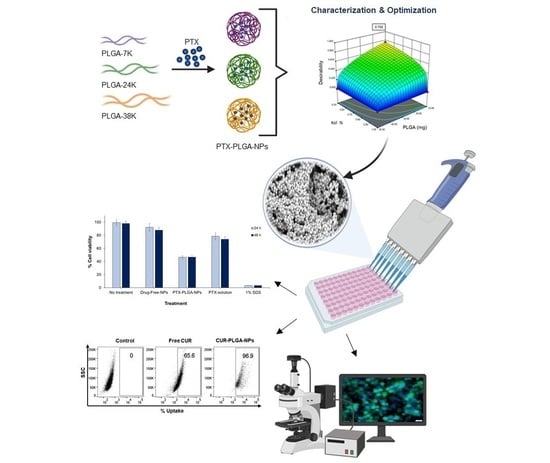
Optimization and Evaluation of Poly(lactide-co-glycolide) Nanoparticles for Enhanced Cellular Uptake and Efficacy of Paclitaxel in the Treatment of Head and Neck Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. QbD Approach for Optimization of PTX-PLGA-NPs
2.3. Preparation of PTX-PLGA-NPs
2.4. Physico-Chemical Characterization of PTX-PLGA-NPs
2.4.1. Determination of PS, PDI, and ZP
2.4.2. Determination of EE%
2.5. Transmission and Scanning Electron Microscopy
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7. In Vitro Release Studies
2.8. Short-Term Stability Study
2.9. Cell Culture
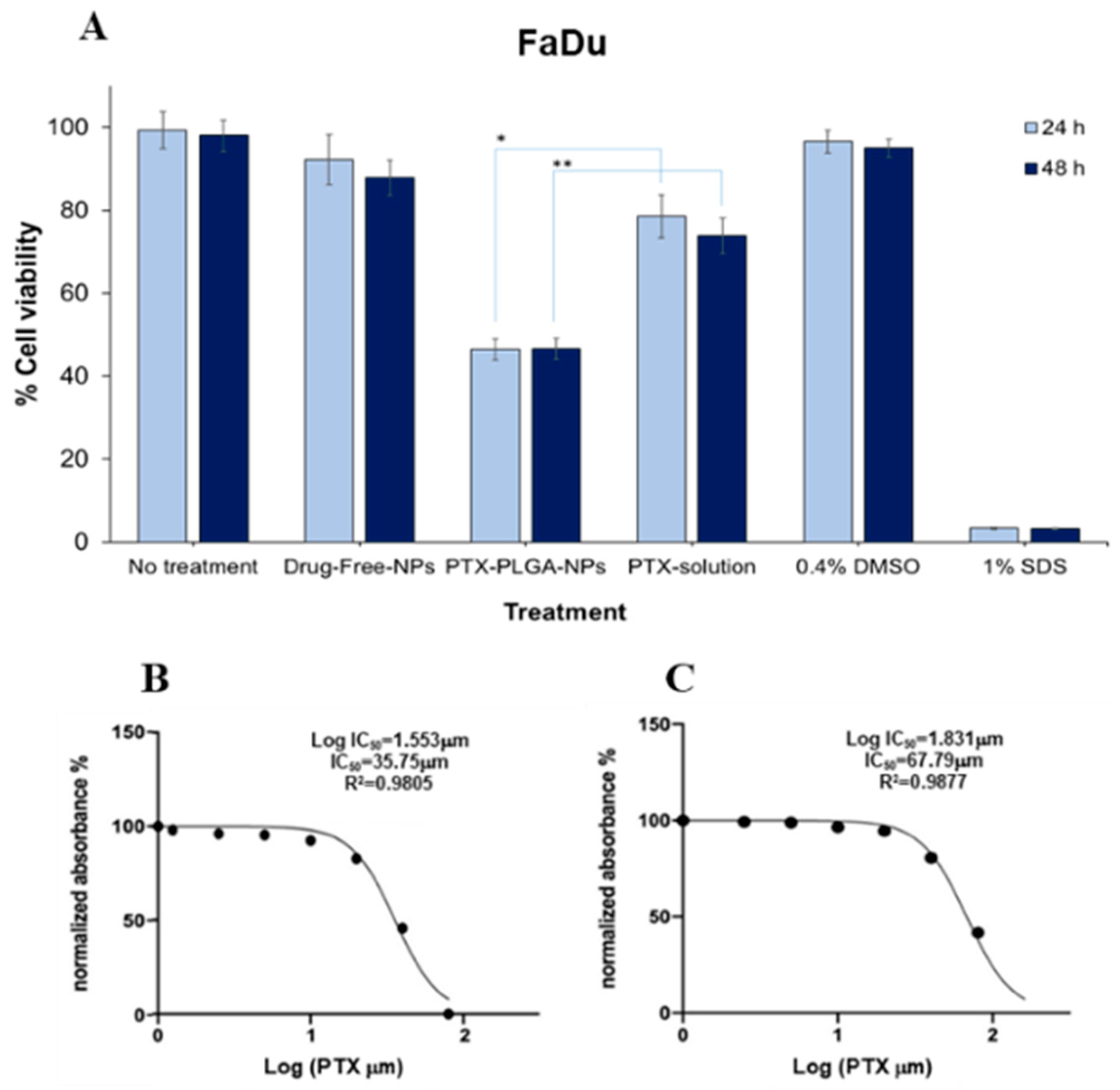
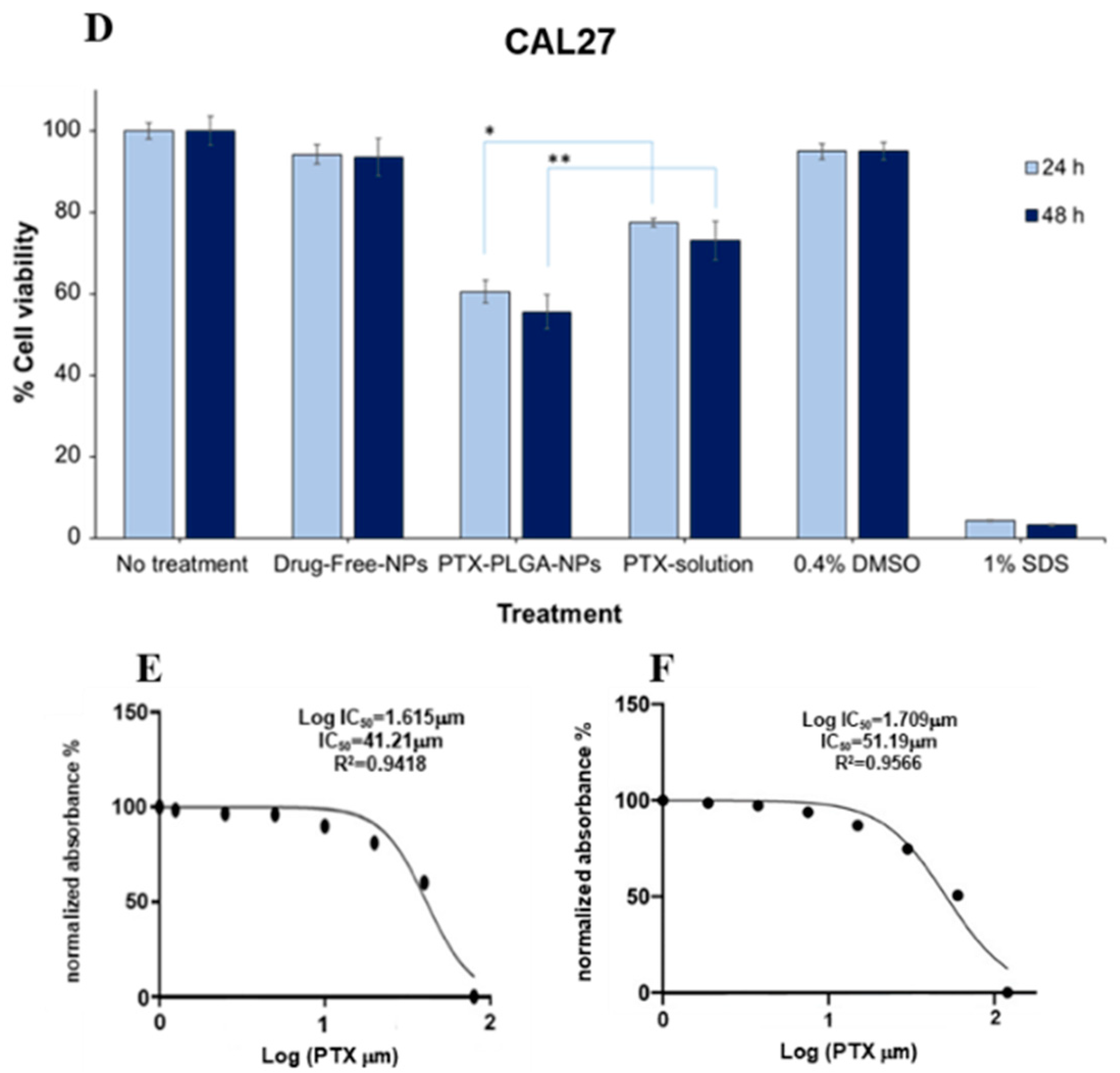
2.10. Cytotoxicity and IC50 Studies
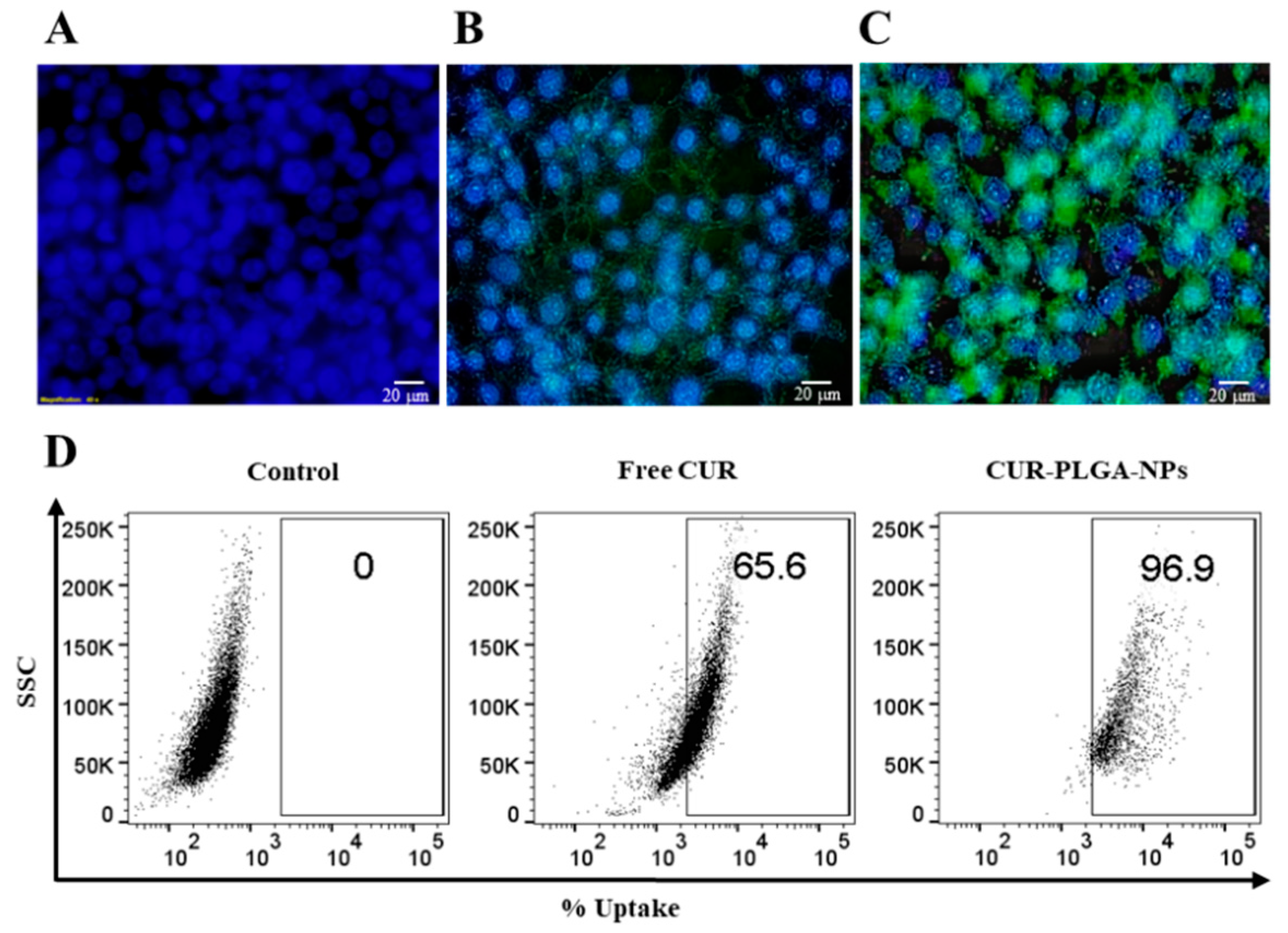
2.11. Cellular Uptake Studies
2.12. Statistical Analysis
3. Results and Discussion
3.1. Design of Experiments and Preparation of PTX-PLGA-NPs
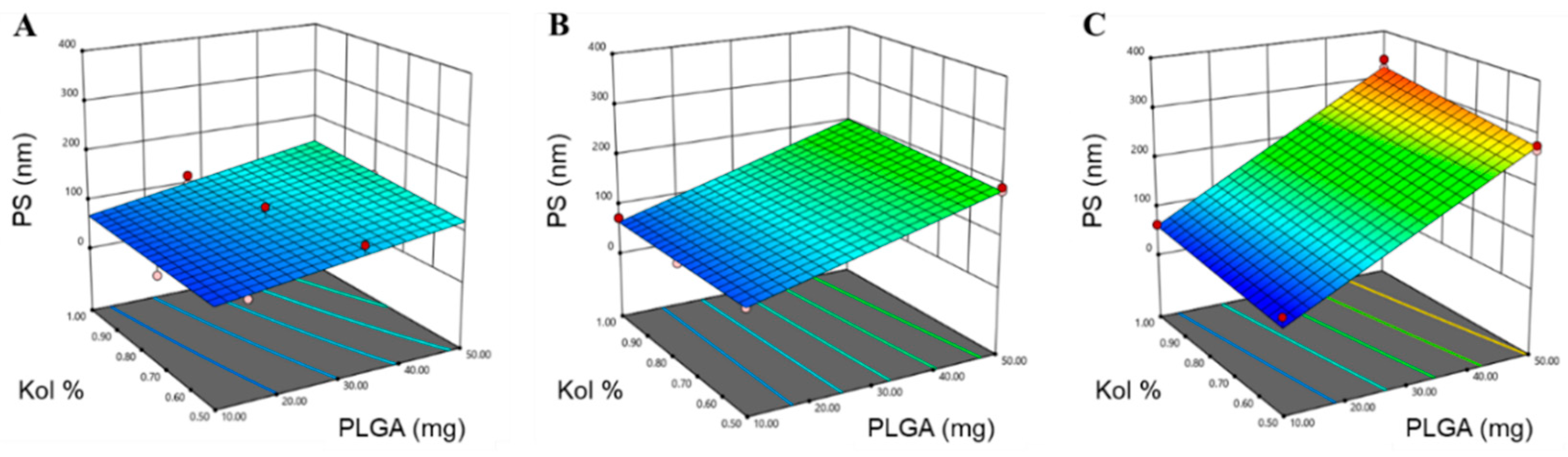
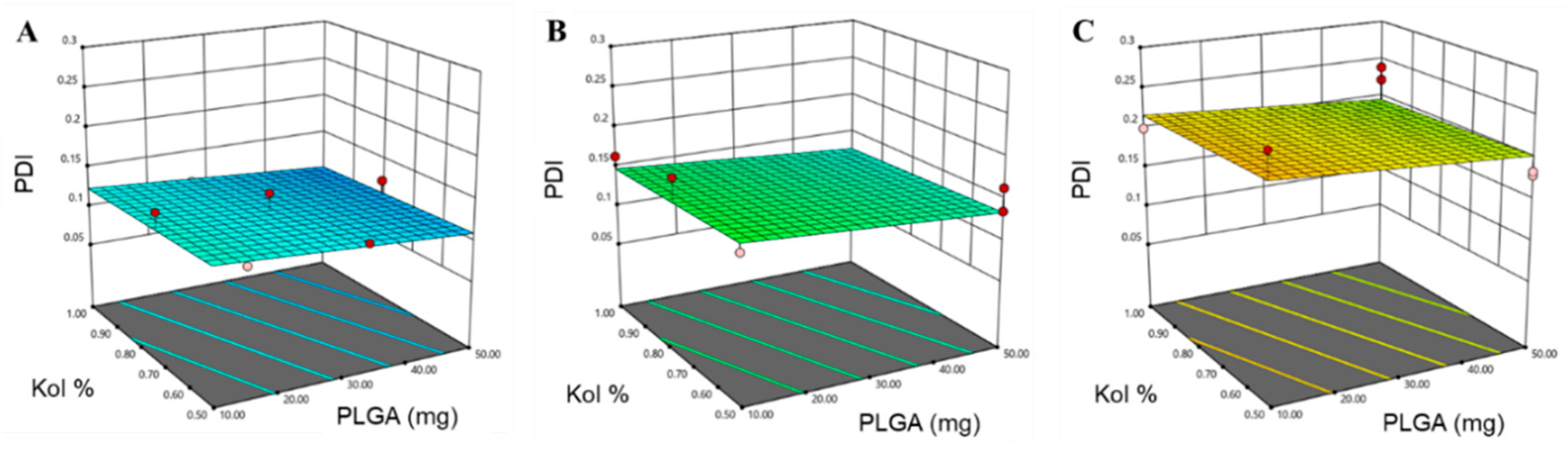
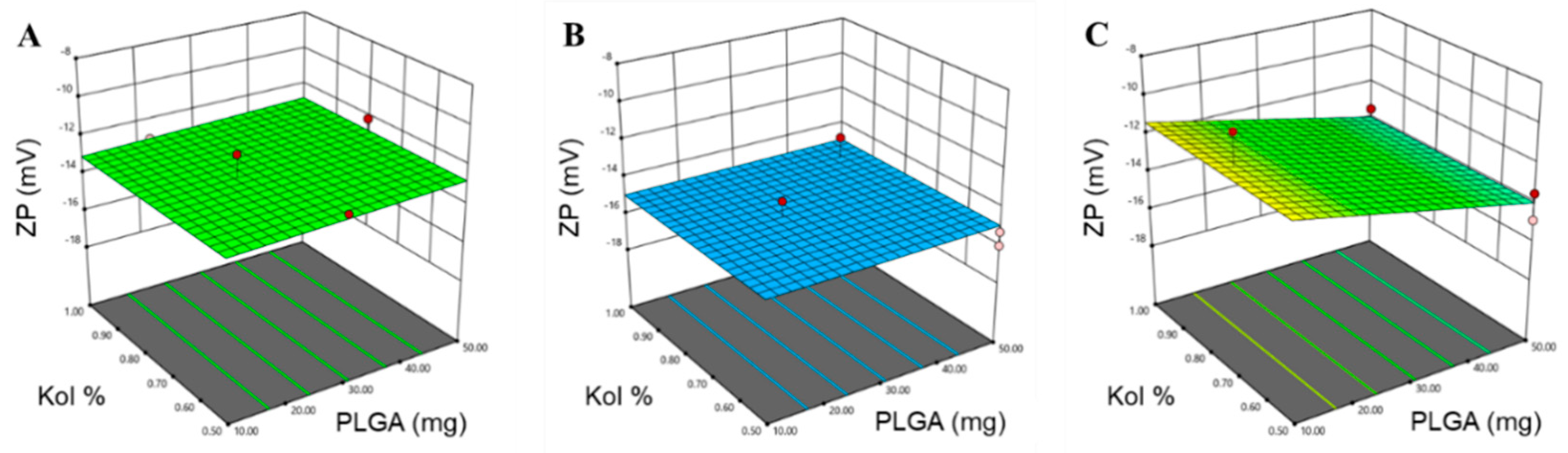
3.1.1. Determination of PS, PDI and ZP
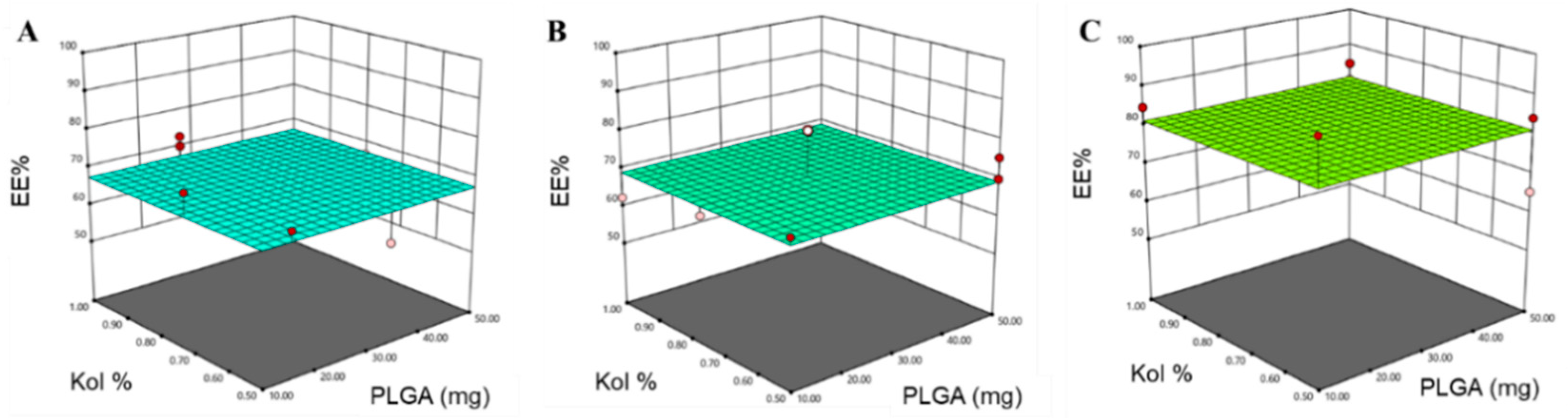
3.1.2. Determination of EE%
3.2. Design Space and Model Verification
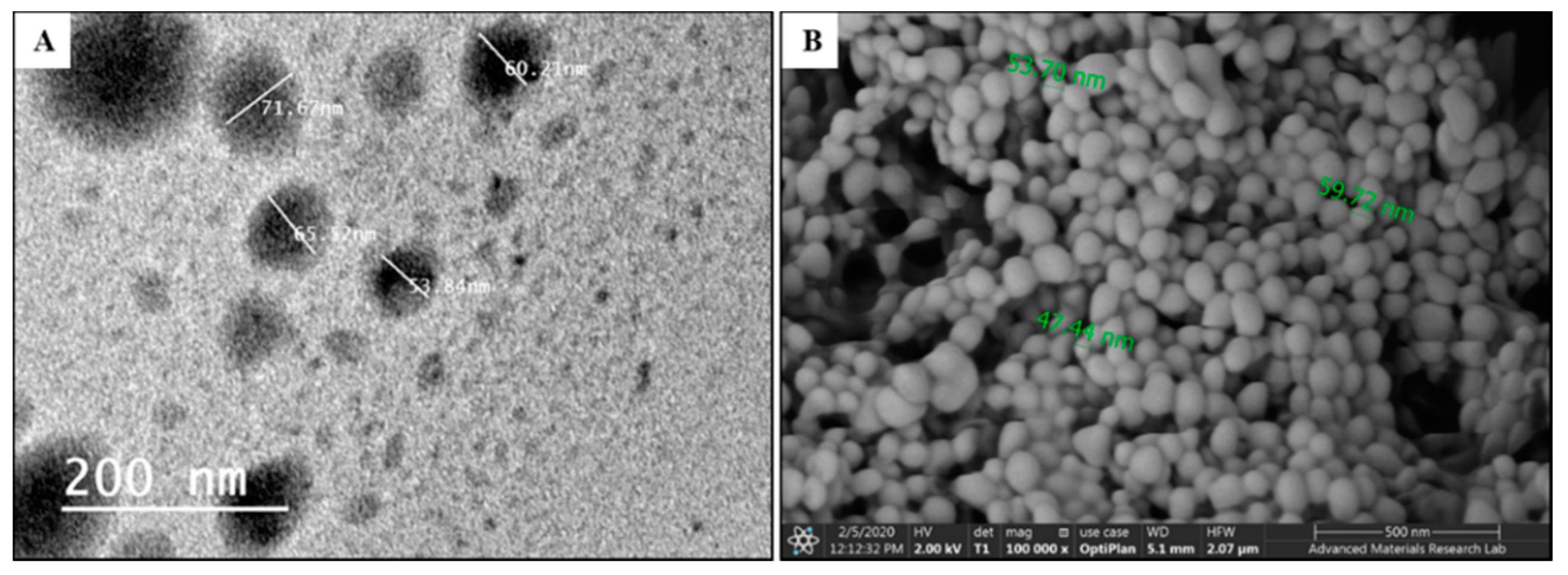
3.3. TEM and SEM Analysis
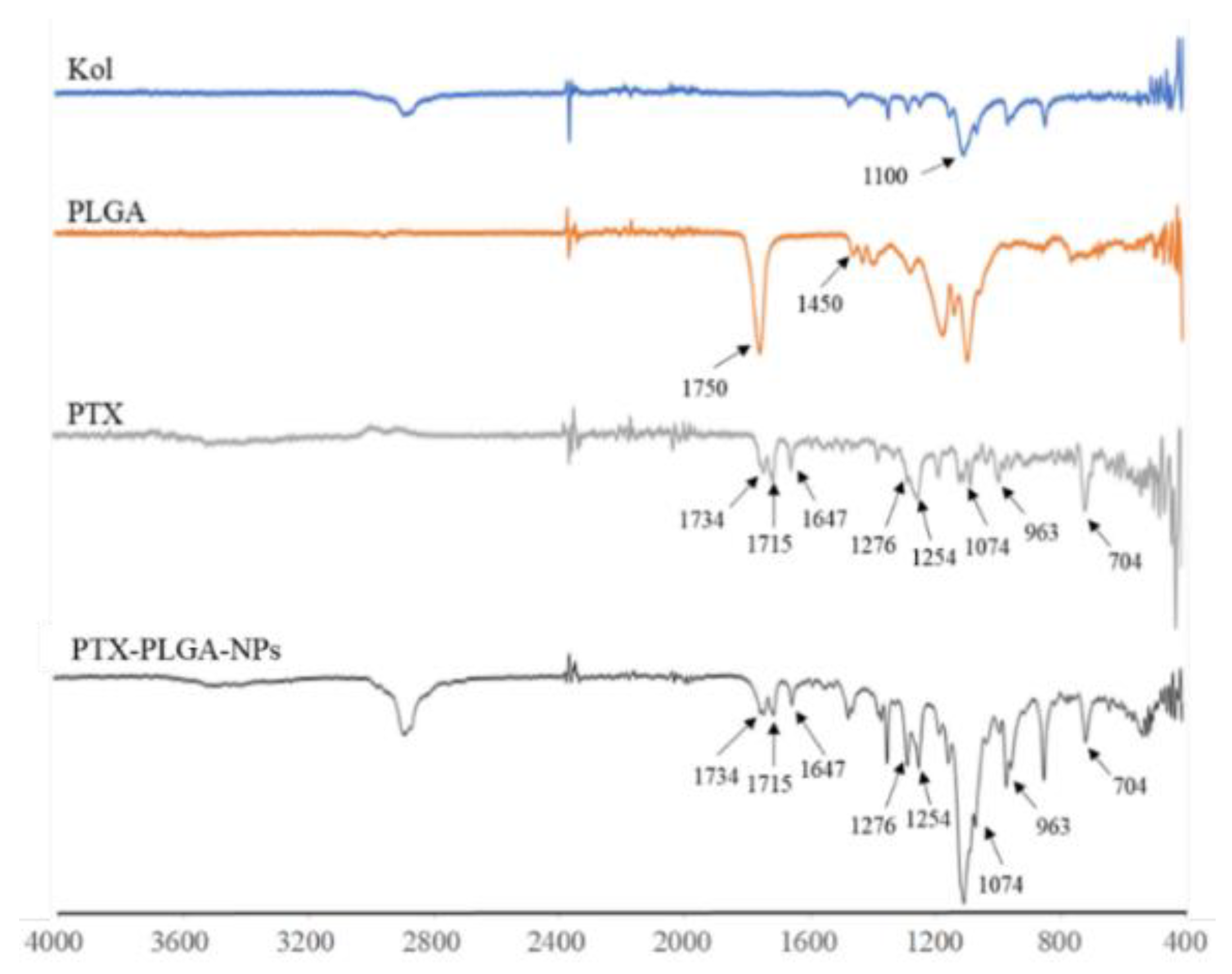
3.4. FT-IR Analysis
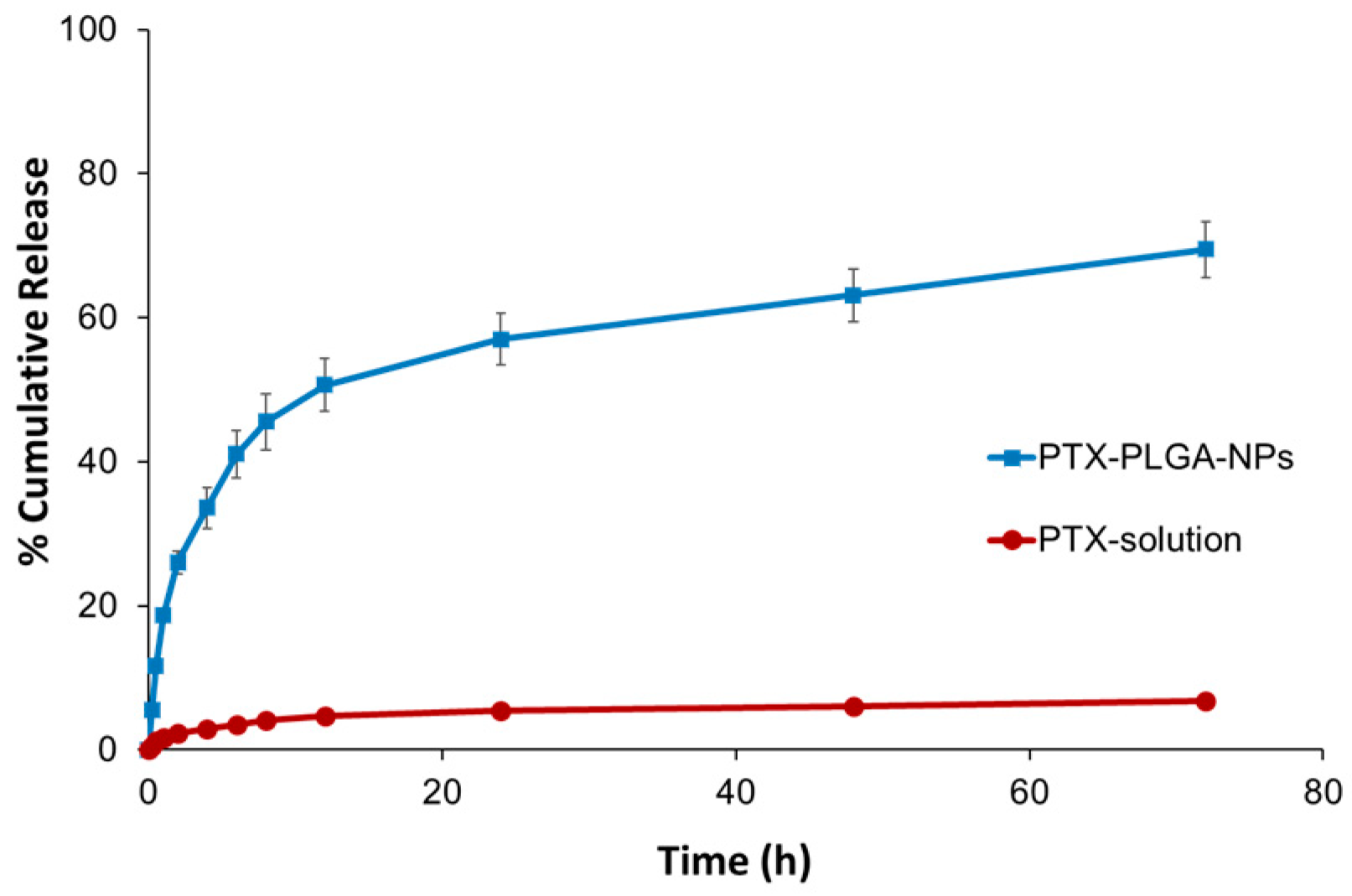
3.5. In Vitro Release Studies
3.6. Short-Term Stability Study
3.7. In Vitro Cytotoxicity and Cellular Uptake Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smirnova, T.; Adomako, A.; Locker, J.; Van Rooijen, N.; Prystowsky, M.B.; Segall, J.E. In vivo invasion of head and neck squamous cell carcinoma cells does not require macrophages. Am. J. Pathol. 2011, 178, 2857–2865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birkeland, A.C.; Swiecicki, P.L.; Brenner, J.C.; Shuman, A.G. A review of drugs in development for the personalized treatment of head and neck squamous cell carcinoma. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, C.; Feng, X.; Zhou, X.; Xu, X.; Xie, L.; Wang, R.; Zhang, D.; Wang, H.; Deng, P.; et al. Biodegradable thermosensitive hydrogel for SAHA and DDP delivery: Therapeutic effects on oral squamous cell carcinoma xenografts. PLoS ONE 2012, 7, e33860. