Abstract
Nanoparticles (NPs) are promising drug delivery systems (DDS) for identifying and treating cancer. Active targeting NPs can be generated by conjugation with ligands that bind overexpressed or mutant cell surface receptors on target cells that are poorly or not even expressed on normal cells. Receptor-mediated endocytosis of the NPs occurs and the drug is released inside the cell or in the surrounding tissue due to the bystander effect. Antibodies are the most frequently used ligands to actively target tumor cells. In this context, antibody-based therapies have been extensively used in HER2+ breast cancer. However, some patients inherently display resistance and in advanced stages, almost all eventually progress. Functionalized NPs through conjugation with antibodies appear to be a promising strategy to optimize targeted therapies due to properties related to biocompatibility, suitable delivery control and efficiency of functionalization. This review is focused on the different strategies to conjugate antibodies into polymeric NPs. Recent antibody conjugation approaches applied to the improvement of breast cancer therapy are highlighted in this review.
1. Introduction
Being a woman is the main risk factor for developing breast cancer and, unfortunately, in advanced stages, the disease remains incurable. According to immunohistochemical and transcriptomic criteria [1], breast cancer can be divided into three subtypes: (1) tumors that express hormonal receptors, so-called luminal; (2) cancers that overexpress the transmembrane tyrosine kinase HER2; and (3) tumors that do not overexpress any of the above-mentioned proteins, the so called triple negative breast cancers (TNBC). Among the different subtypes, those that overexpress HER2 (HER2+) occur in approximately one out of five women diagnosed with breast cancer.
Although HER2 expression has historically been associated with poor outcome, over the last 20 years, different therapies have been approved, improving the prognosis of patients with this type of tumor. Antibodies against the extracellular domain of the receptor (trastuzumab and biosimilars, pertuzumab or T-DM1) and small molecule inhibitors of the kinase activity of the receptor (lapatinib, tucatinib or neratinib) have reached the clinic. Despite the impact of trastuzumab-based therapies, there are patients inherently resistant to the drug. Moreover, in advanced disease states, almost all patients will progress on trastuzumab. The TNBC subtype is characterized by the absence of HER2 overexpression and undetectable levels of estrogen and progesterone receptors. Even though it only represents 15% of breast tumors, the prognosis is poor due to the limited therapeutic options that are currently available. In this context, the development of novel and improved therapeutics is a primary objective and finding targeted therapies is a very promising approach. In fact, antibody drug conjugates (ADCs) are very successful targeted delivery systems (eight ADCs were approved by the United States Food and Drug Administration (FDA) in recent years) [2,3,4,5]. Accordingly, the use of nanomedicines for detecting and targeting transmembrane receptors in cancer cells can also be an attractive approach for the treatment of cancer, as it acts on cancer cells in a specific manner, avoiding undesirable effects to normal tissue. The conjugation of nanocarriers, such as nanoparticles (NPs), with antibodies to generate targeted therapies has been proposed as a novel strategy for the treatment of cancer [6,7,8,9,10].
The antibody conjugated NPs (ACNPs) approach is built on the success of nanotechnology and antibody therapies. When compared with the development of ADCs, ACNPs present many advantages, such as the delivery of the drug in a controlled manner, preservation of the chemical structure of the drug, reduced risk of secondary metabolites (if metabolism is unpredictable), and finally, potentially reduced toxicity [11]. Based on these features, the membrane proteins overexpressed in tumor cells can be used to design the antibodies that will later be implemented as the nanoparticle vector. The mechanism of action of both targeted therapies (ADCs and ACNPs) are very similar. After binding to the target, the complexes are internalized into the cell through receptor-mediated endocytosis [12], resulting in the formation of endosomes. Later, endosomes and lysosomes are coupled to release the drug into the cytoplasm [13]. However, the cargo of the nanoparticle can also diffuse directly through the cellular membrane, augmenting the cellular penetration of the compound.
Polymeric NPs can be generated from biodegradable and biocompatible raw materials, and the release of the drug can be controlled by the polymeric structure. Biodegradable and biocompatible polymers can be naturally or synthetically made. They are configured by ester, amide, and ether functional groups to easily break down in natural environments. Because polycaprolactone (PCL), polylactide (PLA), and poly(lactic-co-glycolic acid) (PLGA) are FDA-approved biocompatible polymers, they are the most used polymers for drug delivery systems (DDS) generation. Other biodegradable polymers such as poly(anhydrides) [14], poly(ortho esters) [15], poly(amides) [16], poly(phosphoesters) [17] and poly(alkyl cyanoacrylates) [18] are great candidates as raw materials to explore nanotechnology. However, their scarce commercial availability hampers their development.
The common ways to obtain biodegradable and biocompatible polyesters are polycondensation processes or physical aggregation. Both methodologies fail in controlling the molecular weight, polydispersities and stereoregularity of the polymer. The physicochemical properties of polymeric NPs as DDS, such as size, molecular weight, molecular weight distributions, crystallinity and polarity, will determine their efficacy and viability. Organometallic catalysis allows the preparation of polymers and copolymers with a precise control in their structure. Currently, structurally well-controlled polyesters, like PCL and PLA, FDA-approved polymers for DDS generation, are catalytically generated via ring opening polymerization (ROP) of lactone or lactide rings using organometallic catalysis. Nowadays, new promising strategies based on high technology and tailor-made building-block materials using chemical catalysis are used to obtain biodegradable and biocompatible polymers with controlled physical properties [19]. Recently, some examples of polyesters or polycarbonate architectures for DDS generation are being synthetized via ROP or ring opening copolymerization (ROCOP) [19,20]. These novel methodologies to generate tailor-made biocompatible polymers are more attractive than the traditional way, facilitating their implementation in the field of nanomedicine.
There are several methodologies to obtain polymeric NPs. All of them can be classified into two main strategies, top-down and bottom-up. In the top-down strategy the NPs are obtained from preformed polymers, while the polymerization is achieved during NP formulation in bottom-up strategies [21]. The most common methods for polymeric NP generation are top-down, multiple emulsion and nanoprecipitation. Such methodologies will interfere in the physical–chemistry properties of DDS, such as efficiency of encapsulation, loading-efficiency, morphology, biodegradation, average size, polydispersities, and surface charge. Whereas nanoprecipitation is more convenient for encapsulating hydrophobic drugs, multiple emulsion allows higher efficiencies for hydrophilic counterparts.
Concerning the release of the drugs from the polymeric NPs, in the first phase, the drug is released abruptly, followed by a second step ruled by diffusion through pores and channels of the NPs, ending with the degradation of the polymer. It is noteworthy to point out that the burst release is dismissed when NPs are prepared from stimuli-triggered polymers. Drug delivery systems (DDS) can be optimized for clinical applications by controlling these above-mentioned steps through modifications in the polymeric structure [22,23]. Modifications on the polymeric NPs can produce several advantages: (1) control of the cargo and the release of the drug, (2) prolongation of their stability in blood circulation, (3) an increase in their capacity to carry high toxicity drugs, and (4) overcoming drug resistance mechanisms.
Although a vast array of materials has been used to formulate ACNPs, this review focuses particularly on polymeric ACNPs for breast cancer therapy.
2. Polymeric Antibody Conjugated Nanoparticles (ACNPs) to Improve Treatments in Breast Cancer
Polymeric NPs appear to be the most promising drug carriers due to their nanoscale size and potential for selective targeting and controlled drug release [24,25,26,27]. NPs in the range of 100–400 nm have been widely reported to accumulate at the tumor site through the enhanced permeability and retention (EPR) effect [28,29]. This favors high accumulation of the drug, facilitating its delivery to the site of interest by convection and diffusion processes [30], reducing, at the same time, the damage to surrounding tissues [31,32]. The passive targeting is drastically influenced by the size and surface charge of the NPs. Both features are important for the retention of the NP in the tumor and for its circulation time.
