CPE-DB: An Open Database of Chemical Penetration Enhancers
Abstract
1. Introduction
2. Materials and Methods
2.1. CPE Database Collection
- PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) for articles, books, and other literature sources;
- PubChem (https://pubchem.ncbi.nlm.nih.gov/) for structural information and trivial names;
- CAS (https://www.cas.org/) for CAS ID;
- DrugBank (https://go.drugbank.com) for compound status as a drug; and
- DrugInfo database (https://druginfo.nlm.nih.gov/drugportal/) for substances used as surface-active agents.
2.2. Scaffold Content and Classification
2.3. Molecular Properties and Descriptors
3. Results and Discussion
3.1. CPE-DB Structure and Content
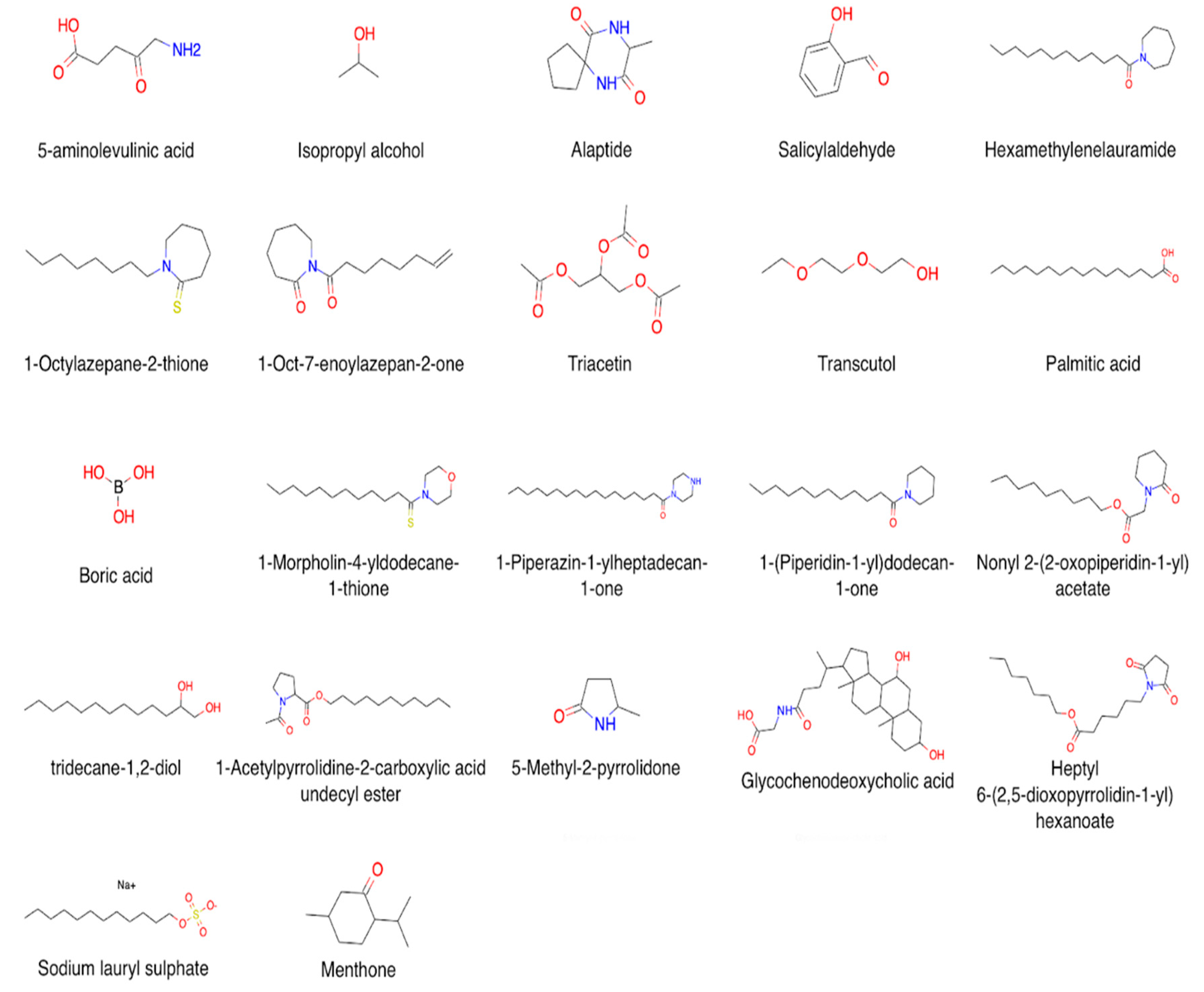
3.2. CPEs: Mechanisms of Action and Chemical Diversity
3.2.1. Alcohols and Polyols
3.2.2. Lactams and Their Analogs
3.2.3. Esters and Ethers
3.2.4. Surfactants
3.2.5. Terpenes, Steroids, and Fatty Acids
3.2.6. Miscellaneous
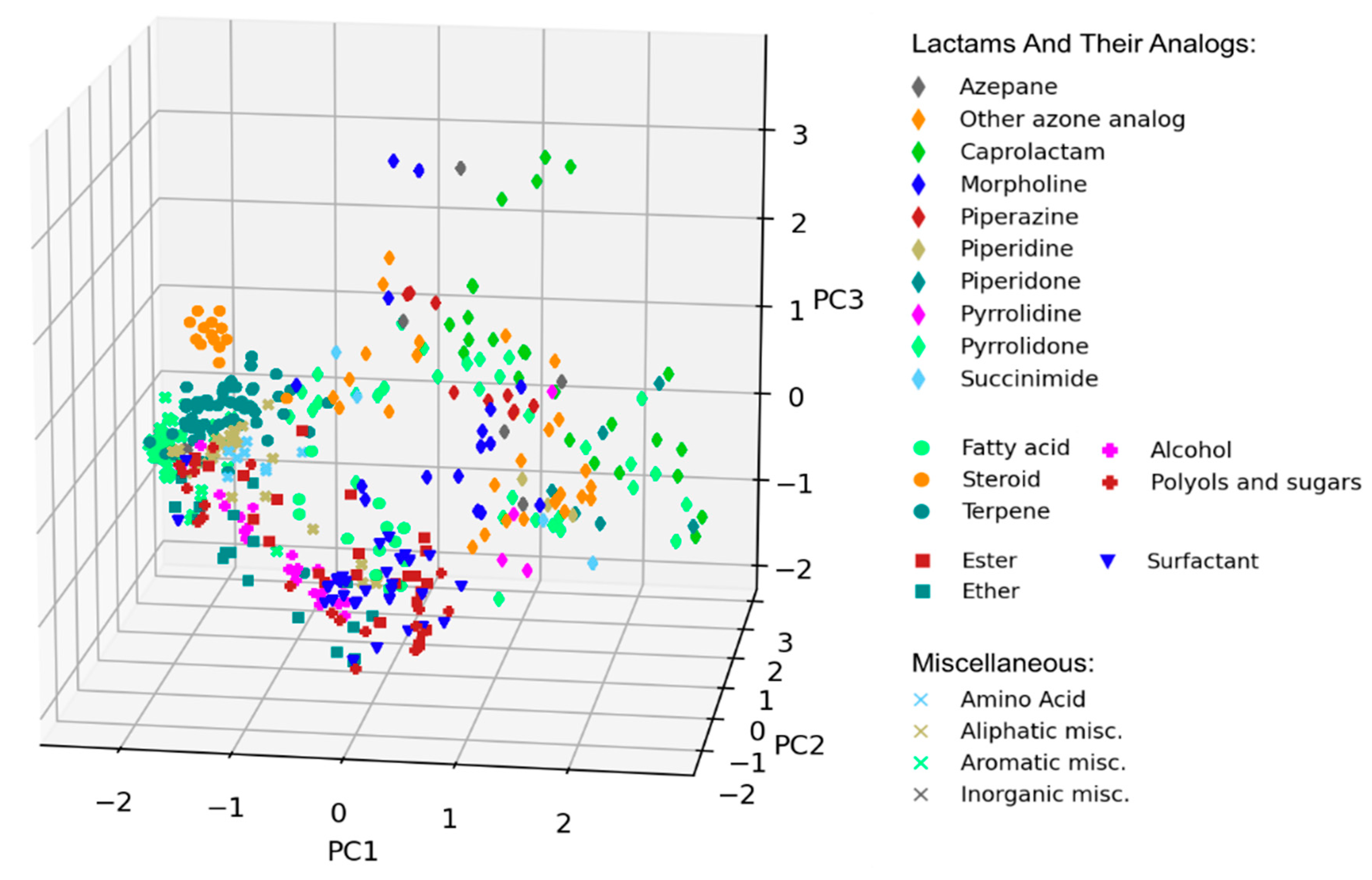
3.3. Scaffold Analysis
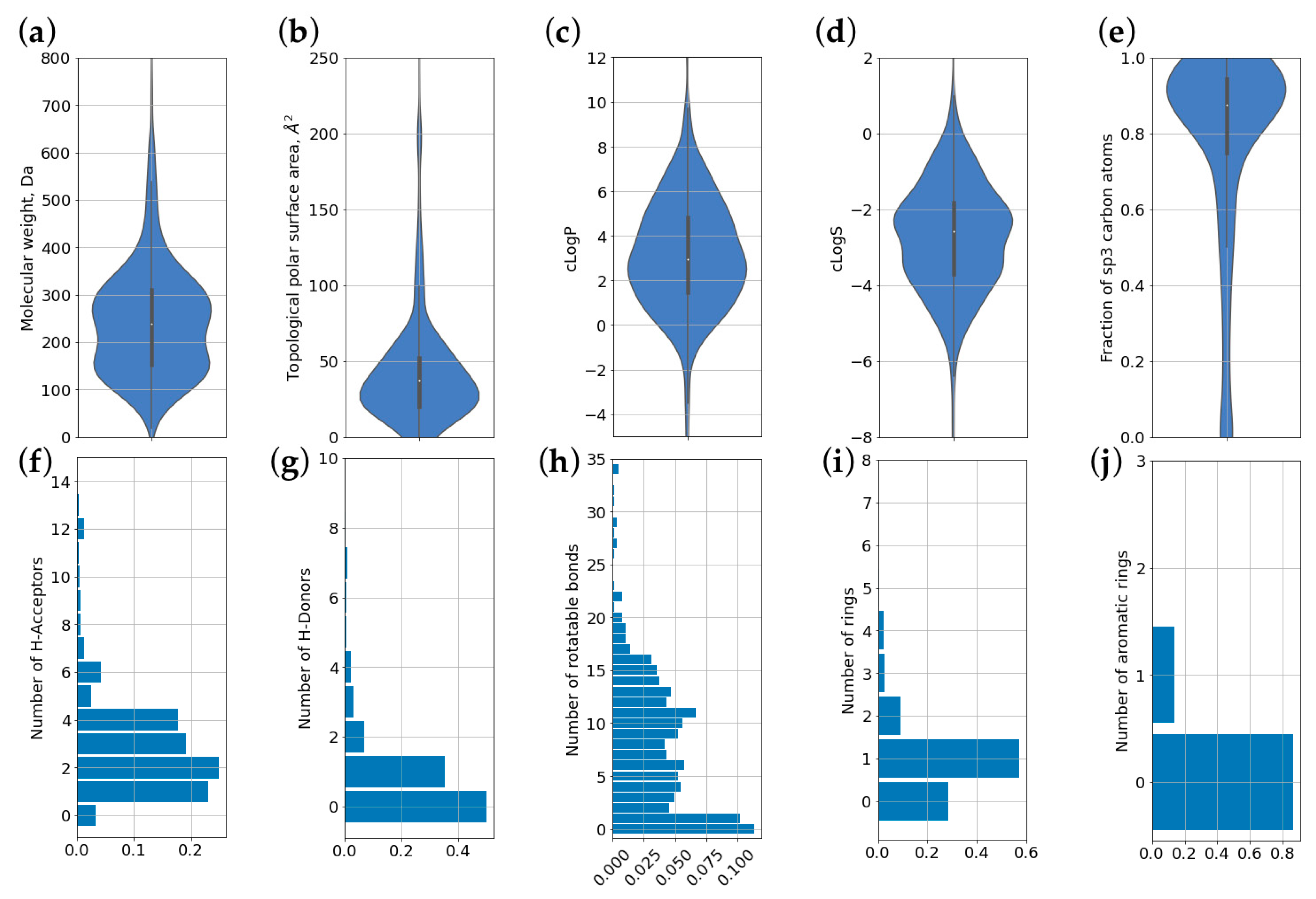
3.4. Molecular Properties and Descriptors
3.5. Using CPE-DB to Predict Skin Permeability of Chemical Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, P.P.; Bastikar, V.A.; Chhajed, S.S. Chemical Structure Databases in Drug Discovery. In Computer Applications in Drug Discovery and Development; Puratchikody, A., Prabu, S.L., Umamaheswari, A., Eds.; Advances in Medical Technologies and Clinical Practice; IGI Global: Hershey, PA, USA, 2019; pp. 47–61. ISBN 9781522573265. [Google Scholar]
- Formulus. Available online: https://formulus.cas.org (accessed on 22 November 2020).