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Peyser, N.D.; Grandis, J.R. Integration of molecular targeted therapy with radiation in head and neck cancer. Pharmacol. Ther. 2014, 142, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Guigay, J.; Fayette, J.; Mesia, R.; Lafond, C.; Saada-Bouzid, E.; Geoffrois, L.; Martin, L.; Cupissol, D.; Capitain, O.; Castanie, H.; et al. TPExtreme randomized trial: TPEx versus Extreme regimen in 1st line recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J. Clin. Oncol. 2019, 37, 6002. [Google Scholar] [CrossRef]
- Xavier-Jr, F.H.; Gueutin, C.; Chacun, H.; Vauthier, C.; Egito, E.S.T. Mucoadhesive paclitaxel-loaded chitosan-poly (isobutyl cyanoacrylate) core-shell nanocapsules containing copaiba oil designed for oral drug delivery. J. Drug Deliv. Sci. Technol. 2019, 53, 101194. [Google Scholar] [CrossRef]
- Khalifa, A.M.; Elsheikh, M.A.; Khalifa, A.M.; Elnaggar, Y.S.R. Current strategies for different paclitaxel-loaded Nano-delivery Systems towards therapeutic applications for ovarian carcinoma: A review article. J. Control. Release 2019, 311–312, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Montagner, I.M.; Banzato, A.; Zuccolotto, G.; Renier, D.; Campisi, M.; Bassi, P.F.; Zanovello, P.; Rosato, A. Paclitaxel-hyaluronan hydrosoluble bioconjugate: Mechanism of action in human bladder cancer cell lines. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Calleja, P.; Espuelas, S.; Corrales, L.; Pio, R.; Irache, J.M. Pharmacokinetics and antitumor efficacy of paclitaxel-cyclodextrin complexes loaded in mucus-penetrating nanoparticles for oral administration. Nanomedicine 2014, 9, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Sharma, S.; Khan, I.; Gothwal, A.; Sharma, A.K.; Singh, Y.; Chourasia, M.K.; Kumar, V. Enhanced apoptotic and anticancer potential of paclitaxel loaded biodegradable nanoparticles based on chitosan. Int. J. Biol. Macromol. 2017, 98, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, I.; Cohen, K.; Koffler, J.; Melikhov, D.; Peer, D.; Margalit, R. Paclitaxel-clusters coated with hyaluronan as selective tumor-targeted nanovectors. Biomaterials 2010, 31, 7106–7114. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Lv, Q.; Lu, J.; Yao, H.; Lv, X.; Jiang, F.; Lu, A.; Zhang, G. Prodrug strategies for paclitaxel. Int. J. Mol. Sci. 2016, 17, 796. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Asghar, S.; Li, H.; Chen, M.; Su, Z.; Xu, Y.; Ping, Q.; Xiao, Y. Preparation of a paclitaxel-loaded cationic nanoemulsome and its biodistribution via direct intratumoral injection. Colloids Surfaces B Biointerfaces 2016, 142, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Aluri, S.; Janib, S.M.; Mackay, J.A. Environmentally responsive peptides as anticancer drug carriers. Adv. Drug Deliv. Rev. 2009, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Shikanov, A.; Shikanov, S.; Vaisman, B.; Golenser, J.; Domb, A.J. Paclitaxel tumor biodistribution and efficacy after intratumoral injection of a biodegradable extended release implant. Int. J. Pharm. 2008, 358, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Meng, X.; Wu, Z.; Wu, Z.; Qi, X. Dynamic imine bond cross-linked self-healing thermosensitive hydrogels for sustained anticancer therapy via intratumoral injection. Mater. Sci. Eng. C 2018, 93, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Calderó, G.; Fornaguera, C.; Zadoina, L.; Dols-Perez, A.; Solans, C. Design of parenteral MNP-loaded PLGA nanoparticles by a low-energy emulsification approach as theragnostic platforms for intravenous or intratumoral administration. Colloids Surf. B 2017, 160, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, C.; Ren, T.; Chen, S.; Ye, X.; Guo, H.; He, H.; Zhang, Y.; Yin, T.; Liang, X.J.; et al. Poly(vinyl methyl ether/maleic anhydride)-Doped PEG-PLA Nanoparticles for Oral Paclitaxel Delivery to Improve Bioadhesive Efficiency. Mol. Pharm. 2017, 14, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Lukyanov, A.N.; Torchilin, V.; Tournier, H.; Schneider, A.N.; Goldberg, S.N. Combined Radiofrequency Ablation and Adjuvant Liposomal Chemotherapy: Effect of Chemotherapeutic Agent, Nanoparticle Size, and Circulation Time. J. Vasc. Interv. Radiol. 2005, 16, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle characterization: State of the art, challenges, and emerging technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Bai, L.; Wu, H.; Song, W.; Guo, G.; Dou, K. Cytotoxicity of paclitaxel incorporated in plga nanoparticles on hypoxic human tumor cells. Pharm. Res. 2009, 26, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Orunoğlu, M.; Kaffashi, A.; Pehlivan, S.B.; Şahin, S.; Söylemezoğlu, F.; Karlı-Oğuz, K.; Mut, M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater. Sci. Eng. C 2017, 78, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Graves, R.A.; Pamujula, S.; Moiseyev, R.; Freeman, T.; Bostanian, L.A.; Mandal, T.K. Effect of different ratios of high and low molecular weight PLGA blend on the characteristics of pentamidine microcapsules. Int. J. Pharm. 