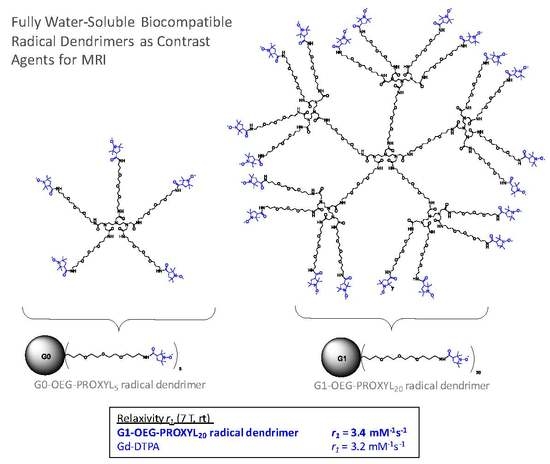
Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
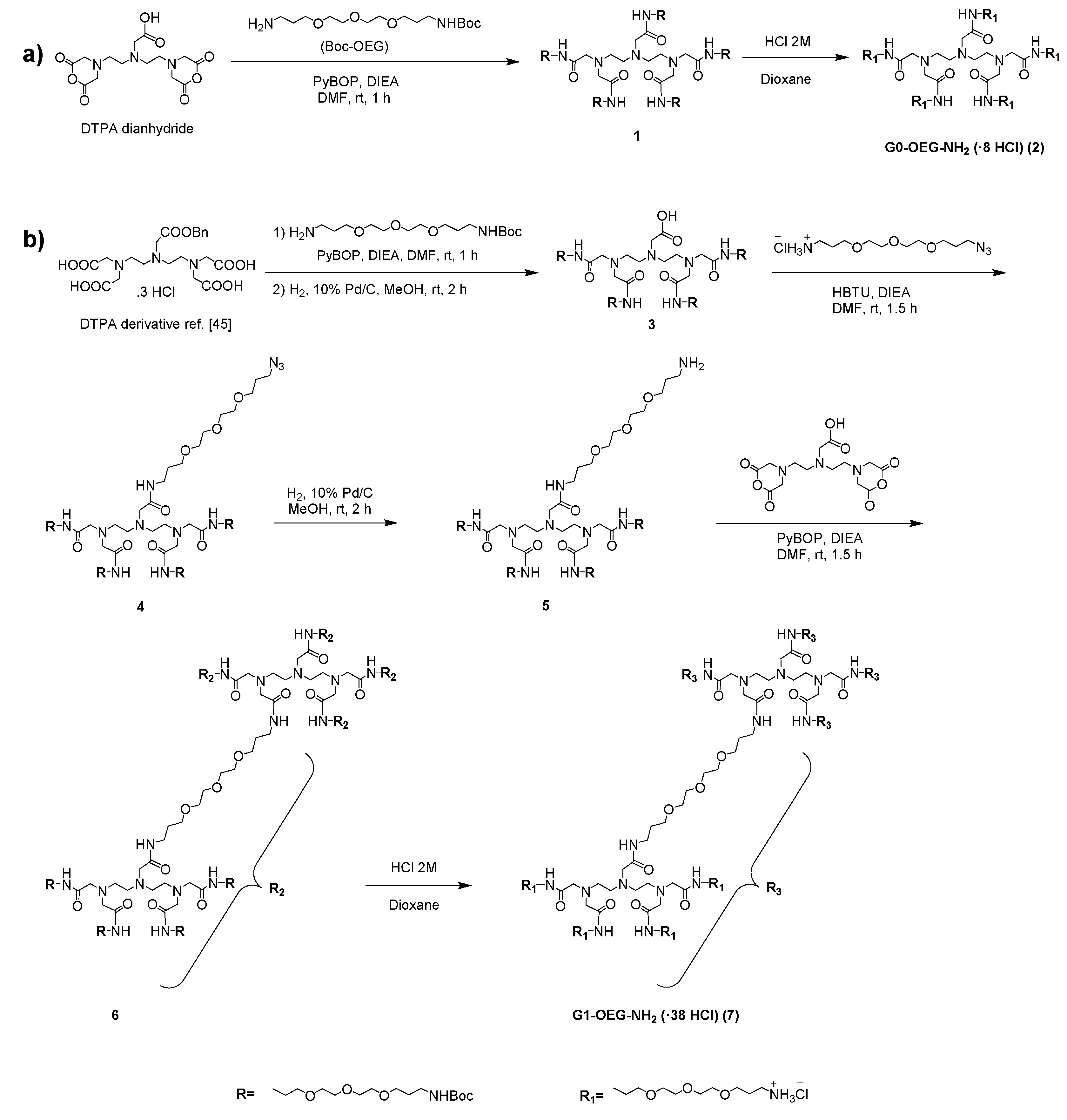
3.1. Synthesis of G0- and G1-OEG-NH2 Dendrimers
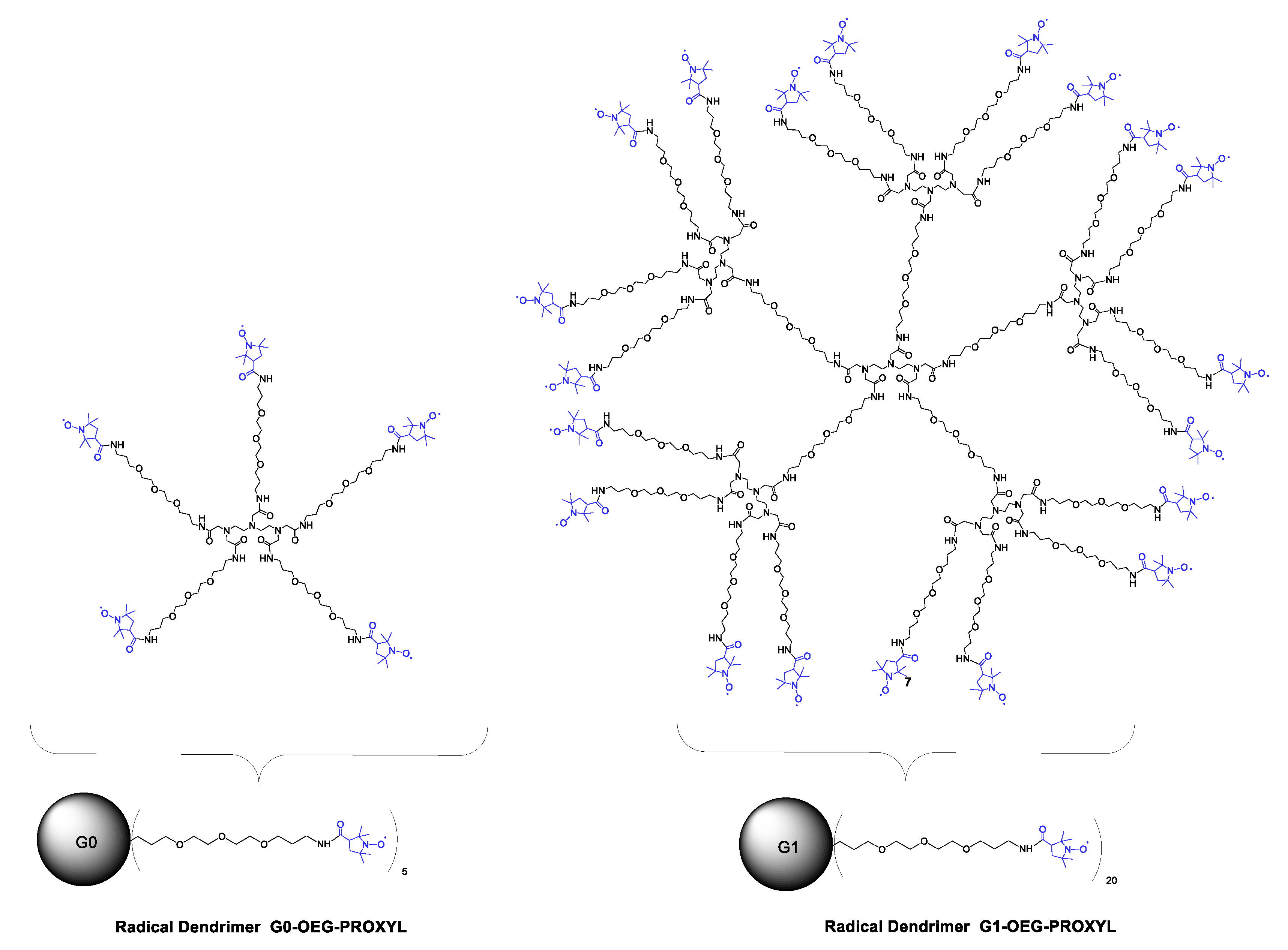
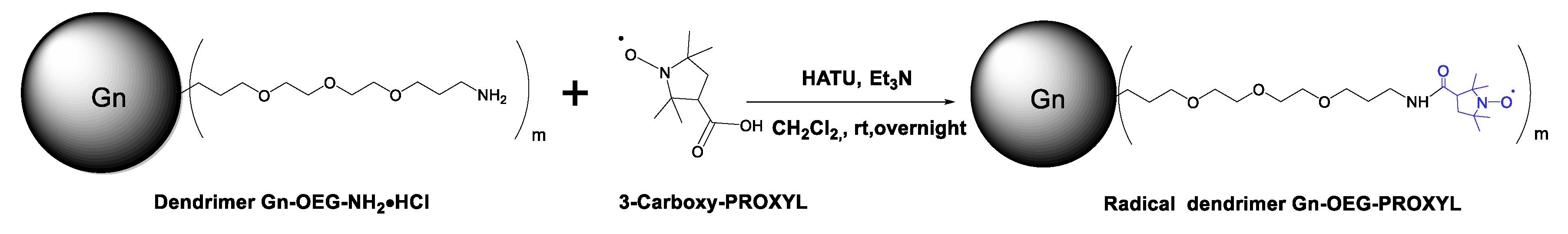
3.2. Synthesis and Characterization of G0- and G1-OEG-PROXYL Radical Dendrimers
3.3. EPR Study of G0-OEG-PROXYL and G1-OEG-PROXYL
3.4. Relaxivity Measurements and Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoerr, V.; Faber, C. Magnetic resonance imaging characterization of microbial infections. J. Pharm. Biomed. Anal. 2014, 93, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Palestro, C.J.; Love, C.; Miller, T.T. Diagnostic imaging tests and microbial infections. Cell. Microbiol. 2007, 9, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Jelicks, L.A.; Lisanti, M.P.; Machado, F.S.; Weiss, L.M.; Tanowitz, H.B.; Desruisseaux, M.S. Imaging of small-animal models of infectious diseases. Am. J. Pathol. 2013, 182, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Niska, J.A.; Meganck, J.A.; Pribaz, J.R.; Shahbazian, J.H.; Lim, E.; Zhang, N.; Rice, B.W.; Akin, A.; Ramos, R.I.; Berntal, N.M.; et al. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and μCT imaging in an orthopaedic implant infection in mice. PLoS ONE 2012, 7, e47397. [Google Scholar] [CrossRef]
- Gildehaus, F.J.; Haasters, F.; Drosse, I.; Wagner, E.; Zach, C.; Mutschler, W.; Cumming, P.; Bartenstein, P.; Schieker, M. Impact of indium-111 oxine labelling on viability of human mesenchymal stem cells in vitro, and 3D cell-tracking using SPECT/CT in vivo. Mol. Imaging Biol. 2011, 13, 1204–1214. [Google Scholar] [CrossRef]
- Gemmel, F.; Dumarey, N.; Welling, M. Future diagnostic agents. Semin. Nucl. Med. 2009, 39, 11–26. [Google Scholar] [CrossRef]
- Soldatos, T.; Durand, D.J.; Subhawong, T.K.; Carrino, J.A.; Chhabra, A. Magnetic resonance imaging of musculoskeletal infections: Systematic diagnostic assessment and key points. Acad. Radiol. 2012, 19, 1434–1443. [Google Scholar] [CrossRef]
