F.; Furre, T.; Borgen, E.; Nesland, J.M.; Peng, Q. Hexaminolevuli-nate-mediated photodynamic purging of marrow grafts with murine breast carcinoma. Bone Marrow Transplant. 2010, 46, 1118–1127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shahzidi, S.; Čunderlíková, B.; Więdłocha, A.; Zhen, Y.; Vasovič, V.; Nesland, J.M.; Peng, Q. Simultaneously targeting mito-chondria and endoplasmic reticulum by photodynamic therapy induces apoptosis in human lymphoma cells. Photochem. Photobiol. Sci. 2011, 10, 1773–1782. [Google Scholar] [CrossRef]
- Regula, J.; MacRobert, A.J.; Gorchein, A.; Buonaccorsi, G.A.; Thorpe, S.M.; Spencer, G.M.; Hatfield, A.R.; Bown, S.G. Photo-sensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid in-duced protoporphyrin IX—A pilot study. Gut 1995, 36, 67–75. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yoon, H.-K.; Lee, H.-C.; Park, H.-P.; Park, C.-K.; Dho, Y.-S.; Hwang, J.-W. Preoperative 5-aminolevulinic acid administration for brain tumor surgery is associated with an increase in postoperative liver enzymes: A retrospective cohort study. Acta Neurochir. 2019, 161, 2289–2298. [Google Scholar] [CrossRef]
- Scarisbrick, J.; Taylor, P.; Holtick, U.; Makar, Y.; Douglas, K.; Berlin, G.; Juvonen, E.; Marshall, S.; on behalf of the Photopheresis Expert Group U.K. Consensus statement on the use of extracorporeal photopheresis for treatment of cutaneous T-cell lymphoma and chronic graft-versus-host disease. Br. J. Dermatol. 2008, 158, 659–678. [Google Scholar] [CrossRef]
- Scarisbrick, J. Extracorporeal photopheresis: What is it and when should it be used? Clin. Exp. Dermatol. 2009, 34, 757–760. [Google Scholar] [CrossRef]
- Knobler, R.; Berlin, G.; Calzavara-Pinton, P.; Greinix, H.; Jaksch, P.; Laroche, L.; Ludvigsson, J.; Quaglino, P.; Reinisch, W.; Scarisbrick, J.; et al. Guidelines on the use of extracorporeal photopheresis. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 1–37. [Google Scholar] [CrossRef]
- Shimoni, A.; Shem-Tov, N.; Chetrit, A.; Volchek, Y.; Tallis, E.; Avigdor, A.; Sadetzki, S.; Yerushalmi, R.; Nagler, A. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia 2013, 27, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.L.; Wolfe, J.T.; Fox, F.E.; DeNardo, B.J.; Macey, W.H.; Bromley, P.G.; Lessin, S.R.; Rook, A.H. Treatment of cutaneous T-cell lymphoma with extracorporeal photopheresis monotherapy and in combination with recombinant interferon alfa: A 10-year experience at a single institution. J. Am. Acad. Dermatol. 1996, 35, 946–957. [Google Scholar] [CrossRef]
- Ackroyd, R.; Brown, N.; Vernon, D.; Roberts, D.; Stephenson, T.; Marcus, S.; Stoddard, C.; Reed, M. 5-Aminolevulinic Acid Photosensitization of Dysplastic Barrett’s Esophagus: A Pharmacokinetic Study. Photochem. Photobiol. 1999, 70, 656. [Google Scholar] [CrossRef] [PubMed]

| Time Point | ||||||
|---|---|---|---|---|---|---|
| Investigation | Screening (Baseline) | ECP, Day 1 | ECP, Day 2 | Post-ECP, Day 4 ± 1 | Post-ECP, Day 16 ± 2 | Controls, Every 3 Months |
| Vital signs | x | x | x | x | ||
| ECG | x | x | ||||
| Haematology | x | x * | x | |||
| Clinical chemistry | x | x * | x | |||
| Urine analyses | x | x * | x | |||
| Conceivable Adverse Events | x | x | ||||
| Adverse Events | x | x | x | x | ||
| Organ, performance, and QoL assessments | x | x * | x |
| Type of Adverse Event | Number of Patients | Number of Grade 1 | Number of Grade 2 | Number of Grade 3 | Relation to Study Medication |
|---|---|---|---|---|---|
| Clotting in buffy | 4 | 0 | 4 | 0 | Possibly |
| Cold like symptoms | 3 | 3 | 1 | 0 | Unlikely |
| Dysuria | 1 | 0 | 3 | 0 | Possible |
| Respiratory infection | 2 | 0 | 1 | 1 | Possible |
| Skin worsening | 1 | 0 | 1 | 0 | Possible |
