Working within the Design Space: Do Our Static Process Characterization Methods Suffice?
Abstract
1. Introduction
2. Design Space
2.1. The Current View on the Development and Representation of the Design Space
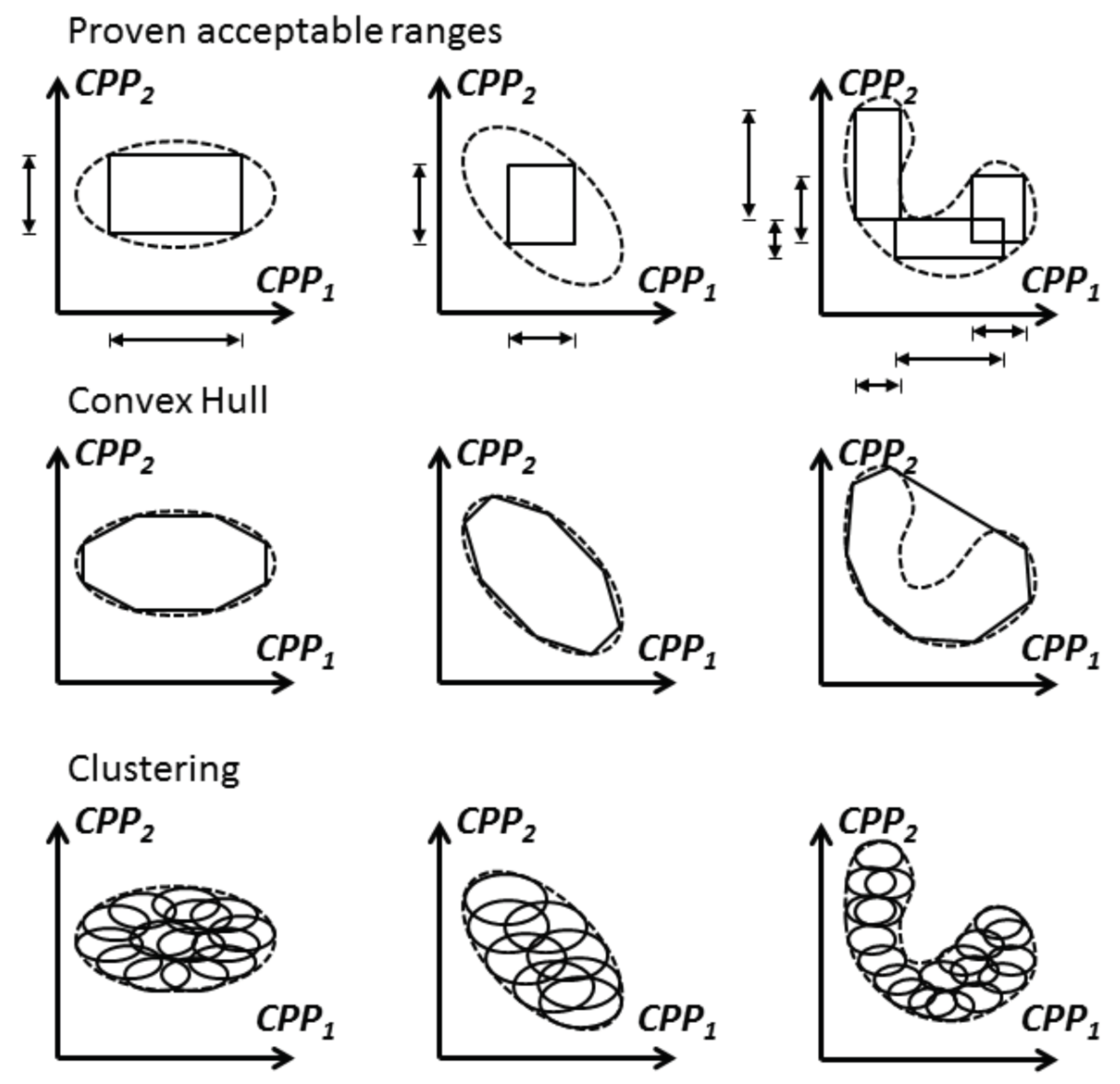
2.2. Alternative Representations of Multidimensional Spaces
2.3. Operation within the Design Space
2.3.1. Uncertainty and Sensitivity Analysis
2.3.2. Control and Systems Theory
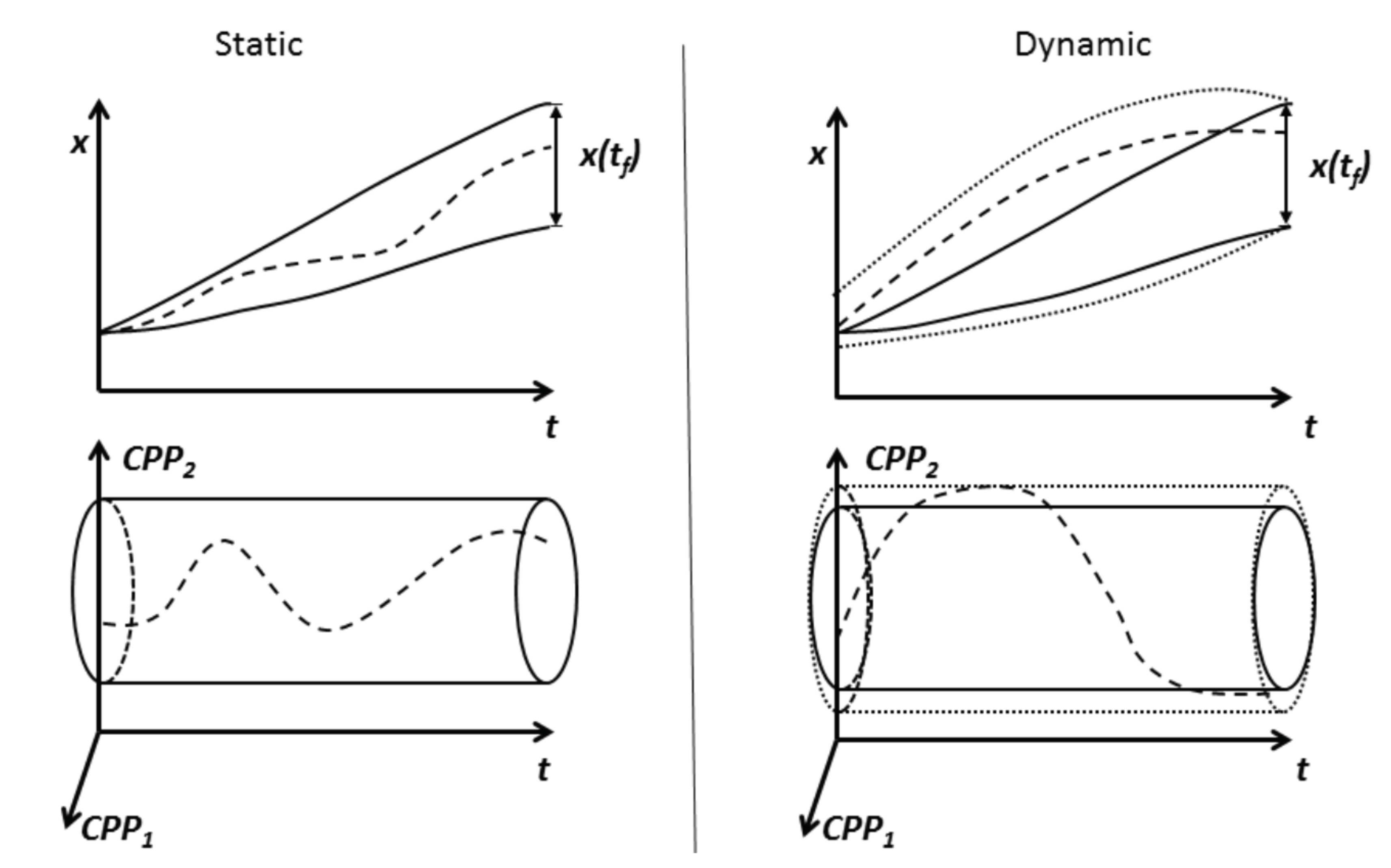
3. A Dynamic Design Space Model
3.1. Suitable Representations of the Dynamic Process Design Space for Process Operation and Approval Filing
3.2. Approaches to Dynamic Design Space Exploration
3.3. Determination of the Design Space Boundaries—Edge of Failure
3.4. Verification of the Design Space
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PAT | Process Analytic Technology |
| FDA | U.S. Food and Drug Administration |
| ICH | International Conference on Harmonization |
| QbD | Quality by Design |
| QbC | Quality by Control |
| DoE | Design of Experiments |
| iDoE | intensified Design of Experiments |
| DoDE | Design of Dynamic Experiments |
| QTPP | Quality Target Product Profile |
| CQA | Critical Quality Attributes |
| CPP | Critical Process Parameters |
| CMA | Critical Material Attributes |
| ANOVA | Analysis of Variance |
| MPC | Model Predictive Control |
| ODEs | Ordinary Differential Equations |
| SA | Sensitivity Analysis |
| PCA | Principal Component Analysis |
| PLS | Partial Least Square |
References
- Rathore, A.S. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009, 27, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.K.; French, J.L.; Kowalski, K.G.; Hutmacher, M.M.; Ewy, W. Quality by Design for Biopharmaceutical Drug Product Development; Springer: Berlin/Heidelberg, Germany, 2015; Volume 18, p. 710. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Hakemeyer, C.; McKnight, N.; St. John, R.; Meier, S.; Trexler-Schmidt, M.; Kelley, B.; Zettl, F.; Puskeiler, R.; Kleinjans, A.; Lim, F.; et al. Process characterization and Design Space definition. Biologicals 2016, 44, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Diab, S.; Gerogiorgis, D.I. Design Space Identification and Visualization for Continuous Pharmaceutical Manufacturing. Pharmaceutics 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- ICH Expert Working Group. Pharmaceutical development Q8(R2). ICH Harmon. Tripart. Guidel. 2009. [Google Scholar] [CrossRef]
- Debevec, V.; Srčič, S.; Horvat, M. Scientific, statistical, practical, and regulatory considerations in design space development. Drug Dev. Ind. Pharm. 2018, 44, 349–364. [Google Scholar] [CrossRef]
- Kahrs, O.; Marquardt, W. The validity domain of hybrid models and its application in process optimization. Chem. Eng. Process. Process Intensif. 2007, 46, 1054–1066. [Google Scholar] [CrossRef]