After accumulation in the tumor region, drug efficiency can even be increased by the so-called active targeting. Binding to overexpressed receptors on target cells [33] that are poorly or not even expressed on normal cells [34,35,36,37] affords active targeting of NPs to tumor cells. Actively targeted NPs allow the delivery of the drugs at the desired location, avoiding normal tissues and, therefore, enhancing the therapeutic efficiency. For example, NPs bearing the monoclonal antibody trastuzumab, which interacts with the surface protein HER2, are expected to accumulate at sites where HER2 is overexpressed, such as in breast or gastric tumors. These particles can be loaded with other agents such as taxanes, which synergize with trastuzumab in the treatment of breast tumors. NP internalization occurs via receptor-mediated endocytosis [37,38]. Antibodies are the most frequently used ligands to target tumor cells [39,40]. Antibody fragments, such as antigen-binding fragments (Fab) may also be conjugated to reach higher diffusion rates and improve tumor uptake [41,42]. Trastuzumab is the most widely explored monoclonal antibody for generating novel nanomedicines for breast cancer therapy by far.
3. Conjugation Strategies for ACNP Generation
NP functionalization with antibodies or antibody fragments can be carried out mainly via the adsorption phenomenon, covalent-nature binding, or binding by the use of adapter molecules (Figure 1). The immobilization of antibodies into the nanoparticle surface must guarantee both the desired amount of antibodies per nanoparticle and the proper antibody orientation [43,44]. Additionally, the immobilization method must generate a stable bond and preserve the biological activity of the antibody [45].
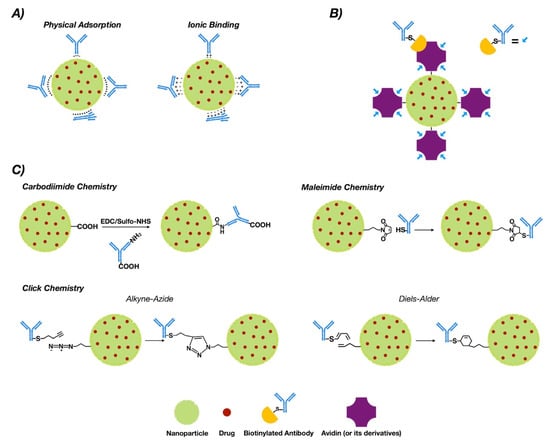
Figure 1.
Conjugation strategies for antibody conjugated nanoparticle (ACNP) generation: (A) adsorption; (B) use of adapters; and (C) covalent binding.
3.1. Adsorption
This phenomenon is a non-covalent immobilization strategy that comprises physical adsorption and ionic binding [46] (see Figure 1A). Physical adsorption occurs via weak interactions such as hydrogen bonding, electrostatic, hydrophobic and Van der Waals attractive forces [47], while ionic binding occurs between the opposite charges of the antibodies and NP surfaces [48]. However, when compared to other methodologies such as covalent binding, adsorption provides less stability [49]. On the other hand, the fact that the interaction is non-covalent may allow release of the antibody in the tumor tissue, allowing deploy of its antitumoral properties. In this regard, three modified methodologies of functionalized PLGA NPs with trastuzumab were generated via adsorption, charged interactions and covalent binding. The results obtained via covalent binding demonstrated its higher stability versus charge interactions and adsorption [50].
3.2. Covalent Strategies
Covalent binding requires prior activation of the NPs [51]. Commonly, covalent strategies occur via carbodiimide chemistry, maleimide chemistry or “click chemistry”. Table 1 compiles a collection of works for breast cancer therapy based on covalent strategies for antibody bio-conjugation, including bio-conjugation using aptamers.
3.2.1. Chemistry of Carbodiimide
The most common covalent conjugation corresponds with the binding through the primary amines of the antibodies [52] (see Figure 1C), without performing any chemical modification [53]. Previously, carboxyl groups of the NPs must be activated by the addition of cross-linking agents, where 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide (EDC) is the most used [54,55]. Even though it is not required, N-hydroxysuccinimide (NHS) or N-hydroxysulfosuccinimide (sulfo-NHS) are usually added to improve the coupling efficiency [56,57].
This methodology is easy but the coupling between functional groups and crosslinkers is not selective. This absence of control over antibody orientation into the NP surface is a primary disadvantage [58,59].
ACNPs prepared via this methodology have been used for detection and treatment of breast cancer. Table 1 shows a few works based on polymeric ACNPs where the antibody was conjugated via carbodiimide chemistry. The polymers mainly used were biodegradable polymers approved by the FDA, such as PLGA. Trastuzumab was the most frequently used antibody in these works [59,60,61,62,63,64,65,66]. As a representative example, monoclonal antibodies recognizing the specific profile of the cytokeratins expressed by breast cancer cells were conjugated to modify PLGA-based NPs via carbodiimide chemistry. Such ACNPs effectively delivered drugs into specific cells [67]. Acharya et al. prepared biodegradable PLGA NPs loaded with rapamycin and conjugated with cetuximab. These ACNPs were able to recognize the extracellular ligand-binding domain of epidermal growth factor receptor (EGFR). The latter receptor is expressed in a subset of breast tumors [68]. The therapeutic effects of docetaxel were greatly enhanced by the formulation of cetuximab conjugated ACNPs. The nanoparticle system demonstrated ~200 fold higher efficiency for the MDA MB 468 and MDA MB 231 cell lines respectively, which express EGFR [69,70].
3.2.2. Maleimide Chemistry
This approach occurs through sulfhydryl groups (–SH) of antibodies (Figure 1C). Sulfhydryl groups are much less abundant than primary amines or thiols. They are present in the side chain of cysteine [71]. Free sulfhydryl, the de-protonated form of thiol at physiological pH, is a better nucleophile than thiol groups [72]. The –SH groups are generated on the antibodies via reaction with primary amines or by reduction of native disulfide bonds of antibodies [73,74]. Furthermore, this chemical group can be easily obtained by modification of the ε-amino of lysine residues with sulfhydryl-addition reagents. 2-Iminothiolane (Traut’s reagent) and N-succinimidyl S-acetylthioacetate (SATA) are the most common [75].
The reaction of maleimide groups towards free sulfhydryls is a thousand times faster than with primary amines at neutral pH, which improves selectivity [76,77]. A stable thioether linkage is formed after alkylation reaction with sulfhydryl groups [78,79] (see Figure 1C). The most used maleimide cross-linking reagents are the NHS/maleimide heterobifunctional linkers, PEGylated analogues (NHS-PEG-maleimide), succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) and sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC) [80,81,82]. NHS-PEG-maleimide has been the most extensively used crosslinker in antibody conjugation strategies in polymeric NPs for breast cancer treatment (Table 1). SMCC is water-insoluble [83] but sulfo-SMCC is soluble in water due to a negatively charged sulfonate group on the NHS ring [84]. Therefore, using sulfo-SMCC as crosslinker, the free sulfhydryls of the antibody can be linked to the primary amines on the surface of the NPs via maleimide chemistry [85]. In this way, trastuzumab was attached to self-assembled chitosan-doxorubicin NPs via thiolation of lysine residues and subsequent linking of the thiols through sulfo-SMCC crosslinker to chitosan [86]. These ACNPS discriminated between HER2+ and HER2− cells in their mechanism of action. Heterobifunctional PEGylated linkers can also be used in the conjugation of thiolated antibodies to NPs through maleimide chemistry [87,88].
Non-selectivity of maleimide to cysteines has been reported. This lack of selectivity is due to exchange reactions with thiol-containing proteins in serum (e.g., albumin). Thus, non-homogenous conjugates and poorly defined yielding off-target cytotoxicity were obtained [89,90]. Antibody engineering techniques can be applied to increase homogeneity but the high cost and complexity of the strategy limits its use [3,91]. Alternative approaches to be explored are the use of next generation maleimides (NGM) or pyridazinediones and site-specific conjugation strategies (“click chemistry”) [92,93,94,95].
3.2.3. Click Chemistry
“Click chemistry” reactions occur efficiently at room temperature, under mild and in aqueous solvents. These reactions, which require no or minimal purification, resulted in irreversible chemical bonds with the absence of cytotoxic byproducts [96,97,98,99].
[3+2] Azide–alkyne cycloaddition (AAC) reactions catalyzed by copper (I) (CuAAC), strain-promoted [3+2] azide–alkyne cycloaddition (SPAAC) reactions, typical [4+2] Diels–Alder (DA), and inverse electron demand hetero Diels–Alder (iEDDA) reactions [100,101,102,103,104] configure the “click chemistry” strategy. The advantage of using azide and alkyne groups relys on their low existence in biological systems and inertness towards the majority of functional groups and biomolecules [105]. Functionalization of NPs with azide or alkyne moieties goes through EDC/NHS coupling or maleimide–thiol conjugation. In this context, NHS ester or maleimide must be present at one side of the linker, while azide or alkyne groups must be located at the other [106].