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. A review. Ski. Pharm. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Prashar, M.; Aggarwal, G.; Harikumar, S.L. Synergistic Action of penetration enhancers in transdermal drug delivery. J. Drug Deliv. Ther. 2014, 4, 41–45. [Google Scholar] [CrossRef]
- Kapoor, A.; Mishra, S.K.; Verma, D.K.; Pandey, P. Chemical penetration enhancers for transdermal drug delivery system. J. Drug Deliv. Ther. 2018, 8, 62–66. [Google Scholar] [CrossRef]
- Ghafourian, T.; Nokhodchi, A.; Kaialy, W. Surfactants as Penetration Enhancers for Dermal and Transdermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 207–230. [Google Scholar]
- Amjadi, M.; Mostaghaci, B.; Sitti, M. Recent Advances in Skin Penetration Enhancers for Transdermal Gene and Drug Delivery. Curr. Gene Ther. 2017, 17, 139–146. [Google Scholar] [CrossRef]
- Mitragotri, S. Discovery of Transdermal Penetration Enhancers for Drug Delivery. Biophys. J. 2010, 98, 436a. [Google Scholar] [CrossRef][Green Version]
- Priyanka, K.; Singh, S. A review on skin targeted delivery of bioactives as ultradeformable vesicles: Overcoming the penetration problem. Curr. Drug Targets 2014, 15, 184–198. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Dragicevic, N.; Maibach, H.I. (Eds.) Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Roberts, M.S.; Anderson, R.A.; Swarbrick, J. Permeability of human epidermis to phenolic compounds. J. Pharm. Pharmacol. 1977, 29, 677–683. [Google Scholar] [CrossRef]
- Xu, X.; Mariano, T.M.; Laskin, J.D.; Weisel, C.P. Percutaneous absorption of trihalomethanes, haloacetic acids, and haloketones. Toxicol. Appl. Pharm. 2002, 184, 19–26. [Google Scholar] [CrossRef]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Kevin Li, S. Efficiency of Fatty Acids as Chemical Penetration Enhancers: Mechanisms and Structure Enhancement Relationship. Pharm. Res. 2010, 27, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Blank, I.H.; Scheuplein, R.J.; Macfarlane, D.J. Mechanism of Percutaneous Absorption. J. Investig. Dermatol. 1967, 49, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A. Dermatological and Transdermal Formulations; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780824743239. [Google Scholar]
- Barry, B.W.; Harrison, S.M.; Dugard, P.H. Vapour and liquid diffusion of model penetrants through human skin; correlation with thermodynamic activity. J. Pharm. Pharmacol. 1985, 37, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Bronaugh, R.L.; Congdon, E.R. Percutaneous Absorption of Hair Dyes: Correlation with Partition Coefficients. J. Investig. Dermatol. 1984, 83, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Coufalová, L.; Mrózek, L.; Rárová, L.; Plaček, L.; Opatřilová, R.; Dohnal, J.; Král’ová, K.; Paleta, O.; Král, V.; Drašar, P.; et al. New propanoyloxy derivatives of 5β-cholan-24-oic acid as drug absorption modifiers. Steroids 2013, 78, 435–453. [Google Scholar] [CrossRef]
- Lifeng, K. Skin Permeation Enhancement by Terpenes for Transdermal Drug Delivery. Ph.D. Thesis, National University of Singapore, Singapore, 2006. [Google Scholar]
- Nava-Arzaluz, M.G.; Piñón-Segundo, E.; Ganem-Rondero, A. Sucrose Esters as Transdermal Permeation Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 273–290. [Google Scholar]
- Scheuplein, R.J.; Blank, I.H. Permeability of the skin. Physiol. Rev. 1971, 51, 702–747. [Google Scholar] [CrossRef]
- Yasir, M.; Som, I.; Bhatia, K. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2. [Google Scholar] [CrossRef]
