Development and In Vitro Evaluation of 5-Fluorouracil-Eluting Stents for the Treatment of Colorectal Cancer and Cancer-Related Obstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Fabrication of 5FU-Loaded Drug-Eluting GI Stents and Substrates
2.3. Characterisation
2.3.1. Photoacoustic Fourier-Transform Infrared (PA-FTIR) Spectroscopy
2.3.2. X-Ray Diffraction (XRD)
2.3.3. X-Ray Photoelectron Spectroscopy (XPS)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Thermal Analysis
2.3.6. HPLC Determination of 5FU
2.3.7. In Vitro Drug Release
2.4. In Vitro Anticancer Activity of 5FU-Loaded GI Stents
2.4.1. Cell Culture and Maintenance
2.4.2. Cytotoxicity Study
2.4.3. Cell Cycle Analysis and Detection of Apoptosis by Flow Cytometry
3. Results and Discussion
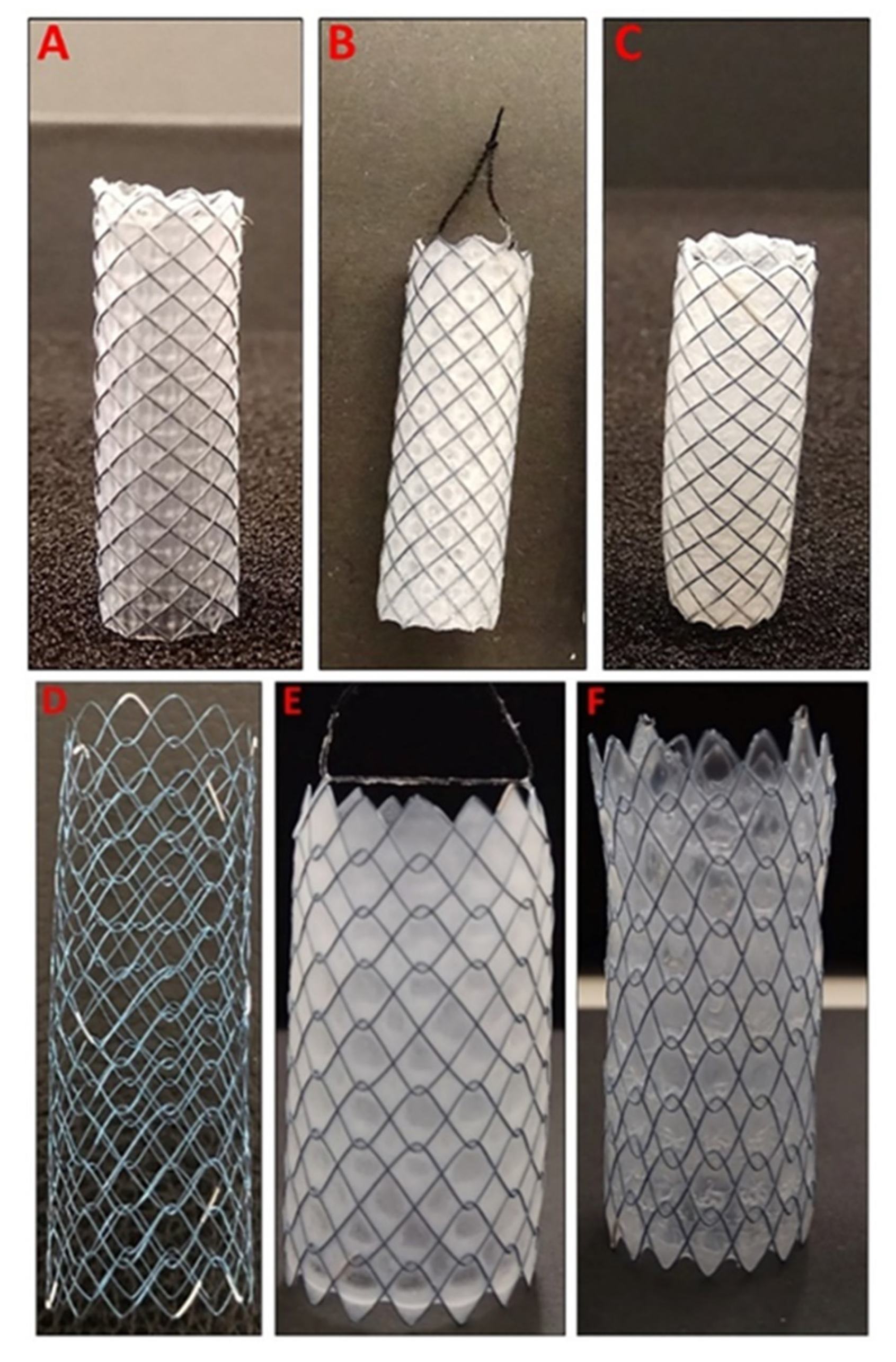
3.1. Preparation of Dip-Coated 5FU-Loaded GI Stents
3.2. Characterisation
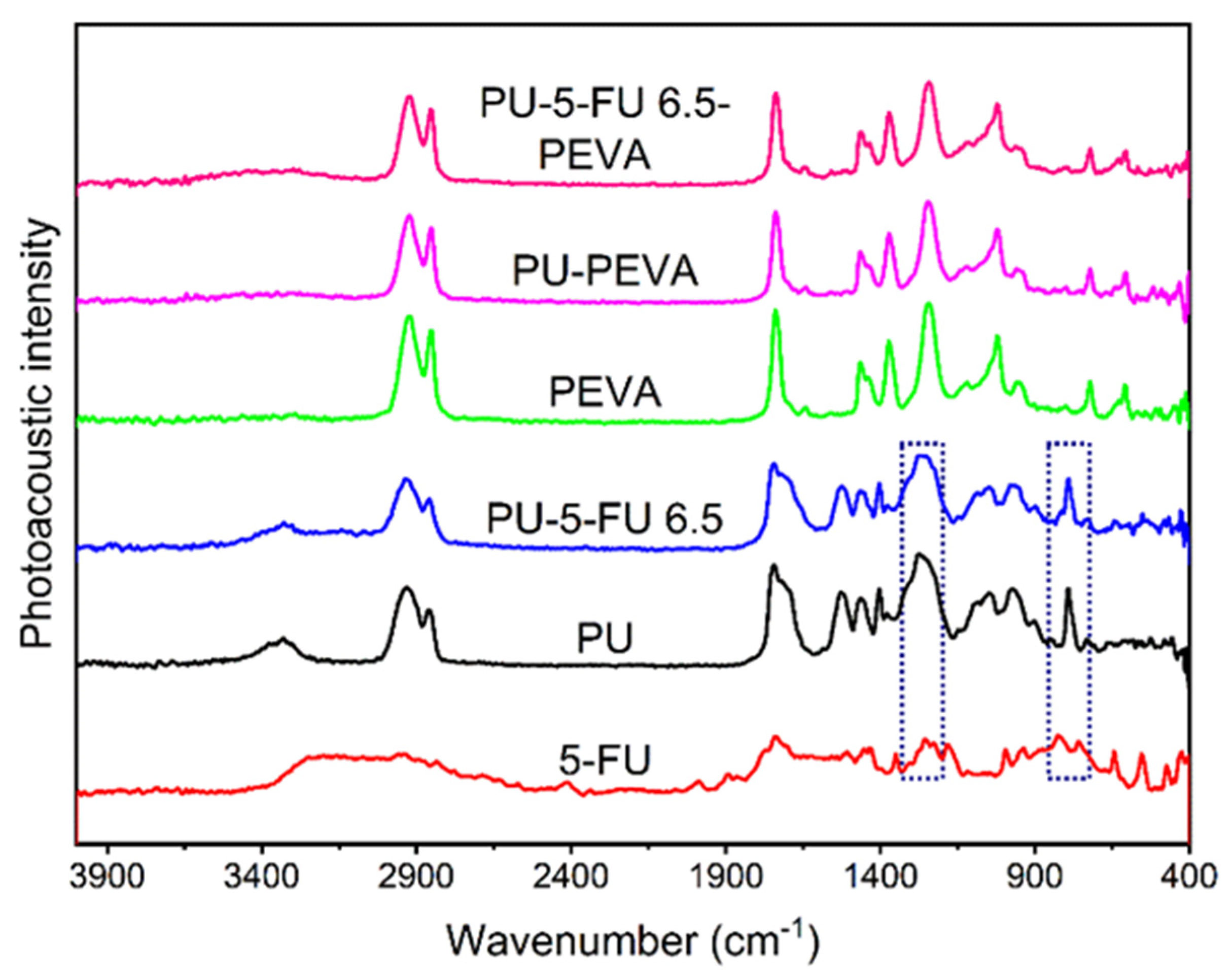
3.2.1. Photoacoustic FTIR Spectroscopy of Coatings
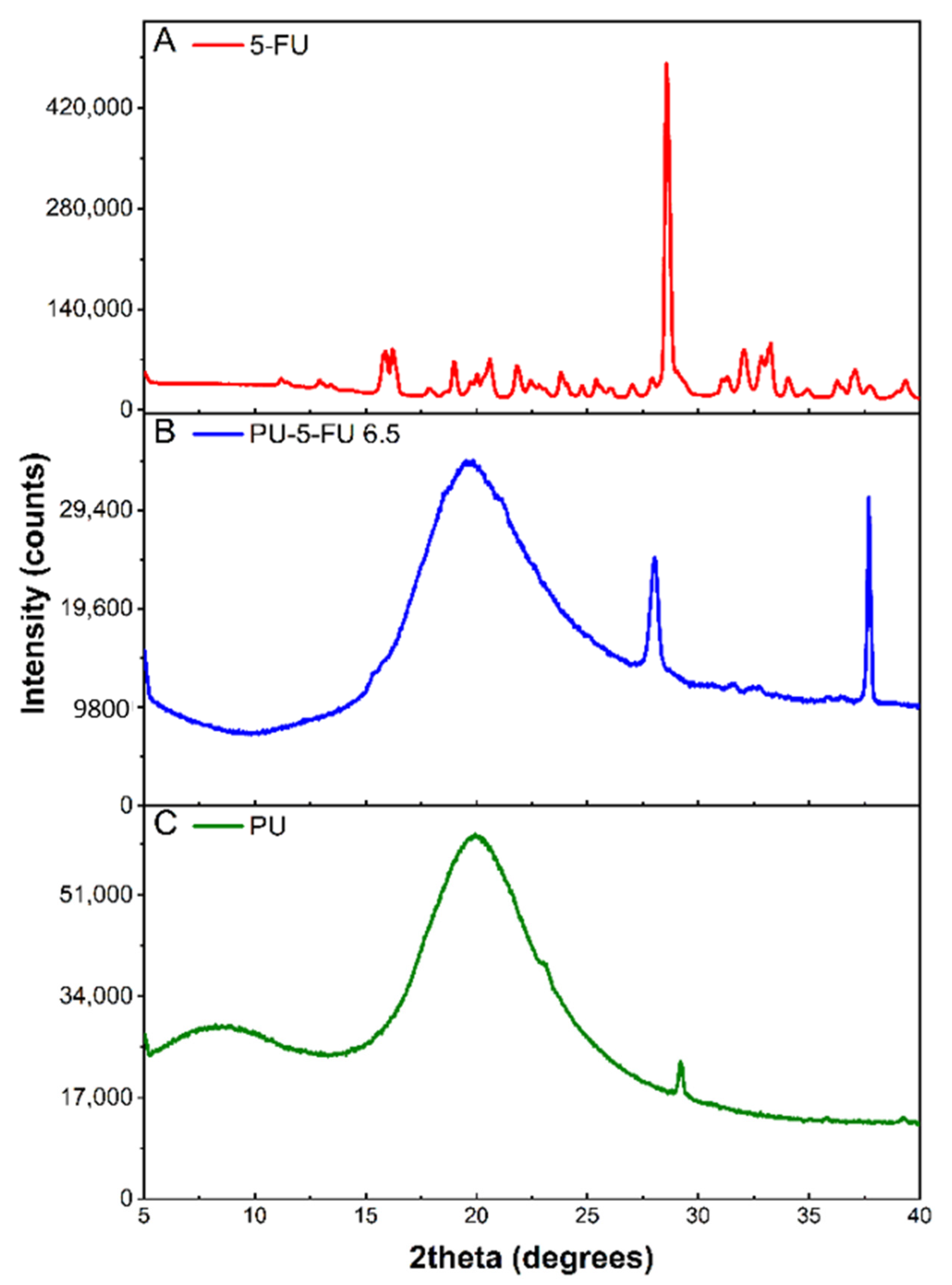
3.2.2. X-Ray Diffraction (XRD) of Coatings
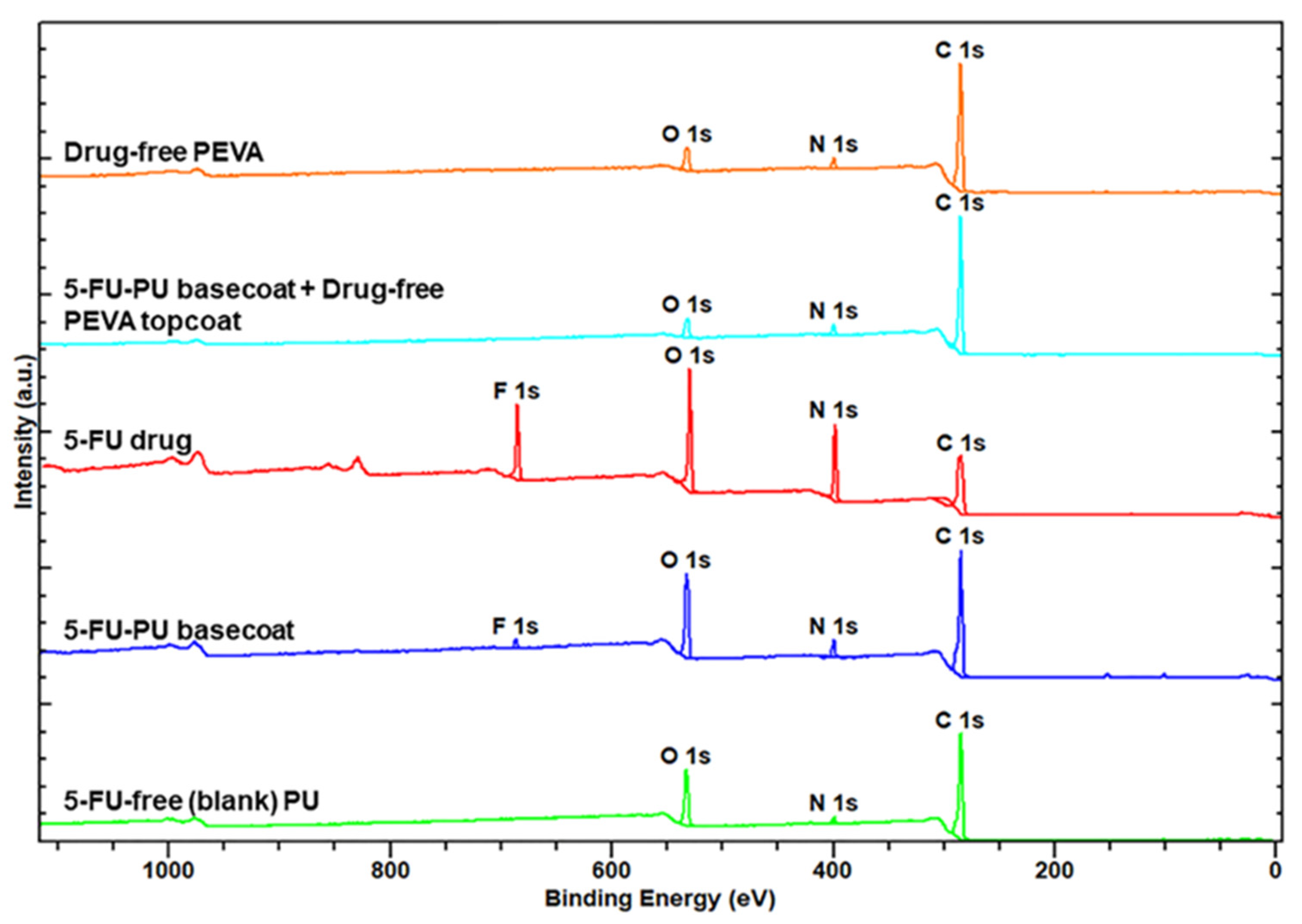
3.2.3. X-Ray Photoelectron Spectroscopy (XPS) of Coatings
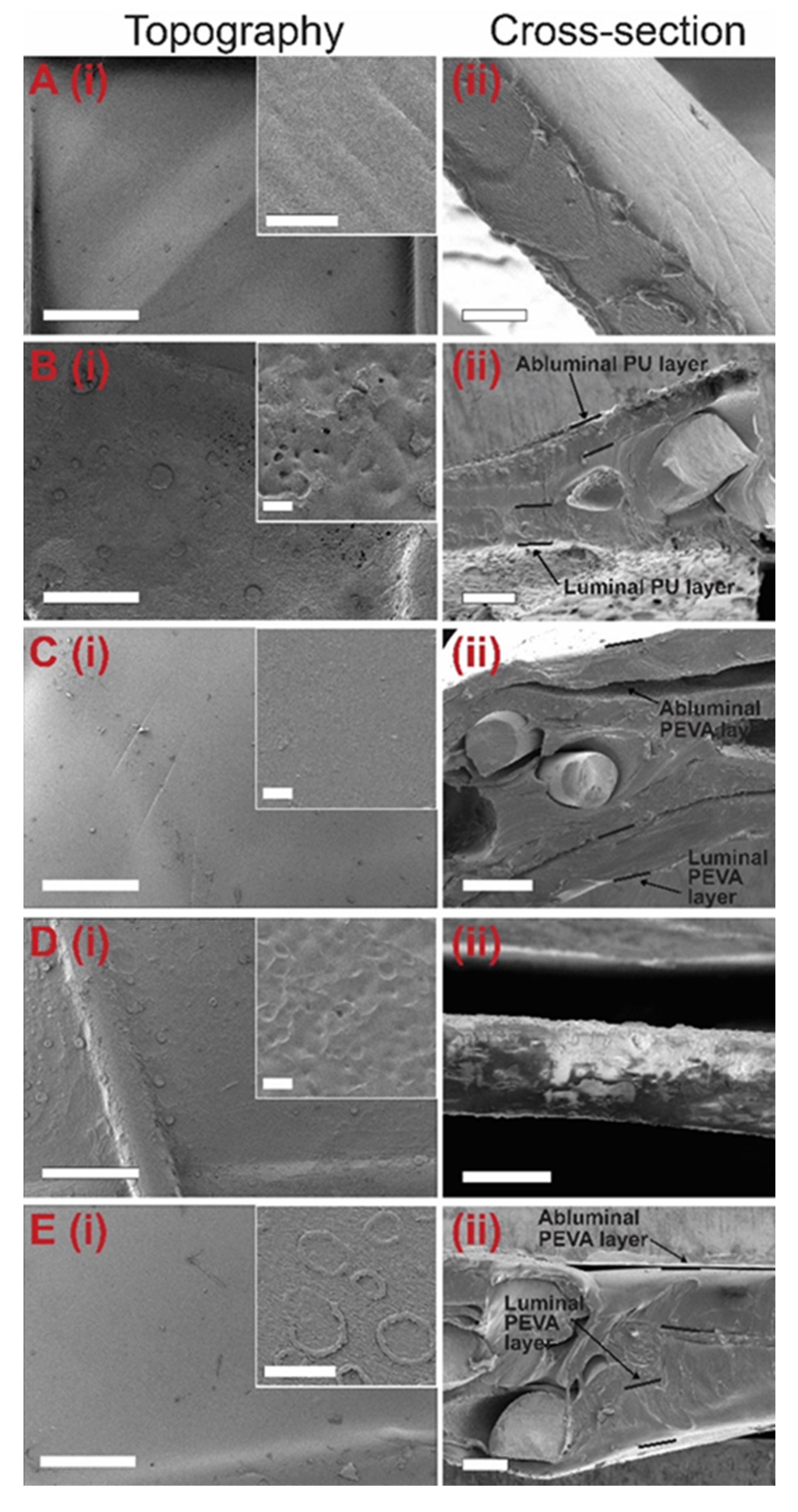
3.2.4. Coating Surface Topography and Thickness by SEM
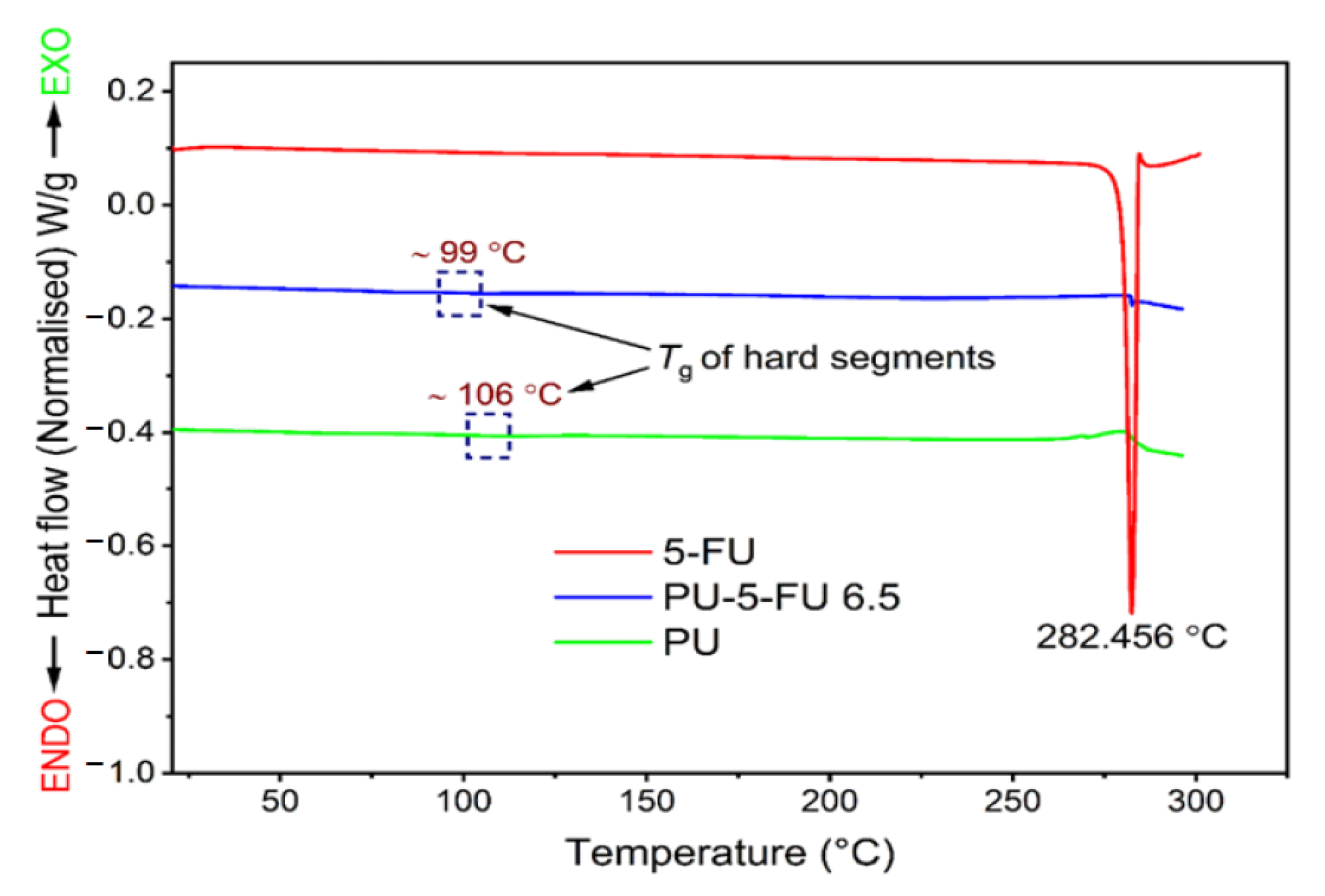
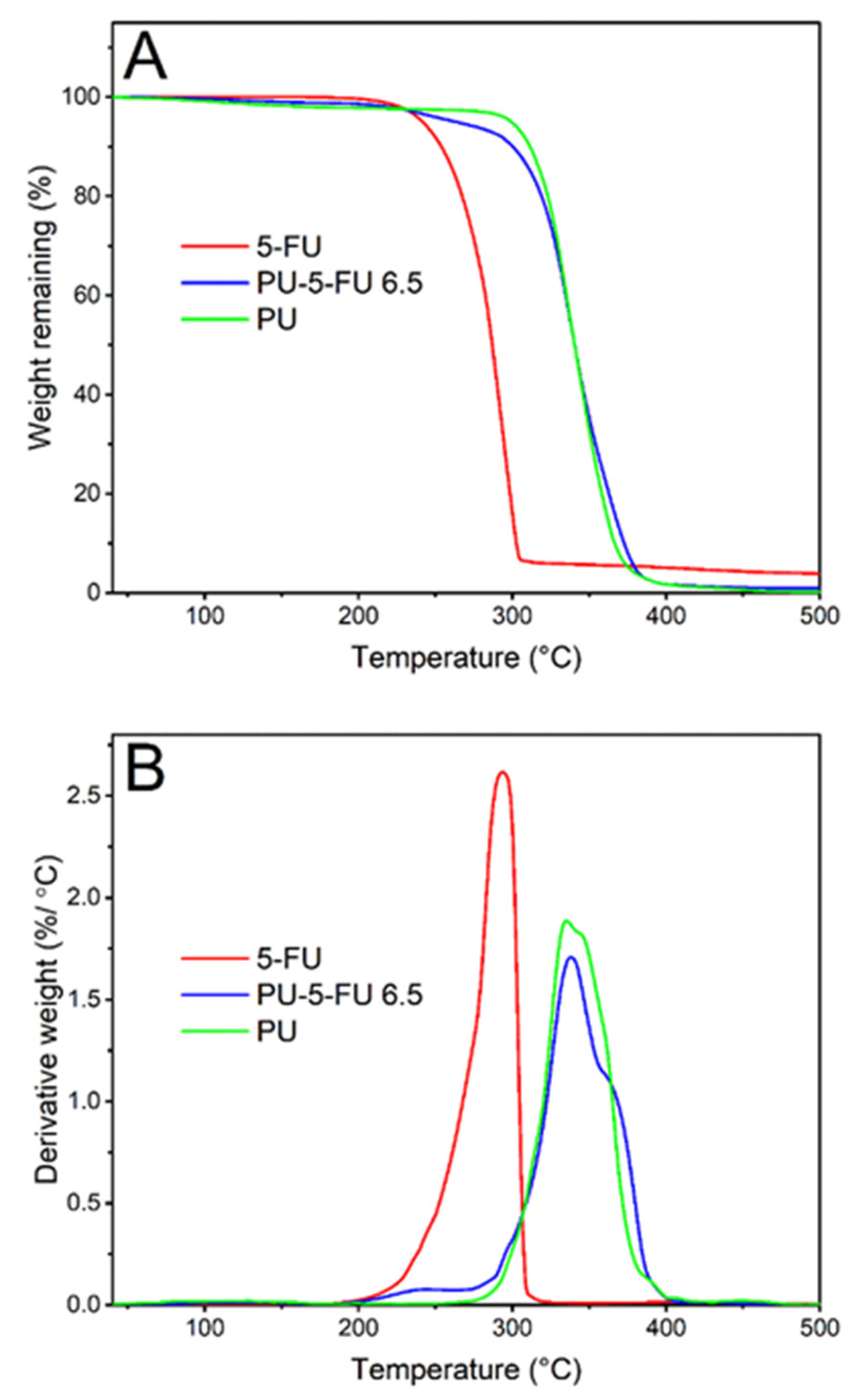
3.2.5. Thermal Analysis of Coatings
