Catechol Containing Polyelectrolyte Complex Nanoparticles as Local Drug Delivery System for Bortezomib at Bone Substitute Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
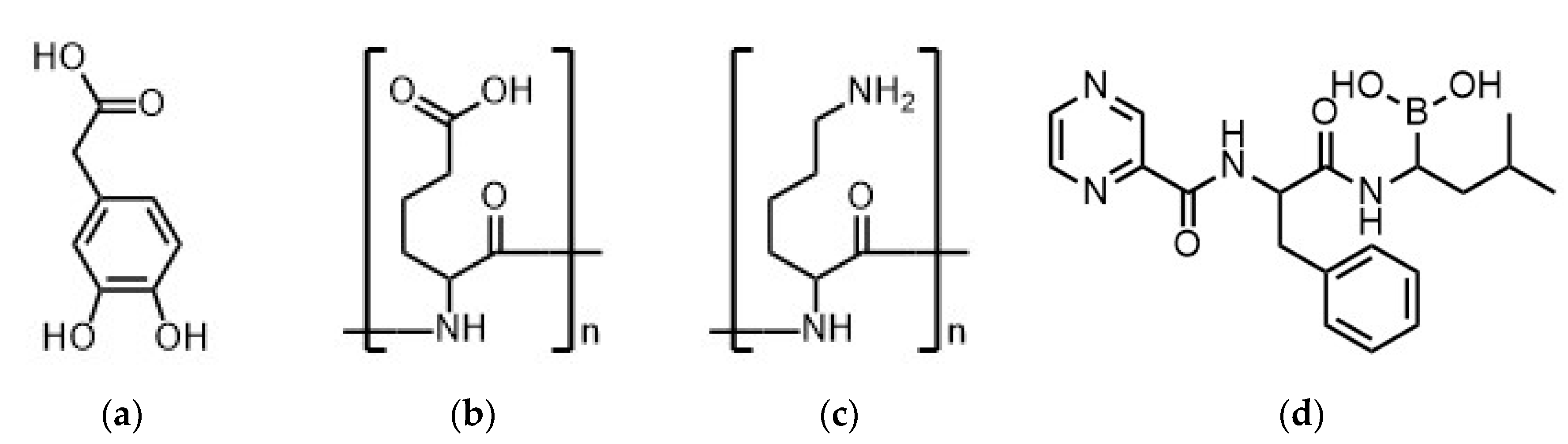
2.2. Synthesis of Catechol Containing PDOPAC
2.3. Preparation of BZM-Loaded Polyelectrolyte Complex (PEC) Nanoparticles (NP)
- –molar mixing ratio (net charge)
- –molar charge factor of PA (F−) and PC (F+)
- –molar amount of anionic (n−) and cationic (n+) repeating units
2.4. Bone Substitute Materials (BSM)
2.5. Colloid Titration
2.6. Quantitative UV/VIS Spectroscopy
- –concentration of adsorbed BZM in PEC NP
- –concentration of BZM before loading
- –concentration of BZM after loading (supernatant)
- –standard deviation of BZM concentration (calculated out of all calibration lines in the wavelength range between 250–300 nm) [mM]
- –absorption at a defined wavelength [-]
- –average of all absorptions of all wavelength (250–300 nm) [-]
- –absorption coefficient for a defined wavelength [1/mM]
- –average of all absorption coefficients of all wavelength (250–300 nm) [1/mM]
- A(t) –drug content [%/mM]
- T –time [h]
- A0 –amplitude [%/mM]
- B –exponent [-]
2.7. Quantitative FTIR Spectroscopy
