New Protein-Coated Silver Nanoparticles: Characterization, Antitumor and Amoebicidal Activity, Antiproliferative Selectivity, Genotoxicity, and Biocompatibility Evaluation
Abstract
1. Introduction
2. Materials and Methods
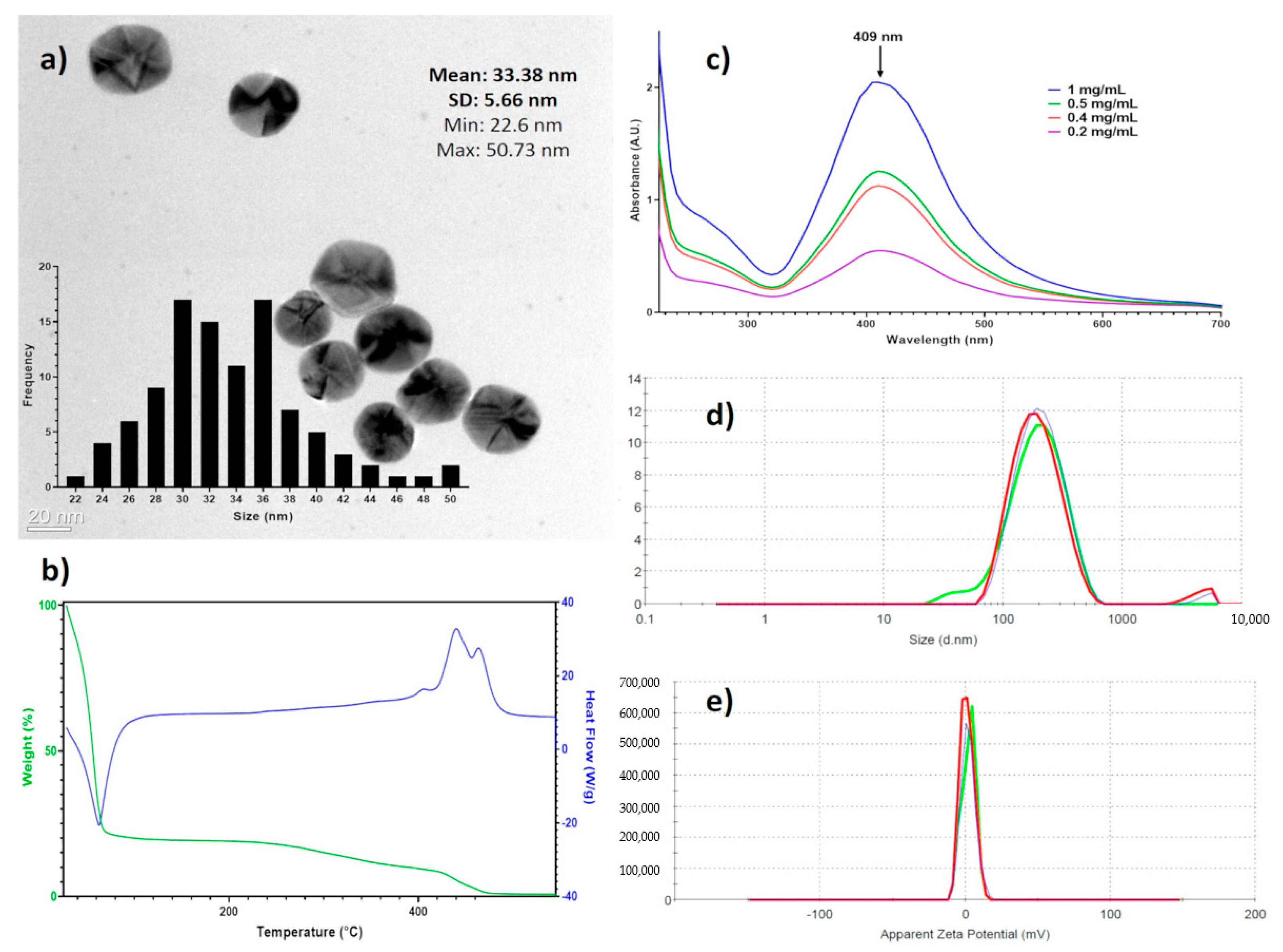
2.1. Silver Nanoparticles (AgNPs) Characterization
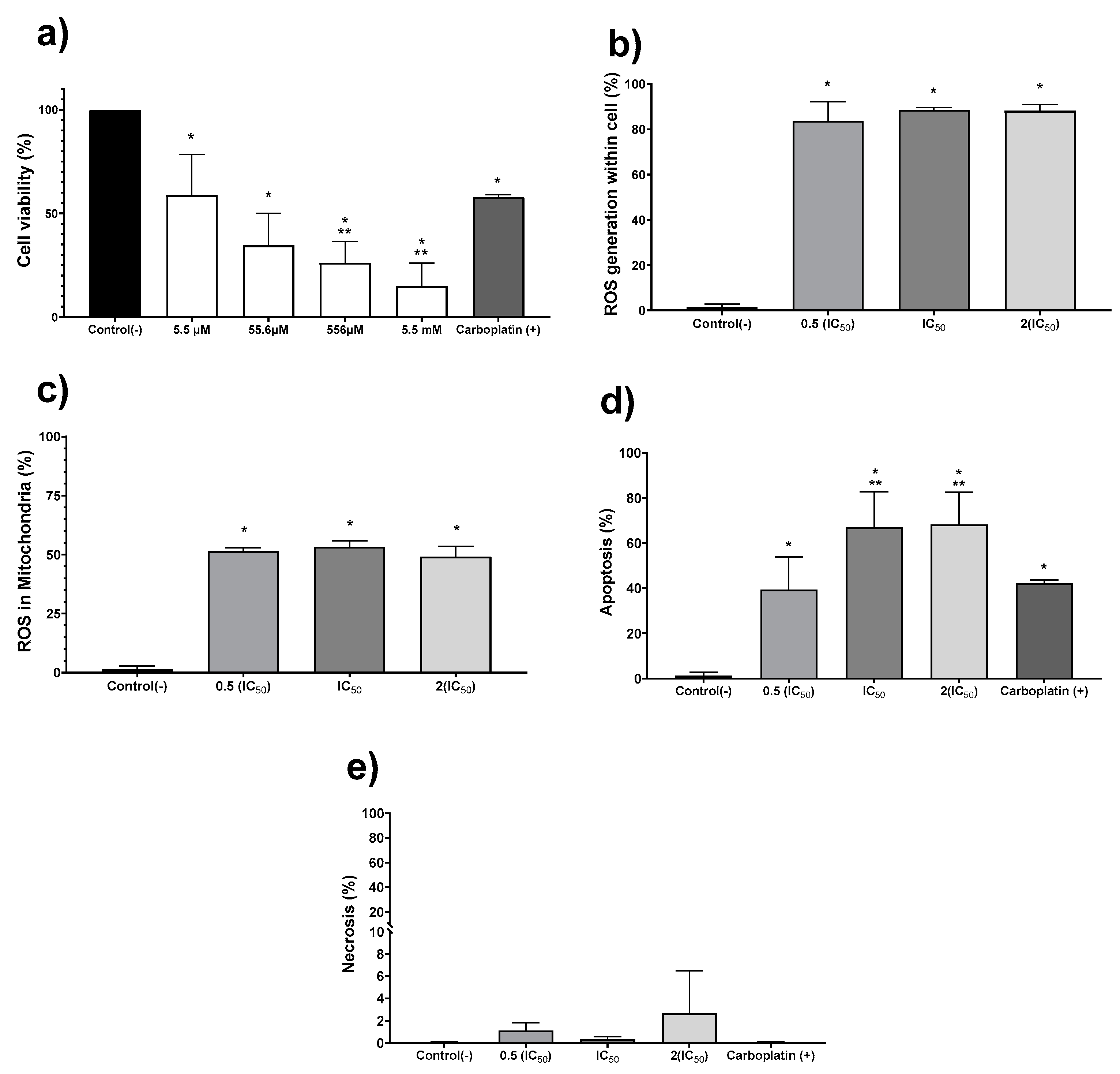
2.2. In Vitro Antitumor Activity
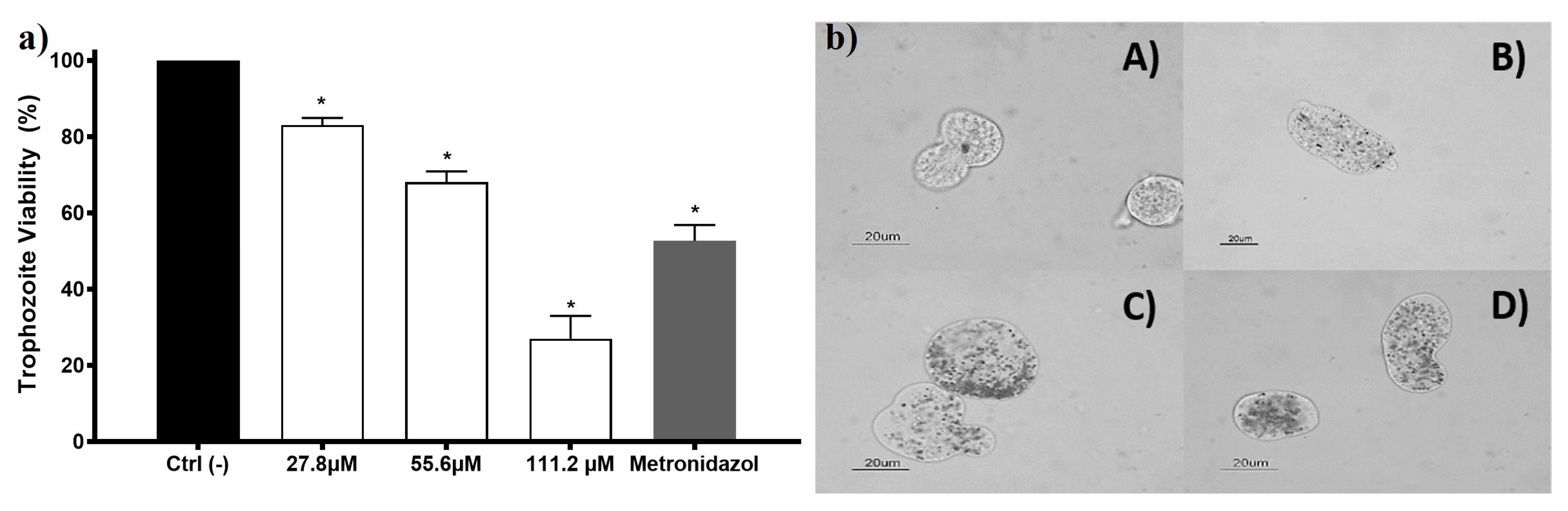
2.3. In Vitro Amoebicidal Activity
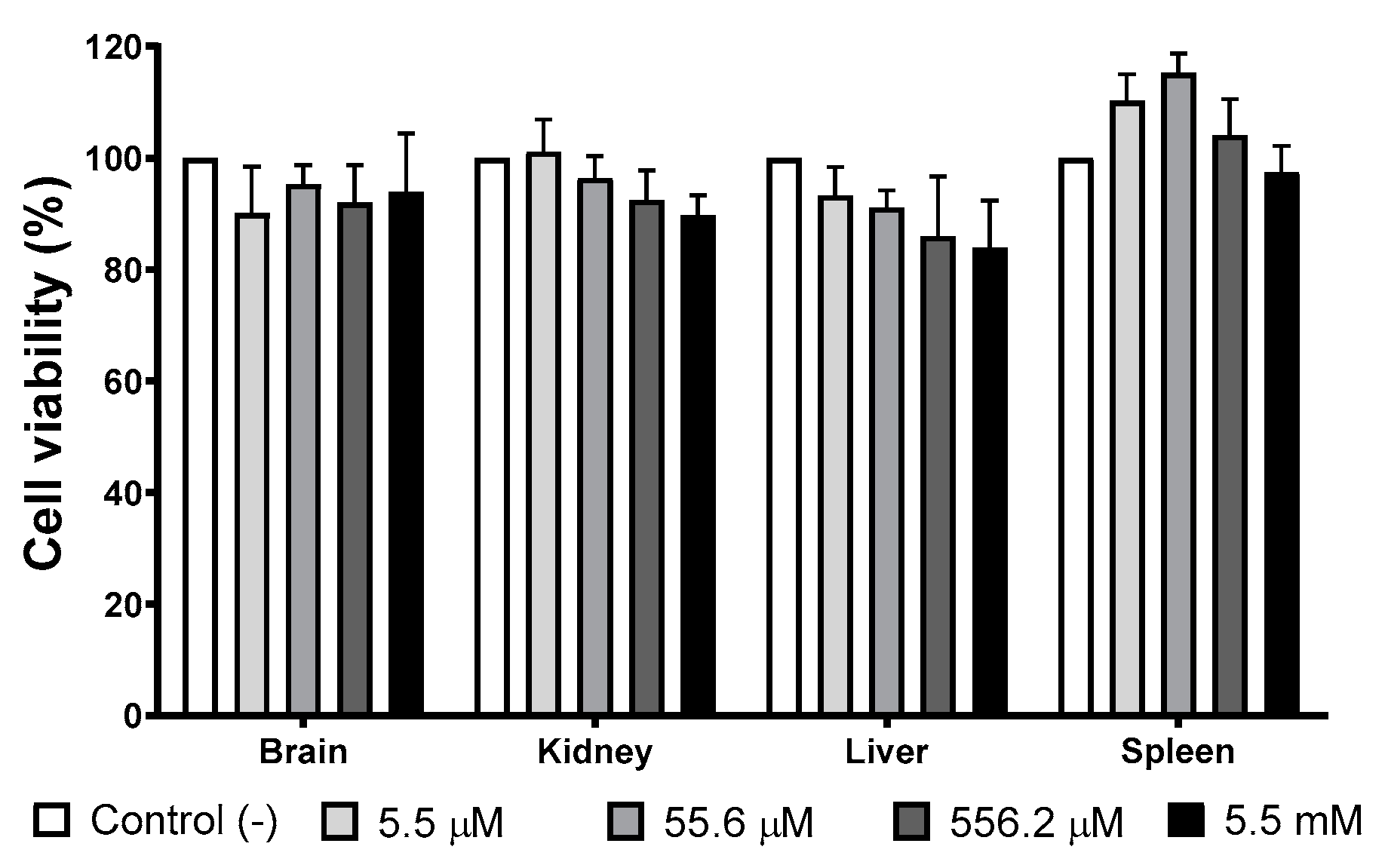
2.4. Toxicity Evaluation
2.4.1. In Vitro Studies
Primary Cell Culture Isolation and Viability
Micronuclei Frequency on Human Peripheral Blood Lymphocytes
2.5. In Vivo Studies
2.5.1. Lethal Dose
2.5.2. Pathology
2.6. Statistics
3. Results
3.1. Characterization of Silver Nanoparticles
3.2. In Vitro Antitumor Activity on HCT-15
3.3. Amoebicidal Activity
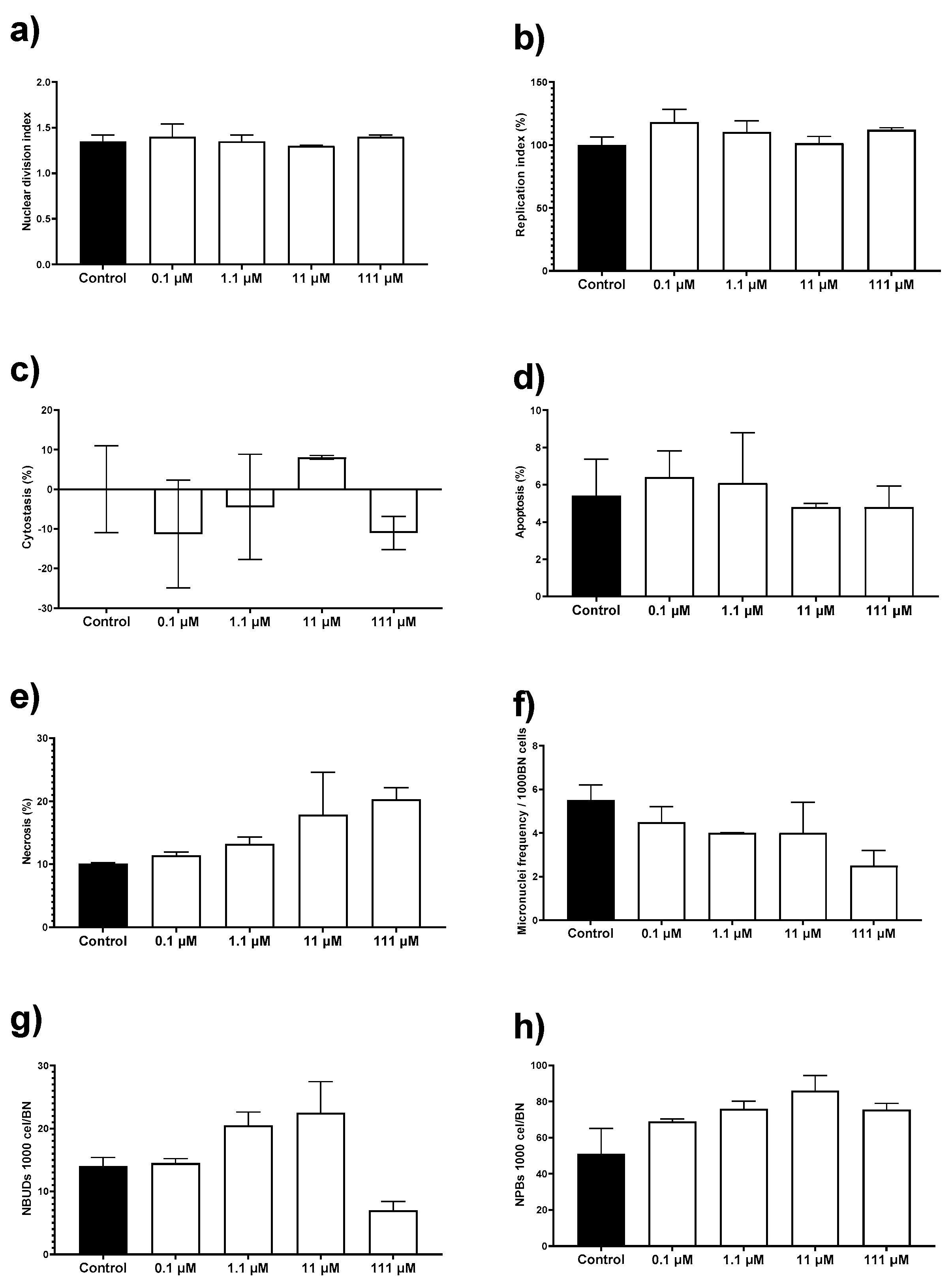