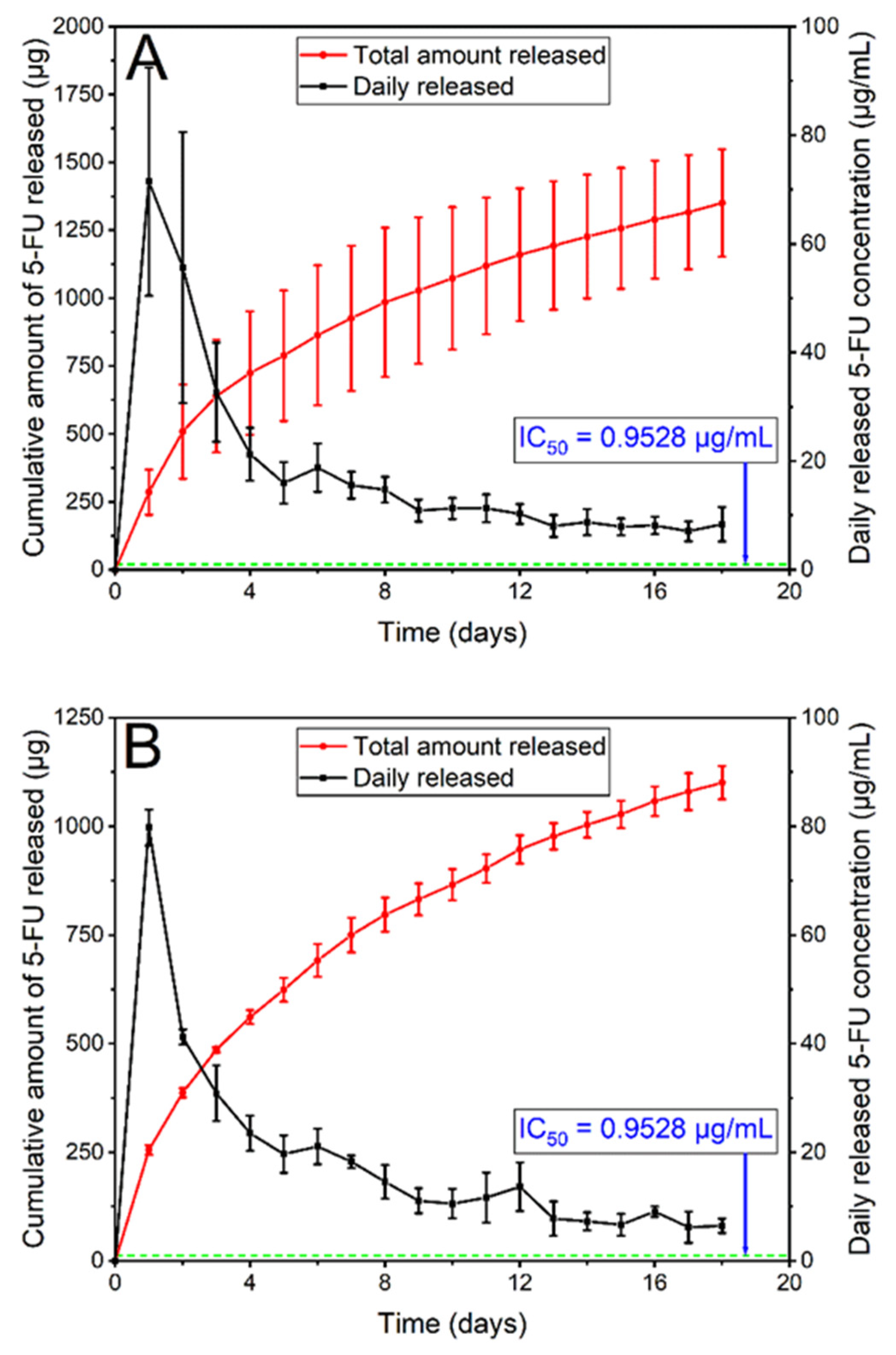
3.2.6. In Vitro Drug Release
3.3. In Vitro Anticancer Activity of the 5FU-Loaded GI Stents
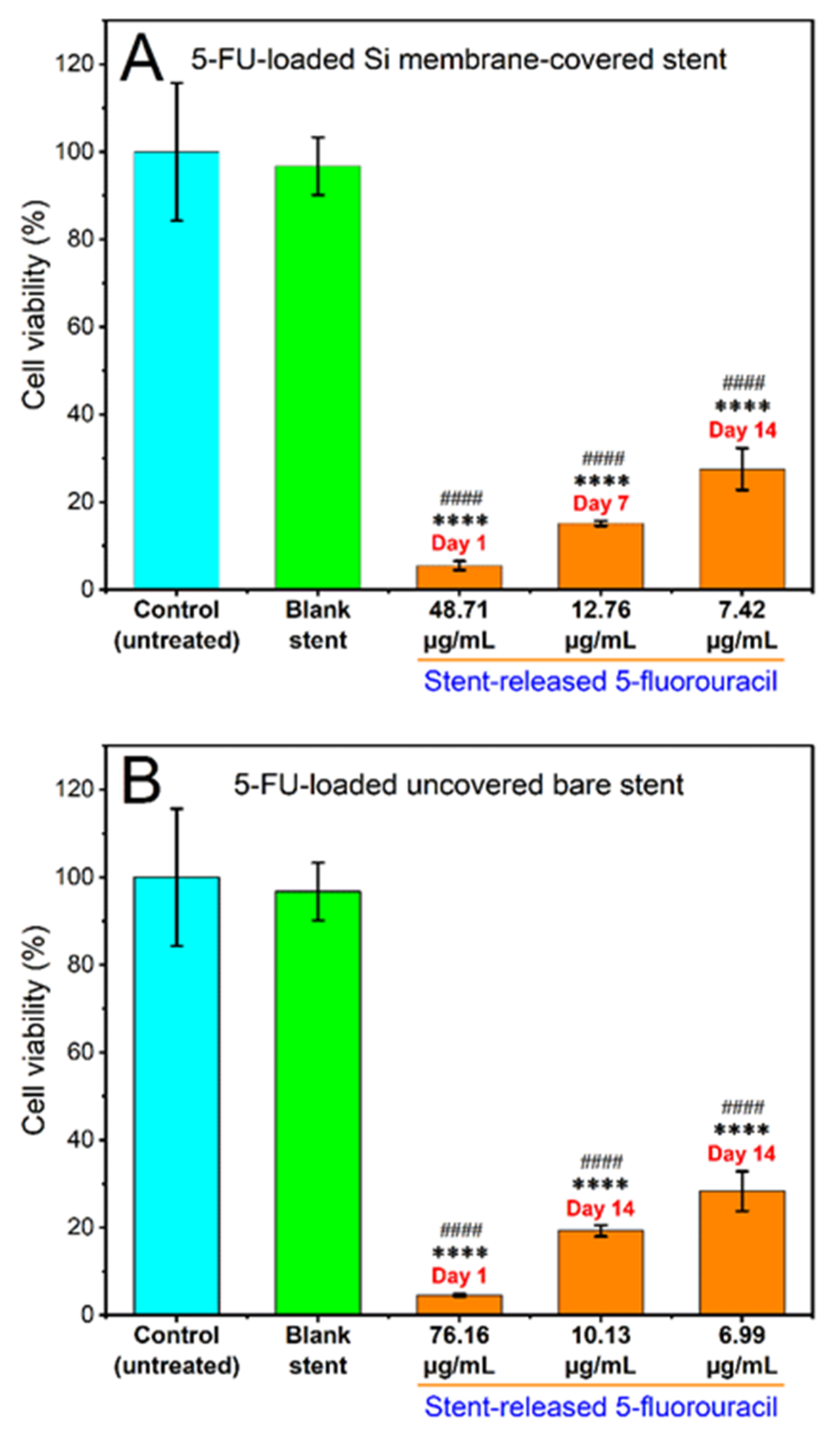
3.3.1. Cytotoxicity of Stent Released 5FU
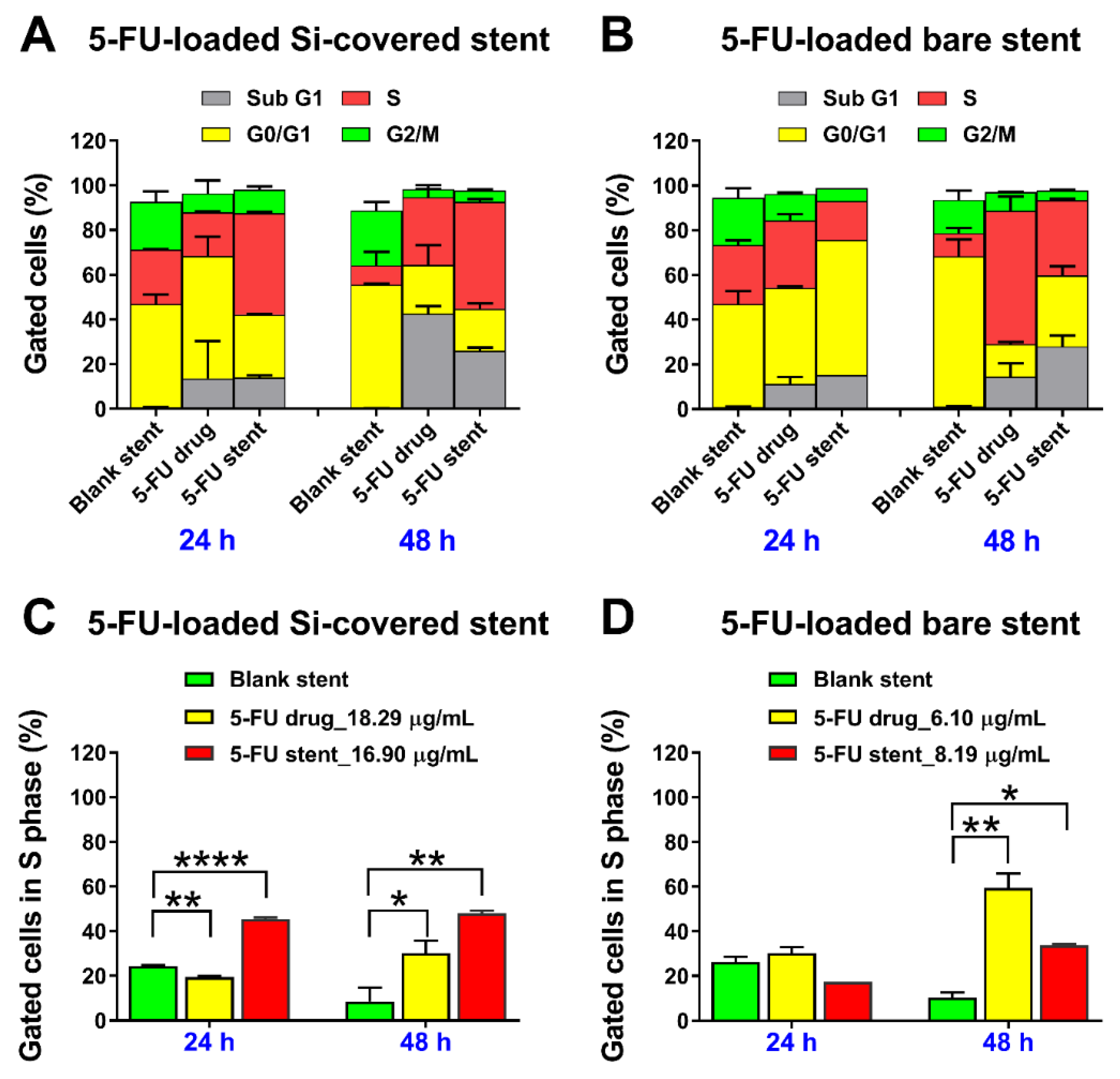
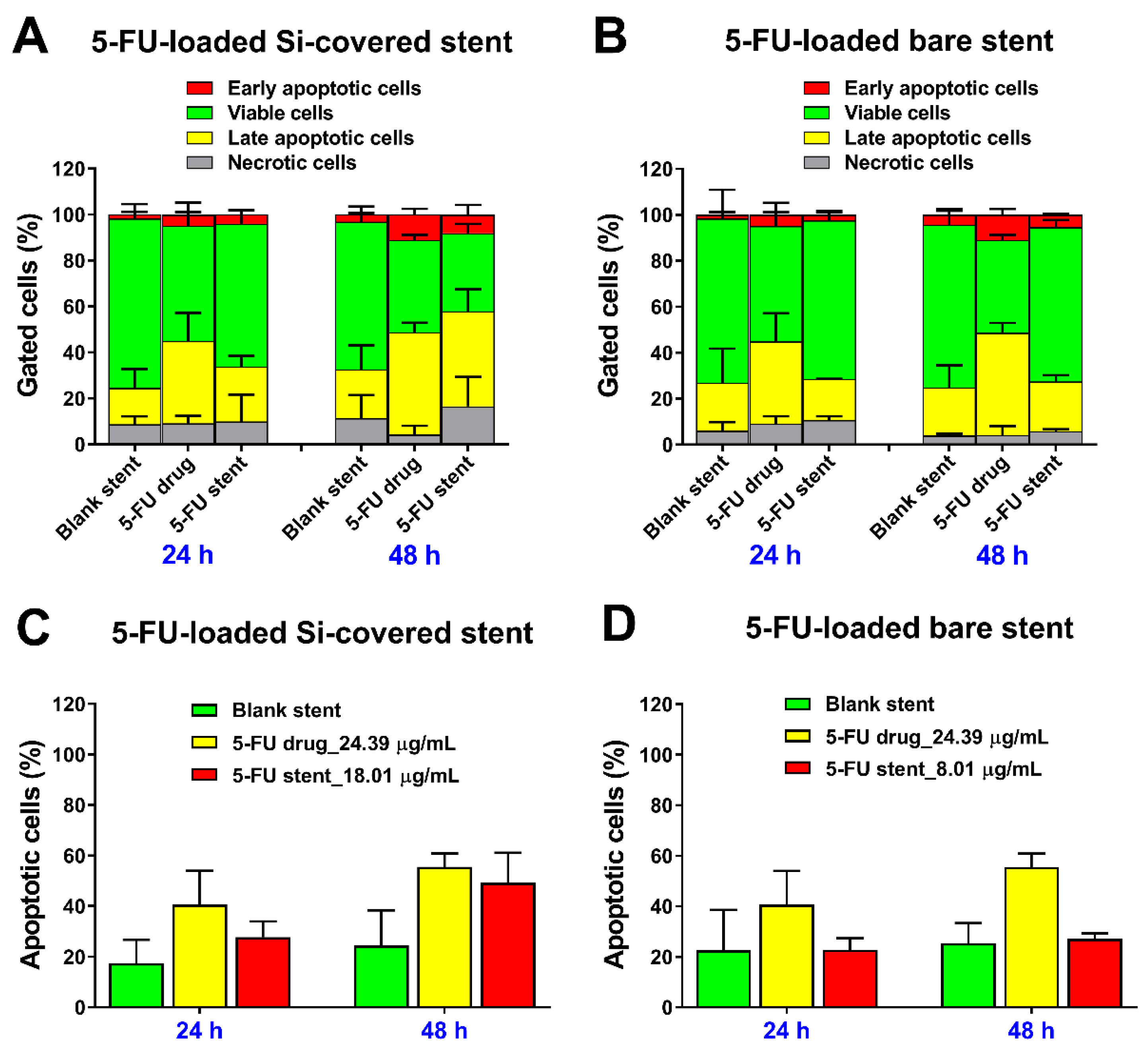
3.3.2. Cell Cycle Analysis and Detection of Apoptosis by Flow Cytometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Samaraee, A.; McCallum, I.J.; Kenny, L.; Isreb, S.; MacDougall, L.; Hayat, M.; Kelly, S. Colorectal stents: Do we have enough evidence? Int. J. Surg. 2011, 9, 595–599. [Google Scholar] [CrossRef]
- Feo, L.; Schaffzin, D.M. Colonic stents: The modern treatment of colonic obstruction. Adv. Ther. 2011, 28, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, Y.J. Stents for colorectal obstruction: Past, present, and future. World J. Gastroenterol. 2016, 22, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Kim, S.W.; Kim, T.I.; Lee, J.-H.; Lee, B.-I.; Keum, B.; Cheung, D.Y.; Yang, C.H. Evidence-Based Recommendations on Colorectal Stenting: A Report from the Stent Study Group of the Korean Society of Gastrointestinal Endoscopy. Clin. Endosc. 2013, 46, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.H. Enteral Stents for the Management of Malignant Colorectal Obstruction. Available online: https://www.uptodate.com/contents/enteral-stents-for-the-management-of-malignant-colorectal-obstruction?source=search_result&search=enteral%20stent&selectedTitle=2~22 (accessed on 30 June 2017).
