The Influence of Co-Surfactants on Lamellar Liquid Crystal Structures Formed in Creams
Abstract
1. Introduction
2. Materials
3. Methods
3.1. Method of Sample Preparation
3.2. Microscopy
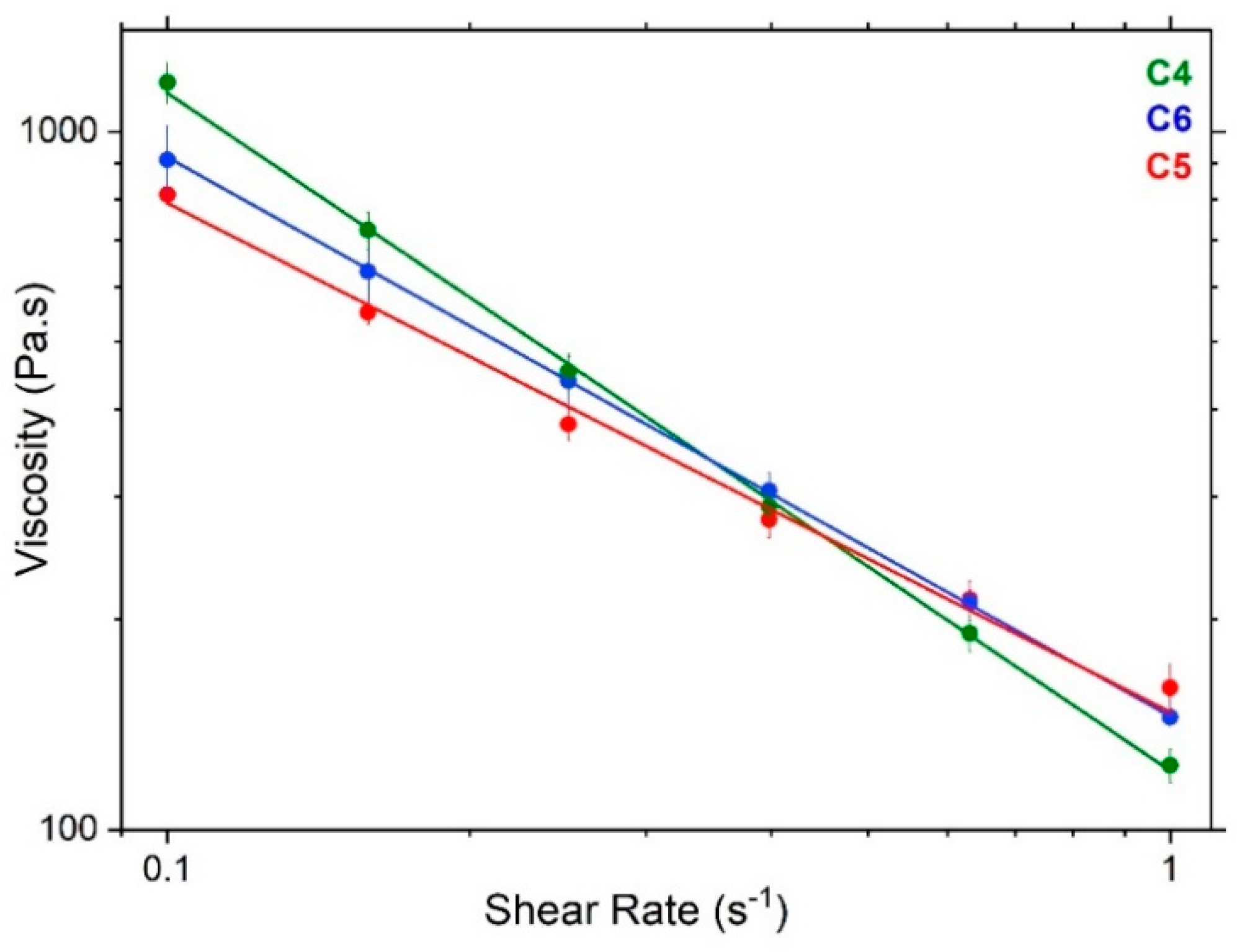
3.3. Rheology
3.4. Small and Wide-Angle X-ray Scattering (SAXS and WAXS)
3.5. Small-Angle Neutron Scattering (SANS)
4. Results
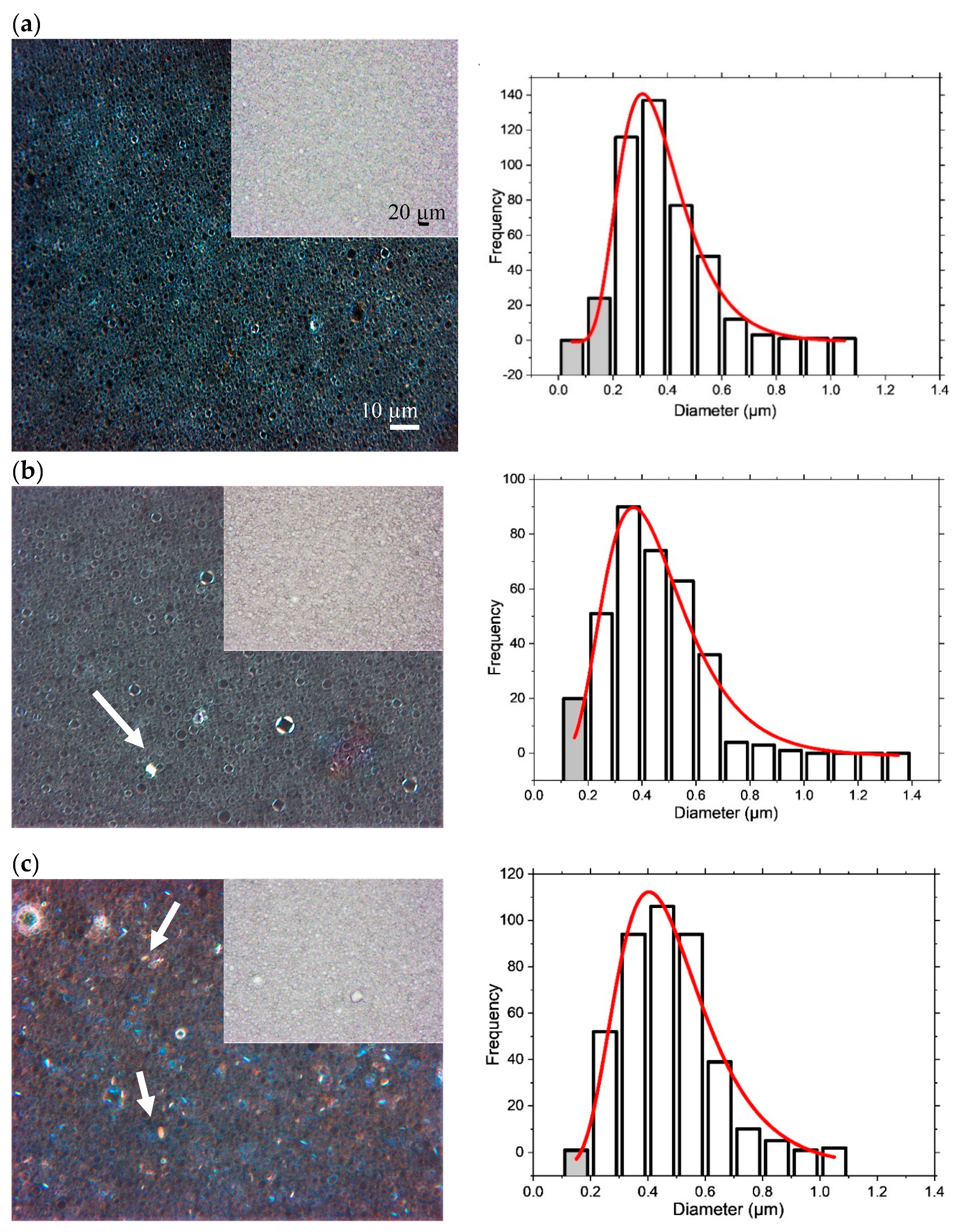
4.1. Macroscopic Structure
4.2. Microscopic Structure
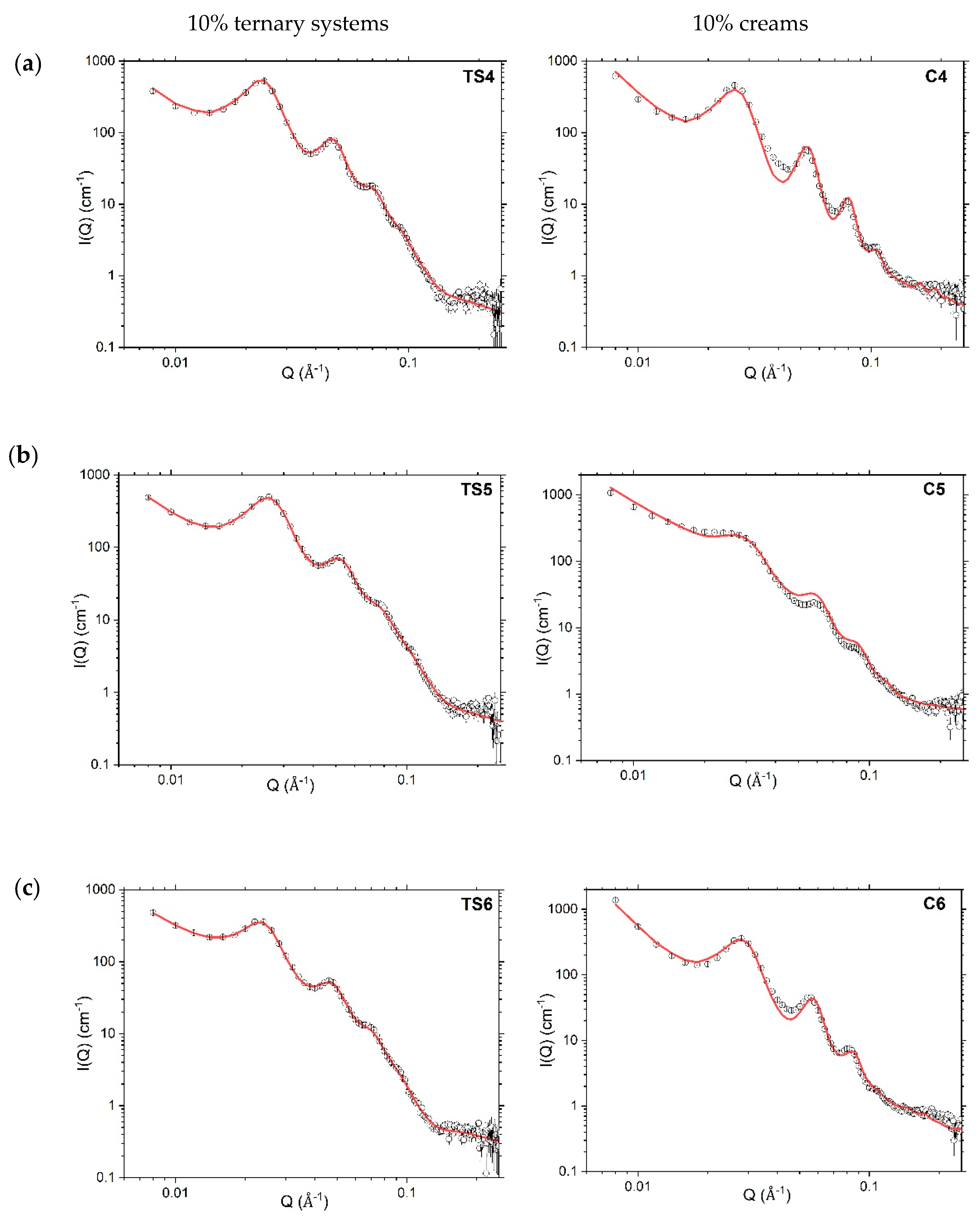
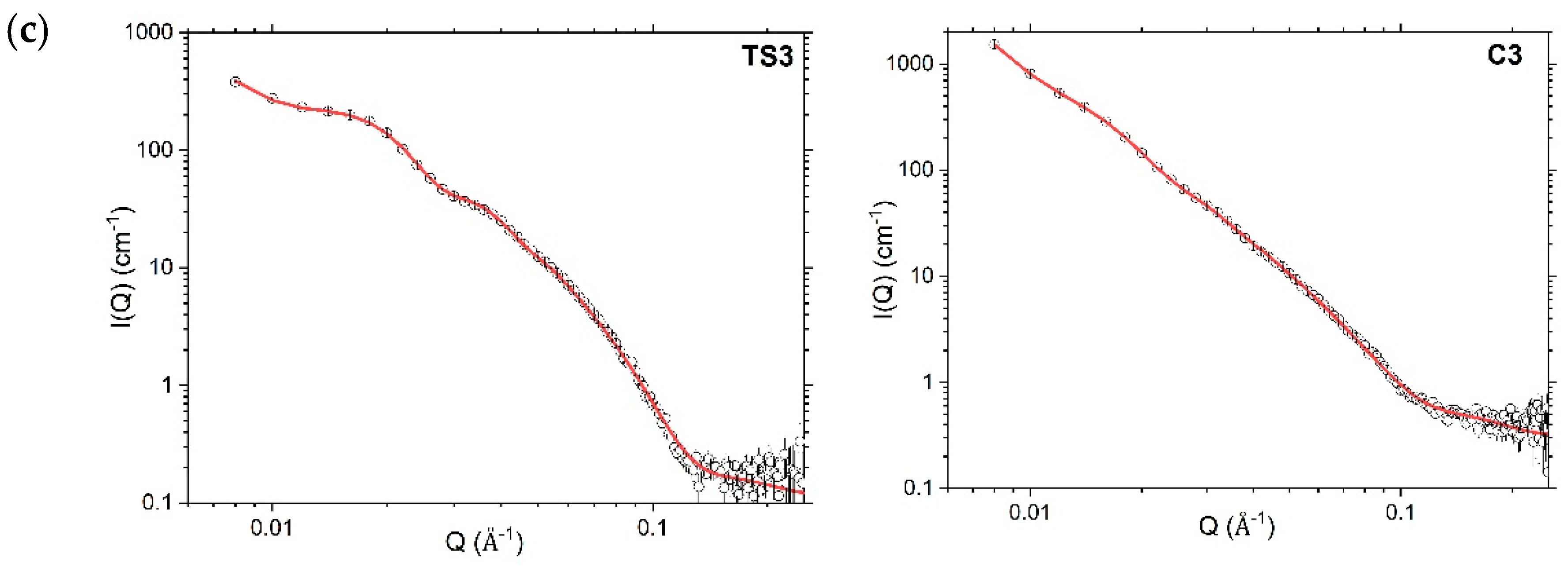
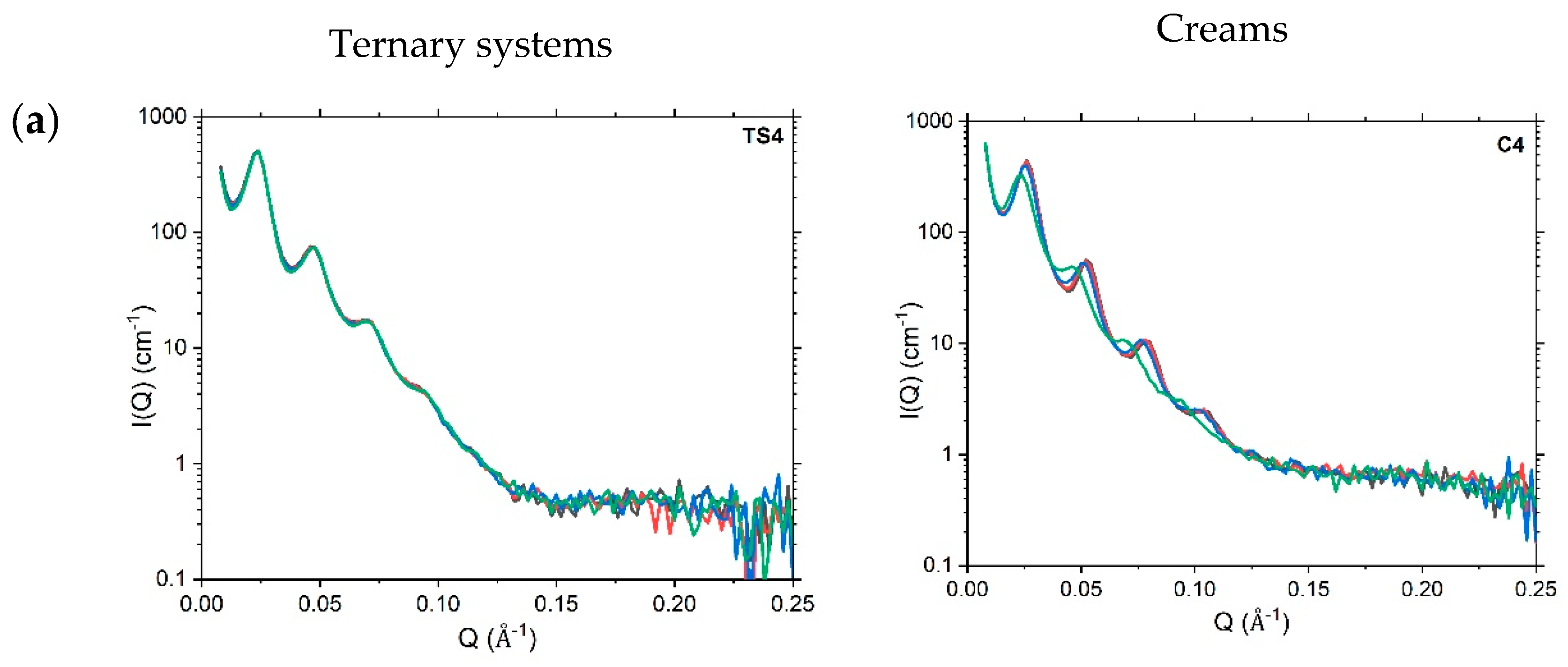
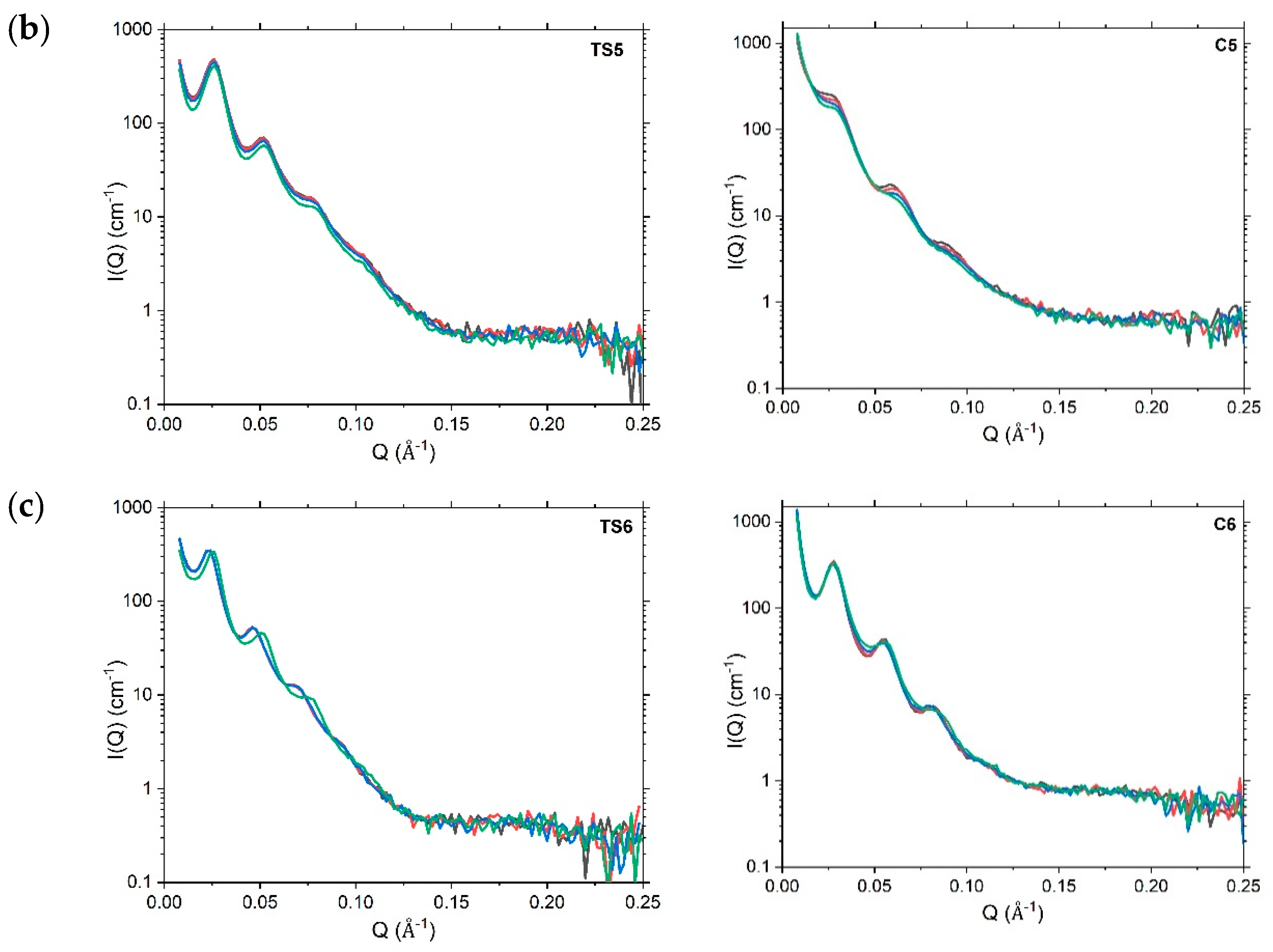
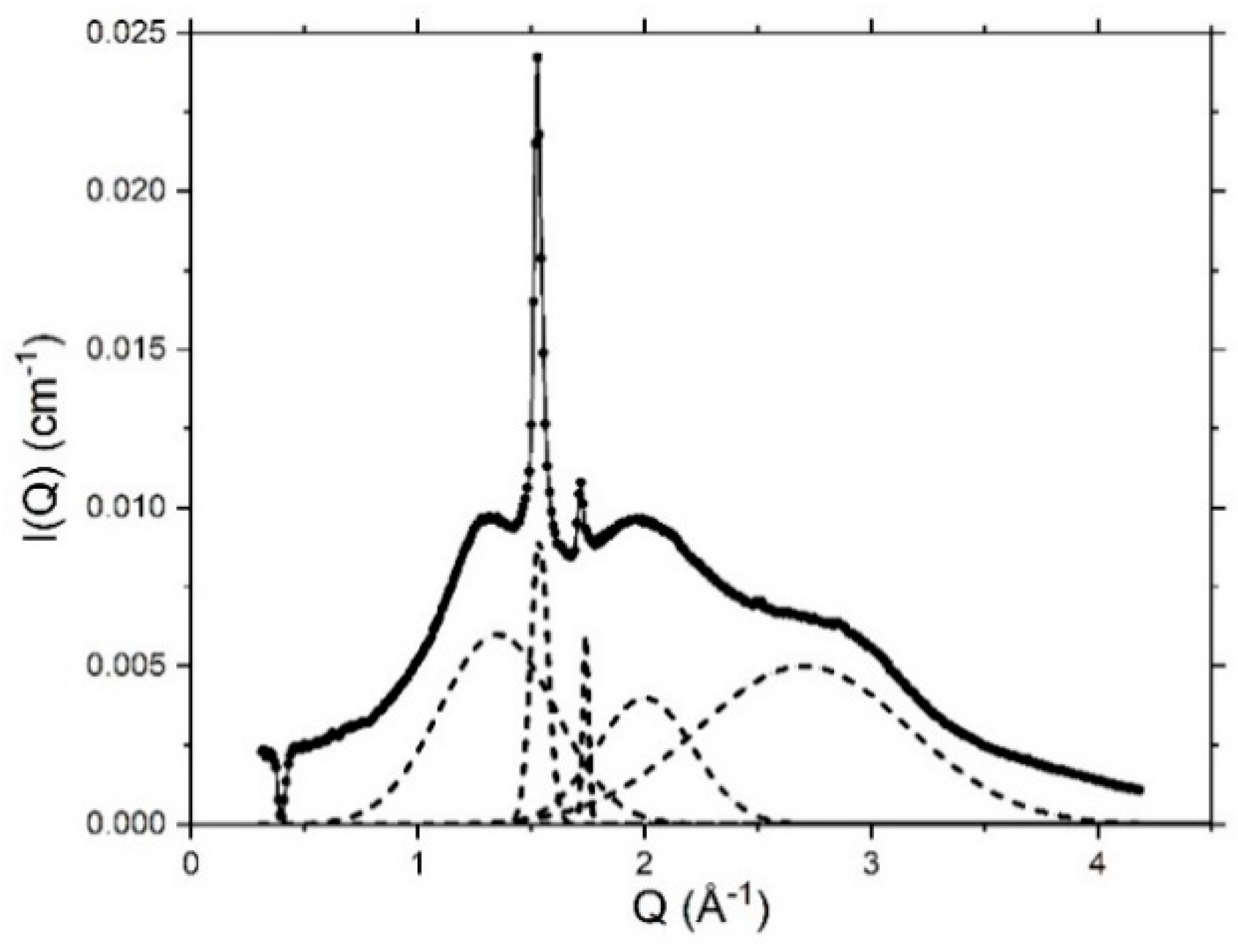
4.3. Nanoscopic Structure
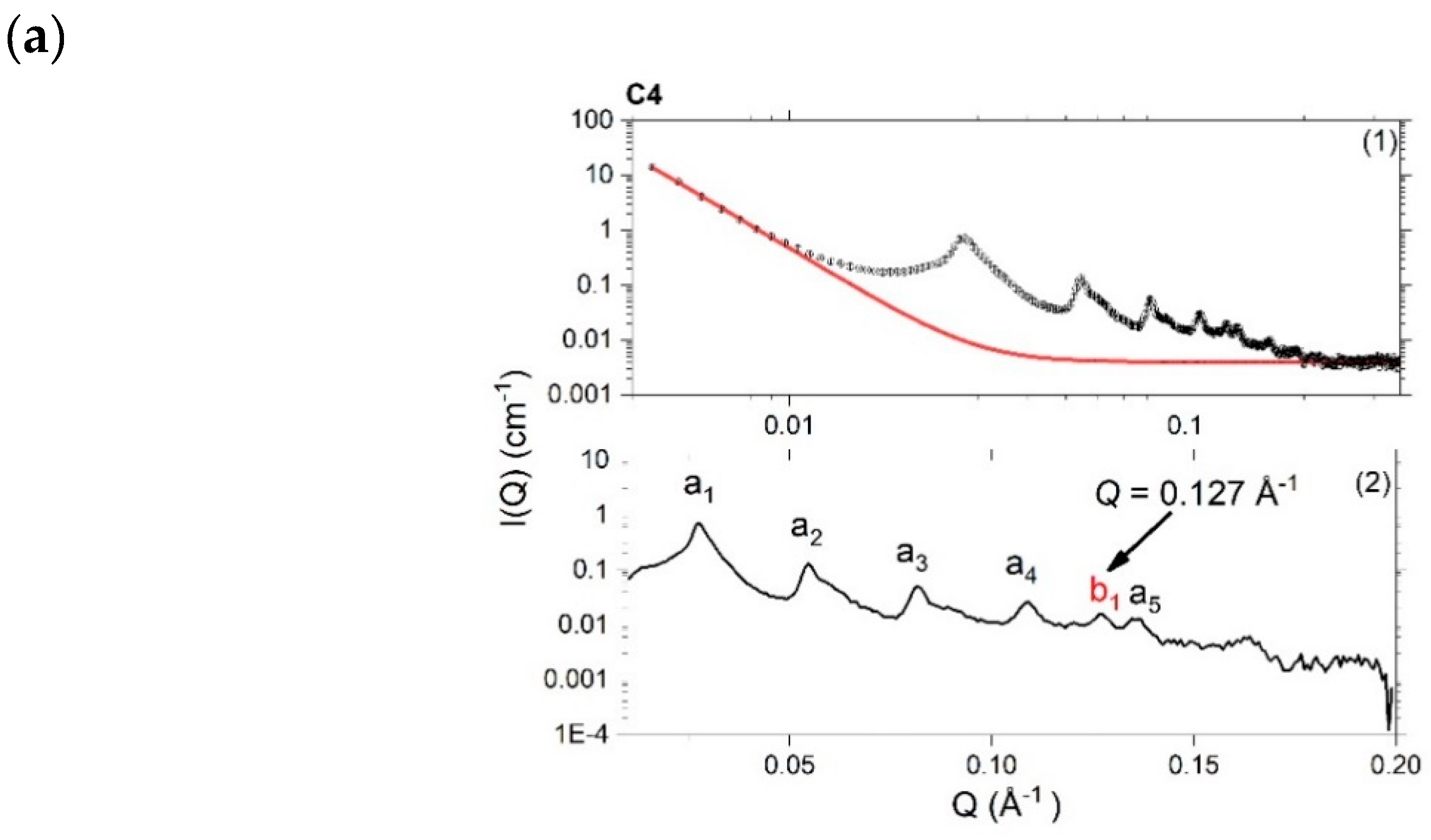
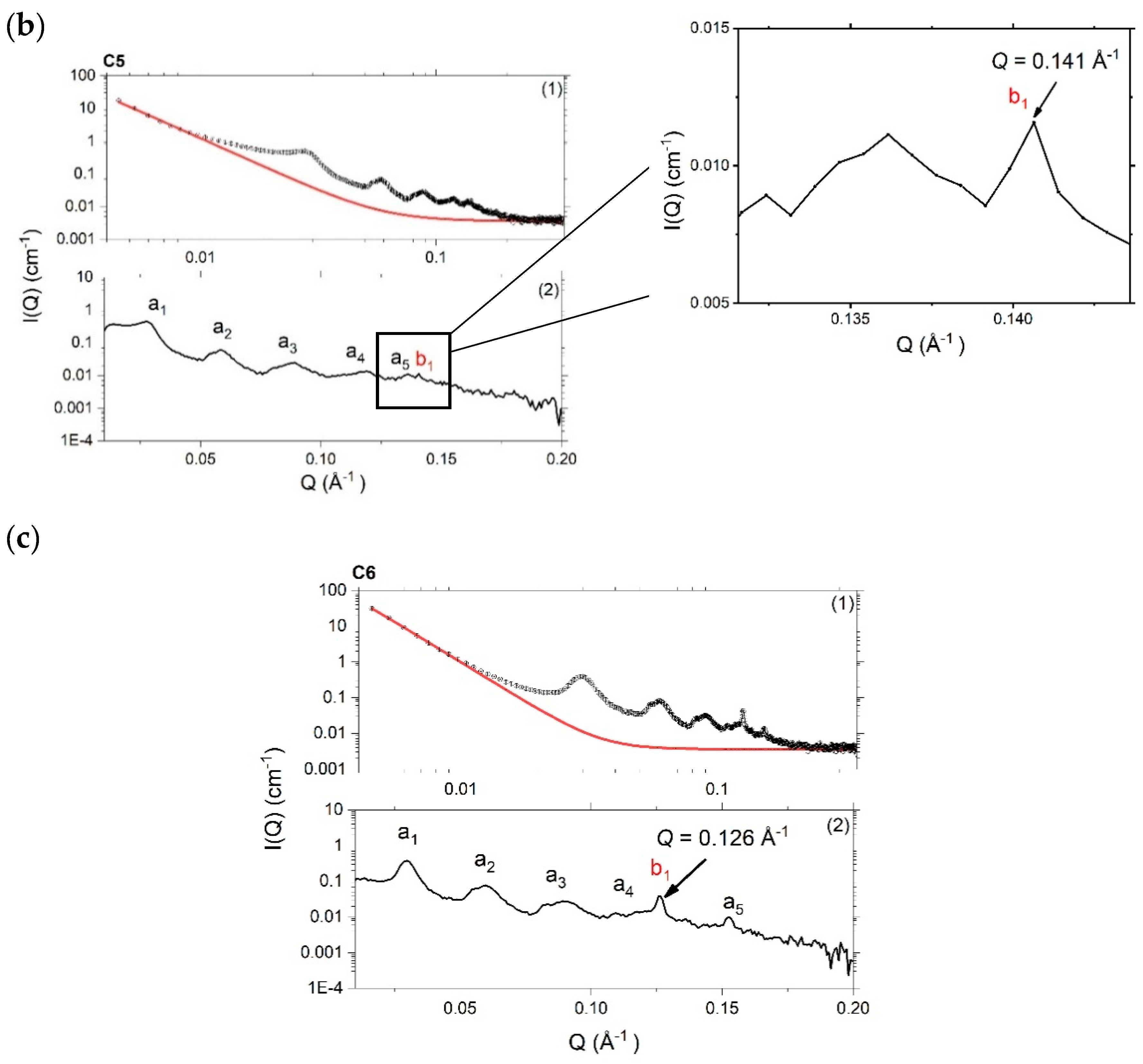
4.3.1. Small-Angle X-ray Scattering (SAXS)
4.3.2. Small-Angle Neutron Scattering
4.3.3. Variations in SANS Profiles with Temperature