Cu(I) catalysts accelerate AAC reactions [107,108]. However, the toxicity associated with these catalysts holds back their use in living systems [109]. Thus, CuAAC reactions were used to develop a HER2 targeted ACNP for delivering DOX to breast cancer cells [110]. These ACNPs included a pH sensitive block polymer, poly(L-histidine). A NHS-PEG-alkyne linker was introduced to trastuzumab to provide an alkyne group into the antibody and thus further improve cellular uptake and toxicity for MCF7 and SKBR3 breast cancer cells. In the same way, optimum polyethylenglycol (PEG) coverage over trastuzumab conjugated ACNPs was also prepared via CuAAC conjugation to improve the pharmacokinetics [111]. Of note, developments in copper-free variants of AAC were achieved by introducing a strain-promoted azide–alkyne cycloaddition (SPAAC) reaction to overcome the CuAAC limitations [112,113,114].
The SPAAC variant introduces ring-strained alkynes (cycloalkynes) to create stable triazoles [115]. Conjugated PLGA–PEG NPs with Fab fragments of trastuzumab or cetuximab for the treatment of breast cancer were obtained via SPAAC [89]. The evaluation of these ACNPs using HER2+ breast cancer lines (HCC1954) demonstrated that site-specific conjugation by SPAAC increased the antigen binding capacity and the conjugation efficiency [89].
Shi et al., developed amphiphilic furan-functionalized self-assembling copolymers (poly(TMCC-co-LA)-g-PEG-furan) to form polymeric NPs and DA cycloadditions were used to conjugate anti-HER2 antibodies [116]. On the other hand, polymeric ACNPs comprised of surface furan groups were used to bind, by DA coupling chemistry, both anti-HER2 antibodies and chemotherapeutic doxorubicin for intracellular delivery of doxorubicin [117]. The cytotoxicity, specificity and intracellular uptake were ascertained in SKBR cells with this type of ACNP.
Thereafter, Logie et al. designed ACNPs of docetaxel to improve the efficacy in a NOD-SCID- IL-2Rgnull mouse model of breast cancer [118]. These ACNPs were generated with the incorporation of a novel HER2 fragment antibody, Fab 73J. The lower toxicity of the ACNPs successfully allowed for higher dosing regimens.
3.3. Binding by Adapter Molecules
Non-covalent approaches by using adapter biomolecules avoid randomly oriented antibodies [119]. The most relevant binding strategy exploits the biotin–avidin interaction, which is based on the strong binding affinity between biotin and a biotin-binding protein, such as avidin or its analogues [120,121] (See Figure 1B).
Biotin is a potential tumor-targeting moiety because it is a water-soluble vitamin (vitamin H), it is essential for growth and cell signaling, and their receptors are overexpressed on a broad range of cancer cells [122,123,124]. As an example of this non-covalent approach, the biotin-decorated PEG-PCL NPs were co-loaded with doxorubicin and quercetin to overcome doxorubicin-resistant breast cancer. In the same way, 7-ethyl-10-hydroxycamptothecin (SN-38) was successfully loaded into PLGA-PEG-biotin NPs to treat breast cancer. Compared to non-biotin conjugated NPs, these conjugated NPs increased the in vivo antitumor drug efficacy [125,126].
On the other hand, three N-acetyl glucosamine and four mannose residues constitute the oligosaccharide side chain of the tetrameric glycoprotein avidin [127,128]. Each of the four avidin monomers can bind to biotin [129]. However, because avidin is a basic protein with a high isoelectric point (pI) (~10.5) [130], both positively charged residues at physiological pH and glycosylation may lead to nonspecific binding of avidin to other molecules [131]. Thus, other forms of avidin, like streptavidin or neutravidin, are preferred [132]. Contrary to avidin, streptavidin is not a glycoprotein [133,134] and its pI is much lower, which prevents interactions with sugar receptors [135]. One of the most elegant strategies applied consisted in engineering monomeric avidin/streptavidin to monovalent biotin binding [136] and nanosized poly-avidins named Avidin-Nucleic-Acid-Nano-Assemblies (ANANAS) [137]. Notably, the ANANAS technology along with the use of an antibody (cetuximab) to target a ligand demonstrated an in vivo therapeutic efficacy at a dose of doxorubicin below the one clinically used [138].
To minimize the immunogenicity, a recombinant non-glycosylated form of avidin (neutravidin), was developed [139,140,141]. Warlick et al. prepared anti-HER2-modified NPs by the use of biotin/neutravidin aptamers to efficiently bind to and internalize in HER2-overexpressing cells. Trastuzumab was biotinylated and human serum albumin NPs were functionalized with neutravidin via NHS-PEG-Mal heterobifunctional crosslinker conjugation. This work clearly proved ACNPs combine specific tumor targeting with the drug delivery properties [141].
Finally, the use of aptamers was extended by Powell et al. into the development of a targeted delivery of siRNA specific to the multidrug resistance (MDR) gene of metastatic breast cancer. The use of a cholesterol, DOTAP and PLGA or PLGA-PEG lipid-polymer hybrid liposomes nanocarrier system overcame resistances of breast cancer cells generated against therapeutic drugs. The mechanism consists in enhancing the knockdown of the MDR gene (P-gp) by the use of aptamer-labeled P-gp siRNA encapsulated NPs [142].
4. Challenges for Clinical Implementation
The myriad of antibody conjugation strategies to generate polymeric ACNPs, along with the elicited benefits, in terms of site-selectivity and decreased off-target cytotoxicity, open attractive opportunities for the development of novel therapeutics in breast cancer.
There are several challenges in the clinical development of this kind of therapeutic agent. Firstly, identification of a suitable target and availability of a specific antibody play a significant role. The design of an effective antibody against a protein that is overexpressed in the tumor cell, but not in non-transformed tissue, is desirable. This finding has been shown to increase the therapeutic index of ADCs and should also apply to ACNPs [143]. However, not many proteins have been described as overexpressed in breast cancer. Table 2 shows a list of ADCs in clinical development. Of note, most of the antibodies used to build these ADCs were not designed to face overexpressed proteins due to gene amplification, as most of them are immunotherapy-based antibodies. In this regard, ADCs can be based on antibodies that lack tumor specificity, like the recently approved ADC, sacituzumab-govitecan, against the trophoblast cell surface antigen 2 (Trop-2) [144,145]. This protein is not only expressed in triple negative breast cancer but in many other epithelial tumors [145]. Nonetheless, this ADC is still active as it maintains an adequate therapeutic index. Indeed, the Trop-2 protein is mainly expressed during germline differentiation and this type of protein is generally expressed in adult mature tissue but not in tumors [145].
Considering the difficulty in finding specific proteins within the tumor, in the case of ADCs, the diffusion of the payload to target not only tumor cells but also the tumor stroma, affecting the tumor environment, emerges as a desirable effect. This is the so-called bystander effect and this has been demonstrated as key for the development of some therapeutics like trastuzumab-deruxtecan, an ADC with a cleavable linker against HER2 [146]. In addition, the bystander effect can revert resistance caused by cancer cells that do not express the target protein [147,148]. ACNPs can be designed to inherently possess a bystander effect, therefore reverting drug resistance due to tumor heterogeneity. In line with this, resistance to some ADCs has been described as related to the lysosomal processing of the antibody [149,150,151]. In this context, ACNPs could overcome this limitation. Finally, polymeric NPs own the ability to release the payload when a specific pH, mainly acidic, is produced in the tumor, particularly in areas of poor vascularization, therefore acting on areas where cancer drugs have limited penetration [152].