2004, 270, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Moradi, S.; Shahlaei, M. A molecular dynamics simulation study on the mechanism of loading of gemcitabine and camptothecin in poly lactic-co-glycolic acid as a nano drug delivery system. J. Mol. Liq. 2018, 269, 110–118. [Google Scholar] [CrossRef]
- Tóth, T.; Kiss, É. A method for the prediction of drug content of poly(lactic-co-glycolic)acid drug carrier nanoparticles obtained by nanoprecipitation. J. Drug Deliv. Sci. Technol. 2019, 50, 42–47. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Si, S.; Li, H.; Han, X. Sustained release olmesartan medoxomil loaded PLGA nanoparticles with improved oral bioavailability to treat hypertension. J. Drug Deliv. Sci. Technol. 2020, 55, 101422. [Google Scholar] [CrossRef]
- Almeida, K.B.; Ramos, A.S.; Nunes, J.B.B.; Silva, B.O.; Ferraz, E.R.A.; Fernandes, A.S.; Felzenszwalb, I.; Amaral, A.C.F.; Roullin, V.G.; Falcão, D.Q. PLGA nanoparticles optimized by Box-Behnken for efficient encapsulation of therapeutic Cymbopogon citratus essential oil. Colloids Surfaces B Biointerfaces 2019, 181, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Tefas, L.R.; Tomuţă, I.; Achim, M.; Vlase, L. Development and optimization of quercetin-loaded plga nanoparticles by experimental design. Clujul Med. 2015, 88, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Escalona-Rayo, O.; Fuentes-Vázquez, P.; Jardon-Xicotencatl, S.; García-Tovar, C.G.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Rapamycin-loaded polysorbate 80-coated PLGA nanoparticles: Optimization of formulation variables and in vitro anti-glioma assessment. J. Drug Deliv. Sci. Technol. 2019, 52, 488–499. [Google Scholar] [CrossRef]
- Fonseca, C.; Simões, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 83, 273–286. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J. Control. Release 2003, 86, 33–48. [Google Scholar] [CrossRef]