3.4. Cell Viability of Mice Primary Cultures Exposed to AgNP
3.5. Cytokinesis-Block Micronucleus Assay (CBMN)
3.6. In Vivo Studies
3.6.1. Determination of Lethal Dose
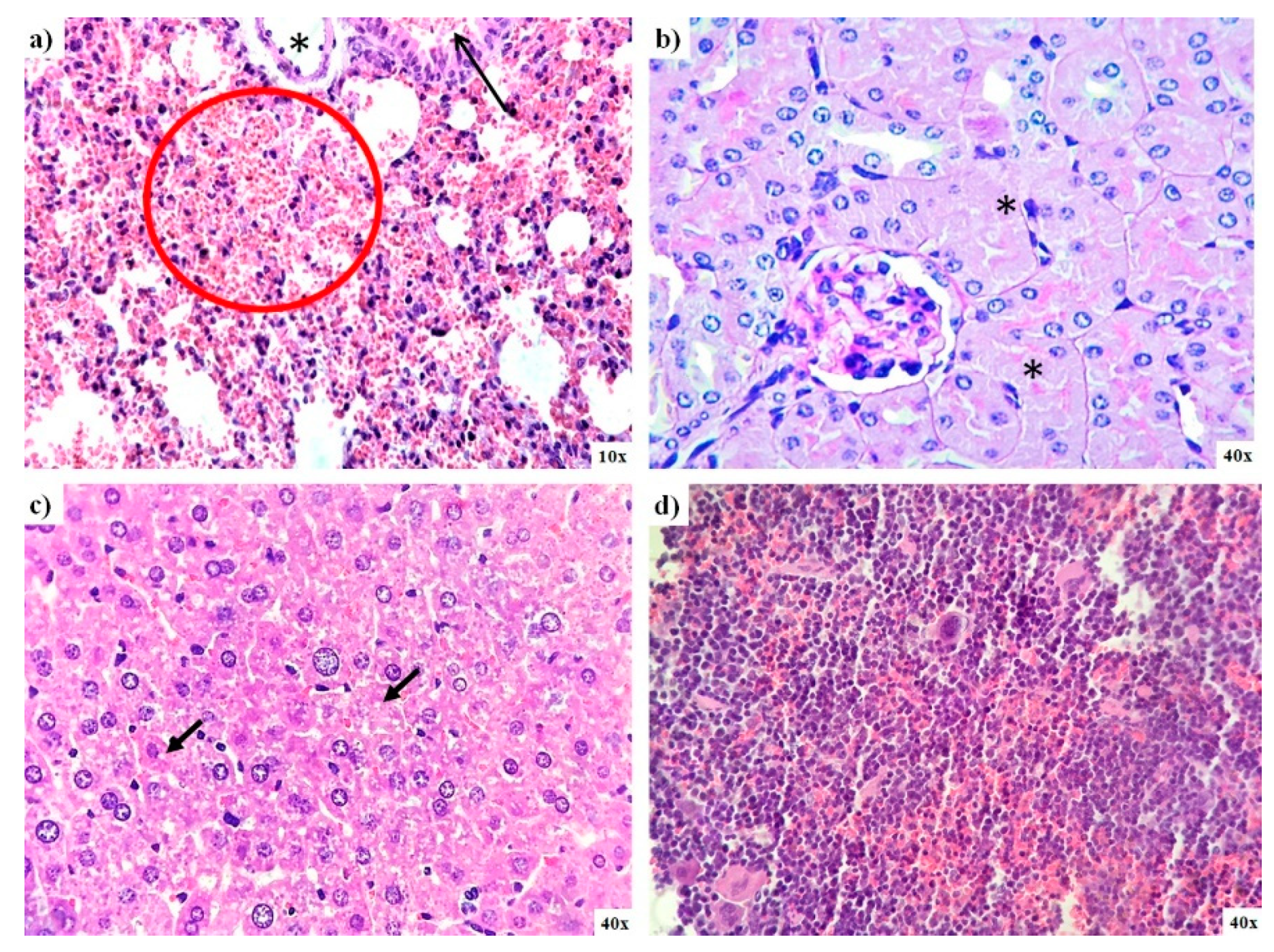
3.6.2. Pathology Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Roseblade, A.; Hansbro, P.M.; Rathbone, M.J.; Dua, K.; Bebawy, M. Nanoparticles in Cancer Treatment: Opportunities and Obstacles. Curr. Drug Targets 2018, 19, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Kailasa, S.K.; Park, T.J. Recent advances of upconversion nanoparticles in theranostics and bioimaging applications. TrAC-Trends Anal. Chem. 2019, 120, 115646. [Google Scholar] [CrossRef]
- Saji, V.S.; Choe, H.C.; Yeung, K.W.K. Nanotechnology in biomedical applications: A review. Int. J. Nano Biomater. 2010, 3, 119–139. [Google Scholar] [CrossRef]