4.3.4. Wide-Angle X-ray Scattering (WAXS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tiddy, G. Surfactant-water liquid crystal phases. Phys. Rep. 1980, 57, 1–46. [Google Scholar] [CrossRef]
- Engels, T.; von Rybinski, W. Liquid crystalline surfactant phases in chemical applications. J. Mater. Chem. 1998, 8, 1313–1320. [Google Scholar] [CrossRef]
- Ahmadi, D.; Mahmoudi, N.; Li, P.; Ma, K.; Doutch, J.; Foglia, F.; Heenan, R.K.; Barlow, D.; Lawrence, M.J. Revealing the Hidden Details of Nanostructure in a Pharmaceutical Cream. Sci. Rep. 2020, 10, 4082. [Google Scholar] [CrossRef]
- Eccleston, G.M. Functions of mixed emulsifiers and emulsifying waxes in dermatological lotions and creams. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123–124, 169–182. [Google Scholar] [CrossRef]
- Kónya, M.; Dékány, I.; Erõs, I. X-ray investigation of the role of the mixed emulsifier in the structure formation in o/w creams. Colloid Polym. Sci. 2007, 285, 657–663. [Google Scholar] [CrossRef]
- Friberg, S.; Mandell, L. Phase equilibria and their influence on the properties of emulsions. J. Am. Oil Chem. Soc. 1970, 47, 149–152. [Google Scholar] [CrossRef]
- Friberg, S. Liquid crystalline phases in emulsions. J. Colloid Interface Sci. 1971, 37, 291–295. [Google Scholar] [CrossRef]
- Friberg, S.E.; Solans, C. Surfactant association structures and the stability of emulsions and foams. Langmuir 1986, 2, 121–126. [Google Scholar] [CrossRef]
- Vilasau, J.; Solans, C.; Gómez, M.J.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Phase behaviour of a mixed ionic/nonionic surfactant system used to prepare stable oil-in-water paraffin emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 473–481. [Google Scholar] [CrossRef]