- Farrell, J.J. Preoperative colonic stenting: How, when and why? Curr. Opin. Gastroenterol. 2007, 23, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Byeon, J.S. Colorectal Stents: Current Status. Clin. Endosc. 2015, 48, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Van Hooft, J.E.; Veld, J.V.; Arnold, D.; Beets-Tan, R.G.H.; Everett, S.; Götz, M.; van Halsema, E.E.; Hill, J.; Manes, G.; Meisner, S.; et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2020. Endoscopy 2020, 52, 389–407. [Google Scholar]
- Park, J.S.; Jeong, S.; Lee, D.H. Recent Advances in Gastrointestinal Stent Development. Clin. Endosc. 2015, 48, 209–215. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, S.; Wang, Z. A type of esophageal stent coating composed of one 5-fluorouracil-containing EVA layer and one drug-free protective layer: In vitro release, permeation and mechanical properties. J. Control. Release 2007, 118, 318–324. [Google Scholar] [CrossRef]
- Lee, J.W.; Yang, S.G.; Na, K. Gemcitabine-releasing polymeric films for covered self-expandable metallic stent in treatment of gastrointestinal cancer. Int. J. Pharm. 2012, 427, 276–283. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.; Hu, J.; Wu, X.; Hu, J.; He, X.; Li, J.; Zhao, Z.; Chen, Z.; Li, Y.; et al. A 5-fluorouracil-loaded polydioxanone weft-knitted stent for the treatment of colorectal cancer. Biomaterials 2013, 34, 9451–9461. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Kim, H.; Kim, K.S.; Park, S.; Chung, J.B.; Park, S.W. Safety evaluation of self-expanding metallic biliary stents eluting gemcitabine in a porcine model. J. Gastroenterol. Hepatol. 2012, 27, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-R.; Wang, Z.-M.; Zhang, Y.-Q.; Lei, L.; Shi, J.-M.; Chen, K.-M.; Yu, Z. In Vivo Evaluation of 5-Fluorouracil-Containing Self-Expandable Nitinol Stent in Rabbits: Efficiency in Long-Term Local Drug Delivery. J. Pharm. Sci. 2010, 99, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Kim, J.H.; Kim, M.; Yang, S.; Jo, E.A.; Lee, J.W.; Na, K.; Kim, J.M.; Jeong, S.; Lee, D.H.; et al. Porcine feasibility and safety study of a new paclitaxel-eluting biliary stent with a Pluronic-containing membrane. Endoscopy 2012, 44, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Lee, D.H.; Lee, D.K.; Na, K. Nonvascular drug-eluting stent coated with sodium caprate-incorporated polyurethane for the efficient penetration of paclitaxel into tumor tissue. J. Biomater. Appl. 2015, 29, 1133–1144. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, Y.-I.; Chung, C.