- Williams, A.C. Urea and Derivatives as Penetration Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 301–308. [Google Scholar]
- Bode, J.W. Reactor ChemAxon Ltd., Maramaros koz 2/a, Budapest, 1037 Hungary. www.chemaxon.com. Contact ChemAxon for pricing information. J. Am. Chem. Soc. 2004, 126, 15317. [Google Scholar] [CrossRef]
- Bemis, G.W.; Murcko, M.A. The Properties of Known Drugs. 1. Molecular Frameworks. J. Med. Chem. 1996, 39, 2887–2893. [Google Scholar] [CrossRef]
- Landrum, G. RDKit. Available online: http://www.rdkit.org (accessed on 11 November 2020).
- López-López, E.; Naveja, J.J.; Medina-Franco, J.L. DataWarrior: An evaluation of the open-source drug discovery tool. Expert Opin. Drug Discov. 2019, 14, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.; Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Model. 2010, 50, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Osolodkin, D.I.; Radchenko, E.V.; Orlov, A.A.; Voronkov, A.E.; Palyulin, V.A.; Zefirov, N.S. Progress in visual representations of chemical space. Expert Opin. Drug Discov. 2015, 10, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Rupp, M.; Teetz, W.; Brandmaier, S.; Abdelaziz, A.; Prokopenko, V.V.; Tanchuk, V.Y.; et al. Online chemical modeling environment (OCHEM): Web platform for data storage, model development and publishing of chemical information. J. Comput. Aided Mol. Des. 2011, 25, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Samaras, E.G.; Riviere, J.E.; Ghafourian, T. The effect of formulations and experimental conditions on in vitro human skin permeation-Data from updated EDETOX database. Int. J. Pharm. 2012, 434, 280–291. [Google Scholar] [CrossRef]
- Wilschut, A.; Berge, W.F.T.; Robinson, P.J.; McKone, T.E. Estimating skin permeation. The validation of five mathematical skin permeation models. Chemosphere 1995, 30, 1275–1296. [Google Scholar] [CrossRef]
- Scheler, S.; Fahr, A.; Liu, X. Linear combination methods for prediction and interpretation of drug skin permeation. ADMET DMPK 2014, 2, 199–220. [Google Scholar] [CrossRef]
- Chantasart, D.; Li, S.K. Structure Enhancement Relationship of Chemical Penetration Enhancers in Drug Transport across the Stratum Corneum. Pharmaceutics 2012, 4, 71–92. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Dermatological Formulations: Percutaneous Absorption; Informa Health Care: New York, NY, USA, 1983; ISBN 9780824717292. [Google Scholar]
- Maibach, H. Dermatological formulations: Percutaneous absorption. By Brian W. Barry. Marcel Dekker, 270 Madison Avenue, New York, NY 10016. 1983. 479pp. 16 × 23.5 cm. Price $55.00 (2070 higher outside the US. and Canada). J. Pharm. Sci. 1984, 73, 573. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Rai, B.; Mitragotri, S. Molecular mechanism of the skin permeation enhancing effect of ethanol: A molecular dynamics study. RSC Adv. 2020, 10, 12234–12248. [Google Scholar] [CrossRef]
- Panchagnula, R.; Salve, P.S.; Thomas, N.S.; Jain, A.K.; Ramarao, P. Transdermal delivery of naloxone: Effect of water, propylene glycol, ethanol and their binary combinations on permeation through rat skin. Int. J. Pharm. 2001, 219, 95–105. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kanikkannan, N.; Singh, M. Skin permeation enhancement effect and skin irritation of saturated fatty alcohols. Int. J. Pharm. 2002, 248, 219–228. [Google Scholar] [CrossRef]