- Ullah Khan, S.; Saleh, T.A.; Wahab, A.; Ullah Khan, M.H.; Khan, D.; Ullah Khan, W.; Rahim, A.; Kamal, S.; Ullah Khan, F.; Fahad, S. Nanosilver: New ageless and versatile biomedical therapeutic scaffold. Int. J. Nanomed. 2018, 13, 733–762. [Google Scholar] [CrossRef]
- Gomes, A.; Sengupta, J.; Datta, P.; Ghosh, S.; Gomes, A. Physiological interactions of nanoparticles in energy metabolism, immune function and their biosafety: A review. J. Nanosci. Nanotechnol. 2016, 16, 92–116. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A. Challenges facing nanotoxicology and nanomedicine due to cellular diversity. Clin. Chim. Acta 2018, 487, 186–196. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Voelcker, N.H.; Seabrook, S.A.; Hor, M.; Kirby, J.K.; Fenech, M.; Davis, T.P.; Ke, P.C. DNA Melting and Genotoxicity Induced by Silver Nanoparticles and Graphene. Chem. Res. Toxicol. 2015, 28, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Milošić, A.; Domijan, A.M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, B.; Arellano-García, M.E.; Radilla-Chávez, P.; Salas-Vargas, D.S.; Toledano-Magaña, Y.; Casillas-Figueroa, F.; Luna Vazquez-Gomez, R.; Pestryakov, A.; García-Ramos, J.C.; Bogdanchikova, N. Cytokinesis-Block Micronucleus Assay Using Human Lymphocytes as a Sensitive Tool for Cytotoxicity/Genotoxicity Evaluation of AgNPs. ACS Omega 2020, 5, 12005–12015. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Figueroa, F.; Arellano-García, M.E.; Leyva-Aguilera, C.; Ruíz-Ruíz, B.; Vázquez-Gómez, R.L.; Radilla-Chávez, P.; Chávez-Santoscoy, R.A.; Pestryakov, A.; Toledano-Magaña, Y.; García-Ramos, J.C.; et al. ArgovitTM silver nanoparticles effects on allium cepa: Plant growth promotion without cyto genotoxic damage. Nanomaterials 2020, 10, 1386. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Scapozza, L.; Altaba, A.R.I. Drug repurposing in oncology: Compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim. Biophys. Acta-Rev. Cancer 2019, 1871, 434–454. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs according to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
- Toledano-Magaña, Y.; García-Ramos, J.C.; Torres-Gutiérrez, C.; Vázquez-Gasser, C.; Esquivel-Sánchez, J.M.; Flores-Alamo, M.; Ortiz-Frade, L.; Galindo-Murillo, R.; Nequiz, M.; Gudiño-Zayas, M.; et al. Water-Soluble Ruthenium (II) Chiral Heteroleptic Complexes with Amoebicidal in Vitro and in Vivo Activity. J. Med. Chem. 2017, 60, 899–912. [Google Scholar] [CrossRef]
- INEGI. Comunicado De Prensa Núm. 480/20. (29/10/2020). Características De Las Defunciones Registradas. 2020. Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2020/EstSociodemo/DefuncionesRegistradas2019.pdf (accessed on 14 November 2020).