- Barber, C.B.; Dobkin, D.P.; Huhdanpaa, H. The Quickhull Algorithm for Convex Hulls. ACM Trans. Math. Softw. 1996, 22, 469–483. [Google Scholar] [CrossRef]
- A-Mab: A Case Study in Bioprocess Development; Technical Report; CMC Biotech Working Group: Emeryville, CA, USA, 2009.
- Castagnoli, C.; Yahyah, M.; Cimarosti, Z.; Peterson, J.J. Application of quality by design principles for the definition of a robust crystallization process for casopitant mesylate. Org. Process Res. Dev. 2010, 14, 1407–1419. [Google Scholar] [CrossRef]
- Quiñones, L.; Obregón, L.; Velázquez, C. A Perspective on the Implementation of QbD on Manufacturing through Control System. In Comprehensive Quality by Design for Pharmaceutical Product Development and Manufacture; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 339–359. [Google Scholar] [CrossRef]
- Reklaitis, G.V.; Seymour, C.; García-Munoz, S. (Eds.) Comprehensive Quality by Design for Pharmaceutical Product Development and Manufacture; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Fissore, D.; Pisano, R.; Barresi, A.A. A Model-Based Framework to Optimize Pharmaceuticals Freeze Drying. Dry. Technol. 2012, 30, 946–958. [Google Scholar] [CrossRef]
- Harinath, E.; Foguth, L.C.; Braatz, R.D. A robust dual-mode MPC approach to ensuring critical quality attributes in Quality-by-Design. In Proceedings of the American Control Conference, Boston, MA, USA, 6–8 July 2016; Volume 2016, pp. 2041–2046. [Google Scholar] [CrossRef]
- Lee, J.H. Model predictive control: Review of the three decades of development. Int. J. Control. Autom. Syst. 2011, 9, 415. [Google Scholar] [CrossRef]
- Mortier, S.T.F.; Van Bockstal, P.J.; Corver, J.; Nopens, I.; Gernaey, K.V.; De Beer, T. Uncertainty analysis as essential step in the establishment of the dynamic Design Space of primary drying during freeze-drying. Eur. J. Pharm. Biopharm. 2016, 103, 71–83. [Google Scholar] [CrossRef]
- Xie, X.; Schenkendorf, R. Stochastic back-off-based robust process design for continuous crystallization of ibuprofen. Comput. Chem. Eng. 2019, 124, 80–92. [Google Scholar] [CrossRef]
- Rantanen, J.; Khinast, J. The Future of Pharmaceutical Manufacturing Sciences. J. Pharm. Sci. Publ. Wiley Period. Inc. Am. Pharm. Assoc. J. Pharm. Sci. 2015, 104, 3612–3638. [Google Scholar] [CrossRef] [PubMed]
- Kusumo, K.P.; Gomoescu, L.; Paulen, R.; Garciá Munõz, S.; Pantelides, C.C.; Shah, N.; Chachuat, B. Bayesian Approach to Probabilistic Design Space Characterization: A Nested Sampling Strategy. Ind. Eng. Chem. Res. 2020, 59, 2396–2408. [Google Scholar] [CrossRef]
- Xie, X.; Schenkendorf, R. Robust Process Design in Pharmaceutical Manufacturing under Batch-to-Batch Variation. Processes 2019, 7, 509. [Google Scholar] [CrossRef]