- Radermacher, K.A.; Beghein, N.; Boutry, S.; Laurent, S.; Vander Elst, L.; Muller, R.N.; Jordan, B.F.; Gallez, B. In vivo detection of inflammation using pegylated iron oxide particles targeted at E-selectin: A multimodal approach using MR imaging and EPR spectroscopy. Investig. Radiol. 2009, 44, 398–404. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Calcagno, C.; Ramachandran, S.; Millon, A.; Robson, P.M.; Mani, V.; Fayad, Z. Gadolinium-based contrast agents for vessel wall magnetic resonance imaging (MRI) of atherosclerosis. Curr. Cardiovasc. Imaging Rep. 2013, 6, 11–24. [Google Scholar] [CrossRef]
- Wermuth, P.J.; Jimenez, S.A. Gadolinium compounds signaling through TLR4 and TLR7 in normal human macrophages: Establishment of a proinflammatory phenotype and implications for the pathogenesis of nephrogenic systemic fibrosis. J. Immunol. 2012, 189, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Baraki, H.; Zinne, N.; Wedekind, D.; Meier, M.; Bleich, A.; Glage, S.; Hedrich, H.J.; Kutschka, I.; Haverich, A. Magnetic resonance imaging of soft tissue infection with iron oxide labeled granulocytes in a rat model. PLoS ONE 2012, 7, e51770. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lee, S.H.; Kang, H.Y.; Baek, S.Y.; Kim, S.M.; Shin, M.J. Assessment of musculoskeletal infection in rats to determine usefulness of SPIO-enhanced MRI. AJR Am. J. Roentgenol. 2007, 189, 542–548. [Google Scholar] [CrossRef]
- Lin, C.; Cai, S.; Feng, J. Positive Contrast Imaging of SPIO Nanoparticles. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Margerum, L.D.; Campion, B.K.; Koo, M.; Shargill, N.; Lai, J.J.; Marumoto, A.; Sontum, P.C. Gadolinium(III) DO3A macrocycles and polyethylene glycol coupled to dendrimers. Effect of molecular weight on physical and biological properties of macromolecular magnetic resonance imaging contrast agents. J. Alloys Comp. 1997, 249, 185–190. [Google Scholar] [CrossRef]