- Lee, C.K.; Uchida, T.; Noguchi, E.; Kim, N.-S.; Goto, S. Skin Permeation Enhancement of Tegafur by Ethanol/Panasate 800 or Ethanol/Water Binary Vehicle and Combined Effect of Fatty Acids and Fatty Alcohols. J. Pharm. Sci. 1993, 82, 1155–1159. [Google Scholar] [CrossRef]
- Andega, S.; Kanikkannan, N.; Singh, M. Comparison of the effect of fatty alcohols on the permeation of melatonin between porcine and human skin. J. Control. Release 2001, 77, 17–25. [Google Scholar] [CrossRef]
- Atef, E.; Altuwaijri, N. Using Raman Spectroscopy in Studying the Effect of Propylene Glycol, Oleic Acid, and Their Combination on the Rat Skin. AAPS PharmSciTech 2018, 19, 114–122. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; de Vries, M.A.; Gooris, G.S.; Bras, W.; Brussee, J.; Ponec, M. Thermodynamic and structural aspects of the skin barrier. J. Control. Release 1991, 15, 209–219. [Google Scholar] [CrossRef]
- Moghadam, S.H.; Saliaj, E.; Wettig, S.D.; Dong, C.; Ivanova, M.V.; Huzil, J.T.; Foldvari, M. Effect of chemical permeation enhancers on stratum corneum barrier lipid organizational structure and interferon alpha permeability. Mol. Pharm. 2013, 10, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin I. Influence of neat solvents. Eur. J. Pharm. Sci. 2017, 104, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Szűts, A.; Szabó-Révész, P. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef] [PubMed]
- El-Laithy, H.M. Novel transdermal delivery of Timolol maleate using sugar esters: Preclinical and clinical studies. Eur. J. Pharm. Biopharm. 2009, 72, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Bravo, H.A.; Quintanar-Guerrero, D.; Naik, A.; Kalia, Y.N.; Cornejo-Bravo, J.M.; Ganem-Quintanar, A. Effects of sucrose oleate and sucrose laureate on in vivo human stratum corneum permeability. Pharm. Res. 2003, 20, 1267–1273. [Google Scholar] [CrossRef]
- Cornwell, P.A.; Barry, B.W.; Bouwstra, J.A.; Gooris, G.S. Modes of action of terpene penetration enhancers in human skin; Differential scanning calorimetry, small-angle X-ray diffraction and enhancer uptake studies. Int. J. Pharm. 1996, 127, 9–26. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Urea analogues in propylene glycol as penetration enhancers in human skin. Int. J. Pharm. 1989, 56, 43–50. [Google Scholar] [CrossRef]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Influence of penetration enhancer on drug permeation from volatile formulations. Int. J. Pharm. 2012, 439, 260–268. [Google Scholar] [CrossRef]
- Rajadhyaksha, V.J. Novel N-bis-Azacyclopentan-2-Onyl Alkanes. U.S. Patent US3989815A, 2 December 1976. [Google Scholar]
- Hadgraft, J.; Peck, J.; Williams, D.G.; Pugh, W.J.; Allan, G. Mechanisms of action of skin penetration enhancers/retarders: Azone and analogues. Int. J. Pharm. 1996, 141, 17–25. [Google Scholar] [CrossRef]
- Jampilek, J.; Brychtova, K. Azone analogues: Classification, design, and transdermal penetration principles. Med. Res. Rev. 2012, 32, 907–947. [Google Scholar] [CrossRef]
- Jampílek, J. Azone® and Its Analogues as Penetration Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 69–105. [Google Scholar]
- Kim, C.-K.; Hong, M.-S.; Kim, Y.-B.; Han, S.-K. Effect of penetration enhancers (pyrrolidone derivatives) on multilamellar liposomes of stratum corneum lipid: A study by UV spectroscopy and differential scanning calorimetry. Int. J. Pharm. 1993, 95, 43–50. [Google Scholar] [CrossRef]