-W.; Kim, C.H.; Kwak, T.W.; Lee, H.M.; Kang, D.H. Preclinical evaluation of sorafenib-eluting stent for suppression of human cholangiocarcinoma cells. Int. J. Nanomed. 2013, 8, 1697–1711. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, M.; Kim, M.-k.; Lee, H.; Lee, D.K.; Lee, D.-H.; Yang, S.-G. Paclitaxel-eluting nanofiber-covered self-expanding nonvascular stent for palliative chemotherapy of gastrointestinal cancer and its related stenosis. Biomed. Microdevices 2014, 16, 897–904. [Google Scholar] [CrossRef]
- Kwak, T.W.; Lee, H.L.; Song, Y.H.; Kim, C.; Kim, J.; Seo, S.-J.; Jeong, Y.-I.; Kang, D.H. Vorinostat-eluting poly(DL-lactide-co-glycolide) nanofiber-coated stent for inhibition of cholangiocarcinoma cells. Int. J. Nanomed. 2017, 12, 7669–7680. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, H.S.; Kim, K.-S.; Lee, W.J.; Kim, H.K.; Won, Y.H.; Byun, Y.R.; Kim, M.Y.; Baik, S.K.; Kwon, S.O. The effect on porcine bile duct of a metallic stent covered with a paclitaxel-incorporated membrane. Gastrointest. Endosc. 2005, 61, 296–301. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Wu, K.; Li, J.; Chen, W.; Shen, Y.; Guo, S. Paclitaxel or 5-fluorouracil/esophageal stent combinations as a novel approach for the treatment of esophageal cancer. Biomaterials 2015, 53, 592–599. [Google Scholar] [CrossRef]