- Kojima, C.; Turkbey, B.; Ogawa, M.; Bernardo, M.; Regino, C.A.S.; Bryant, L.H.; Choyke, P.L.; Kono, K.; Kobayashi, H. Dendrimer-based MRI contrast agents: The effects of PEGylation on relaxivity and pharmacokinetics. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 1001–1008. [Google Scholar] [CrossRef]
- Brandt, C.T.; Simonsen, H.; Liptrot, M.; Søgaard, L.V.; Lundgren, J.D.; Ostergaard, C.; Frimodt-Møller, N.; Rowland, I.J. In vivo study of experimental pneumococcal meningitis using magnetic resonance imaging. BMC Med. Imaging 2008, 8, 1. [Google Scholar] [CrossRef]
- Rodgers, J.; McCabe, C.; Gettinby, G.; Bradley, B.; Condon, B.; Kennedy, P.G. Magnetic resonance imaging to assess blood–brain barrier damage in murine trypanosomiasis. Am. J. Trop. Med. Hyg. 2011, 84, 344–350. [Google Scholar] [CrossRef]
- Lelievre, B.; Legras, P.; Godon, C.; Franconi, F.; Saint-Andre, J.P.; Bouchara, J.P.; Diquet, B. Experimental models of disseminated scedosporiosis with cerebral involvement. J. Pharmacol. Exp. Ther. 2013, 345, 198–205. [Google Scholar] [CrossRef]
- Choi, K.S.; Kim, S.H.; Cai, Q.Y.; Kim, S.Y.; Kim, H.O.; Lee, H.J.; Kim, E.A.; Yoon, S.E.; Yun, K.J.; Yoon, K.H. Inflammation-specific T1 imaging using anti-intercellular adhesion molecule 1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid. Mol. Imaging 2007, 6, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Dear, J.W.; Kobayashi, H.; Jo, S.K.; Holly, M.K.; Hu, X.; Yuen, P.S.; Brechbiel, M.W.; Star, R.A. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int. 2005, 67, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Tang, Z.; Wang, S.; Hui, H.; Gong, L.; Lu, Y.; Xue, Z.; Liao, H.; Chen, F.; Yang, F.; et al. The role of imaging in the detection and management of COVID-19: A review. IEEE Rev. Biomed. Eng. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.F.; Lloveras, V.; Zhang, S.; Liko, F.; Veciana, J.; Muñoz-Gómez, J.L.; Vidal-Gancedo, J. Fully Water-Soluble Polyphosphorhydrazone-Based Radical Dendrimers Functionalized with Tyr-PROXYL Radicals as Metal-Free MRI T1 Contrast Agents. ACS Appl. Bio Mater. 2020, 3, 369–376. [Google Scholar] [CrossRef]
- Brasch, R.C.; London, D.A.; Wesbey, G.E.; Tozer, T.N.; Nitecki, D.E.; Williams, R.D.; Doemeny, J.; Tuck, L.D.; Lallemand, D.P. Work in progress: Nuclear magnetic resonance study of a paramagnetic nitroxide contrast agent for enhancement of renal structures in experimental animals. Radiology 1983, 147, 773–779. [Google Scholar] [CrossRef]
- Brasch, R.C.; Nitecki, D.E.; Brant-Zawadzki, M.; Enzmann, D.R.; Wesbey, G.E.; Tozer, T.N.; Tuck, L.D.; Cann, C.E.; Fike, J.R.; Sheldon, P. Brain nuclear magnetic resonance imaging enhanced by a paramagnetic nitroxide contrast agent: Preliminary report. Am. J. Roentgenol. 1983, 141, 1019–1023. [Google Scholar] [CrossRef]
- Rosen, G.M.; Griffeth, L.K.; Brown, M.A.; Drayer, B.P. Intrathecal administration of nitroxides as potential contrast agents for MR imaging. Radiology 1987, 163, 239–243. [Google Scholar] [CrossRef]
- Bosman, A.W.; Janssen, R.A.J.; Meijer, E.W. Five Generations of Nitroxyl-Functionalized Dendrimers. Macromolecules 1997, 30, 3606–3611. [Google Scholar] [CrossRef][Green Version]
- Kashiwagi, Y.; Kurashima, F.; Kikuchi, C.; Anzai, J.-i.; Osa, T. Voltammetric behavior of poly (amidoamine) dendrimers containing nitroxyl radical end groups. Electrochem. Commun. 1999, 1, 305–308. [Google Scholar] [CrossRef]
- Winalski, C.S.; Shortkroff, S.; Mulkern, R.V.; Schneider, E.; Rosen, G.M. Magnetic resonance relaxivity of dendrimer-linked nitroxides. Magn. Reson. Med. 2002, 48, 965–972. [Google Scholar] [CrossRef]