- Abdelbary, G.; Haider, M. In vitro characterization and growth inhibition effect of nanostructured lipid carriers for controlled delivery of methotrexate. Pharm. Dev. Technol. 2013, 18, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.; Carrião, M.S.; Pacheco, M.T.; Branquinho, L.C.; de Souza, A.L.R.; Bakuzis, A.F.; Lima, E.M. Triggered release of paclitaxel from magnetic solid lipid nanoparticles by magnetic hyperthermia. Mater. Sci. Eng. C 2018, 92, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010, 351, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; El Hosary, R.; Hassan, M.A.; Haider, M.; Abd-Rabo, M.M. Efficacy and Safety Profiles of Oral Atorvastatin-Loaded Nanoparticles: Effect of Size Modulation on Biodistribution. Mol. Pharm. 2018, 15, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; El-Hosary, R.; Shalaby, S.; Abd-Rabo, M.M.; Elkhateeb, D.G.; Nour, S. PD-PK evaluation of freeze-dried atorvastatin calcium-loaded poly-ε-caprolactone nanoparticles. Int. J. Pharm. 2016, 504, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Rashed, H.M.; Fayez, H.; Farouk, F.; Shamma, R.N. Nanoparticle-mediated dual targeting: An approach for enhanced baicalin delivery to the liver. Pharmaceutics 2020, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Hassan, M.A.; Ahmed, I.S.; Shamma, R. Thermogelling Platform for Baicalin Delivery for Versatile Biomedical Applications. Mol. Pharm. 2018, 15, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; A comparative literature review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Guzmán, M.; Molpeceres, J.; Aberturas, M.R. Freeze-drying of polycaprolactone and poly(D,L-lactic-glycolic) nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. Eur. J. Pharm. Biopharm. 2000, 50, 379–387. [Google Scholar] [CrossRef]
- He, C.; Yin, L.; Tang, C.; Yin, C. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials 2012, 33, 8569–8578. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, C. Tuning the Size of Poly(lactic-co-glycolic Acid) (PLGA) Nanoparticles Fabricated by Nanoprecipitation. Biotechnol. J. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification-diffusion methods: Critical comparison. Adv. Colloid Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Chorny, M.; Fishbein, I.; Danenberg, H.D.; Golomb, G. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: Effect of formulation variables on size, drug recovery and release kinetics. J. Control. Release 2002, 83, 389–400. [Google Scholar] [CrossRef]