- Montes, F.C.C.; Gernaey, K.; Sin, G. Dynamic Plantwide Modeling, Uncertainty, and Sensitivity Analysis of a Pharmaceutical Upstream Synthesis: Ibuprofen Case Study. Ind. Eng. Chem. Res. 2018, 57, 10026–10037. [Google Scholar] [CrossRef]
- Xie, X.; Schenkendorf, R. Robust optimization of a pharmaceutical freeze-drying process under non-Gaussian parameter uncertainties. Chem. Eng. Sci. 2019, 207, 805–819. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Braatz, R.D. Worst-case and distributional robustness analysis of finite-time control trajectories for nonlinear distributed parameter systems. IEEE Trans. Control Syst. Technol. 2003, 11, 694–704. [Google Scholar] [CrossRef]
- Beyer, H.G.; Sendhoff, B. Robust optimization–a comprehensive survey. Comput. Methods Appl. Mech. Eng. 2007, 196, 3190–3218. [Google Scholar] [CrossRef]
- Xie, X.; Schenkendorf, R.; Krewer, U. Toward a Comprehensive and Efficient Robust Optimization Framework for (Bio)chemical Processes. Processes 2018, 6, 183. [Google Scholar] [CrossRef]
- Gernaey, K.V.; Cervera-Padrell, A.E.; Woodley, J.M. A perspective on PSE in pharmaceutical process development and innovation. Comput. Chem. Eng. 2012, 42, 15–29. [Google Scholar] [CrossRef]
- Metta, N.; Ghijs, M.; Schäfer, E.; Kumar, A.; Cappuyns, P.; Van Assche, I.; Singh, R.; Ramachandran, R.; De Beer, T.; Ierapetritou, M.; et al. Dynamic flowsheet model development and sensitivity analysis of a continuous pharmaceutical tablet manufacturing process using the wet granulation route. Processes 2019, 7, 234. [Google Scholar] [CrossRef]
- Wang, Z.; Ierapetritou, M. Global sensitivity, feasibility, and flexibility analysis of continuous pharmaceutical manufacturing processes. In Computer Aided Chemical Engineering, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 41, pp. 189–213. [Google Scholar] [CrossRef]
- Sommeregger, W.; Sissolak, B.; Kandra, K.; von Stosch, M.; Mayer, M.; Striedner, G. Quality by control: Towards model predictive control of mammalian cell culture bioprocesses. Biotechnol. J. 2017, 12, 1600546. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, B.; Borsos, Á.; Pal, K.; Nagy, Z.K. Experimental implementation of a Quality-by-Control (QbC) framework using a mechanistic PBM-based nonlinear model predictive control involving chord length distribution measurement for the batch cooling crystallization of l-ascorbic acid. Chem. Eng. Sci. 2019, 195, 335–346. [Google Scholar] [CrossRef]
- Su, Q.; Ganesh, S.; Moreno, M.; Bommireddy, Y.; Gonzalez, M.; Reklaitis, G.V.; Nagy, Z.K. A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing. Comput. Chem. Eng. 2019, 125, 216–231. [Google Scholar] [CrossRef]
- Djuris, J.; Djuric, Z. Modeling in the quality by design environment: Regulatory requirements and recommendations for design space and control strategy appointment. Int. J. Pharm. 2017. [Google Scholar] [CrossRef]