- Friberg, S.E.; Al-Bawab, A. Micelies, microemulsionsli, quidcrystals, and the structure of stratumcorneumlipids. Adv. Colloid Interface Sci. 2006, 313–322. [Google Scholar]
- Eccleston, G.M. Phase transitions in ternary systems and oil-in-water emulsions containing cetrimide and fatty alcohols. Int. J. Pharm. 1985, 27, 311–323. [Google Scholar] [CrossRef]
- Ahmadi, D.; Barlow, D.J.; Lawrence, M.J.; Mahmoudi, N.; Peixun, L.; Tellam, J. Simple Creams, Complex Structures. In Nanoparticle and Molecular Assemblies; American Chemical Society: Washington, DC, USA, 2020; Chapter 6. [Google Scholar]
- Thomas, R.K.; Penfold, J. Multilayering of Surfactant Systems at the Air–Dilute Aqueous Solution Interface. Langmuir 2015, 31, 7440–7456. [Google Scholar] [CrossRef]
- Tucker, I.; Penfold, J.; Thomas, R.K.; Grillo, I.; Barker, J.G.; Mildner, D.F.R. The Surface and Solution Properties of Dihexadecyl Dimethylammonium Bromide. Langmuir 2008, 24, 6509–6520. [Google Scholar] [CrossRef]