- Moon, S.; Yang, S.G.; Na, K. An acetylated polysaccharide-PTFE membrane-covered stent for the delivery of gemcitabine for treatment of gastrointestinal cancer and related stenosis. Biomaterials 2011, 32, 3603–3610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, Y.; Gu, G.; Wu, J.; Fan, Z.; Dong, H.; Hu, Y. Rational design of drug-eluting stents via electrospray and in vivo evaluation of preventing oesophageal stricture. RSC Adv. 2014, 4, 16885–16892. [Google Scholar] [CrossRef]
- Arafat, M.; Fouladian, P.; Blencowe, A.; Albrecht, H.; Song, Y.; Garg, S. Drug-eluting non-vascular stents for localised drug targeting in obstructive gastrointestinal cancers. J. Control. Release 2019, 308, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Lee, D.K. Stents with specialized functions: Drug-eluting stents and stents with antireflux devices. Gastrointest. Interv. 2015, 4, 50–54. [Google Scholar] [CrossRef]
- Wu, P.; Grainger, D.W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006, 27, 2450–2467. [Google Scholar] [CrossRef]
- Schmidt, W.; Lanzer, P. Instrumentation. In Catheter-Based Cardiovascular Interventions: A Knowledge-Based Approach; Lanzer, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 445–472. [Google Scholar]
- Food and Drug Administration Center for Devices and Radiological Health (CDRH). Guidance for Industry: Coronary Drug-Eluting Stents-Nonclinical and Clinical Studies (Draft); Food and Drug Administration (FDA): Rockville, MD, USA, 2008; p. 89.