- Daoutidis, P.; Lee, J.H.; Harjunkoski, I.; Skogestad, S.; Baldea, M.; Georgakis, C. Integrating operations and control: A perspective and roadmap for future research. Comput. Chem. Eng. 2018, 115, 179–184. [Google Scholar] [CrossRef]
- Rehrl, J.; Karttunen, A.P.; Nicolaï, N.; Hörmann, T.; Horn, M.; Korhonen, O.; Nopens, I.; De Beer, T.; Khinast, J.G. Control of three different continuous pharmaceutical manufacturing processes: Use of soft sensors. Int. J. Pharm. 2018, 543, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Sacher, S.; Celikovic, S.; Rehrl, J.; Poms, J.; Kirchengast, M.; Kruisz, J.; Sipek, M.; Salar-Behzadi, S.; Berger, H.; Stark, G.; et al. Towards a novel continuous HME-Tableting line: Process development and control concept. Eur. J. Pharm. Sci. 2020, 142, 105097. [Google Scholar] [CrossRef]
- Capellades, G.; Neurohr, C.; Azad, M.; Brancazio, D.; Rapp, K.; Hammersmith, G.; Myerson, A.S. A Compact Device for the Integrated Filtration, Drying, and Mechanical Processing of Active Pharmaceutical Ingredients. J. Pharm. Sci. 2020, 109, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, A.; Nagy, Z.K.; Huesman, A.E.M.; Kramer, H.J.M.; Van den Hof, P.M.J. Nonlinear Model-Based Control of a Semi-Industrial Batch Crystallizer Using a Population Balance Modeling Framework. IEEE Trans. Control Syst. Technol. 2012, 20, 1188–1201. [Google Scholar] [CrossRef]
- Berkenkamp, F.; Schoellig, A.P.; Turchetta, M.; Krause, A. Safe Model-based Reinforcement Learning with Stability gurantees. In Proceedings of the 31st Conference Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Mesbah, A.; Paulson, J.A.; Lakerveld, R.; Braatz, R.D. Model Predictive Control of an Integrated Continuous Pharmaceutical Manufacturing Pilot Plant. Org. Process Res. Dev. 2017, 21, 844–854. [Google Scholar] [CrossRef]
- Papathanasiou, M.M.; Burnak, B.; Katz, J.; Shah, N.; Pistikopoulos, E.N. Assisting continuous biomanufacturing through advanced control in downstream purification. Comput. Chem. Eng. 2019, 125, 232–248. [Google Scholar] [CrossRef]
- Kager, J.; Tuveri, A.; Ulonska, S.; Kroll, P.; Herwig, C. Experimental verification and comparison of model predictive, PID and model inversion control in a Penicillium chrysogenum fed-batch process. Process Biochem. 2020, 90, 1–11. [Google Scholar] [CrossRef]
- Mesbah, A. Stochastic Model Predictive Control: An Overview and Perspectives for Future Research. IEEE Control Syst. Mag. 2016, 36, 30–44. [Google Scholar]
- Vanbillemont, B.; NicolaÃ, N.; Leys, L.; De Beer, T. Model-Based Optimisation and Control Strategy for the Primary Drying Phase of a Lyophilisation Process. Pharmaceutics 2020, 12, 181. [Google Scholar] [CrossRef]