- Tucker, I.; Penfold, J.; Thomas, R.K.; Grillo, I.; Barker, J.G.; Mildner, D.F.R. Self-Assembly in Mixed Dialkyl Chain Cationic−Nonionic Surfactant Mixtures: Dihexadecyldimethyl Ammonium Bromide−Monododecyl Hexaethylene Glycol (Monododecyl Dodecaethylene Glycol) Mixtures. Langmuir 2008, 24, 7674–7687. [Google Scholar] [CrossRef]
- Eccleston, G.M.; Behan-Martin, M.K.; Jones, G.R.; Towns-Andrews, E. Synchrotron X-ray investigations into the lamellar gel phase formed in pharmaceutical creams prepared with cetrimide and fatty alcohols. Int. J. Pharm. 2000, 203, 127–139. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Origin (Pro), version 2017; OriginLab Corporation: Northampton, MA, USA, 2017.
- Kwak, M.-S.; Ahn, H.-J.; Song, K.-W. Rheological investigation of body cream and body lotion in actual application conditions. Korea-Aust. Rheol. J. 2015, 27, 241–251. [Google Scholar] [CrossRef]
- Richard, D.; Ferrand, M.; Kearley, G.J. Analysis and Visualisation of Neutron-Scattering Data. J. Neutron Res. 1996, 4, 33–39. [Google Scholar] [CrossRef]
- ISIS Water Baths. Available online: https://www.isis.stfc.ac.uk/Pages/Water-Baths.aspx (accessed on 7 April 2020).
- Heenan, R.K.; Penfold, J.; King, S.M. SANS at Pulsed Neutron Sources: Present and Future Prospects. J. Appl. Crystallogr. 1997, 30, 1140–1147. [Google Scholar] [CrossRef]
- Wignall, G.D.; Bates, F.S. Absolute calibration of small-angle neutron scattering data. J. Appl. Crystallogr. 1987, 20, 28–40. [Google Scholar] [CrossRef]
- Doucet, M.; Cho, J.H.; Alina, G.; Bakker, J.; Bouwman, W.; Butler, P.; Campbell, K.; Gonzales, M.; Heenan, R.; Jackson, A.; et al. SasView, Version 4.2; Zenodo; 2018. Available online: https://zenodo.org/record/1412041#.X1icqosRXIU (accessed on 1 January 2020).