We envision the next steps will be developing new strategies to target specific proteins of non-tumor components of the tumor, including vasculature and the immune system. As it is shown in Table 2, a number of ADCs are formed by antibodies that recognize proteins expressed at the membrane of immune system cells, including anti-LAG3, PD-L1, CD73 or TGF ß, among others. A number of these membrane proteins are involved in the stimulation of an immunosuppressed environment. ACNPs aimed at targeting these immune system proteins, combined with the capability of these DDS to load antitumor drugs, opens the possibility of using a single ACNP to carry out multiple antitumor effects. The latter may substantially increase the antitumor capability and may therefore represent a novel and efficient way of fighting tumors. In some specific subtypes of breast cancer, the expression of a particular protein has shown to be a remarkable target. This is the case for HER3 in luminal and HER2 resistant tumors, where recently published data shows signs of potential activity.
Other strategies are being explored for the generation of more efficient ACNPs. Redox responsive polymeric ACNPs for the treatment of breast cancer have been designed [63]. These ACNPs encapsulated doxorubicin and were decorated by folic acid, trastuzumab and folic acid plus trastuzumab, all of them based on carbodiimide chemistry. By comparing in vivo studies, no significant differences in cytotoxicity were observed between ACNPs formulations, and no toxicity to heart, liver or kidney were reported when compared to free doxorubicin.
The influence on the ACNP shapes are still at an early stage and further investigations are required to determine the effects of NP shape on cellular uptake. As a representative example, worm-like PCL-PEG ACNPs for the controlled release of paclitaxel to HER2+ breast cancer cells were designed [153]. A new conjugation strategy based on the use of –C–N– to covalently bond trastuzumab to the surface of NPs was claimed to improve the stability of the formulation in physiological circulation. In this sense, it is worth noting the use of ultrasound to improve the cell targeting capability in vitro and efficiency in vivo of the paclitaxel-loaded ACNPs in a very recent work [66]. The combination of ACNPs and ultrasound enhanced the paclitaxel targeting and accumulation in breast cancers.
Finally, the use of two different antibodies as a full molecule or using only the Fab fragment working as a bispecific antibody could redirect the nanoparticle to two different targets. For instance, one antibody to a protein contained at the membrane of a tumor cell and the other to an immune suppressive target also expressed in a cancer cell. On top of this dual mechanisms, we will also have the effect of the payload. An example will be the use of an anti-HER2 in combination with an anti-PD-L1 in the same nanoparticle.
5. Conclusions and Outlook
We envision two current challenges: First, the identification of the best polymeric structure to better modulate the payload release profile in a given biological context.
Second, the best choice of the antibody and conjugation strategy to better guide the nanoparticle to the tumor area. Optimization of these two features can improve the pharmacokinetic profile, facilitate target binding, induce a bystander effect, and produce direct antitumor activity in addition to immunologic activation. We are aware that countless information will arise in the future in this field providing evidence of the best development process.

Table 1.
Main reported works on antibody conjugation strategies of polymeric NPs for the breast cancer treatment.

Table 2.
Summary of ongoing clinical trials evaluating ADCs (antibody drug conjugates) in breast cancer describing the target, agent and the clinical stage ent.
Author Contributions
The authors detail their individual contributions as follow: Conceptualization, C.A.-M., A.J., I.B. and A.O.; investigation, A.J.; writing—original draft preparation, A.J. and F.J.C.; writing—review and editing, A.P., C.A.-M. and A.J.; supervision, C.A.-M.; funding acquisition, A.O. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge financial support from Ministerio de Ciencia e Innovación, Spain (Grant Nos. CTQ2017-84131-R and CTQ2016-81797-REDC Programa Redes Consolider) (to C. Alonso-Moreno), and Instituto de Salud Carlos III (PI19/00808), CIBERONC and CRIS Cancer Foundation (to A. Ocaña).
Acknowledgments
Alberto Juan and Francisco Cimas acknowledge ACEPAIN Cancer Foundation for the fellowships.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yeo, S.K.; Guan, J.L. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody–drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef]
- Chudasama, V.; Maruani, A.; Caddick, S. Recent advances in the construction of antibody-drug conjugates. Nat. Chem. 2016, 8, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-drug conjugates: A comprehensive review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody–drug conjugates for cancer therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, 1902604. [Google Scholar] [CrossRef]
- Heath, T.D.; Fraley, R.T.; Papahdjopoulos, D. Antibody targeting of liposomes: Cell specificity obtained by conjugation of F (ab’) 2 to vesicle surface. Science 1980, 210, 539–541. [Google Scholar] [CrossRef]
- Leserman, L.D.; Barbet, J.; Kourilsky, F.; Weinstein, J.N. Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature 1980, 288, 602–604. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Mamot, C.; Ritschard, R.; Wicki, A.; Stehle, G.; Dieterle, T.; Bubendorf, L.; Hilker, C.; Deuster, S.; Herrmann, R.; Rochlitz, C. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: A phase 1 dose-escalation study. Lancet Oncol. 2012, 13, 1234–1241. [Google Scholar] [CrossRef]
- Johnston, M.C.; Scott, C.J. Antibody Conjugated Nanoparticles as a Novel Form of Antibody Drug Conjugate Chemotherapy. Drug Discov. Today: Technol. 2018, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Chari, R.V.J. Targeted cancer therapy: Conferring specificity to cytotoxic drugs. Acc. Chem. Res. 2008, 41, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-specific antibody drug conjugates for cancer therapy. MAbs 2014, 6, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Pachence, J.M.; Bohrer, M.P.; Kohn, J. Biodegradable polymers. Princ. Tissue Eng. 2007, 3, 323–339. [Google Scholar] [CrossRef]
- Casalini, T.; Perale, G. Types of bioresorbable polymers for medical applications. In Durability and Reliability of Medical Polymers; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–29. [Google Scholar] [CrossRef]
- Seth, A.; Heo, M.B.; Lim, Y.T. Poly (γ-glutamic acid) based combination of water-insoluble paclitaxel and TLR7 agonist for chemo-immunotherapy. Biomaterials 2014, 35, 7992–8001. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Mao, H.Q.; Leong, K.W. Polyphosphoesters in drug and gene delivery. Adv. Drug Deliv. Rev. 2003, 55, 483–499. [Google Scholar] [CrossRef]
- Fusser, M.; Øverbye, A.; Pandya, A.D.; Mørch, Ý.; Borgos, S.E.; Kildal, W.; Snipstad, S.; Sulheim, E.; Fleten, K.G.; Askautrud, H.A.; et al. Cabazitaxel-loaded Poly(2-ethylbutyl cyanoacrylate) nanoparticles improve treatment efficacy in a patient derived breast cancer xenograft. J. Control. Release 2019, 293, 183–192. [Google Scholar] [CrossRef]
- Martínez, J.; Martínez De Sarasa Buchaca, M.; De La Cruz-Martínez, F.; Alonso-Moreno, C.; Sánchez-Barba, L.F.; Fernandez-Baeza, J.; Rodríguez, A.M.; Rodríguez-Diéguez, A.; Castro-Osma, J.A.; Otero, A.; et al. Versatile organoaluminium catalysts based on heteroscorpionate ligands for the preparation of polyesters. Dalton Trans. 2018, 47, 7471–7479. [Google Scholar] [CrossRef]
- Martínez, J.; Castro-Osma, J.A.; Alonso-Moreno, C.; Rodríguez-Diéguez, A.; North, M.; Otero, A.; Lara-Sánchez, A. One-Component Aluminum (Heteroscorpionate) Catalysts for the Formation of Cyclic Carbonates from Epoxides and Carbon Dioxide. ChemSusChem 2017, 10, 1175–1185. [Google Scholar] [CrossRef]
- Nanotechnology. In Nano-and Microscale Drug Delivery Systems: Design and Fabrication; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–32. [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted Polymeric Therapeutic Nanoparticles: Design, Development and Clinical Translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the Microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, D.C.; Longo, G.; Szleifer, I. Behavior of Ligand Binding Assays with Crowded Surfaces: Molecular Model of Antigen Capture by Antibody-Conjugated Nanoparticles. PLoS ONE 2017, 12, e0185518. [Google Scholar] [CrossRef] [PubMed]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A Review of Current Nanoparticle and Targeting Moieties for the Delivery of Cancer Therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To Exploit the Tumor Microenvironment: Passive and Active Tumor Targeting of Nanocarriers for Anti-Cancer Drug Delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bawa, R. FDA and Nanotech: Baby Steps Lead to Regulatory Uncertainty. In Bio-Nanotechnology; Blackwell Publishing Ltd.: Oxford, UK, 2013; pp. 720–732. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. Odyssey of a Cancer Nanoparticle: From Injection Site to Site of Action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yin, M.; Zhao, L.; Meng, F.; Luo, L. Recent Progress on Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Cancer Biol. Med. 2017, 14, 228–241. [Google Scholar] [CrossRef]