- Sharifzadeh, M. Integration of process design and control: A review. Chem. Eng. Res. Des. 2013, 91, 2515–2549. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, Y.; Su, H.; Wozny, G.; Pan, H. A survey on applications of optimization-based integrating process design and control for chemical processes. Chem. Eng. Commun. 2018, 205, 1365–1383. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Huusom, J.K.; Gani, R.; Sales-Cruz, M. Systematic integrated process design and control of binary element reactive distillation processes. AIChE J. 2016, 62, 3137–3154. [Google Scholar] [CrossRef]
- Burnak, B.; Diangelakis, N.A.; Pistikopoulos, E.N. Towards the Grand Unification of Process Design, Scheduling, and Control—Utopia or Reality? Processes 2019, 7, 461. [Google Scholar] [CrossRef]
- Bano, G.; Facco, P.; Ierapetritou, M.; Bezzo, F.; Barolo, M. Design space maintenance by online model adaptation in pharmaceutical manufacturing. Comput. Chem. Eng. 2019, 127, 254–271. [Google Scholar] [CrossRef]
- MacGregor, J.F.; Bruwer, M.J. A framework for the development of design and control spaces. J. Pharm. Innov. 2008, 3, 15–22. [Google Scholar] [CrossRef]
- Bano, G.; Facco, P.; Bezzo, F.; Barolo, M. Probabilistic Design space determination in pharmaceutical product development: A Bayesian/latent variable approach. AIChE J. 2018, 64, 2438–2449. [Google Scholar] [CrossRef]
- Bano, G.; Wang, Z.; Facco, P.; Bezzo, F.; Barolo, M.; Ierapetritou, M. A novel and systematic approach to identify the design space of pharmaceutical processes. Comput. Chem. Eng. 2018, 115, 309–322. [Google Scholar] [CrossRef]
- Gorban, A.N. Model reduction in chemical dynamics: Slow invariant manifolds, singular perturbations, thermodynamic estimates, and analysis of reaction graph. Curr. Opin. Chem. Eng. 2018, 21, 48–59. [Google Scholar] [CrossRef]
- Bonvin, D.; Georgakis, C.; Pantelides, C.C.; Barolo, M.; Grover, M.A.; Rodrigues, D.; Schneider, R.; Dochain, D. Linking Models and Experiments. Ind. Eng. Chem. Res. 2016, 55, 6891–6903. [Google Scholar] [CrossRef]
- Bhosekar, A.; Ierapetritou, M. Advances in surrogate based modeling, feasibility analysis, and optimization: A review. Comput. Chem. Eng. 2018, 108, 250–267. [Google Scholar] [CrossRef]
- Stosch, M.V.; Hamelink, J.M.; Oliveira, R. Hybrid modeling as a QbD / PAT tool in process development: An industrial E. coli case study. Bioprocess Biosyst. Eng. 2016, 39, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Pisano, R.; Fissore, D.; Barresi, A.A. In-Line and Off-Line Optimization of Freeze-Drying Cycles for Pharmaceutical Products. Dry. Technol. 2013, 31, 905–919. [Google Scholar] [CrossRef][Green Version]
- Burt, J.L.; Braem, A.D.; Ramirez, A.; Mudryk, B.; Rossano, L.; Tummala, S. Model-guided design space development for a drug substance manufacturing process. J. Pharm. Innov. 2011, 6, 181–192. [Google Scholar] [CrossRef]