2.8. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
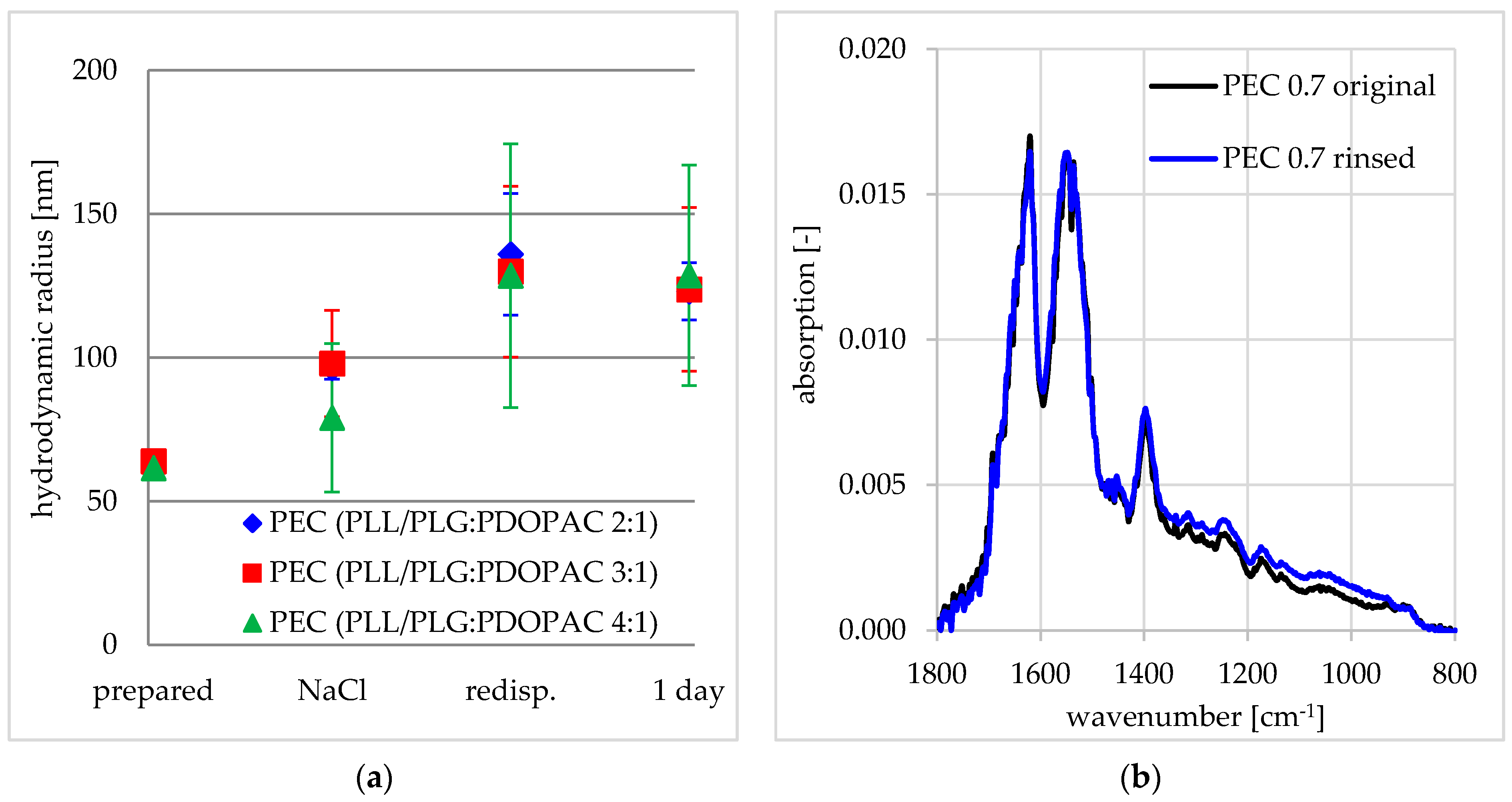
2.9. Dynamic Light Scattering (DLS)
2.10. Zeta Potential (ZP)
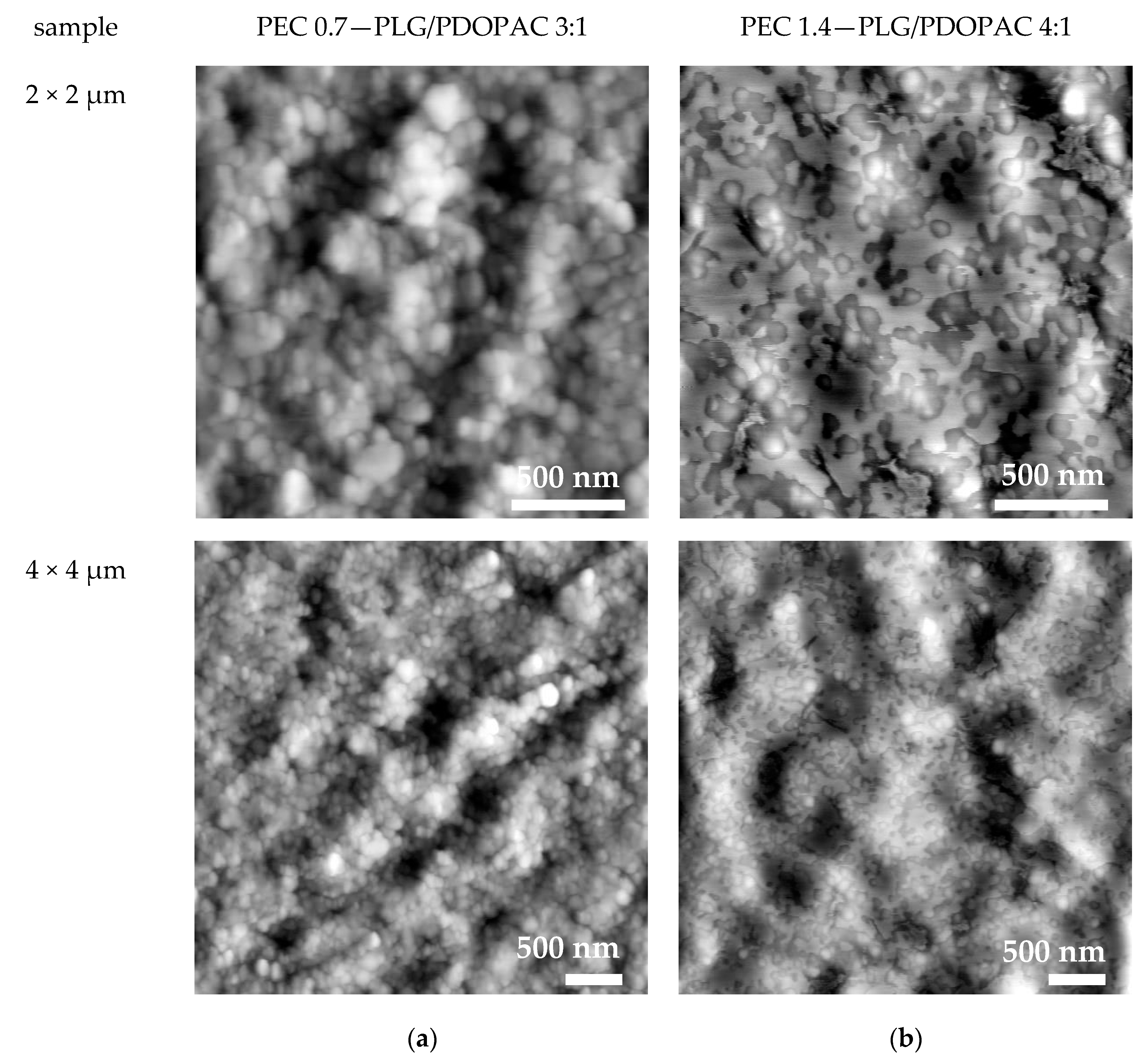
2.11. Scanning Force Microscopy (SFM)
3. Results
3.1. Colloid and Adhesive Properties of PDOPAC/PEC NP
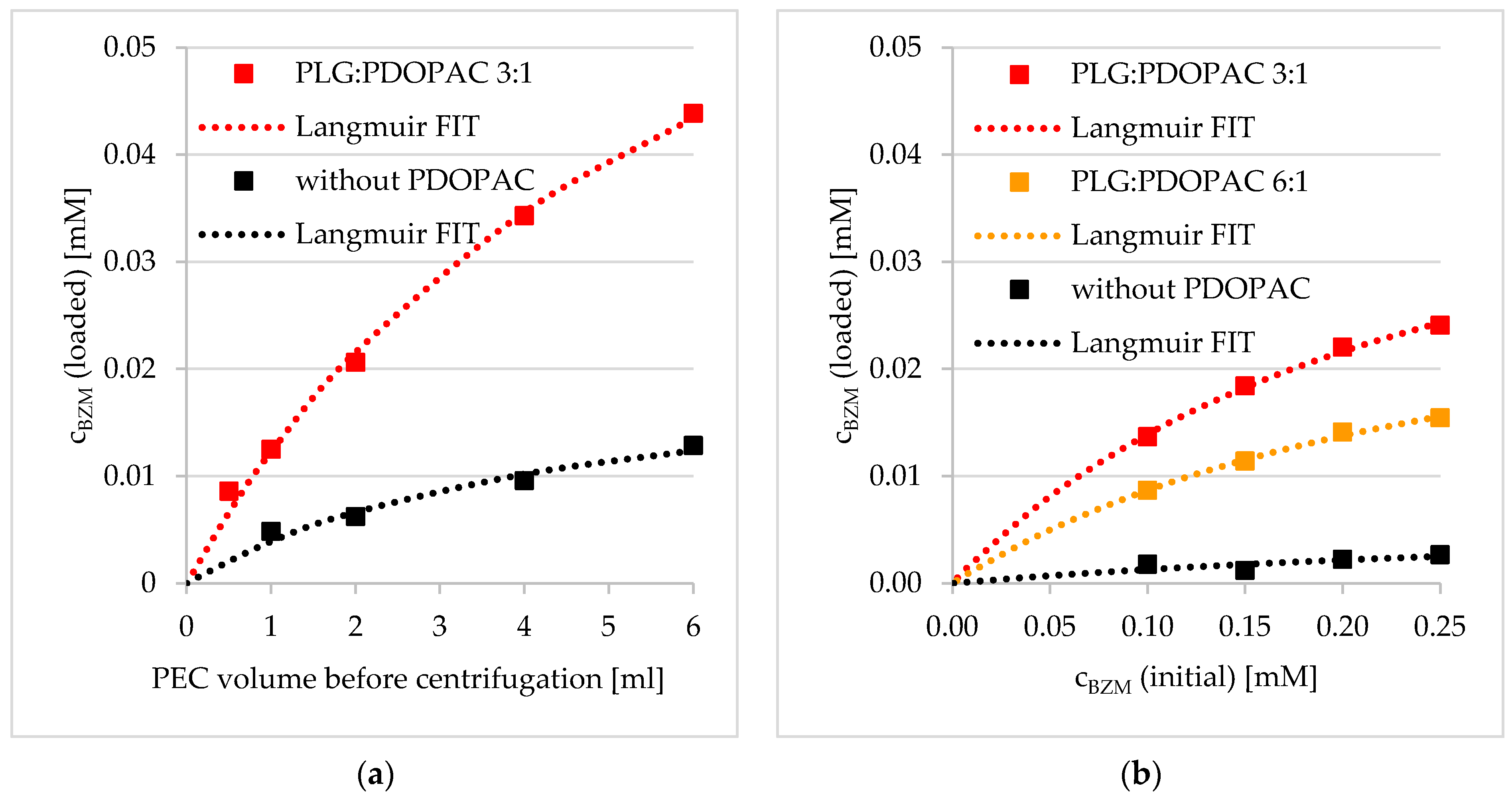
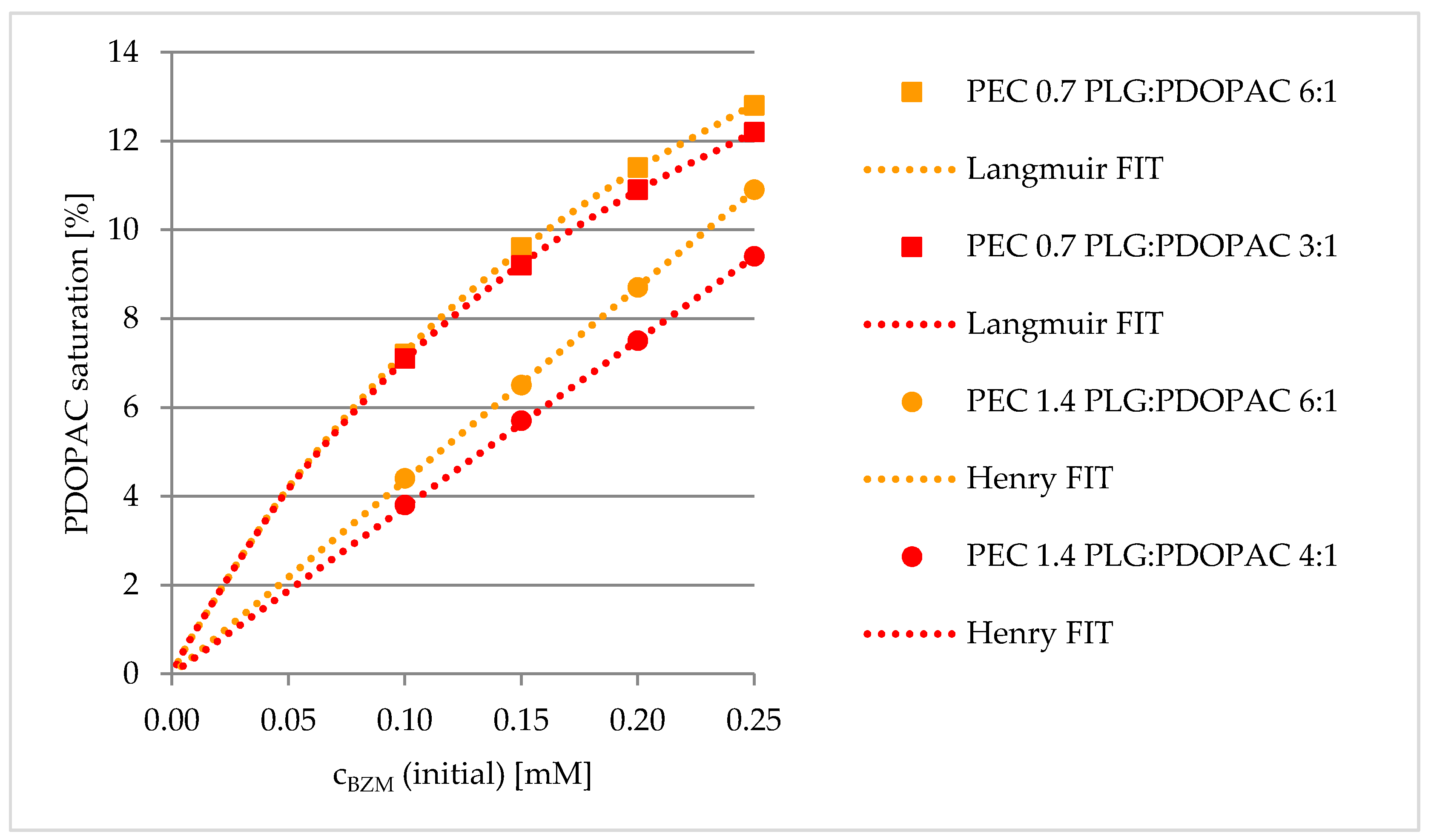
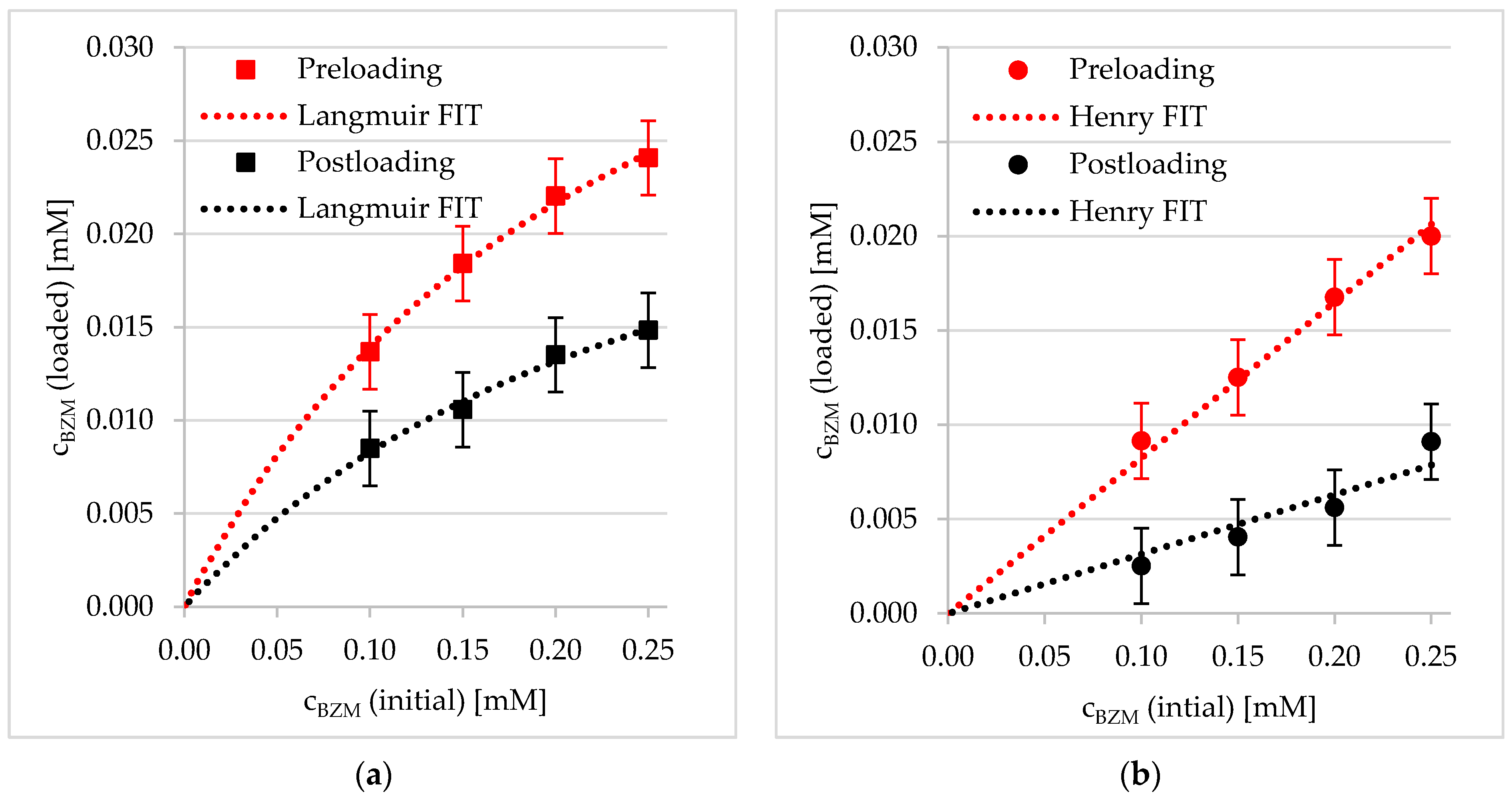
3.2. Loading of BZM into PDOPAC/PEC NP Dispersions and Coatings
3.2.1. Preloading into PDOPAC/PEC Dispersions
Influence of Ionic Strength
Influence of PDOPAC Amount
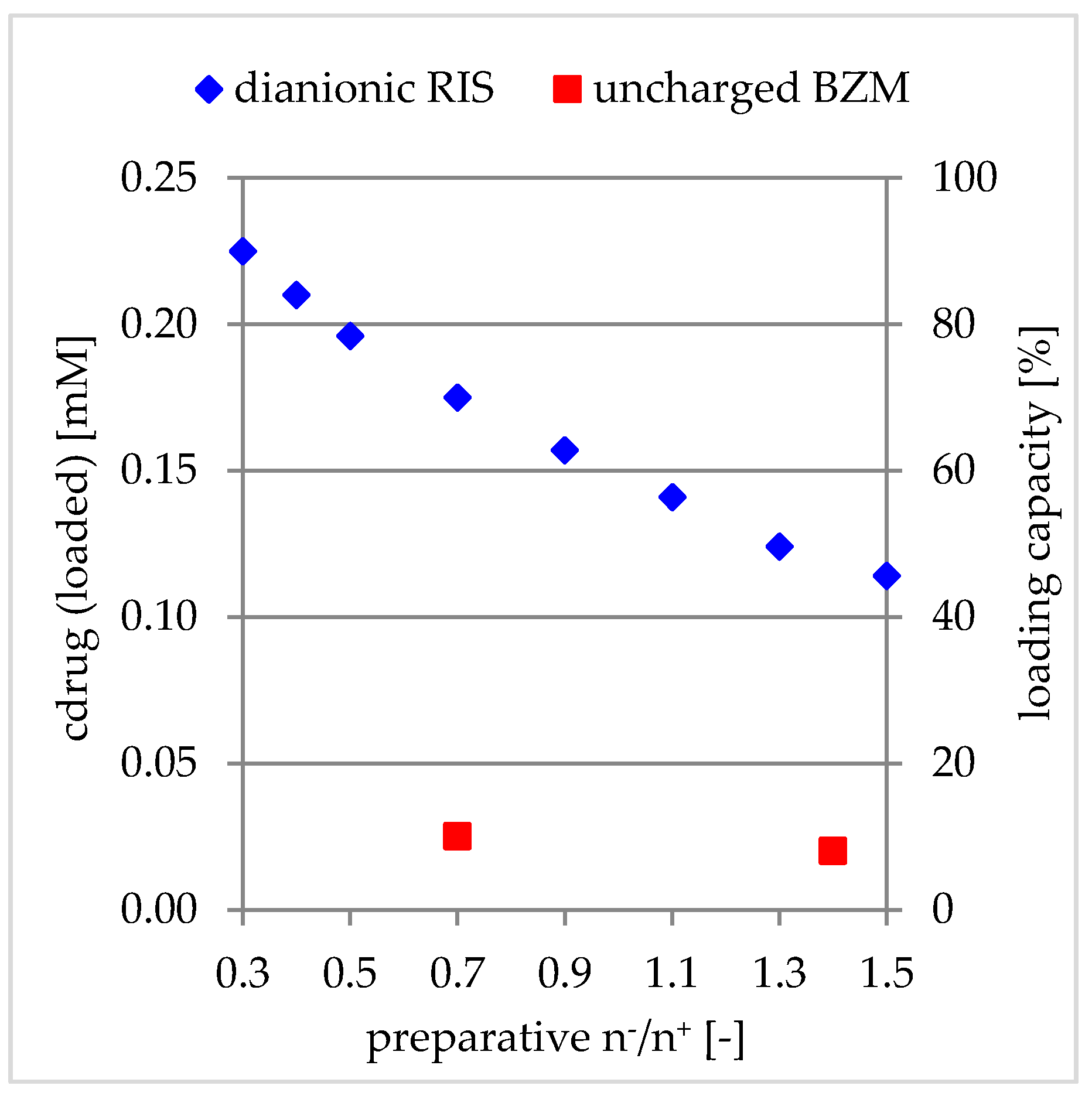
Influence of Net Charge
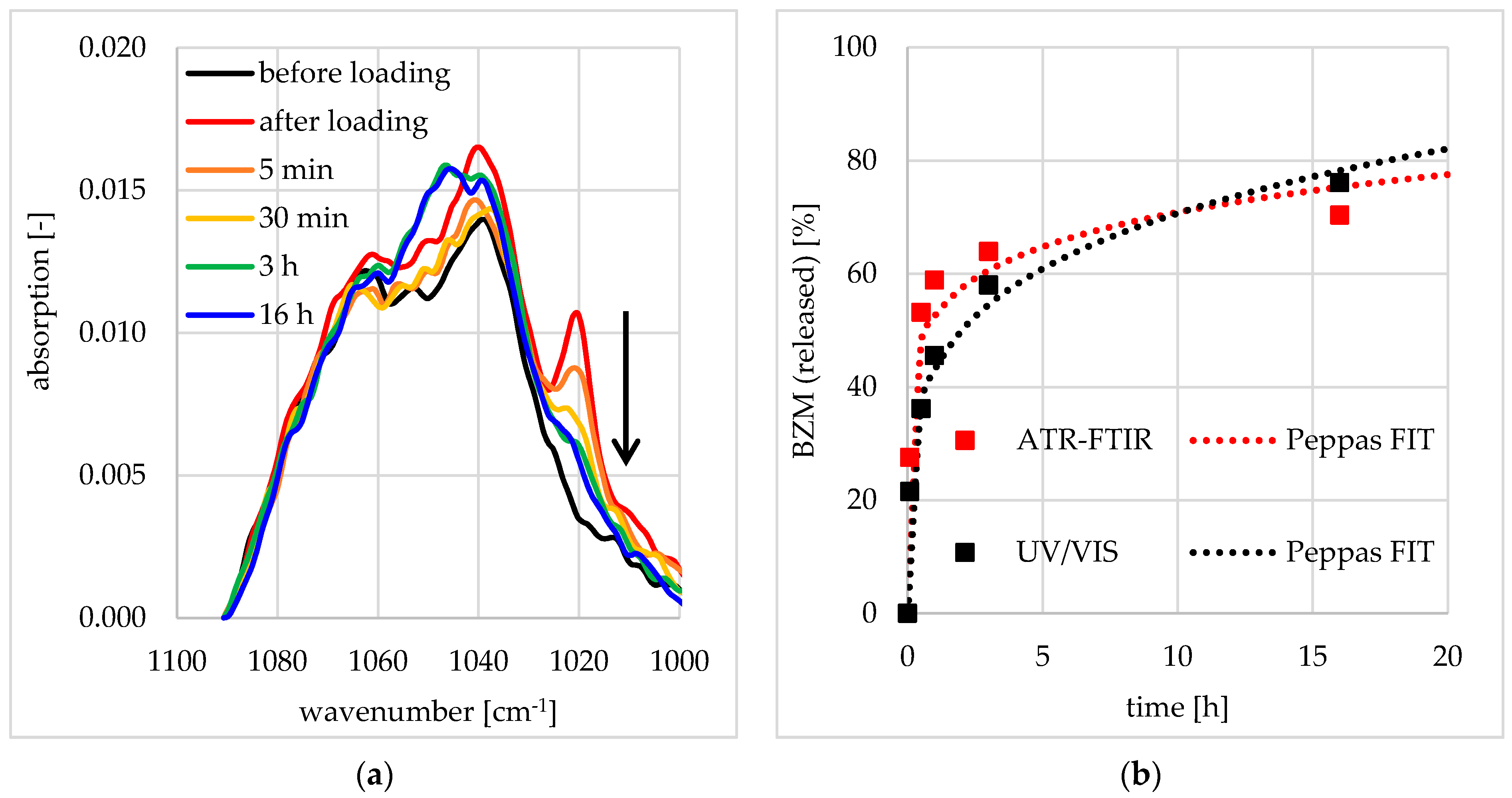
3.2.2. Postloading of PDOPAC/PEC Coatings at Ti40Nb
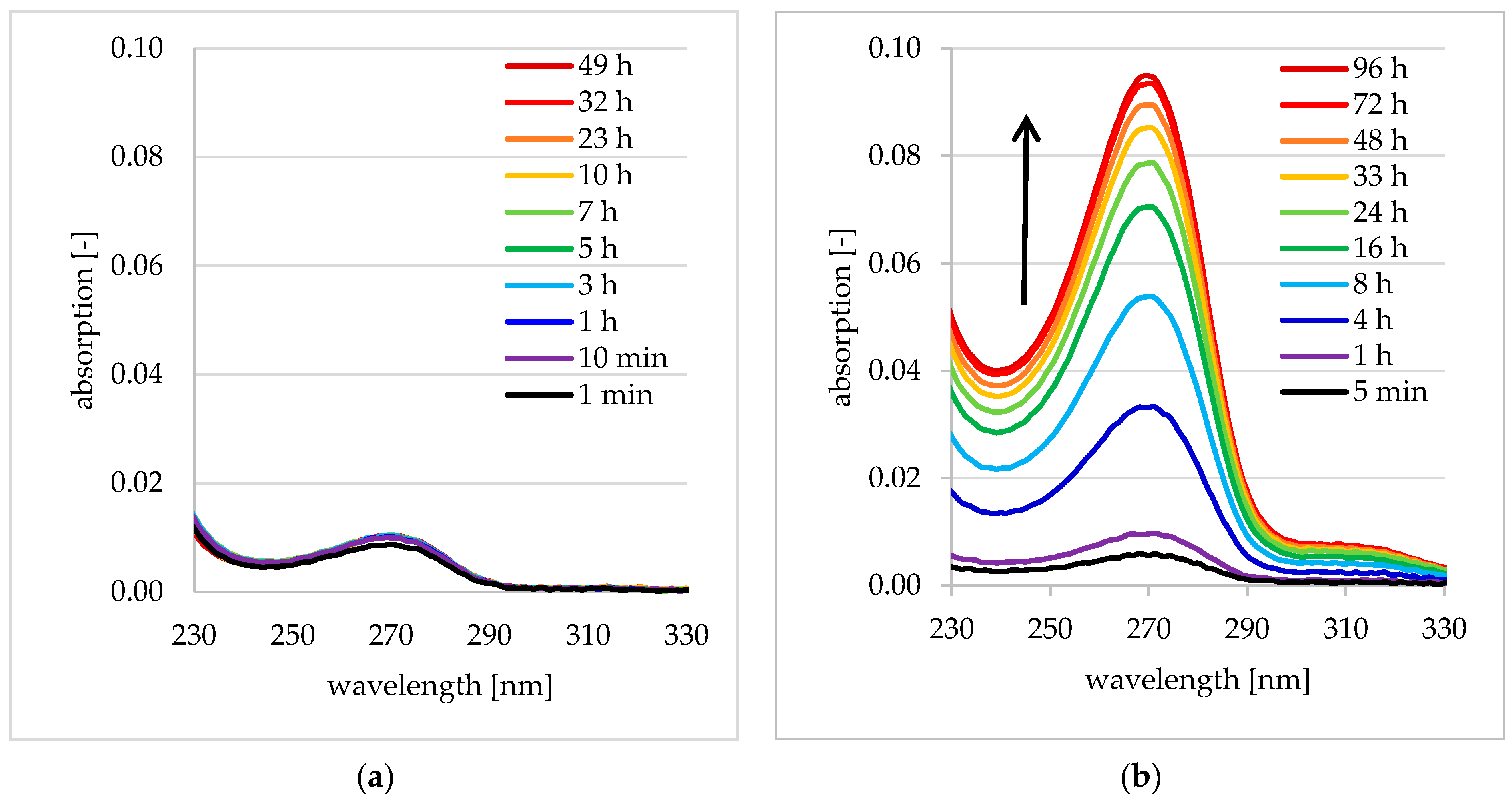
3.2.3. Kinetics of Drug Loading
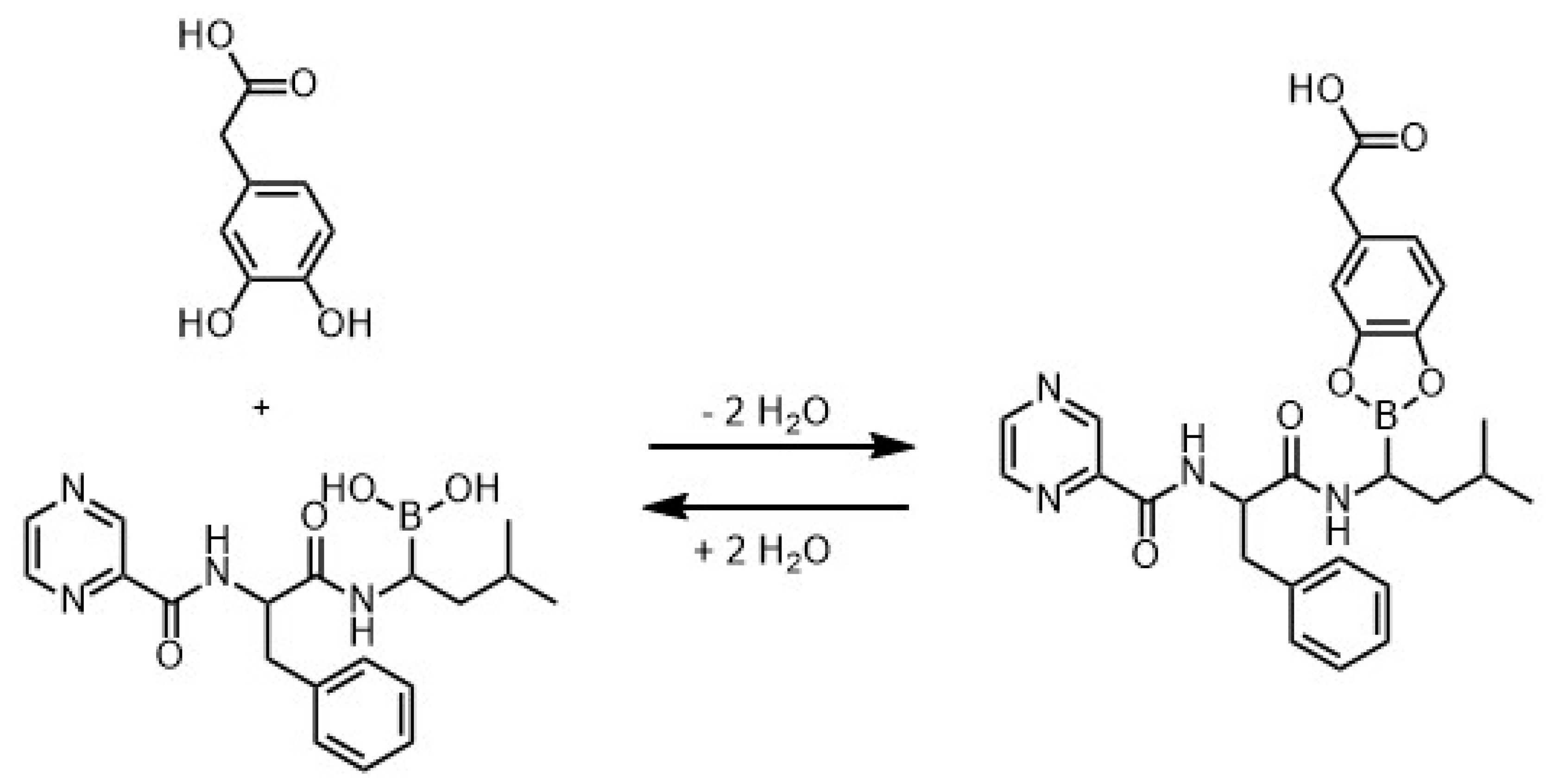
3.2.4. Comparison to Charged Drugs
3.3. Release of BZM from PDOPAC/PEC Coatings
3.3.1. Release Performance from Germanium Model Substrates
3.3.2. Release Performance at Bone Substitute Materials (BSM)
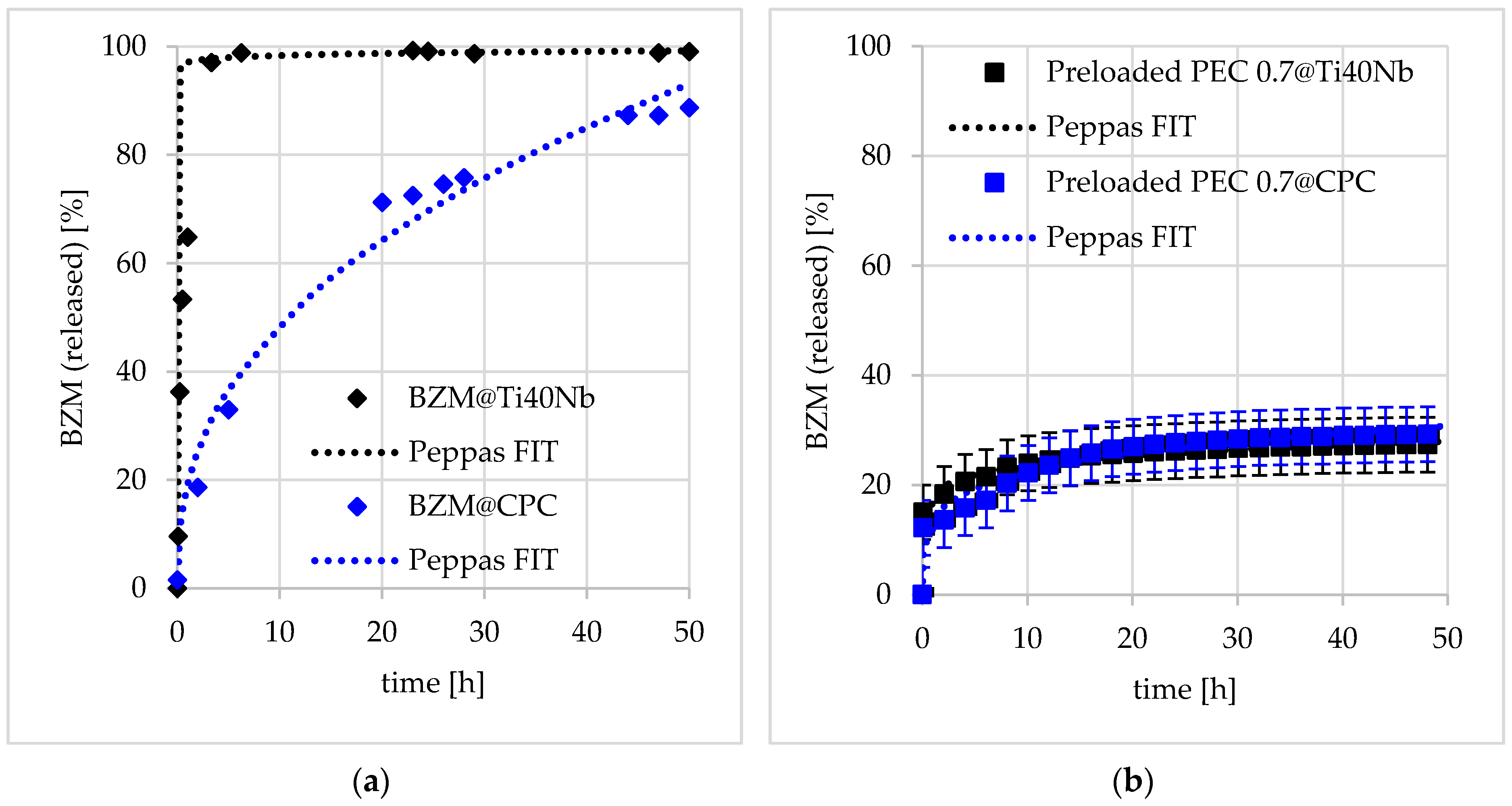
Influence of Substrate
Influence of Net Charge and Type of Loading
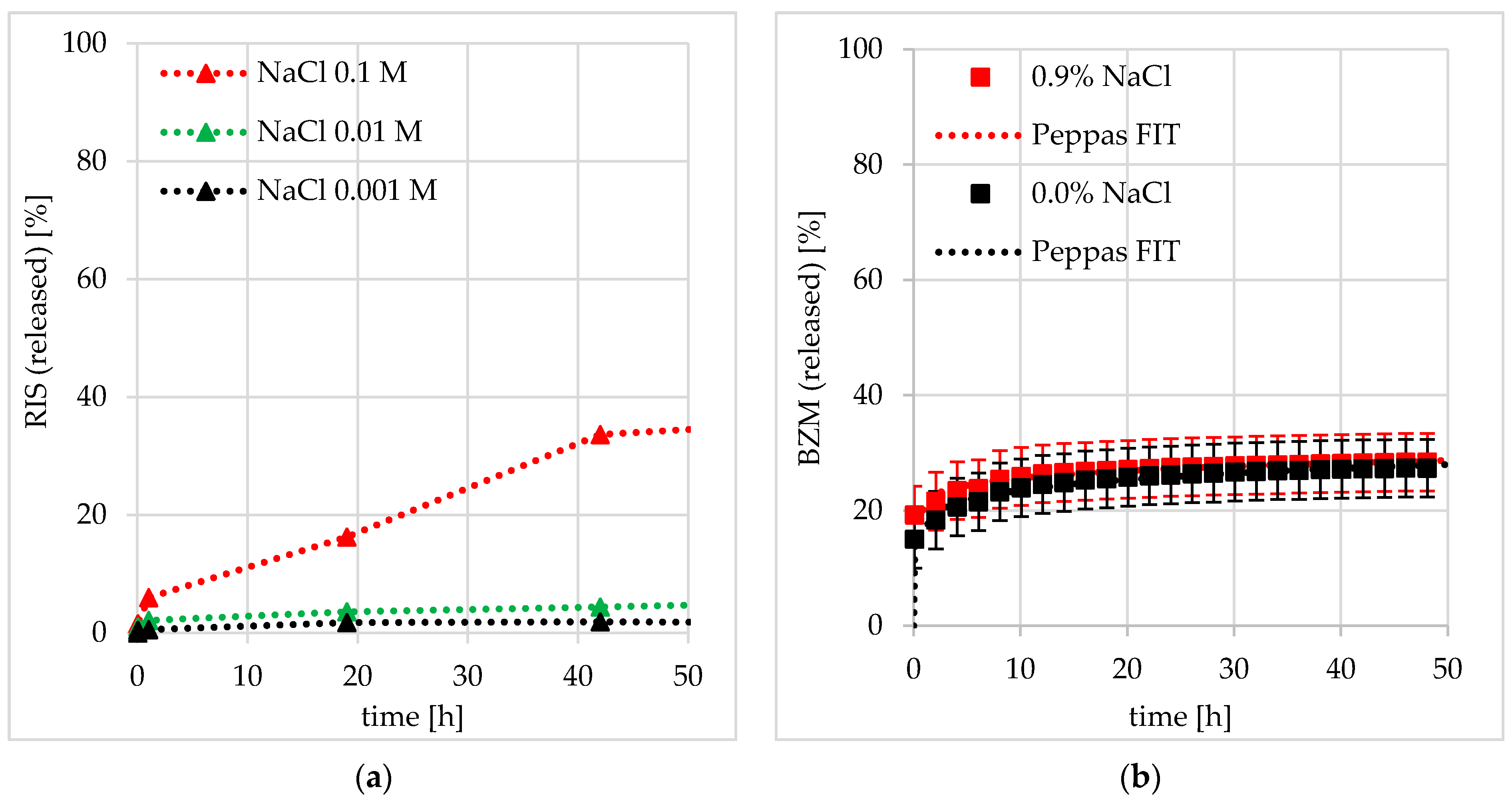