- Clark, J.W.; Grothey, A. Systemic Chemotherapy for Metastatic Colorectal Cancer. Available online: https://www.uptodate.com/contents/systemic-chemotherapy-for-metastatic-colorectal-cancer-completed-clinical-trials/print?source=see_link (accessed on 3 July 2017).
- National Cancer Institute. Drugs Approved for Colon and Rectal Cancer. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/colorectal (accessed on 3 July 2017).
- European Medicines Agency: EMEA/CHMP/EWP/110540/2007. Guideline on the Clinical and Non Clinical Evaluation during the Consultation Procedure on Medicinal Subatances Contained in Drug-Eluting (Medicinal Substance-Eluting) Coronary Stents. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003275.pdf (accessed on 15 July 2017).
- Olukman, M.O.; Şanlı, O.; Solak, E.K. Release of Anticancer Drug 5-Fluorouracil from Different Ionically Crosslinked Alginate Beads. J. Biomater. Nanobiotechnol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Waltenberger, J.; Rogers, J.H. Percutaneous coronary intervention in patients with diabetes: Current concepts and future directions. J. Diabetes Sci. Technol. 2014, 8, 581–589. [Google Scholar] [CrossRef]
- Kwon, H.; Park, S. Local Delivery of Antiproliferative Agents via Stents. Polymers 2014, 6, 755–775. [Google Scholar] [CrossRef]
- Rizas Konstantinos, D.; Mehilli, J. Stent Polymers. Circ. Cardiovasc. Interv. 2016, 9, e002943. [Google Scholar]
- Chappa, R.A.; Hergenrother, R.W.; Wadman, S.A.; Wormuth, K.R. Coating Systems for the Controlled Delivery of Hydrophilic Bioactive Agents. U.S. Patent WO2009US02418, 17 April 2009. [Google Scholar]
- Wang, K.; Deng, Q. The Thermal and Mechanical Properties of Poly(ethylene-co-vinyl acetate) Random Copolymers (PEVA) and its Covalently Crosslinked Analogues (cPEVA). Polymers 2019, 11, 1055. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Wu, K.; Shen, Y.; Mao, A.; Li, J.; Chen, Z.; Guo, S. Nitinol stents loaded with a high dose of antitumor 5-fluorouracil or paclitaxel: Esophageal tissue responses in a porcine model. Gastrointest. Endosc. 2015, 82, 153–160. [Google Scholar] [CrossRef]
- Lee, S.S.; Shin, J.H.; Han, J.M.; Cho, C.H.; Kim, M.-H.; Lee, S.-K.; Kim, J.-H.; Kim, K.-R.; Shin, K.M.; Won, Y.H.; et al. Histologic influence of paclitaxel-eluting covered metallic stents in a canine biliary model. Gastrointest. Endosc. 2009, 69, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.H.; Na, K. Polyurethane membrane with porous surface for controlled drug release in drug eluting stent. Biomater. Res. 2014, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.V.; Kevadiya, B.D.; Patel, H.A.; Bajaj, H.C.; Jasra, R.V. Montmorillonite as a drug delivery system: Intercalation and in vitro release of timolol maleate. Int. J. Pharm. 2009, 374, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.B.; Manjula, B.; Jayaramudu, T.; Sadiku, E.R.; Anand Babu, P.; Periyar Selvam, S. 5-Fluorouracil Loaded Chitosan–PVA/Na+MMT Nanocomposite Films for Drug Release and Antimicrobial Activity. Nano-Micro Lett. 2016, 8, 260–269. [Google Scholar] [CrossRef]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database. NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000.
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy; Wanger, C.D., Riggs, W.M., Davis, L.E., Moulder, J.F., Muilenberg, G.E., Eds.; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979; pp. 190–195. [Google Scholar]
- Beamson, G.; Briggs, D. XPS of Polymers Database; SurfaceSpectra Ltd.: Manchester, UK, 1992. [Google Scholar]
- The United States Pharmacopeia-National Formulary (USP-NF). Fluorouracil; United States Pharmacopeial Convention: Rockville, MD, USA, 2019; p. 1910. [Google Scholar]
- Biswas, S.; Reddy, N.D.; Jayashree, B.S.; Rao, C.M. Evaluation of Novel 3-Hydroxyflavone Analogues as HDAC Inhibitors against Colorectal Cancer. Adv. Pharmacol. Sci. 2018, 2018, 4751806. [Google Scholar] [CrossRef]
- Lam, F.; Abbas, A.Y.; Shao, H.; Teo, T.; Adams, J.; Li, P.; Bradshaw, T.D.; Fischer, P.M.; Walsby, E.; Pepper, C.; et al. Targeting RNA transcription and translation in ovarian cancer cells with pharmacological inhibitor CDKI-73. Oncotarget 2014, 5, 7691–7704. [Google Scholar] [CrossRef]
- Shaikh, M.; Zhang, H.; Wang, H.; Guo, X.; Song, Y.; Kanwar, J.R.; Garg, S. In Vitro and In Vivo Assessment of Docetaxel Formulation Developed for Esophageal Stents. AAPS PharmSciTech 2017, 18, 130–137. [Google Scholar] [CrossRef][Green Version]
- Yi, H.-G.; Choi, Y.-J.; Kang, K.S.; Hong, J.M.; Pati, R.G.; Park, M.N.; Shim, I.K.; Lee, C.M.; Kim, S.C.; Cho, D.-W. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J. Control. Release 2016, 238, 231–241. [Google Scholar] [CrossRef]
- Bege, N.; Steinmüller, S.O.; Kalinowski, M.; Reul, R.; Klaus, S.; Petersen, H.; Curdy, C.; Janek, J.; Kissel, T. Drug eluting stents based on Poly(ethylene carbonate): Optimization of the stent coating process. Eur. J. Pharm. Biopharm. 2012, 80, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Raval, A.; Mandal, R.; Parmar, S.; Jariwala, A.; Tailor, J.; Mehta, A. Development and Evaluation of Drug Eluting Stent Having Biphasic Release from a Single Layer of Biodegradable Polymer. J. Med. Devices 2013, 7, 011005. [Google Scholar] [CrossRef]
- Whitbourne, R.J.; Chamberlain, A.M.; Hullihen, D.G.; Rosebrough, S.F.; Calistri-Yeh, M. Medicated Stent Having Multi-Layer Polymer Coating. U.S. Patent 828,759,0B2, 16 October 2012. [Google Scholar]
- AdvanSource Biomaterials. ChronoFlex AL. Available online: http://www.advbiomaterials.com/products/polycarbonate/chronoflex_al.html (accessed on 22 September 2017).