- Fissore, D.; Pisano, R.; Barresi, A.A. Advanced approach to build the design space for the primary drying of a pharmaceutical freeze-drying process. J. Pharm. Sci. 2011, 100, 4922–4933. [Google Scholar] [CrossRef]
- Adam, S.; Suzzi, D.; Radeke, C.; Khinast, J.G. An integrated Quality by Design (QbD) approach towards design space definition of a blending unit operation by Discrete Element Method (DEM) simulation. Eur. J. Pharm. Sci. 2011, 42, 106–115. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, S.; Luciani, C.V.; Vaidyaraman, S.; Seibert, K.D. Definition of Design Spaces Using Mechanistic Models and Geometric Projections of Probability Maps. Org. Process Res. Dev. 2015, 19, 1012–1023. [Google Scholar] [CrossRef]
- Sun, F.; Xu, B.; Dai, S.; Zhang, Y.; Lin, Z.; Qiao, Y. A Novel Framework to Aid the Development of Design Space across Multi-Unit Operation Pharmaceutical Processes—A Case Study of Panax Notoginseng Saponins Immediate Release Tablet. Pharmaceutics 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Von Stosch, M.; Hamelink, J.M.; Oliveira, R. Toward intensifying design of experiments in upstream bioprocess development: An industrial Escherichia coli feasibility study. Biotechnol. Prog. 2016, 32, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Von Stosch, M.; Willis, M.J. Intensified design of experiments for upstream bioreactors. Eng. Life Sci. 2017, 17, 1173–1184. [Google Scholar] [CrossRef]
- Georgakis, C. Design of dynamic experiments: A data-driven methodology for the optimization of time-varying processes. Ind. Eng. Chem. Res. 2013, 52, 12369–12382. [Google Scholar] [CrossRef]
- Cruz Bournazou, M.N.; Barz, T.; Nickel, D.B.; Lopez Cárdenas, D.C.; Glauche, F.; Knepper, A.; Neubauer, P. Online optimal experimental re-design in robotic parallel fed-batch cultivation facilities. Biotechnol. Bioeng. 2017, 114, 610–619. [Google Scholar] [CrossRef]
- Glassey, J.; von Stosch, M. (Eds.) Hybrid Modeling in Process Industries; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Kishida, M.; Braatz, R.D. Skewed structured singular value-Based approach for the construction of design spaces: Theory and applications. IET Control Theory Appl. 2014, 8, 1321–1327. [Google Scholar] [CrossRef]
- Harinath, E.; Foguth, L.C.; Braatz, R.D. Maximization of ellipsoidal design space for continuous-time systems: A robust optimal control approach. In Proceedings of the American Control Conference, Boston, MA, USA, 6–8 July 2016; Volume 2016, pp. 3850–3855. [Google Scholar] [CrossRef]
- Stockdale, G.W.; Cheng, A. Finding Design Space and a Reliable Operating Region Using a Multivariate Bayesian Approach with Experimental Design. Qual. Technol. Quant. Manag. 2009, 6, 391–408. [Google Scholar] [CrossRef]