Comparison to Charged Drugs and Influence of Salt (Ionic Strength)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hameed, A.; Brady, J.J.; Dowling, P.; Clynes, M.; O’Gorman, P. Bone Disease in Multiple Myeloma: Pathophysiology and Management. Cancer Growth Metastasis 2014, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Paramore, A.; Frantz, S. Bortezomib. Market analysis. Nat. Rev. Drug Discov. 2003, 2, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Accardi, F.; Toscani, D.; Bolzoni, M.; Dalla Palma, B.; Aversa, F.; Giuliani, N. Mechanism of action of bortezomib and the new proteasome inhibitors on myeloma cells and the bone microenvironment: Impact on myeloma-induced alterations of bone remodeling. Biomed. Res. Int. 2015, 2015, 172458. [Google Scholar] [CrossRef]
- Hideshima, T.; Richardson, P.; Chauhan, D.; Palombella, V.J.; Elliott, P.J.; Adams, J.; Anderson, K.C. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001, 61, 3071–3076. [Google Scholar]
- Koushik, O.S.; Rao, Y.V.; Kumar, P.; Karthikeyan, R. Nano Drug Delivery Systems to Overcome Cancer Drug Resistance—A Review. J. Nanomed. Nanotechnol. 2016, 7, 7. [Google Scholar]