- Maliakal, A.J.; Turro, N.J.; Bosman, A.W.; Cornel, J.; Meijer, E.W. Relaxivity studies on dinitroxide and polynitroxyl functionalized dendrimers: Effect of electron exchange and structure on paramagnetic relaxation enhancement. J. Phys. Chem. A 2003, 107, 8467–8475. [Google Scholar] [CrossRef]
- Francese, G.; Dunand, F.A.; Loosli, C.; Merbach, A.E.; Decurtins, S. Functionalization of PAMAM dendrimers with nitronyl nitroxide radicals as models for the outer-sphere relaxation in dentritic potential MRI contrast agents. Magn. Reson. Chem. 2003, 41, 81–83. [Google Scholar] [CrossRef]
- Sebby, K.B.; Walter, E.D.; Usselman, R.J.; Cloninger, M.J.; Singel, D.J. End-Group Distributions of Multiple Generations of Spin-Labeled PAMAM Dendrimers. J. Phys. Chem. B 2011, 115, 4613–4620. [Google Scholar] [CrossRef] [PubMed]
- Shimono, S.; Tamura, R.; Ikuma, N.; Takahashi, H.; Sakai, N.; Yamauchi, J. Characterization of the Chiral Paramagnetic Multispin System Built on a Cyclotriphosphazene Scaffold. Chem. Lett. 2004, 33, 932–933. [Google Scholar] [CrossRef]
- Shimono, S.; Takahashi, H.; Sakai, N.; Tamura, R.; Ikuma, N.; Yamauchi, J. Use of Cyclotriphosphazene as a Molecular Scaffold for Building Chiral Multispin Systems. Mol. Cryst. Liq. Cryst. 2005, 440, 37–52. [Google Scholar] [CrossRef]
- Fidan, I.; Önal, E.; Yerli, Y.; Luneau, D.; Ahsen, V.; Hirel, C. Synthethic Acces to a Pure Polyradical Architecture: Nucleophilic Insertion of Nitronyl Nitroxide on a Cyclotriphosphazene Scaffold. ChemPlusChem 2017, 82, 1384–1389. [Google Scholar] [CrossRef]
- Fidan, I.; Önal, E.; Yerli, Y.; Luneau, D.; Ahsen, V.; Hirel, C. Synthesis and Straightforward Quantification Methods of Imino Nitroxide-Based Hexaradical Architecture on a Cyclotriphosphazene Scaffold. Inorg. Chem. 2016, 55, 11447–11453. [Google Scholar] [CrossRef]
- Lloveras, V.; Badetti, E.; Wurst, K.; Vidal-Gancedo, J. Synthesis, X-Ray Structure, Magnetic Properties, and a Study of Intra/Intermolecular Radical-Radical Interactions of a Triradical TEMPO Compound. ChemPhysChem 2015, 16, 3302–3307. [Google Scholar] [CrossRef]