- Gong, X.; Li, Y.; Chen, H.; Qu, H. Design space development for the extraction process of Danhong injection using a Monte Carlo simulation method. PLoS ONE 2015, 10, e0128236. [Google Scholar] [CrossRef]
- Peterson, J.J.; Yahyah, M.; Lief, K.; Hodnett, N. Predictive Distributions for Constructing the ICH Q8 Design Space. In Comprehensive Quality by Design for Pharmaceutical Product Development and Manufacture; John and Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 55–70. [Google Scholar] [CrossRef]
- Althoff, M.; Krogh, B.H. Reachability analysis of nonlinear differential-algebraic systems. IEEE Trans. Autom. Control 2014, 59, 371–383. [Google Scholar] [CrossRef]
- Apgar, J.F.; Toettcher, J.E.; Endy, D.; White, F.M.; Tidor, B. Stimulus design for model selection and validation in cell signaling. PLoS Comput. Biol. 2008, 4. [Google Scholar] [CrossRef] [PubMed]
- Close, E.J.; Salm, J.R.; Bracewell, D.G.; Sorensen, E. A model based approach for identifying robust operating conditions for industrial chromatography with process variability. Chem. Eng. Sci. 2014, 116, 284–295. [Google Scholar] [CrossRef]
- Neubauer, P.; Junne, S. Scale-Up and Scale-Down Methodologies for Bioreactors. In Bioreactors; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 323–354. [Google Scholar] [CrossRef]
- Neubauer, P.; Junne, S. Scale-down simulators for metabolic analysis of large-scale bioprocesses. Curr. Opin. Biotechnol. 2010, 21, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Mandenius, C.F.; Titchener-Hooker, N.J. Measurement, Monitoring, Modelling and Control of Bioprocesses; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 132. [Google Scholar] [CrossRef]
- Ten Have, R.; Reubsaet, K.; Van Herpen, P.; Kersten, G.; Amorij, J.P. Demonstrating functional equivalence of pilot and production scale freeze-drying of BCG. PLoS ONE 2016, 11, e0151239. [Google Scholar] [CrossRef] [PubMed]
- Grant, Y.; Matejtschuk, P.; Dalby, P.A. Rapid optimization of protein freeze-drying formulations using ultra scale-down and factorial design of experiment in microplates. Biotechnol. Bioeng. 2009, 104, 957–964. [Google Scholar] [CrossRef] [PubMed]
- FDA/EMA. Questions and Answers on Design Space Verification; Technical Report; European Medicines Agency: Amsterdam, The Netherlands, 2013. [Google Scholar]
| Modeling Approach | Area of Application | Design Space Characterization and Representation | |
|---|---|---|---|
| Burt et al. [58] | Combination of a material balance based set of ordinary differential equations with a statistical model describing the impurities | Chemical drug substance manufacturing process | DynoChem used for design space exploration, which seems to perform a grid-evaluation for design space characterization. Rectangular design space representation. |