- Jiang, Z.; Guan, J.; Qian, J.; Zhan, C. Peptide Ligand-Mediated Targeted Drug Delivery of Nanomedicines. Biomater. Sci. 2019, 7, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Ligand-Targeted Therapeutics in Anticancer Therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Stefanick, J.F.; Omstead, D.T.; Kiziltepe, T.; Bilgicer, B. Dual-Receptor Targeted Strategy in Nanoparticle Design Achieves Tumor Cell Selectivity through Cooperativity. Nanoscale 2019, 11, 4414–4427. [Google Scholar] [CrossRef] [PubMed]
- Ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Pirollo, K.F.; Chang, E.H. Does a Targeting Ligand Influence Nanoparticle Tumor Localization or Uptake? Trends Biotechnol. 2008, 26, 552–558. [Google Scholar] [CrossRef]
- Bareford, L.M.; Swaan, P.W. Endocytic Mechanisms for Targeted Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine Applied to Translational Oncology: A Future Perspective on Cancer Treatment. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 81–103. [Google Scholar] [CrossRef]
- Farahavar, G.; Abolmaali, S.S.; Gholijani, N.; Nejatollahi, F. Antibody-Guided Nanomedicines as Novel Breakthrough Therapeutic, Diagnostic and Theranostic Tools. Biomater. Sci. 2019, 7, 4000–4016. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, Y.; Pang, Z. Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery. Front. Pharmacol. 2017, 8, 952. [Google Scholar] [CrossRef]
- Xenaki, K.T.; Oliveira, S.; van Bergen en Henegouwen, P.M.P. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol. 2017, 8, 1287. [Google Scholar] [CrossRef]
- Quarta, A.; Manna, L.; Pellegrino, T. Antibody-Functionalized Inorganic NPs: Mimicking Nature for Targeted Diagnosis and Therapy. In Bioinspired Approaches for Human-Centric Technologies; Springer International Publishing: New York, NY, USA, 2014; pp. 1–28. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Antunes, P.W.P.; Yapuchura, E.R.; Guimarães, M.C.C. Impact of Conjugation Strategies for Targeting of Antibodies in Gold Nanoparticles for Ultrasensitive Detection of 17β-Estradiol. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Cardoso, M.M.; Peça, I.N.; Roque, A.C.A. Antibody-Conjugated Nanoparticles for Therapeutic Applications. Curr. Med. Chem. 2012, 19, 3103–3127. [Google Scholar] [CrossRef] [PubMed]
- Liébana, S.; Drago, G.A. Bioconjugation and Stabilisation of Biomolecules in Biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar] [CrossRef]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Grazia Cascone, M.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the Chemical and Biological Functionalization of Scaffolds for Cardiac Tissue Engineering: A Review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Sein, H.; Lu, S.; Radwanska, M.; Muyldermans, S.; Sterckx, Y.G.J.; Magez, S. Functionalization of Gold Nanoparticles with Nanobodies through Physical Adsorption. Anal. Methods 2017, 9, 3430–3440. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jang, W.S.; Park, J.S. Comparison of Adsorption and Conjugation of Herceptin on Poly(Lactic--Glycolic Acid) Nanoparticles–Effect on Cell Internalization in Breast Cancer Cells. Mater. Sci. Eng. C 2018, 92, 496–507. [Google Scholar] [CrossRef]
- Parracino, M.A.; Martín, B.; Grazú, V. State-of-the-Art Strategies for the Biofunctionalization of Photoactive Inorganic Nanoparticles for Nanomedicine. In Photoactive Inorganic Nanoparticles: Surface Composition and Nanosystem Functionality; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–257. [Google Scholar] [CrossRef]
- Sivaram, A.J.; Wardiana, A.; Howard, C.B.; Mahler, S.M.; Thurecht, K.J. Recent Advances in the Generation of Antibody-Nanomaterial Conjugates. Adv. Healthc. Mater. 2018, 7, 1700607. [Google Scholar] [CrossRef]
- Polo, E.; Puertas, S.; Moros, M.; Batalla, P.; Guisán, J.M.; De La Fuente, J.M.; Grazú, V. Tips for the Functionalization of Nanoparticles with Antibodies. Methods Mol. Biol. 2013, 1051, 149–163. [Google Scholar] [CrossRef]
- Fager, C.; Olsson, E. Understanding and Utilizing the Biomolecule/Nanosystems Interface: Soft Materials and Coatings for Controlled Drug Release. In Nanotechnologies in Preventive and Regenerative Medicine: An Emerging Big Picture; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 244–260. [Google Scholar] [CrossRef]
- Saha, B.; Songe, P.; Evers, T.H.; Prins, M.W.J. The Influence of Covalent Immobilization Conditions on Antibody Accessibility on Nanoparticles. Analyst 2017, 142, 4247–4256. [Google Scholar] [CrossRef]
- Yao, V.J.; D’Angelo, S.; Butler, K.S.; Theron, C.; Smith, T.L.; Marchiò, S.; Gelovani, J.G.; Sidman, R.L.; Dobroff, A.S.; Brinker, C.J.; et al. Ligand-Targeted Theranostic Nanomedicines against Cancer. J. Control. Release 2016, 240, 267–286. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 Years of Biofunctionalization and Surface Chemistry of Inorganic Nanoparticles for Nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Rusling, J.F.; Dixit, C.K. Site-Selective Orientated Immobilization of Antibodies and Conjugates for Immunodiagnostics Development. Methods 2017, 116, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, C.H.; Park, J.; Seo, S.; Lim, E.K.; Song, Y.J.; Suh, J.S.; Yoon, H.G.; Huh, Y.M.; Haam, S. Antibody Conjugated Magnetic PLGA Nanoparticles for Diagnosis and Treatment of Breast Cancer. J. Mater. Chem. 2007, 17, 2695–2699. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, X.; Zhao, J.; Ding, J.; Feng, S.S. Multimodality Treatment of Cancer with Herceptin Conjugated, Thermomagnetic Iron Oxides and Docetaxel Loaded Nanoparticles of Biodegradable Polymers. Biomaterials 2012, 33, 7519–7529. [Google Scholar] [CrossRef]
- Sun, B.; Feng, S.S. Trastuzumab-Functionalized Nanoparticles of Biodegradable Copolymers for Targeted Delivery of Docetaxel. Nanomedicine 2009, 4, 431–445. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Nipunbabu, V.; Rejeeth, C.; Sivasubramanian, S.; Gunasekaran, P.; Muthuchelian, K.; Kannan, S. Multifunctional HER2-Antibody Conjugated Polymeric Nanocarrier-Based Drug Delivery System for Multi-Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2014, 6, 6469–6480. [Google Scholar] [CrossRef]
- Kumar, A.; Lale, S.V.; Alex, M.R.A.; Choudhary, V.; Koul, V. Folic Acid and Trastuzumab Conjugated Redox Responsive Random Multiblock Copolymeric Nanocarriers for Breast Cancer Therapy: In-Vitro and in-Vivo Studies. Colloids Surf. B Biointerfaces 2017, 149, 369–378. [Google Scholar] [CrossRef]
- Fathian, F.; Derakhshandeh, K.; Khaleseh, F.; Hemmati, A.; Mansouri, K.; Khazaei, M. Active Targeting Carrier for Breast Cancer Treatment: Monoclonal Antibody Conjugated Epirubicin Loaded Nanoparticle. J. Drug Deliv. Sci. Technol. 2019, 53, 101136. [Google Scholar] [CrossRef]
- Niza, E.; Noblejas-lópez, M.D.M.; Bravo, I.; Nieto-jiménez, C.; Castro-osma, J.A.; Canales-vázquez, J.; Lara-sanchez, A.; Moya, E.M.G.; Burgos, M.; Ocaña, A.; et al. Trastuzumab-Targeted Biodegradable Nanoparticles for Enhanced Delivery of Dasatinib in HER2+ Metastasic Breast Cancer. Nanomaterials 2019, 9, 1793. [Google Scholar] [CrossRef]
- Zhong, S.; Ling, Z.; Zhou, Z.; He, J.; Ran, H.; Wang, Z.; Zhang, Q.; Song, W.; Zhang, Y.; Luo, J. Herceptin-Decorated Paclitaxel-Loaded Poly(Lactide-Co-Glycolide) Nanobubbles: Ultrasound-Facilitated Release and Targeted Accumulation in Breast Cancers. Pharm. Dev. Technol. 2020, 25, 454–463. [Google Scholar] [CrossRef]
- Kocbek, P.; Obermajer, N.; Cegnar, M.; Kos, J.; Kristl, J. Targeting Cancer Cells Using PLGA Nanoparticles Surface Modified with Monoclonal Antibody. J. Control. Release 2007, 120, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Dilnawaz, F.; Sahoo, S.K. Targeted Epidermal Growth Factor Receptor Nanoparticle Bioconjugates for Breast Cancer Therapy. Biomaterials 2009, 30, 5737–5750. [Google Scholar] [CrossRef] [PubMed]
- Kutty, R.V.; Feng, S.-S. Cetuximab Conjugated Vitamin E TPGS Micelles for Targeted Delivery of Docetaxel for Treatment of Triple Negative Breast Cancers. Biomaterials 2013, 34, 10160–10171. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chang, D.S. Fabrication, Characterization, and Biological Evaluation of Anti-HER2 Indocyanine Green-Doxorubicinencapsulated PEG-b-PLGA Copolymeric Nanoparticles for Targeted Photochemotherapy of Breast Cancer Cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific-ES. Sulfhydryl-Reactive Crosslinker Chemistry. Available online: https://www.thermofisher.com/es/es/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/sulfhydryl-reactive-crosslinker-chemistry.html (accessed on 19 August 2020).