- Southwell, D.; Barry, B.W. Penetration enhancers for human skin: Mode of action of 2-pyrrolidone and dimethylformamide on partition and diffusion of model compounds water, n-alcohols, and caffeine. J. Invest. Dermatol. 1983, 80, 507–514. [Google Scholar] [CrossRef]
- Jungbauer, F.H.; Coenraads, P.J.; Kardaun, S.H. Toxic hygroscopic contact reaction to N-methyl-2-pyrrolidone. Contact Dermat. 2001, 45, 303–304. [Google Scholar] [CrossRef]
- Leopold, C.S.; Lippold, B.C. An attempt to clarify the mechanism of the penetration enhancing effects of lipophilic vehicles with differential scanning calorimetry (DSC). J. Pharm. Pharm. 1995, 47, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, I.; Müller-Goymann, C.C. Role of isopropyl myristate, isopropyl alcohol and a combination of both in hydrocortisone permeation across the human stratum corneum. Ski. Pharm. Appl. Ski. Physiol. 2003, 16, 393–404. [Google Scholar] [CrossRef]
- Panchagnula, R.; Ritschel, W.A. Development and evaluation of an intracutaneous depot formulation of corticosteroids using Transcutol as a cosolvent: In-vitro, ex-vivo and in-vivo rat studies. J. Pharm. Pharm. 1991, 43, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F. Applied Surfactants: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9783527604531. [Google Scholar]
- Fox, L.T.; Gerber, M.; Du Plessis, J.; Hamman, J.H. Transdermal Drug Delivery Enhancement by Compounds of Natural Origin. Molecules 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Smith, E.W.; Maibach, H.I. Percutaneous Penetration Enhancers; CRC Press: Boca Raton, FL, USA, 1995; ISBN 9780849326059. [Google Scholar]
- Chen, J.; Jiang, Q.-D.; Chai, Y.-P.; Zhang, H.; Peng, P.; Yang, X.-X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Terpenes and the lipid-protein-partitioning theory of skin penetration enhancement. Pharm. Res. 1991, 8, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, P.A.; Barry, B.W.; Stoddart, C.P.; Bouwstra, J.A. Wide-angle X-ray diffraction of human stratum corneum: Effects of hydration and terpene enhancer treatment. J. Pharm. Pharm. 1994, 46, 938–950. [Google Scholar] [CrossRef]
- Golden, G.M.; McKie, J.E.; Potts, R.O. Role of stratum corneum lipid fluidity in transdermal drug flux. J. Pharm. Sci. 1987, 76, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharm. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Notman, R.; Anwar, J.; Briels, W.J.; Noro, M.G.; den Otter, W.K. Simulations of skin barrier function: Free energies of hydrophobic and hydrophilic transmembrane pores in ceramide bilayers. Biophys. J. 2008, 95, 4763–4771. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Li, S.K. Chemical enhancer solubility in human stratum corneum lipids and enhancer mechanism of action on stratum corneum lipid domain. Int. J. Pharm. 2010, 383, 89–98. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; de Graaff, A.; Gooris, G.S.; Nijsse, J.; Wiechers, J.W.; van Aelst, A.C. Water distribution and related morphology in human stratum corneum at different hydration levels. J. Investig. Dermatol. 2003, 120, 750–758. [Google Scholar] [CrossRef]
- Tsakovska, I.; Pajeva, I.; Al Sharif, M.; Alov, P.; Fioravanzo, E.; Kovarich, S.; Worth, A.P.; Richarz, A.-N.; Yang, C.; Mostrag-Szlichtyng, A.; et al. Quantitative structure-skin permeability relationships. Toxicology 2017, 387, 27–42. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Lian, G. Recent advances in predicting skin permeability of hydrophilic solutes. Adv. Drug Deliv. Rev. 2013, 65, 295–305. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Riviere, J.E.; Brooks, J.D. Predicting skin permeability from complex chemical mixtures. Toxicol. Appl. Pharmacol. 2005, 208, 99–110. [Google Scholar] [CrossRef]