| Fissore et al. [59] | Material and Energy balance based Ordinary Differential Equation | Freeze drying: Primary drying step | A grid evaluation technique for characterizing the design space and visual representation of the limits. |
| Adam et al. [60] | Material, Momentum and Energy balances solved using Discrete Element Method and Computational Fluid Dynamics | Pharmaceutical Blending Process | The model is exploited to create examples of contour plots for some CPPs but cannot be used for advanced process control. |
| García-Muñoz et al. [61] | (1) Mass balance in the packed bed column and scavenger particle level in form of Partial differential Equations (2) Mass balance of the Suzuki coupling reaction components in form of Ordinary Differential Equations | (1) Continuous Pd Removal in Packed Bed Columns (2) Suzuki Reaction | Grid-evaluation for design space characterization and uncertainty evaluation. Geometric representation of the Design Space. |
| Mortier et al. [17] | Material and Energy balance based Ordinary Differential Equation | Freeze drying: Primary drying step | Grid-evaluation for design space characterization and uncertainty evaluation. |
| Sun et al. [62] | Statistical modeling of a multi-unit operation process | Panax Notoginseng Saponins immediate release tablet | Multi-block partial least squares path model. |
| Vanbillemont et al. [44] | Material and energy balance based Ordinary Differential Equation | Freeze drying: Primary drying step | Supervisor process control for dynamic design space implementation under uncertainty. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Stosch, M.; Schenkendorf, R.; Geldhof, G.; Varsakelis, C.; Mariti, M.; Dessoy, S.; Vandercammen, A.; Pysik, A.; Sanders, M. Working within the Design Space: Do Our Static Process Characterization Methods Suffice? Pharmaceutics 2020, 12, 562. https://doi.org/10.3390/pharmaceutics12060562
von Stosch M, Schenkendorf R, Geldhof G, Varsakelis C, Mariti M, Dessoy S, Vandercammen A, Pysik A, Sanders M. Working within the Design Space: Do Our Static Process Characterization Methods Suffice? Pharmaceutics. 2020; 12(6):562. https://doi.org/10.3390/pharmaceutics12060562
Chicago/Turabian Stylevon Stosch, Moritz, René Schenkendorf, Geoffroy Geldhof, Christos Varsakelis, Marco Mariti, Sandrine Dessoy, Annick Vandercammen, Alexander Pysik, and Matthew Sanders. 2020. "Working within the Design Space: Do Our Static Process Characterization Methods Suffice?" Pharmaceutics 12, no. 6: 562. https://doi.org/10.3390/pharmaceutics12060562
APA Stylevon Stosch, M., Schenkendorf, R., Geldhof, G., Varsakelis, C., Mariti, M., Dessoy, S., Vandercammen, A., Pysik, A., & Sanders, M. (2020). Working within the Design Space: Do Our Static Process Characterization Methods Suffice? Pharmaceutics, 12(6), 562. https://doi.org/10.3390/pharmaceutics12060562