- Lei, L.; Liu, X.; Guo, S.; Tang, M.; Cheng, L.; Tian, L. 5-Fluorouracil-loaded multilayered films for drug controlled releasing stent application: Drug release, microstructure, and ex vivo permeation behaviors. J. Control. Release 2010, 146, 45–53. [Google Scholar] [CrossRef]
- Mc Conville, C.; Major, I.; Friend, D.R.; Clark, M.R.; Woolfson, A.D.; Malcolm, R.K. Development of polylactide and polyethylene vinyl acetate blends for the manufacture of vaginal rings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Tallury, P.; Alimohammadi, N.; Kalachandra, S. Poly(ethylene-co-vinyl acetate) copolymer matrix for delivery of chlorhexidine and acyclovir drugs for use in the oral environment: Effect of drug combination, copolymer composition and coating on the drug release rate. Dent. Mater. 2007, 23, 404–409. [Google Scholar] [CrossRef]
- Kanth, V.R.; Kajjari, P.B.; Madalageri, P.M.; Ravindra, S.; Manjeshwar, L.S.; Aminabhavi, T.M. Blend Hydrogel Microspheres of Carboxymethyl Chitosan and Gelatin for the Controlled Release of 5-Fluorouracil. Pharmaceutics 2017, 9, 13. [Google Scholar] [CrossRef]
- Khalaf, A.I.; El Nashar, D.E.; Helaly, F.M.; Soliman, A. Evaluation of controlled release PVC/PEG polymeric films containing 5-fluorouracil for long-term antitumor. Polym. Bull. 2019, 76, 3555–3568. [Google Scholar] [CrossRef]
- Singh, P.; Tyagi, G.; Mehrotra, R.; Bakhshi, A.K. Thermal stability studies of 5-fluorouracil using diffuse reflectance infrared spectroscopy. Drug Test. Anal. 2009, 1, 240–244. [Google Scholar] [CrossRef]
- Abraham, G.A.; Frontini, P.M.; Cuadrado, T.R. Physical and mechanical behavior of sterilized biomedical segmented polyurethanes. J. Appl. Polym. Sci. 1997, 65, 1193–1203. [Google Scholar] [CrossRef]
- Cho, I.H.; Park, S. Preparation of a Microporous Polyurethane Film with Negative Surface Charge for siRNA Delivery via Stent. Int. J. Polym. Sci. 2017, 2017, 2841682. [Google Scholar] [CrossRef]
- Dias, R.C.M.; Góes, A.M.; Serakides, R.; Ayres, E.; Oréfice, R.L. Porous biodegradable polyurethane nanocomposites: Preparation, characterization, and biocompatibility tests. Mater. Res. 2010, 13, 211–218. [Google Scholar] [CrossRef]
- Shin, M.S.; Hong, J.Y.; Park, S. Gemcitabine release behavior of polyurethane matrixes designed for local anti-cancer drug delivery via stent. J. Drug Deliv. Sci. Technol. 2012, 22, 301–306. [Google Scholar] [CrossRef]
- Fouladian, P.; Afinjuomo, F.; Arafat, M.; Bergamin, A.; Song, Y.; Blencowe, A.; Garg, S. Influence of Polymer Composition on the Controlled Release of Docetaxel: A Comparison of Non-Degradable Polymer Films for Oesophageal Drug-Eluting Stents. Pharmaceutics 2020, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.A.P.; do Nascimento, O.L.; Mansur, H.S. Physico-chemical characterization of EVA-modified mortar and porcelain tiles interfaces. Cem. Concr. Res. 2009, 39, 1199–1208. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Aguilar-Flores, C.; Aparicio-Saguilán, A. Fingerprint analysis of FTIR spectra of polymers containing vinyl acetate. DYNA 2019, 86, 198–205. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, G.; Tiwari, R.; Srivastava, R.; Rai, A.K. Enteric coated HPMC capsules plugged with 5-FU loaded microsponges: A potential approach for treatment of colon cancer. Braz. J. Pharm. Sci. 2015, 51, 591–605. [Google Scholar] [CrossRef]
- Jubeen, F.; Liaqat, A.; Sultan, M.; Zafar Iqbal, S.; Sajid, I.; Sher, F. Green synthesis and biological evaluation of novel 5-fluorouracil derivatives as potent anticancer agents. Saudi Pharm. J. 2019, 27, 1164–1173. [Google Scholar] [CrossRef]
- Araujo Borges, R.; Choudhury, D.; Zou, M. 3D printed PCU/UHMWPE polymeric blend for artificial knee meniscus. Tribol. Int. 2018, 122, 1–7. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A. Aliphatic polycarbonate-based thermoplastic polyurethane elastomers containing diphenyl sulfide units. J. Therm. Anal. Calorim. 2016, 126, 225–243. [Google Scholar] [CrossRef][Green Version]
- Todea, M.; Muresan-Pop, M.; Simon, S.; Moisescu-Goia, C.; Simon, V.; Eniu, D. XPS investigation of new solid forms of 5-fluorouracil with piperazine. J. Mol. Struct. 2018, 1165, 120–125. [Google Scholar] [CrossRef]
- Von Birgelen, C. Benchside testing of drug-eluting stent surface and geometry. Interv. Cardiol. 2010, 2, 159–175. [Google Scholar]
- Shimokawa, M.; Yoshida, H.; Komatsu, T.; Omachi, R.; Kudo, K. Emergence of Wrinkles during the Curing of Coatings. Gels 2018, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, M.; Denape, J.; Paris, J.Y.; Ural, A.G.; Alcalá, N.; Martínez, F.J. Wear dynamics of a TPU/steel contact under reciprocal sliding. Wear 2014, 315, 103–114. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Wang, Y.; Cui, F.; Zhang, J.; Huang, Q. Preparation and characterization of 5-fluorouracil-loaded PLLA–PEG/PEG nanoparticles by a novel supercritical CO2 technique. Int. J. Pharm. 2012, 436, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Pavličević, J.; Špírková, M.; Aroguz, A.; Jovičić, M.; Kojić, D.; Govedarica, D.; Ikonić, B. The effect of TiO2 particles on thermal properties of polycarbonate-based polyurethane nanocomposite films. J. Therm. Anal. Calorim. 2019, 138, 2043–2055. [Google Scholar] [CrossRef]
- Gadagkar, S.R.; Call, G.B. Computational tools for fitting the Hill equation to dose–response curves. J. Pharmacol. Toxicol. Methods 2015, 71, 68–76. [Google Scholar] [CrossRef]
- Singh, R.; George, J.; Shukla, Y. Role of senescence and mitotic catastrophe in cancer therapy. Cell Div. 2010, 5, 4. [Google Scholar] [CrossRef]
- Silva, V.R.; Corrêa, R.S.; Santos, L.d.S.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. A ruthenium-based 5-fluorouracil complex with enhanced cytotoxicity and apoptosis induction action in HCT116 cells. Sci. Rep. 2018, 8, 288. [Google Scholar] [CrossRef]
- Tawfik, E.; Ahamed, M.; Almalik, A.; Alfaqeeh, M.; Alshamsan, A. Prolonged exposure of colon cancer cells to 5-fluorouracil nanoparticles improves its anticancer activity. Saudi Pharm. J. 2017, 25, 206–213. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Yoshikawa, R.; Kusunoki, M.; Yanagi, H.; Noda, M.; Furuyama, J.I.; Yamamura, T.; Hashimoto-Tamaoki, T. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: A novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001, 61, 1029–1037. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafat, M.; Fouladian, P.; Wignall, A.; Song, Y.; Parikh, A.; Albrecht, H.; Prestidge, C.A.; Garg, S.; Blencowe, A. Development and In Vitro Evaluation of 5-Fluorouracil-Eluting Stents for the Treatment of Colorectal Cancer and Cancer-Related Obstruction. Pharmaceutics 2021, 13, 17. https://doi.org/10.3390/pharmaceutics13010017
Arafat M, Fouladian P, Wignall A, Song Y, Parikh A, Albrecht H, Prestidge CA, Garg S, Blencowe A. Development and In Vitro Evaluation of 5-Fluorouracil-Eluting Stents for the Treatment of Colorectal Cancer and Cancer-Related Obstruction. Pharmaceutics. 2021; 13(1):17. https://doi.org/10.3390/pharmaceutics13010017
Chicago/Turabian StyleArafat, Mohammad, Paris Fouladian, Anthony Wignall, Yunmei Song, Ankit Parikh, Hugo Albrecht, Clive A. Prestidge, Sanjay Garg, and Anton Blencowe. 2021. "Development and In Vitro Evaluation of 5-Fluorouracil-Eluting Stents for the Treatment of Colorectal Cancer and Cancer-Related Obstruction" Pharmaceutics 13, no. 1: 17. https://doi.org/10.3390/pharmaceutics13010017
APA StyleArafat, M., Fouladian, P., Wignall, A., Song, Y., Parikh, A., Albrecht, H., Prestidge, C. A., Garg, S., & Blencowe, A. (2021). Development and In Vitro Evaluation of 5-Fluorouracil-Eluting Stents for the Treatment of Colorectal Cancer and Cancer-Related Obstruction. Pharmaceutics, 13(1), 17. https://doi.org/10.3390/pharmaceutics13010017