| Migraine | 1 | 0 | 0 | 1 | Unlikely |
| Flue like symptoms | 1 | 1 | 0 | 0 | Unlikely |
| Elevated INR | 1 | 1 | 0 | 0 | Unlikely |
| Prickling around mouth | 1 | 1 | 0 | 0 | Possible |
| UVI | 1 | 0 | 1 | 0 | Possible |
| Malaise | 1 | 1 | 0 | 0 | Unlikely |
| Sore throat, pain toes and hip | 1 | 1 | 0 | 0 | Unlikely |
| Prolonged QTc interval | 1 | 1 | 0 | 0 | Unlikely |
| Type of Adverse Event | Number of Patients | Number of Grade 1 | Number of Grade 2 |
|---|---|---|---|
| Nausea | 4 | 19 | 2 |
| Vomiting | 2 | 1 | 1 |
| Headache | 4 | 10 | 3 |
| Photosensitivity | 0 | 0 | 0 |
| Chills | 2 | 4 | 0 |
| Target | Scoring Tool | Scoring Scale | Baseline (Mean Score, min.- max.) | Last Control (Mean Score, min.- max.) |
|---|---|---|---|---|
| Skin | Modified organ scoring system | 0–3 (0 = no symptom) | 2.6 (1–3) | 1.6 (1–2) |
| Skin | Body surface area % | 0–100% (0 = no area affected) | 15.8 (7–30) | 5.0 (3–7) |
| Pruritus | Visual analogue scale | 0–10 (0 = no symptom) | 3.3 (0–7) | 1.4 (1–2) |
| Mouth | Modified organ scoring system | 0–3 (0 = no symptom) | 1.6 (0–3) | 1.4 (0–2) |
| Eye, right | Schirmer’s test | 1–4 (1 = normal) | 3.0 (1–4) | 4.0 (4–4) |
| Eye, left | Schirmer’s test | 1–4 (1 = normal) | 3.6 (3–4) | 4.0 (4–4) |
| Performance | Modified organ scoring system | 0–3 (0 = no symptom) | 1.2 (1–2) | 0.8 (0–1) |
| Gastrointestinal tract | Modified organ scoring system | 0–3 (0 = no symptom) | 0.4 (0–1) | 0.4 (0–1) |
| Function | Karnovsky’s performance scale | 1–100 (0 = low function) | 74 (70–90) | 82 (70–100) |
| Skindex, emotions | Questionnaire | 0–100 (0 = no symptom) | 22.0 (8–48) | 10.2 (0–30) |
| Skindex, symptoms | Questionnaire | 0–100 (0 = no symptom) | 30.4 (21–39) | 24.2 (17–36) |
| Skindex, function | Questionnaire | 0–100 (0 = no symptom) | 19.6 (2–56) | 13.4 (2–42) |
| Skindex, single item | Questionnaire | 0–100 (0 = no symptom) | 10.0 (0–25) | 10.0 (0–50) |
| EORTC30, functional | Questionnaire | 0–100% (0 = low function) | 76.2 (70–100) | 82.0 (57–100) |
| EORTC30, symptoms | Questionnaire | 0–100% (0 = low level of symptom) | 23.4 (7–40) | 20.0 (3–50) |
| EORTC30, global health status | Questionnaire | 0–100% (0 = low state) | 65.2 (42–75) | 61.4 (16–83) |
| FACT-G, total score | Questionnaire | 0–108 (0 = low state) | 86.8 (63–99) | 88.4 (70–97) |
| FACT-BMT, subscale score | Questionnaire | 0–40 (0 = much concern) | 27.8 (22–35) | 29.6 (18–36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, E.; Foss, O.A.; Quist-Paulsen, P.; Staur, I.; Pettersen, F.; Holien, T.; Juzenas, P.; Peng, Q. Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Patients with Chronic Graft-versus-Host Disease: A First-in-Human Study. Pharmaceutics 2021, 13, 1558. https://doi.org/10.3390/pharmaceutics13101558
Christensen E, Foss OA, Quist-Paulsen P, Staur I, Pettersen F, Holien T, Juzenas P, Peng Q. Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Patients with Chronic Graft-versus-Host Disease: A First-in-Human Study. Pharmaceutics. 2021; 13(10):1558. https://doi.org/10.3390/pharmaceutics13101558
Chicago/Turabian StyleChristensen, Eidi, Olav A. Foss, Petter Quist-Paulsen, Ingrid Staur, Frode Pettersen, Toril Holien, Petras Juzenas, and Qian Peng. 2021. "Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Patients with Chronic Graft-versus-Host Disease: A First-in-Human Study" Pharmaceutics 13, no. 10: 1558. https://doi.org/10.3390/pharmaceutics13101558
APA StyleChristensen, E., Foss, O. A., Quist-Paulsen, P., Staur, I., Pettersen, F., Holien, T., Juzenas, P., & Peng, Q. (2021). Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Patients with Chronic Graft-versus-Host Disease: A First-in-Human Study. Pharmaceutics, 13(10), 1558. https://doi.org/10.3390/pharmaceutics13101558