- Xu, G.; Hughes-Oliver, J.M.; Brooks, J.D.; Baynes, R.E. Predicting skin permeability from complex chemical mixtures: Incorporation of an expanded QSAR model. SAR QSAR Environ. Res. 2013, 24, 711–731. [Google Scholar] [CrossRef]
- Riviere, J.E.; Brooks, J.D. Prediction of dermal absorption from complex chemical mixtures: Incorporation of vehicle effects and interactions into a QSPR framework. SAR QSAR Environ. Res. 2007, 18, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Chittenden, J.T.; Riviere, J.E. The Effects of Vehicle Mixtures on Transdermal Absorption: Thermodynamics, Mechanisms, Assessment, and Prediction. In Percutaneous Penetration Enhancers Drug Penetration into/through the Skin; Springer: Berlin/Heidelberg, Germany, 2017; pp. 95–117. [Google Scholar]
- Ghafourian, T.; Samaras, E.G.; Brooks, J.D.; Riviere, J.E. Modelling the effect of mixture components on permeation through skin. Int. J. Pharm. 2010, 398, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Ghafourian, T.; Samaras, E.G.; Brooks, J.D.; Riviere, J.E. Validated models for predicting skin penetration from different vehicles. Eur. J. Pharm. Sci. 2010, 41, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Karadzovska, D.; Brooks, J.D.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting skin permeability from complex vehicles. Adv. Drug Deliv. Rev. 2013, 65, 265–277. [Google Scholar] [CrossRef]



| Group Name | Total Number of Compounds | Classes and Number of Compounds |
|---|---|---|
| Alcohols and polyols | 62 | Alcohol (37), polyol (25) data |
| Lactams and their analogs | 278 | Azepane (21), azone other (34), caprolactam (57), morpholine (44), piperazine (25), piperidine (12), piperidone (19), pyrrolidine (7), pyrrolidone (49), succinimide (10) |
| Esters and ethers | 53 | Ester (31), ether (22) |
| Surfactants | 39 | Surfactant (39) |
| Fatty acids, terpenes, steroids | 112 | Fatty acid (31), terpene (67), steroid (14) |
| Miscellaneous | 105 | Amino acid (8), aliphatic misc. (41), aromatic misc. (54), inorganic misc. (2) |
| Property | Mean | S.D. | Median |
|---|---|---|---|
| Molecular weight, Da | 251.24 | 139.55 | 239.4 |
| TPSA, Å2 | 43.43 | 42.07 | 37.3 |
| logP | 3.15 | 2.69 | 2.95 |
| logS | −2.75 | 1.48 | −2.59 |
| Fraction of carbon atoms in the sp3 hybridization | 78% | 27% | 88% |
| Number of H-bond acceptor atoms (HBA) | 3.02 | 2.78 | 2.0 |
| Number of number of H-bond donor atoms (HBD) | 0.88 | 1.55 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasyuchenko, E.P.; Orekhov, P.S.; Armeev, G.A.; Bozdaganyan, M.E. CPE-DB: An Open Database of Chemical Penetration Enhancers. Pharmaceutics 2021, 13, 66. https://doi.org/10.3390/pharmaceutics13010066
Vasyuchenko EP, Orekhov PS, Armeev GA, Bozdaganyan ME. CPE-DB: An Open Database of Chemical Penetration Enhancers. Pharmaceutics. 2021; 13(1):66. https://doi.org/10.3390/pharmaceutics13010066
Chicago/Turabian StyleVasyuchenko, Ekaterina P., Philipp S. Orekhov, Grigoriy A. Armeev, and Marine E. Bozdaganyan. 2021. "CPE-DB: An Open Database of Chemical Penetration Enhancers" Pharmaceutics 13, no. 1: 66. https://doi.org/10.3390/pharmaceutics13010066
APA StyleVasyuchenko, E. P., Orekhov, P. S., Armeev, G. A., & Bozdaganyan, M. E. (2021). CPE-DB: An Open Database of Chemical Penetration Enhancers. Pharmaceutics, 13(1), 66. https://doi.org/10.3390/pharmaceutics13010066