- Vazquez-Muñoz, R.; Bogdanchikova, N.; Huerta-Saquero, A. Beyond the Nanomaterials Approach: Influence of Culture Conditions on the Stability and Antimicrobial Activity of Silver Nanoparticles. ACS Omega 2020, 5, 28441–28451. [Google Scholar] [CrossRef]
- Uraskulova, B.B.; Gyusan, A.O. The clinical and bacteriological study of the effectiveness of the application of silver nanoparticle for the treatment of tuberculosis. Vestn. Otorinolaringol. 2017, 82, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Romo-Quiñonez, C.R.; Álvarez-Sánchez, A.R.; Álvarez-Ruiz, P.; Chávez-Sánchez, M.C.; Bogdanchikova, N.; Pestryakov, A.; Mejia-Ruiz, C.H. Evaluation of a new Argovit as an antiviral agent included in feed to protect the shrimp Litopenaeus vannamei against white spot syndrome virus infection. PeerJ 2020, 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Glotov, A.G.; Glotova, T.I.; Sergeev, A.A.; Belkina, T.V.; Sergeev, A.N. [Antiviral activity of different drugs in vitro against viruses of bovine infectious rhinotracheitis and bovine diarrhea]. Vopr. Virusol. 2004, 49, 43–46. [Google Scholar] [PubMed]
- Glotov, A.G.; Glotova, T.I.; Sergeev, A.A.; Sergeev, A.N. Study of antiviral activity of different drugs against bovine herpes and pestivirus. Antibiot. I Khimioterapiia = Antibiot. Chemoterapy [Sic] 2004, 49, 6–9. [Google Scholar]
- Juarez-Moreno, K.; Gonzalez, E.; Girón-Vazquez, N.; Chávez-Santoscoy, R.; Mota-Morales, J.; Perez-Mozqueda, L.; Garcia-Garcia, M.; Pestryakov, A.; Bogdanchikova, N. Comparison of cytotoxicity and genotoxicity effects of silver nanoparticles on human cervix and breast cancer cell lines. Hum. Exp. Toxicol. 2017, 36, 931–948. [Google Scholar] [CrossRef]
- Valenzuela-Salas, L.M.; Girón-Vázquez, N.G.; García-Ramos, J.C.; Torres-Bugarín, O.; Gómez, C.; Pestryakov, A.; Villarreal-Gómez, L.J.; Toledano-Magaña, Y.; Bogdanchikova, N. Antiproliferative and Antitumour Effect of Nongenotoxic Silver Nanoparticles on Melanoma Models. Oxid. Med. Cell. Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Fuentes-Valencia, M.A.; Fajer-Ávila, E.J.; Chávez-Sánchez, M.C.; Martínez-Palacios, C.A.; Martínez-Chávez, C.C.; Junqueira-Machado, G.; Lara, H.H.; Raggi, L.; Gómez-Gil, B.; Pestryakov, A.A.; et al. Silver nanoparticles are lethal to the ciliate model Tetrahymena and safe to the pike silverside Chirostoma estor. Exp. Parasitol. 2020, 209, 107825. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Guerra, C.; Cáceres-Martínez, J.; Vásquez-Yeomans, R.; Pestryakov, A.; Bogdanchikova, N. Lethal effects of silver nanoparticles on Perkinsus marinus, a protozoan oyster parasite. J. Invertebr. Pathol. 2020, 169, 107304. [Google Scholar] [CrossRef]
- Pimentel-Acosta, C.A.; Morales-Serna, F.N.; Chávez-Sánchez, M.C.; Lara, H.H.; Pestryakov, A.; Bogdanchikova, N.; Fajer-Ávila, E.J. Efficacy of silver nanoparticles against the adults and eggs of monogenean parasites of fish. Parasitol. Res. 2019, 118, 1741–1749. [Google Scholar] [CrossRef]
- Ochoa-Meza, A.R.; Álvarez-Sánchez, A.R.; Romo-Quiñonez, C.R.; Barraza, A.; Magallón-Barajas, F.J.; Chávez-Sánchez, A.; García-Ramos, J.C.; Toledano-Magaña, Y.; Bogdanchikova, N.; Pestryakov, A.; et al. Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol. 2019, 84, 1083–1089. [Google Scholar] [CrossRef]
- Castro-Gamboa, S.; Garcia-Garcia, M.R.; Piñon-Zarate, G.; Rojas-Lemus, M.; Jarquin-Yañez, K.; Angel Herrera-Enriquez, M.; Fortoul, T.I.; Toledano-Magaña, Y.; Garcia-Iglesias, T.; Pestryakov, A.; et al. Toxicity of silver nanoparticles in mouse bone marrow-derived dendritic cells: Implications for phenotype. J. Immunotoxicol. 2019, 1–9. [Google Scholar] [CrossRef]
- Kitada, N.; Takara, K.; Minegaki, T.; Itoh, C.; Tsujimoto, M.; Sakaeda, T.; Yokoyama, T. Factors affecting sensitivity to antitumor platinum derivatives of human colorectal tumor cell lines. Cancer Chemother. Pharmacol. 2008, 62, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Test No. 420: Acute Oral Toxicity-Fixed Dose Procedure; OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2002; Section 4; ISBN 9789264070943.
- Toledano-Magaña, Y.; Meléndrez-Luévano, R.; Navarro-Olivarria, M.; García-Ramos, J.C.; Flores-Alamo, M.; Ortiz-Frade, L.; Ruiz-Azuara, L.; Cabrera-Vivas, B.M. Synthesis, characterization and evaluation of the substituent effect on the amoebicide activity of new hydrazone derivatives. Medchemcomm 2014, 5, 989. [Google Scholar] [CrossRef]
- Hernández-Ayala, L.F.; Toledano-Magaña, Y.; Ortiz-Frade, L.; Flores-Alamo, M.; Galindo-Murillo, R.; Reina, M.; García-Ramos, J.C.; Ruiz-Azuara, L. Heteroleptic NiII complexes: Synthesis, structural characterization, computational studies and amoebicidal activity evaluation. J. Inorg. Biochem. 2020, 206, 11043. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Brunelli, A.; Zhu, C.; Hristozov, D.; Liu, Y.; Semenzin, E.; Wang, W.; Tao, W.; Liang, J.; Marcomini, A.; et al. Demonstrating approaches to chemically modify the surface of Ag nanoparticles in order to influence their cytotoxicity and biodistribution after single dose acute intravenous administration. Nanotoxicology 2016, 10, 129–139. [Google Scholar] [CrossRef]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-Specific and size-Dependent effects. Part. Fibre Toxicol. 2016, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Globally Harmonized System of Classification and Labelling of Chemicals (GHS); Globally Harmonized System of Classification and Labelling of Chemicals (GHS); United Nations: New York, NY, USA; Geneva, Switzerland, 2019; ISBN 9789210040839.