- Müller, M.; Keßler, B. Release of pamidronate from poly(ethyleneimine)/cellulose sulphate complex nanoparticle films: An in situ ATR-FTIR study. J. Pharm. Biomed. Anal. 2012, 66, 183–190. [Google Scholar] [CrossRef]
- Torger, B.; Vehlow, D.; Urban, B.; Salem, S.; Appelhans, D.; Müller, M. Cast adhesive polyelectrolyte complex particle films of unmodified or maltose-modified poly (ethyleneimine) and cellulose sulphate: Fabrication, film stability and retarded release of zoledronate. Biointerphases 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Li, L.; Srivastava, S.; Andreev, M.; Marciel, A.B.; De Pablo, J.J.; Tirrell, M.V. Phase Behavior and Salt Partitioning in Polyelectrolyte Complex Coacervates. Macromolecules 2018, 51, 2988–2995. [Google Scholar] [CrossRef]
- Vehlow, D.; Schmidt, R.; Gebert, A.; Siebert, M.; Lips, K.S.; Müller, M. Polyelectrolyte complex based interfacial drug delivery system with controlled loading and improved release performance for bone therapeutics. Nanomaterials 2016, 6, 53. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Smith, M.K.; Northrop, B.H. Vibrational properties of boroxine anhydride and boronate ester materials: Model systems for the diagnostic characterization of covalent organic frameworks. Chem. Mater. 