- Awad, T.S.; Johnson, E.S.; Bureiko, A.; Olsson, U. Colloidal Structure and Physical Properties of Gel Networks Containing Anionic Surfactant and Fatty Alcohol Mixture. J. Dispers. Sci. Technol. 2011, 32, 807–815. [Google Scholar] [CrossRef]
- Kolp, D.G.; Lutton, E.S. The Polymorphism of n-Hexadecanol and n-Octadecanol. J. Am. Chem. Soc. 1951, 73, 5593–5595. [Google Scholar] [CrossRef]
- Valoppi, F.; Calligaris, S.; Marangoni, A.G. Phase Transition and Polymorphic Behavior of Binary Systems Containing Fatty Alcohols and Peanut Oil. Cryst. Growth Des. 2016, 16, 4209–4215. [Google Scholar] [CrossRef]
- Tanford, C. Micelle shape and size. J. Phys. Chem. 1972, 76, 3020–3024. [Google Scholar] [CrossRef]
- Hammouda, B. A new Guinier–Porod model. J. Appl. Crystallogr. 2010, 43, 716–719. [Google Scholar] [CrossRef]
- Ahmadi, D.; Ledder, R.; Mahmoudi, N.; Li, P.; Tellam, J.; Robinson, D.; Heenan, R.K.; Smith, P.; Lorenz, C.D.; Barlow, D.J.; et al. Supramolecular Architecture of a Multi-Component Biomimetic Lipid Barrier Formulation. J. Colloid Interface Sci. submitted.
- Niemi, L.; Laine, E. Effect of water content on the microstructure of an o/w cream. Int. J. Pharm. 1991, 68, 205–214. [Google Scholar] [CrossRef]
- Rowe, R.C.; Bray, D. Water distribution in creams prepared using cetostearyl alcohol and cetrimide. J. Pharm. Pharmacol. 1987, 39, 642–643. [Google Scholar] [CrossRef]
- Marsh, D. Lateral order in gel, subgel and crystalline phases of lipid membranes: Wide-angle X-ray scattering. Chem. Phys. Lipids 2012, 165, 59–76. [Google Scholar] [CrossRef]
- Karl, W.; Perla, R.; Gérard, C.; Franck, C.; Luc, N.-M.; Hayat, B.; Denis, F. Effect of surfactant on structure thermal behavior of cetyl stearyl alcohols: DSC and X-ray scattering studies. J. Therm. Anal. Calorim. 2016, 123, 1411–1417. [Google Scholar] [CrossRef]
- Pilgram, G.S.K.; Vissers, D.C.J.; van der Meulen, H.; Koerten, H.K.; Pavel, S.; Lavrijsen, S.P.M.; Bouwstra, J.A. Aberrant Lipid Organization in Stratum Corneum of Patients with Atopic Dermatitis and Lamellar Ichthyosis. J. Investig. Dermatol. 2001, 117, 710–717. [Google Scholar] [CrossRef] [PubMed]
- BP 2016 Monographs Containing Extemporaneous Preparation Information. Available online: https://www.pharmacopoeia.com/file/BP-list-of-substances.pdf (accessed on 25 August 2020).

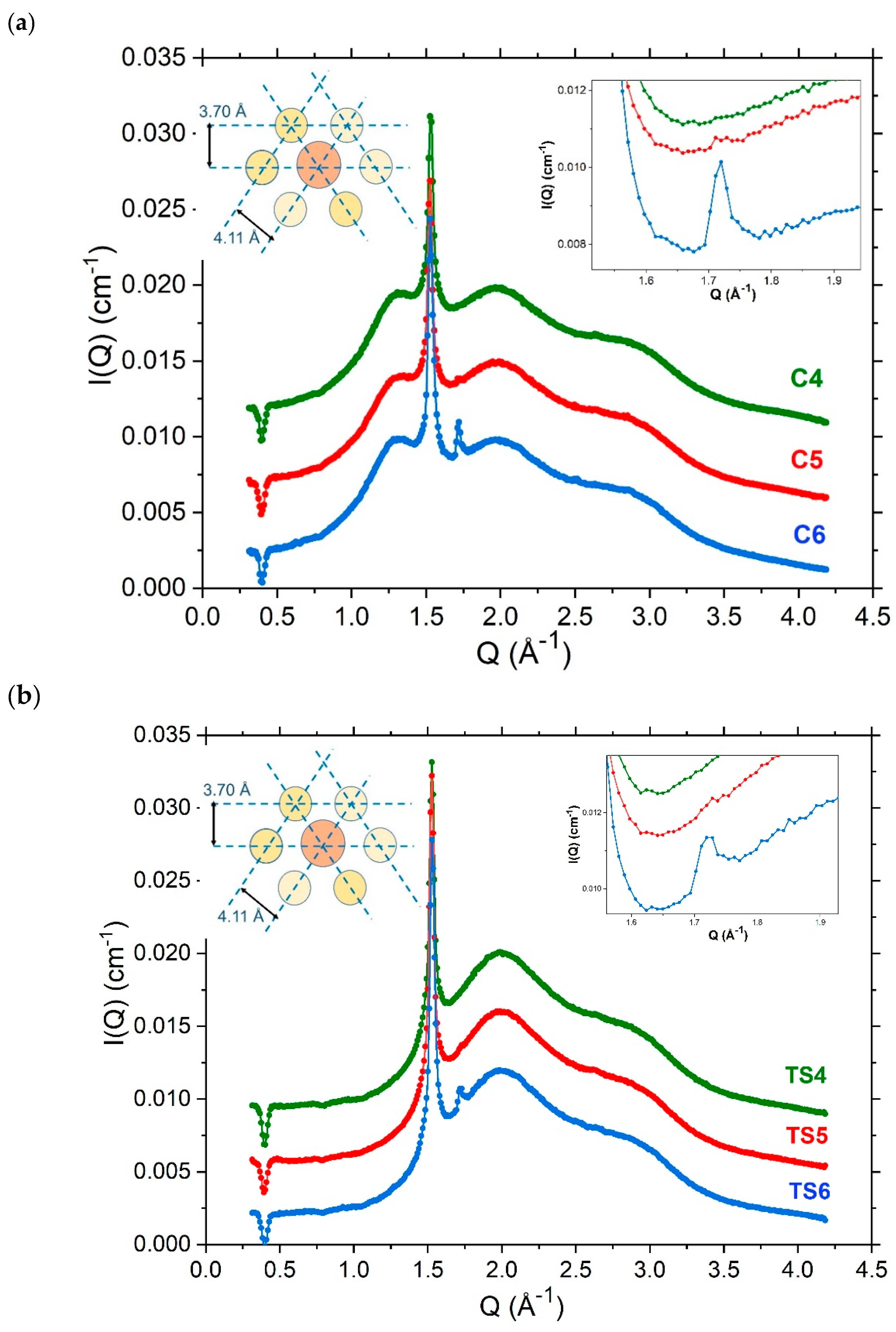
| Formulations | Total Emulsifier Concentration | Hexadecanol | Octadecanol | SDS | Liquid Paraffin | Water b |
|---|---|---|---|---|---|---|
| Percentage % (w/w) | ||||||
| C1 | 4 | 1.70 | 1.90 | 0.40 | 20 | 76 |
| C2 | 4 | 3.60 | -- | 0.40 | 20 | 76 |
| C3 | 4 | -- | 3.60 | 0.40 | 20 | 76 |
| C4 | 10 | 4.25 | 4.75 | 1.00 | 20 | 70 |
| C5 | 10 | 9 | -- | 1.00 | 20 | 70 |
| C6 | 10 | -- | 9 | 1.00 | 20 | 70 |
| (1:0) | (1:1) | (0:1) | ||||
|---|---|---|---|---|---|---|
| TS5 | C5 | TS4 | C4 | TS6 | C6 | |
| Bilayer thickness (Å) b | 45 ± 1 | 47 | 48 ± 1 | 50 | 49 ± 1 | 52 |
| Polydispersity on bilayer thickness | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| d-spacing (Å) | 232 ± 1 | 207 ± 1 | 259 ± 1 | 233 ± 1 | 257 ± 1 | 216 ± 1 |