- Kantner, T.; Watts, A.G. Characterization of Reactions between Water-Soluble Trialkylphosphines and Thiol Alkylating Reagents: Implications for Protein-Conjugation Reactions. Bioconjug. Chem. 2016, 27, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.M.; Rehmann, M.S.; Skeens, K.M.; Maverakis, E.; Kloxin, A.M. Thiol-Ene Click Hydrogels for Therapeutic Delivery. ACS Biomater. Sci. Eng. 2016, 2, 165–179. [Google Scholar] [CrossRef]
- Agarwal, P.; Bertozzi, C.R. Site-Specific Antibody-Drug Conjugates: The Nexus of Bioorthogonal Chemistry, Protein Engineering, and Drug Development. Bioconjug. Chem. 2015, 26, 176–192. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef]
- Akkapeddi, P.; Azizi, S.A.; Freedy, A.M.; Cal, P.M.S.D.; Gois, P.M.P.; Bernardes, G.J.L. Construction of Homogeneous Antibody-Drug Conjugates Using Site-Selective Protein Chemistry. Chem. Sci. 2016, 7, 2954–2963. [Google Scholar] [CrossRef]
- Renault, K.; Fredy, J.W.; Renard, P.Y.; Sabot, C. Covalent Modification of Biomolecules through Maleimide-Based Labeling Strategies. Bioconjugate Chem. 2018, 29, 2497–2513. [Google Scholar] [CrossRef]
- Kim, D.; Herr, A.E. Protein Immobilization Techniques for Microfluidic Assays. Biomicrofluidics 2013, 7, 041501. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.B.; Caspersen, M.B.; Robinson, E.; Morais, M.; Maruani, A.; Nunes, J.P.M.; Nicholls, K.; Saxton, M.J.; Caddick, S.; Baker, J.R.; et al. A Platform for Efficient, Thiol-Stable Conjugation to Albumin’s Native Single Accessible Cysteine. Org. Biomol. Chem. 2015, 13, 7946–7949. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, B.; Afarideh, H.; Ghannadi-Maragheh, M.; Bahrami-Samani, A.; Asgari, M. Preparation and Evaluation of APTES-PEG Coated Iron Oxide Nanoparticles Conjugated to Rhenium-188 Labeled Rituximab. Nucl. Med. Biol. 2017, 48, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Dovgan, I.; Kolodych, S.; Koniev, O.; Wagner, A. 2-(Maleimidomethyl)-1,3-Dioxanes (MD): A Serum-Stable Self-Hydrolysable Hydrophilic Alternative to Classical Maleimide Conjugation. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Markwalter, C.F.; Kantor, A.G.; Moore, C.P.; Richardson, K.A.; Wright, D.W. Inorganic Complexes and Metal-Based Nanomaterials for Infectious Disease Diagnostics. Chem. Rev. 2019, 119, 1456–1518. [Google Scholar] [CrossRef]
- Koniev, O.; Wagner, A. Developments and Recent Advancements in the Field of Endogenous Amino Acid Selective Bond Forming Reactions for Bioconjugation. Chem. Soc. Rev. 2015, 44, 5495–5551. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, T.T.; Huang, Z.F.; Hu, S.W.; Zhao, W.; Xu, J.J.; Chen, H.Y. An Exploration of Nucleic Acid Liquid Biopsy Using a Glucose Meter. Chem. Sci. 2018, 9, 3517–3522. [Google Scholar] [CrossRef]
- Di Marco, M.; Shamsuddin, S.; Razak, K.A.; Aziz, A.A.; Devaux, C.; Borghi, E.; Levy, L.; Sadun, C. Overview of the Main Methods Used to Combine Proteins with Nanosystems: Absorption, Bioconjugation, and Encapsulation. Int. J. Nanomed. 2010, 5, 37–49. [Google Scholar] [CrossRef]
- Yousefpour, P.; Atyabi, F.; Vasheghani-Farahani, E.; Movahedi, A.A.M.; Dinarvand, R. Targeted Delivery of Doxorubicin-Utilizing Chitosan Nanoparticles Surface-Functionalized with Anti-Her2 Trastuzumab. Int. J. Nanomed. 2011, 6, 1977–1990. [Google Scholar] [CrossRef][Green Version]
- Eloy, J.O.; Petrilli, R.; Trevizan, L.N.F.; Chorilli, M. Immunoliposomes: A Review on Functionalization Strategies and Targets for Drug Delivery. Colloids Surf. B Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, Y.; Li, Y.; Sun, X.; Sun, F.; Wang, X.; Mu, H.; Li, J.; Liu, X.; Teng, L.; et al. Comparison of Three Different Conjugation Strategies in the Construction of Herceptin-Bearing Paclitaxel-Loaded Nanoparticles. Biomater. Sci. 2016, 4, 1219. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.K.; Richards, D.A.; Nogueira, J.C.F.; Campbell, K.; Smyth, P.; Fernández, M.; Scott, C.J.; Chudasama, V. Forming Next-Generation Antibody-Nanoparticle Conjugates through the Oriented Installation of Non-Engineered Antibody Fragments. Chem. Sci. 2017, 9, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody-Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.F.U.H.; Wang, R.; Ling, S.; Wang, S. Antibody Engineering for Pursuing a Healthier Future. Front. Microbiol. 2017, 8, 495. [Google Scholar] [CrossRef] [PubMed]
- Forte, N.; Livanos, M.; Miranda, E.; Morais, M.; Yang, X.; Rajkumar, V.S.; Chester, K.A.; Chudasama, V.; Baker, J.R. Tuning the Hydrolytic Stability of Next Generation Maleimide Cross-Linkers Enables Access to Albumin-Antibody Fragment Conjugates and Tri-ScFvs. Bioconjug. Chem. 2018, 29, 486–492. [Google Scholar] [CrossRef]
- Schumacher, F.F.; Sanchania, V.A.; Tolner, B.; Wright, Z.V.F.; Ryan, C.P.; Smith, M.E.B.; Ward, J.M.; Caddick, S.; Kay, C.W.M.; Aeppli, G.; et al. Homogeneous Antibody Fragment Conjugation by Disulfide Bridging Introduces “Spinostics”. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Bahou, C.; Richards, D.A.; Maruani, A.; Love, E.A.; Javaid, F.; Caddick, S.; Baker, J.R.; Chudasama, V. Highly Homogeneous Antibody Modification through Optimisation of the Synthesis and Conjugation of Functionalised Dibromopyridazinediones. Org. Biomol. Chem. 2018, 16, 1359–1366. [Google Scholar] [CrossRef]
- Morais, M.; Ma, M.T. Site-Specific Chelator-Antibody Conjugation for PET and SPECT Imaging with Radiometals. Drug Discov. Today Technol. 2018, 30, 91–104. [Google Scholar] [CrossRef]
- Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Application of Click Chemistry in Nanoparticle Modification and Its Targeted Delivery. Biomater. Res. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Yin, B.; Jiang, X. Click Chemistry-Mediated Nanosensors for Biochemical Assays. Theranostics 2016, 6, 969–985. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.M.; Wang, D. Click Chemistry, a Powerful Tool for Pharmaceutical Sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Click Chemistry as a Tool for Cell Engineering and Drug Delivery. Molecules 2019, 24, 172. [Google Scholar] [CrossRef] [PubMed]
- Presolski, S.I.; Hong, V.P.; Finn, M.G. Copper-Catalyzed Azide–Alkyne Click Chemistry for Bioconjugation. Curr. Protoc. Chem. Biol. 2011, 3, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-Free Click Chemistry for Dynamic in Vivo Imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef]