2014, 26, 3781–3795. [Google Scholar] [CrossRef]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol polymers for pH-responsive, targeted drug delivery to cancer cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef]
- Hasegawa, U.; Moriyama, M.; Uyama, H.; van der Vlies, A.J. Antioxidant micelles for bortezomib delivery. Colloid Polym. Sci. 2015, 293, 1887–1892. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Hu, K.; Shao, N.; Cheng, Y. Tumor extracellular acidity activated “off-on” release of bortezomib from a biocompatible dendrimer. Biomater. Sci. 2015, 3, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Polydopamine nanomaterials: Recent advances in synthesis methods and applications. Front. Bioeng. Biotechnol. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.; Panzella, L.; Oscurato, S.; Salvatore, M.; Avolio, R.; Errico, M.; Maddalena, P.; Napolitano, A.; d’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J.; Mrowcynski, R.; Scheidt, H.A.; Filip, C.; Hadade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Müller, M.; Kessler, B.; Richter, S. Preparation of monomodal polyelectrolyte complex nanoparticles of PDADMAC/poly(maleic acid-alt-α-methylstyrene) by consecutive centrifugation. Langmuir 2005, 21, 7044–7051. [Google Scholar] [CrossRef]
- Schumacher, M.; Henß, A.; Rohnke, M.; Gelinsky, M. A Novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef]
- Helth, A.; Pilz, S.; Kirsten, T.; Giebeler, L.; Freudenberger, J.; Calin, M.; Eckert, J.; Gebert, A. Effect of thermomechanical processing on the mechanical biofunctionality of a low modulus Ti-40Nb alloy. J. Mech. Behav. Biomed. Mater. 2017, 65, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Helth, A.; Gostin, P.F.; Oswald, S.; Wendrock, H.; Wolff, U.; Hempel, U.; Arnhold, S.; Calin, M.; Eckert, J.; Gebert, A. Chemical nanoroughening of Ti40Nb surfaces and its effect on human mesenchymal stromal cell response. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Petzold, R.; Vehlow, D.; Urban, B.; Grab, A.L.; Cavalcanti-Adam, E.A.; Alt, V.; Müller, M. Colloid, adhesive and release properties of nanoparticular ternary complexes between cationic and anionic polysaccharides and basic proteins like bone morphogenetic protein BMP-2. Colloids Surf. B Biointerfaces 2017, 151, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Starchenko, V.; Müller, M.; Lebovka, N. Growth of polyelectrolyte complex nanoparticles: Computer simulations and experiments. J. Phys. Chem. C 2008, 112, 8863–8869. [Google Scholar] [CrossRef]