- Badetti, E.; Lloveras, V.; Wurst, K.; Sebastián, R.M.; Caminade, A.-M.; Majoral, J.-P.; Veciana, J.; Vidal-Gancedo, J. Synthesis and Structural Characterization of a Dendrimer Model Compound Based on a Cyclotriphosphazene Core with TEMPO Radicals as Substituents. Org. Lett. 2013, 15, 3490–3493. [Google Scholar] [CrossRef]
- Badetti, E.; Lloveras, V.; Muñoz-Gómez, J.L.; Sebastián, R.M.; Caminade, A.M.; Majoral, J.P.; Veciana, J.; Vidal-Gancedo, J. Radical Dendrimers: A Family of Five Generations of Phosphorus Dendrimers Functionalized with TEMPO Radicals. Macromolecules 2014, 47, 7717–7724. [Google Scholar] [CrossRef]
- Rajca, A.; Wang, Y.; Boska, M.; Paletta, J.T.; Olankitwanit, A.; Swanson, M.A.; Mitchell, D.G.; Eaton, S.S.; Eaton, G.R.; Rajca, S. Organic Radical Contrast Agents for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2012, 134, 15724–15727. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Gokuden, R.; Watanabe, K.; Mori, T.; Naganuma, T.; Utsumi, H.; Ichikawa, K.; Katayama, Y. Nitroxyl radicals-modified dendritic poly(l-lysine) as a contrast agent for Overhauser-enhanced MRI. J. Biomater. Sci. Polym. Ed. 2014, 25, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Al-Abd, A.M. Thermoresponsive dendrimers based on oligoethylene glycols: Design, synthesis and cytotoxic activity against MCF-7 breast cancer cells. Eur. J. Med. Chem. 2013, 69, 848–854. [Google Scholar] [CrossRef]
- Wang, L.; Kiemle, D.J.; Boyle, C.J.; Connors, E.L.; Gitsov, I. “Click” Synthesis of Intrinsically Hydrophilic Dendrons and Dendrimers Containing Metal Binding Moieties at Each Branching Unit. Macromolecules 2014, 47, 2199–2213. [Google Scholar] [CrossRef]
- Pulido, D.; Albericio, F.; Royo, M. Controlling Multivalency and Multimodality: Up to Pentamodal Dendritic Platforms Based on Diethylenetriaminepentaacetic Acid Cores. Org. Lett. 2014, 16, 1318–1321. [Google Scholar] [CrossRef]