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver nanomaterials for wound dressing applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and nanosafety: Safety-By-Design and testing at a glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, T.; Cunningham, C.K.; Chen, T.; Jones, M.Y.; Abbas, M.; Li, Y.; Mei, N.; Guo, X.; Moore, M.M.; et al. Size- and coating-Dependent cytotoxicity and genotoxicity of silver nanoparticles evaluated using in vitro standard assays. Nanotoxicology 2016, 10, 1373–1384. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Chan, C.; Bharali, D.J.; Mousa, S.A.; Vásquez, E.; Reliene, R. Particle coatings but not silver ions mediate genotoxicity of ingested silver nanoparticles in a mouse model. NanoImpact 2017, 5, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, G.; Fenech, M.; Pompa, P.P.; Voelcker, N.H. Lab-On-A-Chip-Based high-Throughput screening of the genotoxicity of engineered nanomaterials. Small 2014, 10, 2721–2734. [Google Scholar] [CrossRef]
- Ahlberg, S.; Antonopulos, A.; Diendorf, J.; Dringen, R.; Epple, M.; Flöck, R.; Goedecke, W.; Graf, C.; Haberl, N.; Helmlinger, J.; et al. PVP-Coated, negatively charged silver nanoparticles: A multi-Center study of their physicochemical characteristics, cell culture and in vivo experiments. Beilstein J. Nanotechnol. 2014, 5, 1944–1965. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Anh, D.; Jürgen, H.; Autrup, H.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-Coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Jiang, X.; Micləuş, T.; Chen, C.; Autrup, H.; Beer, C. Silver nanoparticles-Wolves in sheep’s clothing? Toxicol. Res. (Camb) 2015, 4, 563–575. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, D.Y. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef]
- Mao, B.H.; Tsai, J.C.; Chen, C.W.; Yan, S.J.; Wang, Y.J. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology 2016, 10, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Joardar, N.; Guevara-Flores, A.; Martínez-González, J.D.J.; Sinha Babu, S.P. Thiol antioxidant thioredoxin reductase: A prospective biochemical crossroads between anticancer and antiparasitic treatments of the modern era. Int. J. Biol. Macromol. 2020, 165, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Kirtonia, A.; Gala, K.; Fernandes, S.G.; Pandya, G.; Pandey, A.K.; Sethi, G.; Khattar, E.; Garg, M. Repurposing of drugs: An attractive pharmacological strategy for cancer therapeutics. Semin. Cancer Biol. 2020, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Borrego, B.; Lorenzo, G.; Mota-Morales, J.D.; Almanza-Reyes, H.; Mateos, F.; López-Gil, E.; de la Losa, N.; Burmistrov, V.A.; Pestryakov, A.N.; Brun, A.; et al. Potential application of silver nanoparticles to control the infectivity of Rift Valley fever virus in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1185–1192. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Vázquez-Muñoz, R.; Huerta-Saquero, A.; Peña-Jasso, A.; Aguilar-Uzcanga, G.; Picos-Díaz, P.L.; Pestryakov, A.; Burmistrov, V.A.; Martynyuk, O.; Luna-Vázquez-Gómez, R.; et al. Silver nanoparticles composition for treatment of distemper in dogs. Int. J. Nanotechnol. 2016, 13, 227–237. [Google Scholar] [CrossRef]
- Guerra, J.D.; Sandoval, G.; Avalos-Borja, M.; Pestryakov, A.; Garibo, D.; Susarrey-Arce, A.; Bogdanchikova, N. Selective antifungal activity of silver nanoparticles: A comparative study between Candida tropicalis and Saccharomyces boulardii. Colloid Interface Sci. Commun. 2020, 37, 100280. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green Biosynthesized Silver Nanoparticles With Aqueous Extracts of Ginkgo Biloba Induce Apoptosis via Mitochondrial Pathway in Cervical Cancer Cells. Front. Oncol. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Cid, M.G.; Mudry, M.; Larripa, I. Chromosome damage induced by carboplatin (CBDCA). Toxicol. Lett. 1995, 76, 97–103. [Google Scholar] [CrossRef]
- Calvert, A.H.; Harland, S.J.; Newell, D.R.; Siddik, Z.H.; Jones, A.C.; Mcelwain, T.J.; Raju, S.; Wiltshaw, E.; Smith, I.E.; Baker, J.M.; et al. Early Clinical Studies with cis-Diammine-1,1-Cyclobutane Dicarboxylate Platinum II. Cancer Chemother. Pharmacol. 1982, 9, 140–147. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Fu, Z.; Zhu, B.; Wang, J.; Guan, S.; Hua, Z. Curcumin activates DNA repair pathway in bone marrow to improve carboplatin-Induced myelosuppression. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Malaikozhundan, B.; Saravanakumar, K.; Durán-Lara, E.F.; Wang, M.H.; Vaseeharan, B. Garlic clove extract assisted silver nanoparticle–Antibacterial, antibiofilm, antihelminthic, anti-inflammatory, anticancer and ecotoxicity assessment. J. Photochem. Photobiol. B Biol. 2019, 198, 111558. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Rademan, S.; Matsabisa, M.G. Effects of Silver Nanoparticle from Dicoma anomala Sond. Root Extract on MCF-7 Cancer Cell Line and NF54 Parasite Strain: An In Vitro Study. Biol. Trace Elem. Res. 2020, 195, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Joksic, G.; Stasic, J.; Filipovic, J.; Sobot, A.V.; Trtica, M.; Joksić, G.; Stašić, J.; Filipović, J.; Šobot, A.V.; Trtica, M. Size of silver nanoparticles determines proliferation ability of human circulating lymphocytes in vitro. Toxicol. Lett. 2016, 247, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Shaniba, V.S.; Aziz, A.A.; Jayasree, P.R.; Kumar, P.R.M. Manilkara zapota (L.) P. Royen Leaf Extract Derived Silver Nanoparticles Induce Apoptosis in Human Colorectal Carcinoma Cells Without Affecting Human Lymphocytes or Erythrocytes. Biol. Trace Elem. Res. 2019, 192, 160–174. [Google Scholar] [CrossRef]
- Chakraborty, B.; Pal, R.; Ali, M.; Singh, L.M.; Rahman, D.S.; Ghosh, S.K.; Sengupta, M. Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell. Mol. Immunol. 2016, 13, 191–205. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Everds, N.E. Evaluation of Clinical Pathology Data:Correlating Changes with Other Study Data. Toxicol. Pathol. 2015, 43, 90–97. [Google Scholar] [CrossRef]
- Kim, J.S.; Song, K.S.; Sung, J.H.; Ryu, H.R.; Choi, B.G.; Cho, H.S.; Lee, J.K.; Yu, I.J. Genotoxicity, acute oral and dermal toxicity, eye and dermal irritation and corrosion and skin sensitisation evaluation of silver nanoparticles. Nanotoxicology 2013, 7, 953–960. [Google Scholar] [CrossRef]
- Maneewattanapinyo, P.; Banlunara, W.; Thammacharoen, C.; Ekgasit, S.; Kaewamatawong, T. An evaluation of acute toxicity of colloidal silver nanoparticles. J. Vet. Med. Sci. 2011, 73, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Melnik, E.A.; Buzulukov, Y.P.; Demin, V.F.; Demin, V.A.; Gmoshinski, I.V.; Tyshko, N.V.; Tutelyan, V.A. Transfer of silver nanoparticles through the placenta and breast milk during in vivo experiments on rats. Acta Nat. 2013, 5, 107–115. [Google Scholar] [CrossRef]
| Values from Limit Test | Reference Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | Units | Mean | SD a | N b | Mean | Low | High | N b |
| ALP | U/L | 305 | 229 | 5 | 187 | 108 | 367 | 138 |
| ALT | U/L | 127 | 193 | 5 | 63 | 40 | 170 | 121 |
| AST | U/L | 552 | 630 | 5 | 154 | 67 | 381 | 130 |
| Glucose | mg/dL | 136 | 18 | 5 | 193 | 85 | 281 | 132 |
| Tissue | Pathological Finding | Incidence |
|---|---|---|
| Kidney | Tubular necrosis data | 6/6 |
| Liver | Micro vesicular steatosis and capillary congestion | 6/6 |
| Lung | Small bleeding patches | 6/6 |
| Spleen | Capillary congestion and hematopoiesis | 6/6 and 4/6, respectively |
| Brain | Signs of ischemia | 4/6 |
| Intestine | Capillary congestion | 2/6 |
| Heart | Near normal | 6/6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Salas, L.M.; Blanco-Salazar, A.; Perrusquía-Hernández, J.D.; Nequiz-Avendaño, M.; Mier-Maldonado, P.A.; Ruiz-Ruiz, B.; Campos-Gallegos, V.; Arellano-García, M.E.; García-Ramos, J.C.; Pestryakov, A.; et al. New Protein-Coated Silver Nanoparticles: Characterization, Antitumor and Amoebicidal Activity, Antiproliferative Selectivity, Genotoxicity, and Biocompatibility Evaluation. Pharmaceutics 2021, 13, 65. https://doi.org/10.3390/pharmaceutics13010065
Valenzuela-Salas LM, Blanco-Salazar A, Perrusquía-Hernández JD, Nequiz-Avendaño M, Mier-Maldonado PA, Ruiz-Ruiz B, Campos-Gallegos V, Arellano-García ME, García-Ramos JC, Pestryakov A, et al. New Protein-Coated Silver Nanoparticles: Characterization, Antitumor and Amoebicidal Activity, Antiproliferative Selectivity, Genotoxicity, and Biocompatibility Evaluation. Pharmaceutics. 2021; 13(1):65. https://doi.org/10.3390/pharmaceutics13010065
Chicago/Turabian StyleValenzuela-Salas, Lucía Margarita, Alberto Blanco-Salazar, Jesús David Perrusquía-Hernández, Mario Nequiz-Avendaño, Paris A. Mier-Maldonado, Balam Ruiz-Ruiz, Verónica Campos-Gallegos, María Evarista Arellano-García, Juan Carlos García-Ramos, Alexey Pestryakov, and et al. 2021. "New Protein-Coated Silver Nanoparticles: Characterization, Antitumor and Amoebicidal Activity, Antiproliferative Selectivity, Genotoxicity, and Biocompatibility Evaluation" Pharmaceutics 13, no. 1: 65. https://doi.org/10.3390/pharmaceutics13010065
APA StyleValenzuela-Salas, L. M., Blanco-Salazar, A., Perrusquía-Hernández, J. D., Nequiz-Avendaño, M., Mier-Maldonado, P. A., Ruiz-Ruiz, B., Campos-Gallegos, V., Arellano-García, M. E., García-Ramos, J. C., Pestryakov, A., Villarreal-Gómez, L. J., Toledano-Magaña, Y., & Bogdanchikova, N. (2021). New Protein-Coated Silver Nanoparticles: Characterization, Antitumor and Amoebicidal Activity, Antiproliferative Selectivity, Genotoxicity, and Biocompatibility Evaluation. Pharmaceutics, 13(1), 65. https://doi.org/10.3390/pharmaceutics13010065