- Wu, H.; Ting, J.M.; Tirrell, M.V. Mechanism of Dissociation Kinetics in Polyelectrolyte Complex Micelles. Macromolecules 2020, 53, 102–111. [Google Scholar] [CrossRef]


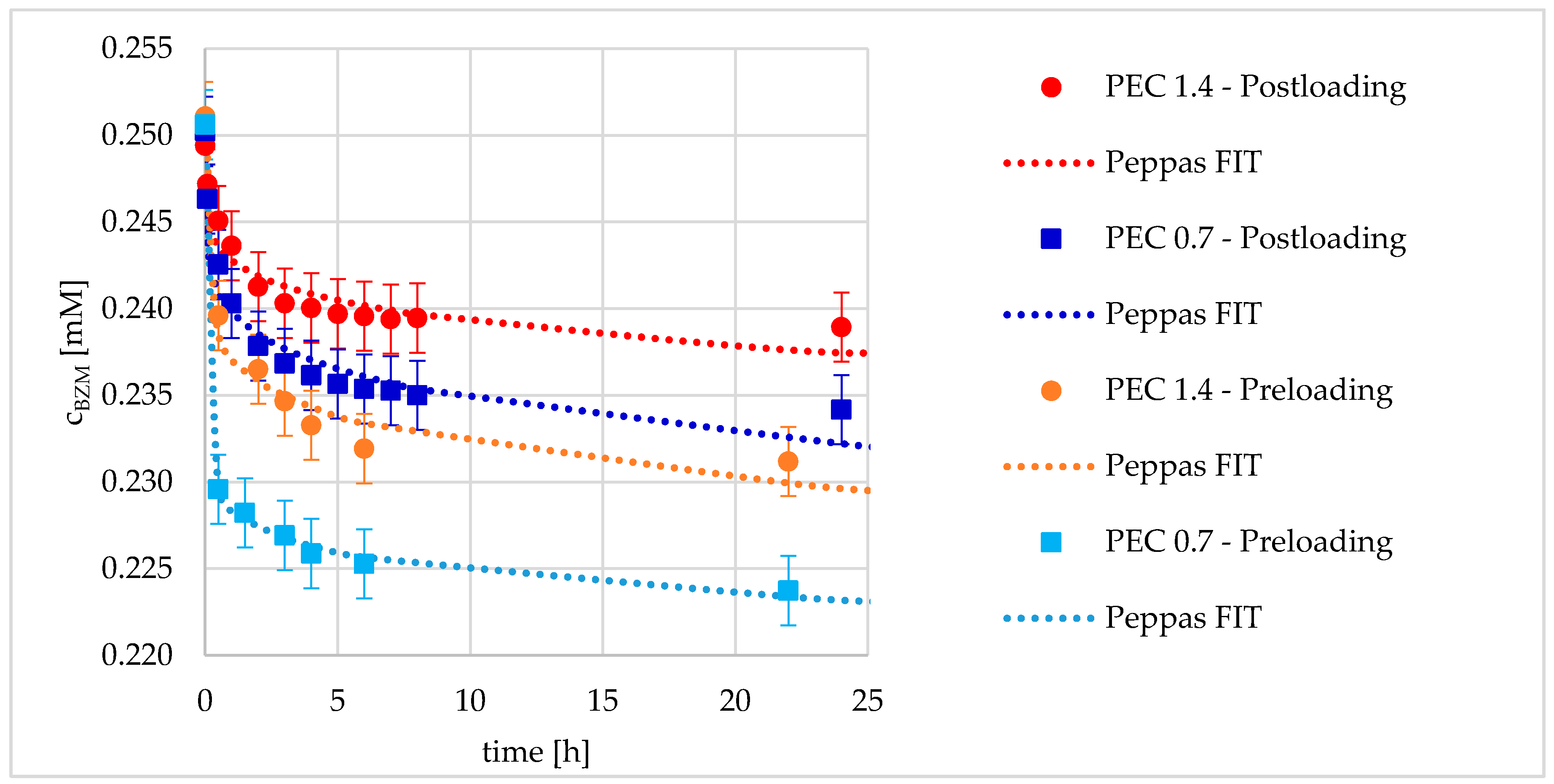


| Kinetic Parameter | Preloading | Postloading | ||
|---|---|---|---|---|
| PEC 0.7 | PEC 1.4 | PEC 0.7 | PEC 1.4 | |
| Amplitude A0 [mM] | 0.02212 ± 0.00020 | 0.01384 ± 0.00067 | 0.01107 ± 0.00059 | 0.00683 ± 0.00039 |
| Exponent B [-] | 0.06702 ± 0.00508 | 0.13697 ± 0.02501 | 0.18037 ± 0.02717 | 0.18923 ± 0.02884 |
| Kinetic Parameter | Preloading | Postloading | ||
|---|---|---|---|---|
| PEC 0.7 | PEC 1.4 | PEC 0.7 | PEC 1.4 | |
| Amplitude A0 [mM] | 20.1102 ± 2.3925 | 3.3194 ± 0.4150 | 23.3205 ± 0.5028 | 21.5088 ± 2.4807 |
| Exponent B [-] | 0.0884 ± 0.0260 | 0.1753 ± 0.0179 | 0.3521 ± 0.0107 | 0.1620 ± 0.0035 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vehlow, D.; Wong, J.P.H.; Urban, B.; Weißpflog, J.; Gebert, A.; Schumacher, M.; Gelinsky, M.; Stamm, M.; Müller, M. Catechol Containing Polyelectrolyte Complex Nanoparticles as Local Drug Delivery System for Bortezomib at Bone Substitute Materials. Pharmaceutics 2020, 12, 799. https://doi.org/10.3390/pharmaceutics12090799