- Simón-Gracia, L.; Pulido, D.; Sevrin, C.; Grandfils, C.; Albericio, F.; Royo, M. Biocompatible, multifunctional, and well-defined OEG-based dendritic platforms for biomedical applications. Org. Biomol. Chem. 2013, 11, 4109–4121. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.K.; Wolf, M.O. Electrodeposition and properties of TEMPO functionalized polythiophene thin films. Polym. Chem. 2011, 2, 640–644. [Google Scholar] [CrossRef]
- Badetti, E.; Caminade, A.-M.; Majoral, J.-P.; Moreno-Mañas, M.; Sebastián, R.M. Palladium(0) Nanoparticles Stabilized by Phosphorus Dendrimers Containing Coordinating 15-Membered Triolefinic Macrocycles in Periphery. Langmuir 2008, 24, 2090–2101. [Google Scholar] [CrossRef]
- Blais, J.-C.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P. MALDI TOF Mass Spectrometry for the Characterization of Phosphorus-Containing Dendrimers. Scope and Limitations. Anal. Chem. 2000, 72, 5097–5105. [Google Scholar] [CrossRef]
- Hudson, A.; Luckhurst, G.R. Electron resonance spectrum of a triradical. Mol. Phys. 1967, 13, 409–416. [Google Scholar] [CrossRef]
- Likhtenstein, G.I. Spin Labeling Methods in Molecular Biology; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Likhtenstein, G.I. Biophysical Labeling Methods in Molecular Biology; Cambridge University Press: New York, NY, USA, 1993. [Google Scholar]
- Lloveras, V.; Badetti, E.; Wurst, K.; Chechik, V.; Veciana, J.; Vidal-Gancedo, J. Magnetic and Electrochemical Properties of a Diradical TEMPO-substituted Disulfide in Solution, in a Crystal and anchored on Au(111) forming a SAM. Chem. Eur. J. 2016, 22, 1805–1815. [Google Scholar] [CrossRef]
- Afzal, V.; Brasch, R.C.; Nitecki, D.E.; Wolff, S. Nitroxyl spin label contrast enhancers for magnetic resonance imaging. Studies of acute toxicity and mutagenesis. Investig. Radiol. 1984, 19, 549–552. [Google Scholar] [CrossRef]