- Menon, J.U.; Kona, S.; Wadajkar, A.S.; Desai, F.; Vadla, A.; Nguyen, K.T. Effects of surfactants on the properties of PLGA nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Pelikh, O.; Stahr, P.L.; Huang, J.; Gerst, M.; Scholz, P.; Dietrich, H.; Geisel, N.; Keck, C.M. Nanocrystals for improved dermal drug delivery. Eur. J. Pharm. Biopharm. 2018, 128, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, P.; Pokharkar, V. Risk assessment and QbD based optimization of an Eprosartan mesylate nanosuspension: In-vitro characterization, PAMPA and in-vivo assessment. Int. J. Pharm. 2019, 567, 118415. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Ma, H.; Huang, K.; Liu, J.; Wei, T.; Jin, S.; Zhang, J.; He, S.; Liang, X.J. Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors. Cancer Res. 2013, 73, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Asai, D.; Xu, D.; Liu, W.; Quiroz, F.G.; Callahan, D.J.; Zalutsky, M.R.; Craig, S.L.; Chilkoti, A. Protein polymer hydrogels by in situ, rapid and reversible self-gelation. Biomaterials 2012, 33, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Termsarasab, U.; Cho, H.-J.; Kim, D.H.; Chong, S.; Chung, S.-J.; Shim, C.-K.; Moon, H.T.; Kim, D.-D. Chitosan oligosaccharide–arachidic acid-based nanoparticles for anti-cancer drug delivery. Int. J. Pharm. 2013, 441, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Z.; Shi, R.J.; Chen, D.; Sun, Y.Y.; Wu, Q.W.; Wang, T.; Wang, P.H. Potential biomarkers for paclitaxel sensitivity in hypopharynx cancer cell. Int. J. Clin. Exp. Pathol. 2013, 6, 2745–2756. [Google Scholar] [PubMed]
- Dreher, M.R.; Liu, W.; Michelich, C.R.; Dewhirst, M.W.; Yuan, F.; Chilkoti, A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 2006, 98, 335–344. [Google Scholar] [CrossRef] [PubMed]

| Numerical Factors | Applied Levels | ||||
|---|---|---|---|---|---|
| Low (−1) | High (+1) | ||||
| X1 | Amount of PLGA (mg) | 10 | 50 | ||
| X2 | Kol concentration (g/100 mL) | 0.5 | 1 | ||
| Categorical Factor | Applied Levels | ||||
| X3 | Mw of PLGA (kD) | 7–17 | 24–38 | 38–54 | |
| Responses | Optimization Goal | ||||
| Y1 | Particle size (PS) (nm) | <60 nm | |||
| Y2 | Polydispersity index (PDI) | Minimize | |||
| Y3 | Zeta potential (ZP) (mV) | Maximize | |||
| Y4 | Encapsulation efficiency (EE) (%) | Maximize | |||