Vehlow D, Wong JPH, Urban B, Weißpflog J, Gebert A, Schumacher M, Gelinsky M, Stamm M, Müller M. Catechol Containing Polyelectrolyte Complex Nanoparticles as Local Drug Delivery System for Bortezomib at Bone Substitute Materials. Pharmaceutics. 2020; 12(9):799. https://doi.org/10.3390/pharmaceutics12090799
Chicago/Turabian StyleVehlow, David, Jeremy P. H. Wong, Birgit Urban, Janek Weißpflog, Annett Gebert, Matthias Schumacher, Michael Gelinsky, Manfred Stamm, and Martin Müller. 2020. "Catechol Containing Polyelectrolyte Complex Nanoparticles as Local Drug Delivery System for Bortezomib at Bone Substitute Materials" Pharmaceutics 12, no. 9: 799. https://doi.org/10.3390/pharmaceutics12090799
APA StyleVehlow, D., Wong, J. P. H., Urban, B., Weißpflog, J., Gebert, A., Schumacher, M., Gelinsky, M., Stamm, M., & Müller, M. (2020). Catechol Containing Polyelectrolyte Complex Nanoparticles as Local Drug Delivery System for Bortezomib at Bone Substitute Materials. Pharmaceutics, 12(9), 799. https://doi.org/10.3390/pharmaceutics12090799