- Samuni, Y.; Gamson, J.; Samuni, A.; Yamada, K.; Russo, A.; Krishna, M.C.; Mitchell, J.B. Factors influencing nitroxide reduction and cytotoxicity in vitro. Antioxid. Redox Signal. 2004, 6, 587–595. [Google Scholar] [CrossRef]
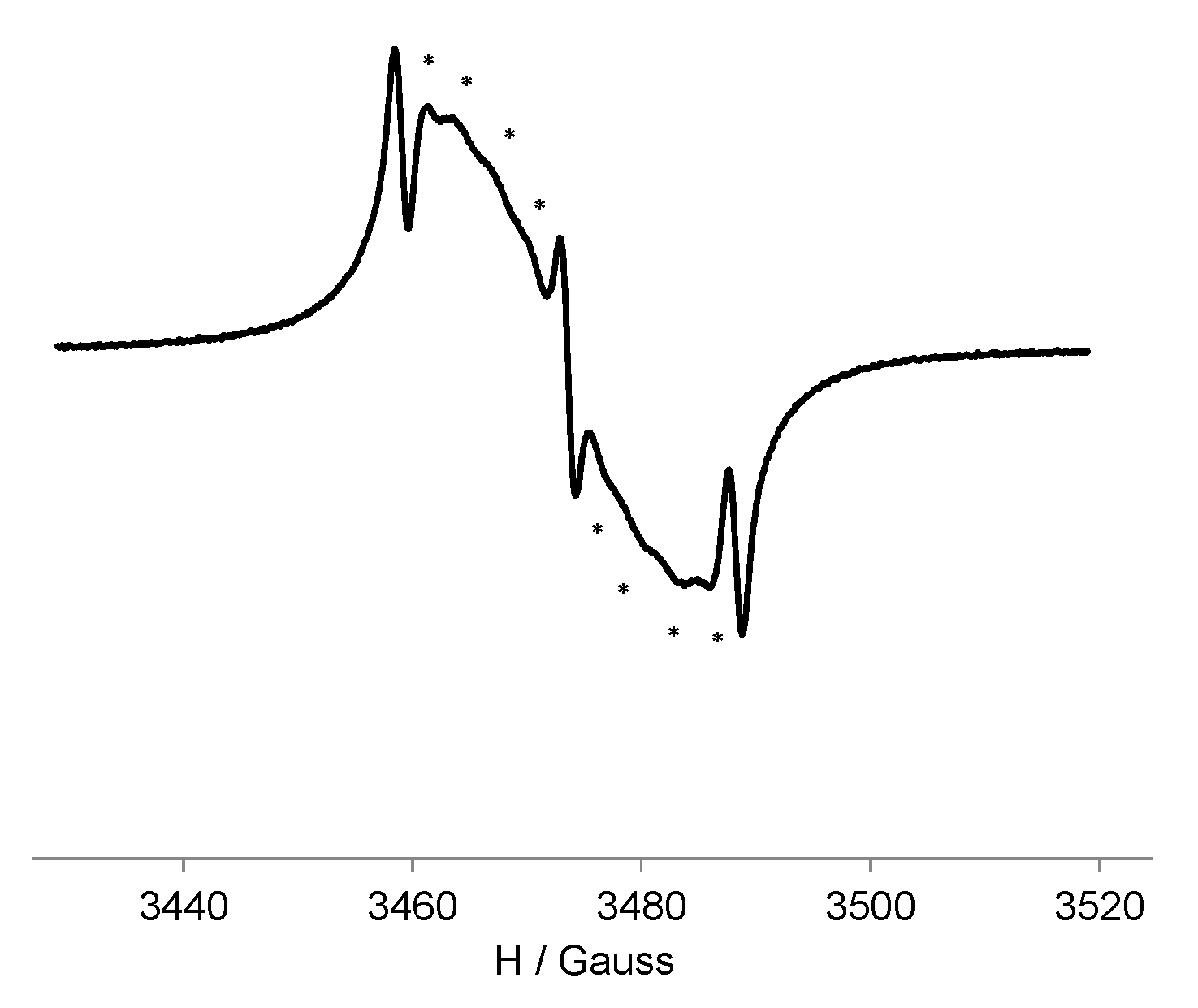
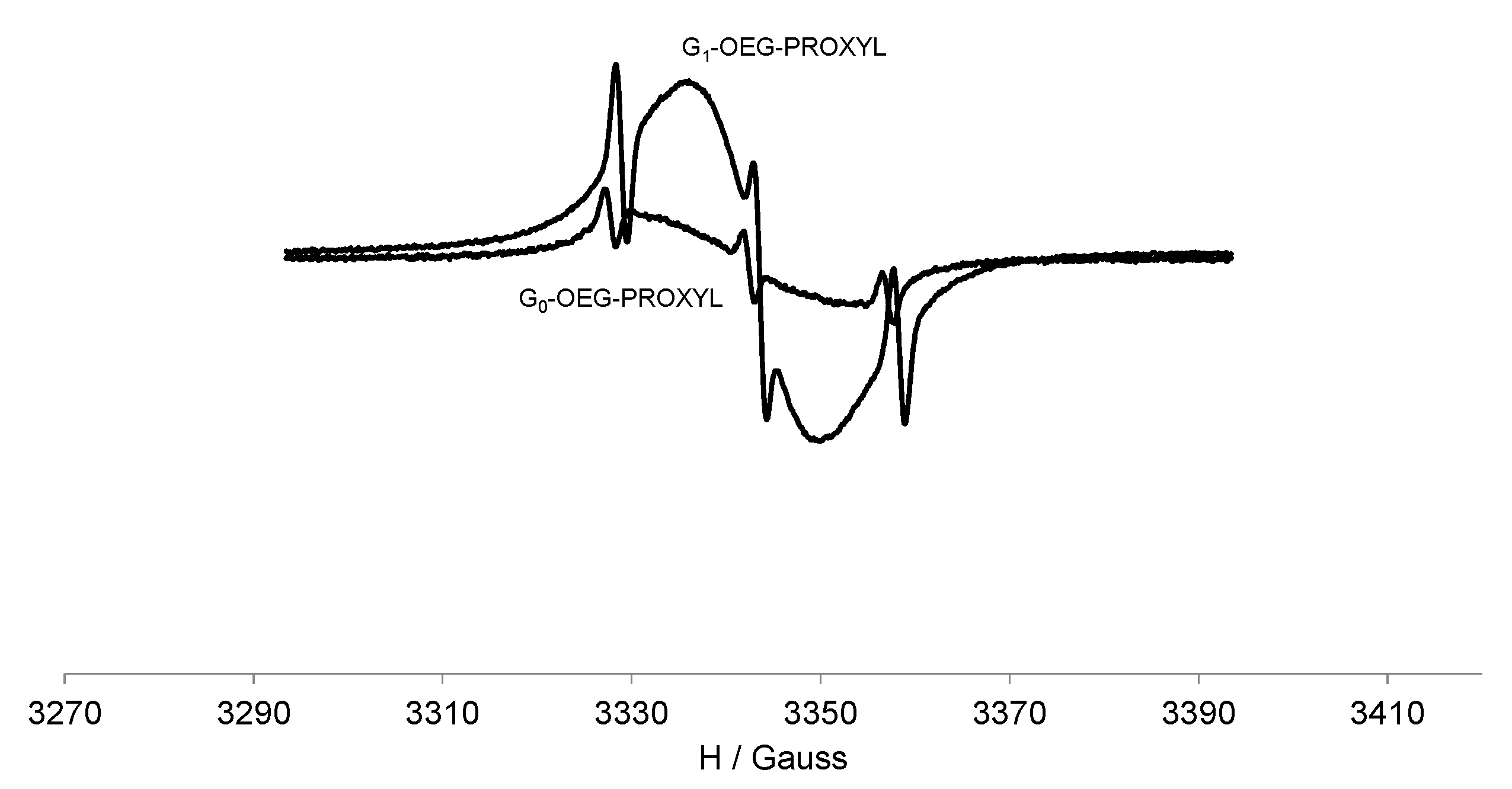
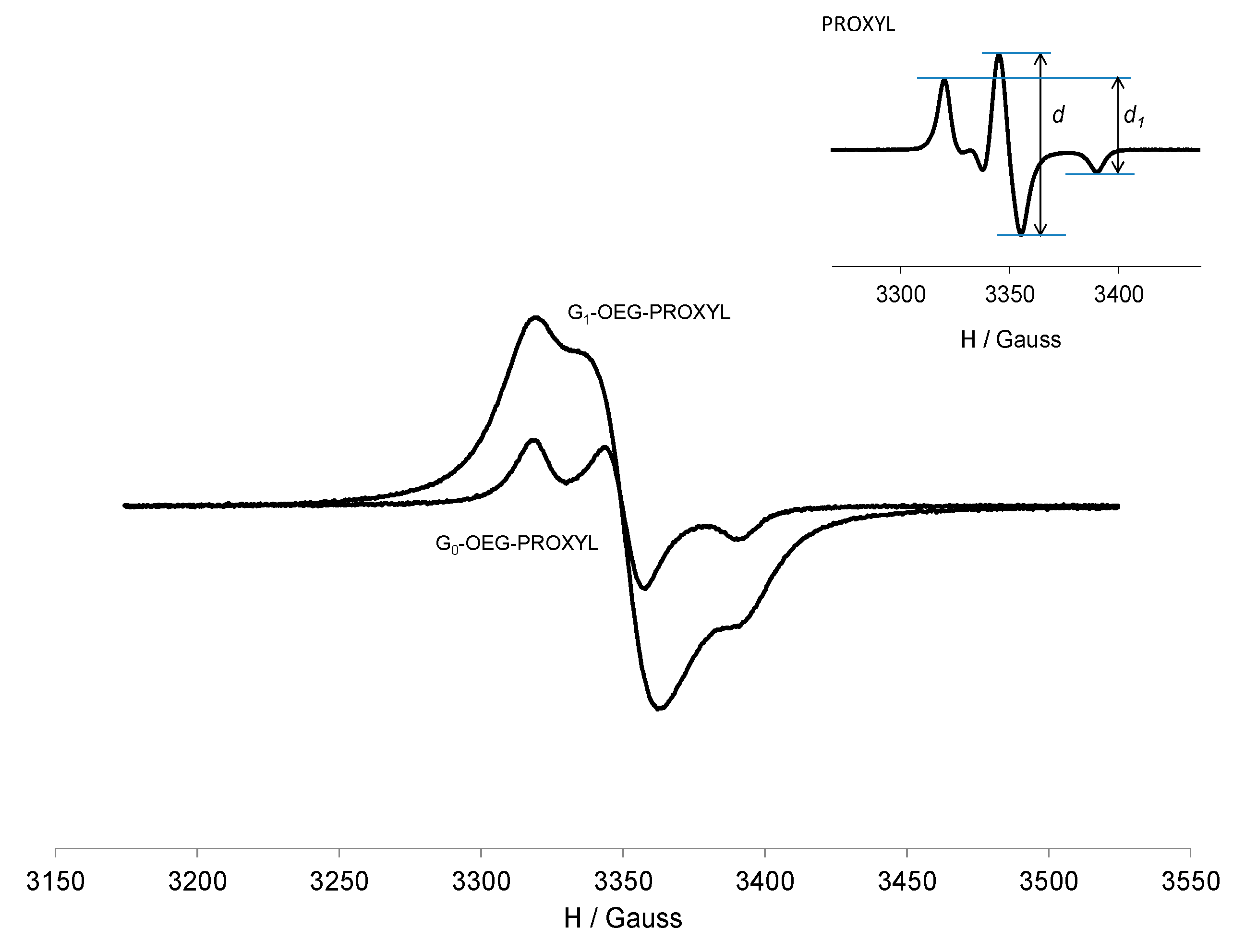
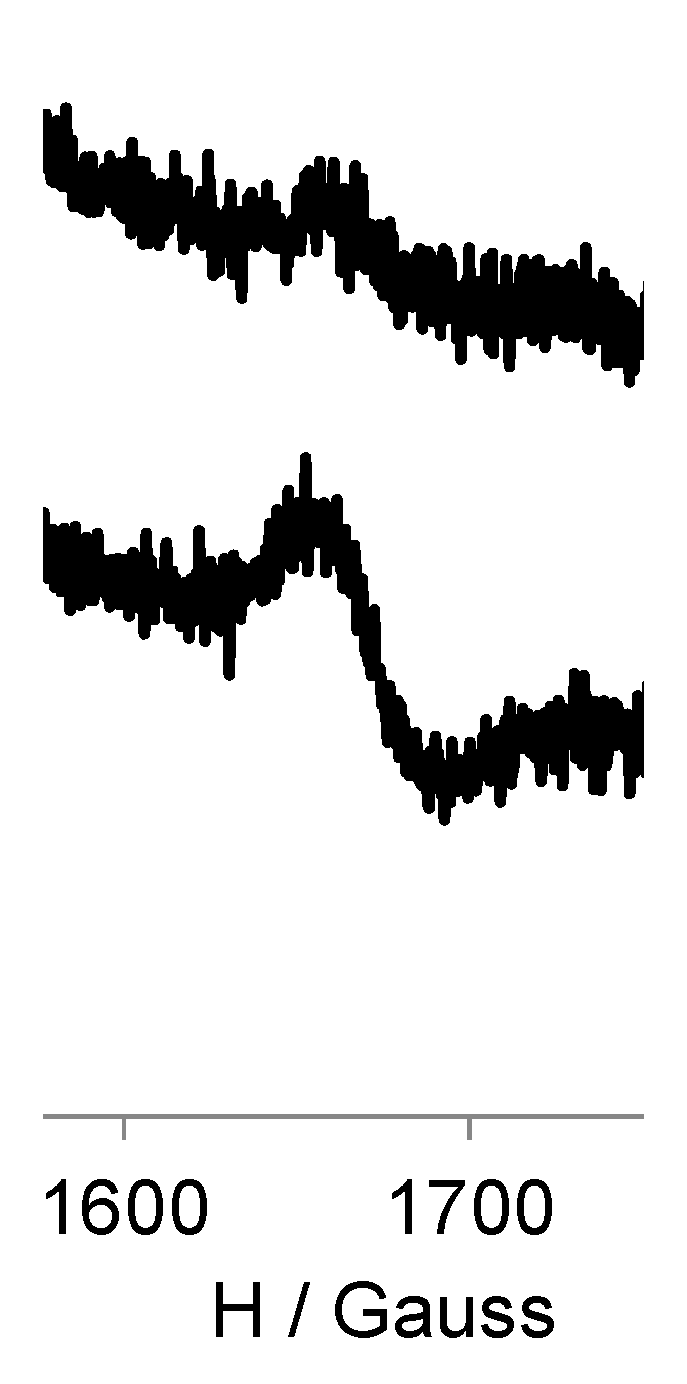
| Dendrimer | Area (Double Integral) Spectra at 300 K a | Ratio G1/G0 | Area (Double Integral) Spectra at 120 K a | Ratio G1/G0 |
|---|---|---|---|---|
| G0-OEG-PROXYL | 1.829 × 105 | 3.96 | 1.231 × 106 | 4.08 |
| G1-OEG-PROXYL | 7.246 × 105 | 5.028 × 106 |
| Compound | r17T (mM−1s−1) per Molecule | r17T (mM−1s−1) per Unit of Radical | r27T (mM−1s−1) per Molecule | r27T (mM−1s−1) per Unit of Radical |
|---|---|---|---|---|
| 3-carboxy PROXYL | 0.18 | 0.18 | 0.20 | 0.20 |
| G0-OEG-PROXYL | 0.91 | 0.18 | 0.95 | 0.19 |
| G1-OEG-PROXYL | 3.39 | 0.17 | 4.02 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Lloveras, V.; Pulido, D.; Liko, F.; Pinto, L.F.; Albericio, F.; Royo, M.; Vidal-Gancedo, J. Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI. Pharmaceutics 2020, 12, 772. https://doi.org/10.3390/pharmaceutics12080772
Zhang S, Lloveras V, Pulido D, Liko F, Pinto LF, Albericio F, Royo M, Vidal-Gancedo J. Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI. Pharmaceutics. 2020; 12(8):772. https://doi.org/10.3390/pharmaceutics12080772
Chicago/Turabian StyleZhang, Songbai, Vega Lloveras, Daniel Pulido, Flonja Liko, Luiz F. Pinto, Fernando Albericio, Miriam Royo, and José Vidal-Gancedo. 2020. "Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI" Pharmaceutics 12, no. 8: 772. https://doi.org/10.3390/pharmaceutics12080772
APA StyleZhang, S., Lloveras, V., Pulido, D., Liko, F., Pinto, L. F., Albericio, F., Royo, M., & Vidal-Gancedo, J. (2020). Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI. Pharmaceutics, 12(8), 772. https://doi.org/10.3390/pharmaceutics12080772