| Formulation | X1 | X2 | X3 | Y1: PS (nm) | Y2: PDI | Y3: ZP (mV) | Y4: EE (%) |
|---|---|---|---|---|---|---|---|
| F1 | 50 | 1 | 38 | 329.4 ± 2.9 | 0.239 ± 0.048 | −13.5 ± 2.1 | 84.9 ± 3.6 |
| F2 | 50 | 1 | 38 | 335.8 ± 3.1 | 0.222 ± 0.015 | −14.4 ± 2.6 | 80.4 ± 4.7 |
| F3 | 29.2 | 0.8 | 7 | 118.7 ± 1.7 | 0.139 ± 0.014 | −11.8 ± 2.2 | 65.4 ± 3.9 |
| F4 | 10 | 1 | 24 | 72.5 ± 0.4 | 0.163 ± 0.011 | −13.5 ± 1.8 | 62.3 ± 5.1 |
| F5 | 10 | 0.8 | 24 | 61.8 ± 0.7 | 0.181 ± 0.016 | −16.0 ± 3.9 | 66.6 ± 2.8 |
| F6 | 15 | 0.5 | 7 | 58.9 ± 0.4 | 0.122 ± 0.011 | −12.5 ± 2.1 | 70.0 ± 4.1 |
| F7 | 50 | 0.5 | 38 | 278.1 ± 2.1 | 0.179 ± 0.015 | −13.5 ± 1.4 | 84.1 ± 3.8 |
| F8 | 20 | 0.8 | 38 | 79.4 ± 0.9 | 0.171 ± 0.013 | −9.9 ± 1.6 | 75.8 ± 2.2 |
| F9 | 50 | 0.8 | 7 | 130.0 ± 1.4 | 0.092 ± 0.008 | −12.1 ± 1.7 | 66.8 ± 3.5 |
| F10 | 46.3 | 0.9 | 24 | 184.2 ± 1.9 | 0.106 ± 0.009 | −13.8 ± 2.1 | 57.3 ± 2.6 |
| F11 | 50 | 0.5 | 24 | 193.7 ± 1.5 | 0.163 ± 0.012 | −16.0 ± 2.3 | 75.3 ± 5.5 |
| F12 | 10 | 1 | 38 | 62.7 ± 0.7 | 0.199 ± 0.014 | −12.2 ± 1.4 | 84.7 ± 5.3 |
| F13 | 26.8 | 1 | 7 | 97.3 ± 1.0 | 0.111 ± 0.011 | −13.0 ± 2.9 | 72.8 ± 3.7 |
| F14 | 33.2 | 0.5 | 7 | 113.2 ± 1.1 | 0.121 ± 0.017 | −12.7 ± 1.6 | 59.4 ± 2.7 |
| F15 | 50 | 0.8 | 7 | 115.3 ± 1.1 | 0.121 ± 0.012 | −13.9 ± 2.9 | 61.1 ± 4.3 |
| F16 | 10 | 0.5 | 38 | 51.1 ± 0.8 | 0.256 ± 0.019 | −10.7 ± 1.9 | 95.0 ± 4.6 |
| F17 | 10 | 0.5 | 24 | 66.2 ± 1.3 | 0.144 ± 0.016 | −15.9 ± 2.3 | 70.9 ± 3.4 |
| F18 | 26.8 | 1 | 7 | 116.4 ± 1.9 | 0.075 ± 0.015 | −13.6 ± 2.1 | 70.1 ± 2.9 |
| F19 | 30 | 0.8 | 24 | 121.9 ± 2.1 | 0.098 ± 0.008 | −14.1 ± 1.9 | 81.2 ± 4.4 |
| F20 | 50 | 0.5 | 24 | 186.8 ± 1.6 | 0.135 ± 0.018 | −15.3 ± 2.4 | 69.8 ± 3.9 |
| F21 | 10 | 0.7 | 7 | 42.3 ± 0.6 | 0.148 ± 0.016 | −14.1 ± 1.3 | 73.5 ± 4.1 |
| F22 | 50 | 0.5 | 38 | 269.4 ± 2.8 | 0.183 ± 0.020 | −14.9 ± 1.1 | 65.3 ± 2.8 |
| Response | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Significant Terms |
|---|---|---|---|---|---|
| PS (nm) | 0.9801 | 0.9651 | 0.9151 | 25.859 | X1 (p < 0.0001) X2 (p = 0.024) X3 (p < 0.0001) X1X3 (p < 0.0001) |
| PDI | 0.7036 | 0.6339 | 0.5160 | 8.222 | X3 (p < 0.0001) |
| ZP (mV) | 0.6623 | 0.5568 | 0.3101 | 7.4293 | X3 (p = 0.0016) X1X3 (p = 0.0298) |
| EE (%) | 0.4416 | 0.3828 | 0.2453 | 5.0564 | X3 (p = 0.0039) |