- Knall, A.C.; Slugovc, C. Inverse Electron Demand Diels-Alder (IEDDA)-Initiated Conjugation: A (High) Potential Click Chemistry Scheme. Chem. Soc. Rev. 2013, 42, 5131–5142. [Google Scholar] [CrossRef]
- Schilling, C.I.; Jung, N.; Biskup, M.; Schepers, U.; Bräse, S. Bioconjugation via Azide-Staudinger Ligation: An Overview. Chem. Soc. Rev. 2011, 40, 4840–4871. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Sun, H.; Cui, C.; Zhang, L.; Jiang, Y.; Wu, Y.; Wang, Y.; Li, J.; Sumerlin, B.S.; et al. Thiol-Ene Click Chemistry: A Biocompatible Way for Orthogonal Bioconjugation of Colloidal Nanoparticles. Chem. Sci. 2017, 8, 6182–6187. [Google Scholar] [CrossRef]
- Spicer, C.D.; Davis, B.G. Selective Chemical Protein Modification. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Pickens, C.J.; Johnson, S.N.; Pressnall, M.M.; Leon, M.A.; Berkland, C.J. Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide-Alkyne Cycloaddition. Bioconjugate Chem. 2018, 29, 686–701. [Google Scholar] [CrossRef]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface Functionalization of Exosomes Using Click Chemistry. Bioconjug. Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-Catalysed Azide-Alkyne Cycloadditions (CuAAC): An Update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gai, Y.; Anderson, C.J.; Zeng, D. Highly-Efficient and Versatile Fluorous-Tagged Cu (i)-Catalyzed Azide-Alkyne Cycloaddition Ligand for Preparing Bioconjugates. Chem. Commun. 2015, 51, 17072–17075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Badkas, A.; Stevenson, M.; Lee, J.Y.; Leung, Y.K. Herceptin Conjugated PLGA-PHis-PEG PH Sensitive Nanoparticles for Targeted and Controlled Drug Delivery. Int. J. Pharm. 2015, 487, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Badkas, A.; Frank, E.; Zhou, Z.; Jafari, M.; Chandra, H.; Sriram, V.; Lee, J.-Y.; Yadav, J.S. Modulation of in Vitro Phagocytic Uptake and Immunogenicity Potential of Modified Herceptin®-Conjugated PLGA-PEG Nanoparticles for Drug Delivery. Colloids Surf. B Biointerfaces 2018, 162, 271–278. [Google Scholar] [CrossRef]
- Hatit, M.Z.C.; Reichenbach, L.F.; Tobin, J.M.; Vilela, F.; Burley, G.A.; Watson, A.J.B. A Flow Platform for Degradation-Free CuAAC Bioconjugation. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Ramil, C.P.; Lin, Q. Bioorthogonal Chemistry: Strategies and Recent Developments. Chem. Commun. 2013, 49, 11007–11022. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A Strain-Promoted [3 + 2] Azide-Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Dommerholt, J.; Rutjes, F.P.J.T.; van Delft, F.L. Strain-Promoted 1, 3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides. In Topics in Current Chemistry; Springer International Publishing: New York, NY, USA, 2016; pp. 1–20. [Google Scholar] [CrossRef]
- Shi, M.; Wosnick, J.H.; Ho, K.; Keating, A.; Shoichet, M.S. Immuno-Polymeric Nanoparticles by Diels-Alder Chemistry. Angew. Chem.-Int. Ed. 2007, 46, 6126–6131. [Google Scholar] [CrossRef]
- Shi, M.; Ho, K.; Keating, A.; Shoichet, M.S. Doxorubicin-Conjugated Immuno-Nanoparticles for Intracellular Anticancer Drug Delivery. Adv. Funct. Mater. 2009, 19, 1689–1696. [Google Scholar] [CrossRef]
- Logie, J.; Ganesh, A.N.; Aman, A.M.; Al-awar, R.S.; Shoichet, M.S. Preclinical Evaluation of Taxane-Binding Peptide-Modified Polymeric Micelles Loaded with Docetaxel in an Orthotopic Breast Cancer Mouse Model. Biomaterials 2017, 123, 39–47. [Google Scholar] [CrossRef]
- Gordon, M.R.; Canakci, M.; Li, L.; Zhuang, J.; Osborne, B.; Thayumanavan, S. Field Guide to Challenges and Opportunities in Antibody-Drug Conjugates for Chemists. Bioconjugate Chem. 2015, 26, 2198–2215. [Google Scholar] [CrossRef] [PubMed]
- Gascón, V.; Márquez-Alvarez, C.; Díaz, I.; Blanco, R.M. Hybrid Ordered Mesoporous Materials as Supports for Permanent Enzyme Immobilization through Non-Covalent Interactions. In Non-covalent Interactions in the Synthesis and Design of New Compounds; Wiley: Hoboken, NJ, USA, 2016; pp. 345–360. [Google Scholar] [CrossRef]
- Huang, H.; Oizumi, S.; Kojima, N.; Niino, T.; Sakai, Y. Avidin-Biotin Binding-Based Cell Seeding and Perfusion Culture of Liver-Derived Cells in a Porous Scaffold with a Three-Dimensional Interconnected Flow-Channel Network. Biomaterials 2007, 28, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Pal, D.; Mitra, A.K. Functional and Molecular Aspects of Biotin Uptake via SMVT in Human Corneal Epithelial (HCEC) and Retinal Pigment Epithelial (D407) Cells. AAPS J. 2012, 14, 832–842. [Google Scholar] [CrossRef]
- Ojima, I.; Wang, X.; Jing, Y.; Wang, C. Quest for Efficacious Next-Generation Taxoid Anticancer Agents and Their Tumor-Targeted Delivery. J. Nat. Prod. 2018, 81, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Chen, J.; Chen, J.; Kuznetsova, L.; Wong, S.S.; Ojima, I. Mechanism-Based Tumor-Targeting Drug Delivery System. Validation of Efficient Vitamin Receptor-Mediated Endocytosis and Drug Release. Bioconjug. Chem. 2010, 21, 979–987. [Google Scholar] [CrossRef]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and Doxorubicin Co-Encapsulated Biotin Receptortargeting Nanoparticles for Minimizing Drug Resistance in Breast Cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Rouhani, H.; Sepehri, N.; Varshochian, R.; Ghahremani, M.H.; Amini, M.; Gharghabi, M.; Ostad, S.N.; Atyabi, F.; Baharian, A.; et al. Biotin Decorated PLGA Nanoparticles Containing SN-38 Designed for Cancer Therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 495–504. [Google Scholar] [CrossRef]
- Krkavcová, E.; Kreisinger, J.; Hyánková, L.; Hyršl, P.; Javůrková, V. The Hidden Function of Egg White Antimicrobials: Egg Weightdependent Effects of Avidin on Avian Embryo Survival and Hatchling Phenotype. Biol. Open 2018, 7, 031518. [Google Scholar] [CrossRef]
- Jain, A.; Barve, A.; Zhao, Z.; Jin, W.; Cheng, K. Comparison of Avidin, Neutravidin, and Streptavidin as Nanocarriers for Efficient SiRNA Delivery. Mol. Pharm. 2017, 14, 1517–1527. [Google Scholar] [CrossRef]
- Verdoliva, A.; Bellofiore, P.; Rivieccio, V.; Catello, S.; Colombo, M.; Albertoni, C.; Rosi, A.; Leoni, B.; Anastasi, A.M.; De Santis, R. Biochemical and Biological Characterization of a New Oxidized Avidin with Enhanced Tissue Binding Properties. J. Biol. Chem. 2010, 285, 9090–9099. [Google Scholar] [CrossRef]