| Polydispersity on d-spacing | 0.1 | 0.08 | 0.1 | 0.06 | 0.1 | 0.09 |
| Lorentz term | 95 ± 1 | 209 ± 2 | 108 ± 1 | 127 ± 6 | 133 ± 2 | 381 ± 7 |
| Unilamellar | 0.20 | 0.57 | 0.20 | 0.23 | 0.35 | 0.16 |
| Bilamellar | 0.40 | 0.28 | 0.38 | 0.38 | 0.32 | 0.40 |
| Trilamellar | 0.22 | 0.15 | 0.19 | 0.19 | 0.17 | 0.25 |
| Quadrilamellar | 0.10 | 0 | 0.10 | 0.08 | 0.10 | 0.09 |
| Pentalamellar | 0.08 | 0 | 0.13 | 0.12 | 0.06 | 0.10 |
| Oil droplet layer thickness (Å) | -- | 24 ± 2 | -- | 26 ± 0.5 | -- | 27 ± 0.3 |
| (1:0) | (1:1) | (0:1) | ||||
|---|---|---|---|---|---|---|
| TS2 | C2 | TS1 | C1 | TS3 | C3 | |
| Bilayer thickness (Å) b | 41 ± 6 | 47 | 44 ± 4 | 50 | 49 ± 6 | 52 |
| Polydispersity on bilayer thickness | 0.15 | -- | 0.11 | -- | 0.14 | -- |
| d-spacing (Å) c | 374 | 383 | 357 | 400 | 328 | 365 |
| Polydispersity on d-spacing | 0.2 | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 |
| Lorentz term | 230 ± 3 | -- | -- | 298 ± 9 | -- | 245 ± 4 |
| Unilamellar | 0.59 | -- | 0.54 | -- | 0.54 | -- |
| Bilamellar | 0.41 | -- | 0.46 | -- | 0.46 | -- |
| Trilamellar | 0 | -- | 0 | -- | 0 | -- |
| Quadrilamellar | 0 | -- | 0 | -- | 0 | -- |
| Pentalamellar | 0 | -- | 0 | -- | 0 | -- |
| Power law exponent | -- | 3.4 | -- | 3.1 | -- | 3.3 |
| (1:0) | (1:1) | (0:1) | ||||
|---|---|---|---|---|---|---|
| TS5 | C5 | TS4 | C4 | TS6 | C6 | |
| Bilayer thickness (Å)b | 45 ± 1 | 47 | 48 ± 1 | 50 | 49 ± 1 | 52 |
| Polydispersity on bilayer thickness | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| d-spacing (Å) | 231 ± 1 | 198 ± 1 | 258 ± 1 | 253 ± 1 | 240 ± 1 | 212 ± 1 |
| Polydispersity on d-spacing | 0.1 | 0.10 | 0.1 | 0.08 | 0.1 | 0.08 |
| Lorentz term | 95 ± 1 | 344 ± 6 | 109 ± 1 | 117 ± 7 | 98 ± 1 | 379 ± 8 |
| Unilamellar | 0.16 | 0.63 | 0.17 | 0.38 | 0.34 | 0.0 |
| Bilamellar | 0.42 | 0.2 | 0.4 | 0.43 | 0.3 | 0.52 |
| Trilamellar | 0.24 | 0.17 | 0.2 | 0.09 | 0.13 | 0.32 |
| Quadrilamellar | 0.09 | 0 | 0.11 | 0.10 | 0.1 | 0.11 |
| Pentalamellar | 0.09 | 0 | 0.12 | 0.0 | 0.13 | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, D.; Mahmoudi, N.; Heenan, R.K.; Barlow, D.J.; Lawrence, M.J. The Influence of Co-Surfactants on Lamellar Liquid Crystal Structures Formed in Creams. Pharmaceutics 2020, 12, 864. https://doi.org/10.3390/pharmaceutics12090864
Ahmadi D, Mahmoudi N, Heenan RK, Barlow DJ, Lawrence MJ. The Influence of Co-Surfactants on Lamellar Liquid Crystal Structures Formed in Creams. Pharmaceutics. 2020; 12(9):864. https://doi.org/10.3390/pharmaceutics12090864
Chicago/Turabian StyleAhmadi, Delaram, Najet Mahmoudi, Richard K. Heenan, David J. Barlow, and M. Jayne Lawrence. 2020. "The Influence of Co-Surfactants on Lamellar Liquid Crystal Structures Formed in Creams" Pharmaceutics 12, no. 9: 864. https://doi.org/10.3390/pharmaceutics12090864
APA StyleAhmadi, D., Mahmoudi, N., Heenan, R. K., Barlow, D. J., & Lawrence, M. J. (2020). The Influence of Co-Surfactants on Lamellar Liquid Crystal Structures Formed in Creams. Pharmaceutics, 12(9), 864. https://doi.org/10.3390/pharmaceutics12090864