| Variables | Values | Response | Predicted Values | Observed Values |
|---|---|---|---|---|
| X1 | 10 mg | Y1 (PS) | 48.5 nm | 53.1 nm |
| X2 | 0.5% (w/v) | Y2 (PDI) | 0.22 | 0.22 |
| X3 | 38–54 kD | Y3 (ZP) | −10.8 mV | −10.1 mV |
| Y4 (EE%) | 81.4% | 92.2% |
| Release Kinetic Model | Equation | k | Unit | n | R2 |
|---|---|---|---|---|---|
| Zero Order | Ct = Co − kt | 2.2156 | %/h | - | 0.713 |
| First Order | ln Ct = ln Co − kt | 0.0343 | h−1 | - | 0.801 |
| Korsmeyer–Peppas | Mt/M∞ = ktn | 15.495 | h−n | 0.4936 | 0.941 |
| Hixson–Crowell | 3√W0 = 3√Wi + kHC t | 0.0457 | (%)1/3/h | - | 0.772 |
| Higuchi | Q = kt0.5 | 12.57 | (%)/h0.5 | - | 0.933 |
| Formulation | Storage Conditions | PS (nm) | PDI | ZP (mV) | EE (%) |
|---|---|---|---|---|---|
| PTX-PLGA-NPs | Fresh | 51.7 ± 1.7 | 0.207 ± 0.024 | −11.6 ± 1.6 | 93.9 ± 3.7 |
| 25 °C | 322.2 * ± 23.8 | 0.608 * ± 0.118 | −8.1 * ± 2.7 | 95.1 ± 4.7 | |
| 4 °C | 54.3 ± 3.4 | 0.249 ± 0.040 | −11.2 ± 1.6 | 91.2 ± 3.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, M.; Elsherbeny, A.; Jagal, J.; Hubatová-Vacková, A.; Saad Ahmed, I. Optimization and Evaluation of Poly(lactide-co-glycolide) Nanoparticles for Enhanced Cellular Uptake and Efficacy of Paclitaxel in the Treatment of Head and Neck Cancer. Pharmaceutics 2020, 12, 828. https://doi.org/10.3390/pharmaceutics12090828
Haider M, Elsherbeny A, Jagal J, Hubatová-Vacková A, Saad Ahmed I. Optimization and Evaluation of Poly(lactide-co-glycolide) Nanoparticles for Enhanced Cellular Uptake and Efficacy of Paclitaxel in the Treatment of Head and Neck Cancer. Pharmaceutics. 2020; 12(9):828. https://doi.org/10.3390/pharmaceutics12090828
Chicago/Turabian StyleHaider, Mohamed, Amr Elsherbeny, Jayalakshmi Jagal, Anna Hubatová-Vacková, and Iman Saad Ahmed. 2020. "Optimization and Evaluation of Poly(lactide-co-glycolide) Nanoparticles for Enhanced Cellular Uptake and Efficacy of Paclitaxel in the Treatment of Head and Neck Cancer" Pharmaceutics 12, no. 9: 828. https://doi.org/10.3390/pharmaceutics12090828
APA StyleHaider, M., Elsherbeny, A., Jagal, J., Hubatová-Vacková, A., & Saad Ahmed, I. (2020). Optimization and Evaluation of Poly(lactide-co-glycolide) Nanoparticles for Enhanced Cellular Uptake and Efficacy of Paclitaxel in the Treatment of Head and Neck Cancer. Pharmaceutics, 12(9), 828. https://doi.org/10.3390/pharmaceutics12090828