- Fahie, M.A.; Chen, M. Electrostatic Interactions between OmpG Nanopore and Analyte Protein Surface Can Distinguish between Glycosylated Isoforms. J. Phys. Chem. B 2015, 119, 10198–10206. [Google Scholar] [CrossRef]
- Jain, A.; Cheng, K. The Principles and Applications of Avidin-Based Nanoparticles in Drug Delivery and Diagnosis. J. Control. Release 2017, 245, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Parak, W.J. Surface Modification, Functionalization and Bioconjugation of Colloidal Inorganic Nanoparticles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.M.; Risse, J.M.; Jussen, D.; Flaschel, E. Development of Fed-Batch Strategies for the Production of Streptavidin by Streptomyces Avidinii Based on Power Input and Oxygen Supply Studies. J. Biotechnol. 2013, 163, 325–332. [Google Scholar] [CrossRef]
- Wu, S.C.; Wang, C.; Hansen, D.; Wong, S.L. A Simple Approach for Preparation of Affinity Matrices: Simultaneous Purification and Reversible Immobilization of a Streptavidin Mutein to Agarose Matrix. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Sly, K.L.; Conboy, J.C. Comparison of the Energetics of Avidin, Streptavidin, NeutrAvidin, and Anti-Biotin Antibody Binding to Biotinylated Lipid Bilayer Examined by Second-Harmonic Generation. Anal. Chem. 2012, 84, 201–208. [Google Scholar] [CrossRef]
- Kroetsch, A.; Chin, B.; Nguyen, V.; Gao, J.; Park, S. Functional Expression of Monomeric Streptavidin and Fusion Proteins in Escherichia Coli: Applications in Flow Cytometry and ELISA. Appl. Microbiol. Biotechnol. 2018, 102, 10079–10089. [Google Scholar] [CrossRef]
- Bigini, P.; Previdi, S.; Casarin, E.; Silvestri, D.; Violatto, M.B.; Facchin, S.; Sitia, L.; Rosato, A.; Zuccolotto, G.; Realdon, N.; et al. In Vivo Fate of Avidin-Nucleic Acid Nanoassemblies as Multifunctional Diagnostic Tools. ACS Nano 2014, 8, 175–187. [Google Scholar] [CrossRef]
- Roncato, F.; Rruga, F.; Porcù, E.; Casarin, E.; Ronca, R.; Maccarinelli, F.; Realdon, N.; Basso, G.; Alon, R.; Viola, G.; et al. Improvement and Extension of Anti-EGFR Targeting in Breast Cancer Therapy by Integration with the Avidin-Nucleic-Acid-Nano-Assemblies. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Elzahhar, P.; Belal, A.S.F.; Elamrawy, F.; Helal, N.A.; Nounou, M.I. Bioconjugation in Drug Delivery: Practical Perspectives and Future Perceptions. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 2000, pp. 125–182. [Google Scholar] [CrossRef]
- Ren, W.X.; Han, J.; Uhm, S.; Jang, Y.J.; Kang, C.; Kim, J.H.; Kim, J.S. Recent Development of Biotin Conjugation in Biological Imaging, Sensing, and Target Delivery. Chem. Commun. 2015, 51, 10403–10418. [Google Scholar] [CrossRef]
- Wartlick, H.; Michaelis, K.; Balthasar, S.; Strebhardt, K.; Langer, K. Highly Specific HER2-Mediated Cellular Uptake of Antibody-Modified Nanoparticles in Tumour Cells. J. Drug Target. 2004, 12, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.; Chandra, S.; Dodson, K.; Shaheen, F.; Wiltz, K.; Ireland, S.; Syed, M.; Dash, S.; Wiese, T.; Mandal, T.; et al. Aptamer-Functionalized Hybrid Nanoparticle for the Treatment of Breast Cancer. Eur. J. Pharm. Biopharm. 2017, 114, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Stein, R.; Sharkey, R.M. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018, 9, 28989. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 2020, 387, 610–621. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, S.; Ocaña, A.; Pandiella, A. Trastuzumab Emtansine: Mechanisms of Action and Resistance, Clinical Progress, and Beyond. Trends Cancer 2020, 6, 130–146. [Google Scholar] [CrossRef]
- Ocaña, A.; Amir, E.; Pandiella, A. HER2 heterogeneity and resistance to anti-HER2 antibody-drug conjugates. Breast Cancer Res. 2020, 22, 1–3. [Google Scholar] [CrossRef]
- García-Alonso, S.; Ocaña, A.; Pandiella, A. Resistance to antibody–drug conjugates. Cancer Res. 2018, 78, 2159–2165. [Google Scholar] [CrossRef]
- Ríos-Luci, C.; García-Alonso, S.; Díaz-Rodríguez, E.; Nadal-Serrano, M.; Arribas, J.; Ocaña, A.; Pandiella, A. Resistance to the antibody–drug conjugate T-DM1 is based in a reduction in lysosomal proteolytic activity. Cancer Res. 2017, 77, 4639–4651. [Google Scholar] [CrossRef]
- Seruga, B.; Ocana, A.; Tannock, I.F. Drug resistance in metastatic castration-resistant prostate cancer. Nat. Rev. Clin. Oncol. 2011, 8, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Gandullo-Sánchez, L.; Capone, E.; Ocaña, A.; Iacobelli, S.; Sala, G.; Pandiella, A. HER3 targeting with an antibody-drug conjugate bypasses resistance to anti-HER2 therapies. EMBO Mol. Med. 2020, 12, e11498. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-conjugated paclitaxel loaded PCL-PEG worm-like nanocrystal micelles for the combinatorial treatment of HER2-positive breast cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, I.; Spa, B.; Strebhardt, K.; Langer, K. Trastuzumab-Modified Nanoparticles: Optimisation of Preparation and Uptake in Cancer Cells. Biomaterials 2006, 27, 4975–4983. [Google Scholar] [CrossRef]
- Koopaei, M.N.; Dinarvand, R.; Amini, M.; Rabbani, H.; Emami, S.; Ostad, S.N.; Atyabi, F. Docetaxel Immunonanocarriers as Targeted Delivery Systems for HER 2-Positive Tumor Cells: Preparation, Characterization, and Cytotoxicity Studies. Int. J. Nanomed. 2011, 6, 1903–1912. [Google Scholar] [CrossRef][Green Version]
- Kouchakzadeh, H.; Shojaosadati, S.A.; Mohammadnejad, J.; Paknejad, M.; Rasaee, M.J. Attachment of an Anti-MUC1 Monoclonal Antibody to 5-FU Loaded BSA Nanoparticles for Active Targeting of Breast Cancer Cells. Hum. Antibodies 2012, 21, 49–56. [Google Scholar] [CrossRef]
- Swaminathan, S.K.; Roger, E.; Toti, U.; Niu, L.; Ohlfest, J.R.; Panyam, J. CD133-Targeted Paclitaxel Delivery Inhibits Local Tumor Recurrence in Amousemodel of Breast Cancer. J. Control. Release 2013, 171, 280–287. [Google Scholar] [CrossRef]
- Khanna, V.; Kalscheuer, S.; Kirtane, A.; Zhang, W.; Panyam, J. Perlecan-Targeted Nanoparticles for Drug Delivery to Triple-Negative Breast Cancer. Future Drug Discov. 2019, 1, FDD8. [Google Scholar] [CrossRef]
- Saqafi, B.; Rahbarizadeh, F. Polyethyleneimine-Polyethylene Glycol Copolymer Targeted by Anti-HER2 Nanobody for Specific Delivery of Transcriptionally Targeted TBid Containing Construct. Artif. Cells Nanomed. Biotechnol. 2019, 47, 501–511. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).