Safety of Nanoclay/Spring Water Hydrogels: Assessment and Mobility of Hazardous Elements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Elemental Composition of Pristine Ingredients
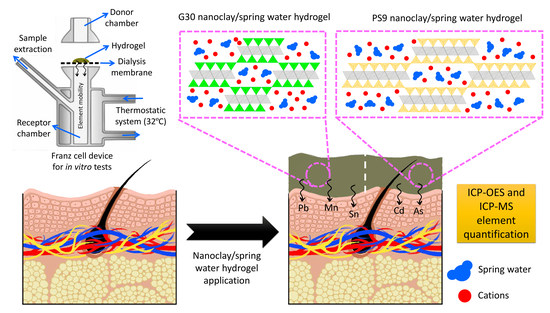
2.2.2. In Vitro Release of Elemental Impurities from Hydrogels
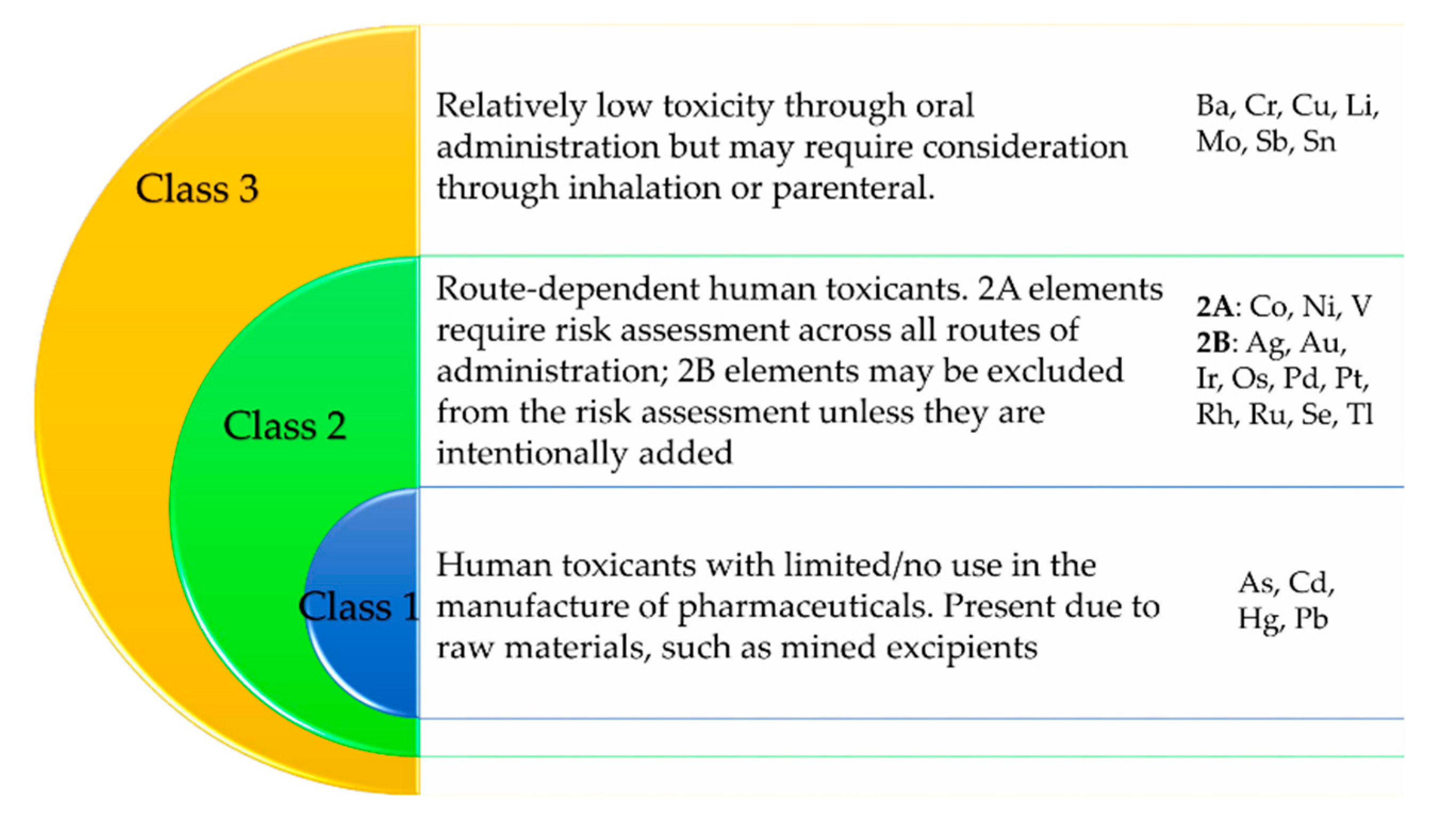
2.3. Result Discussion and Interpretation Bases and Criteria
Dose of Nanoclay/Natural Spring Water Hydrogels
3. Results
3.1. Elemental Composition of Pristine Ingredients
3.2. In Vitro Release of Hazardous Elements from Hydrogels
4. Discussion
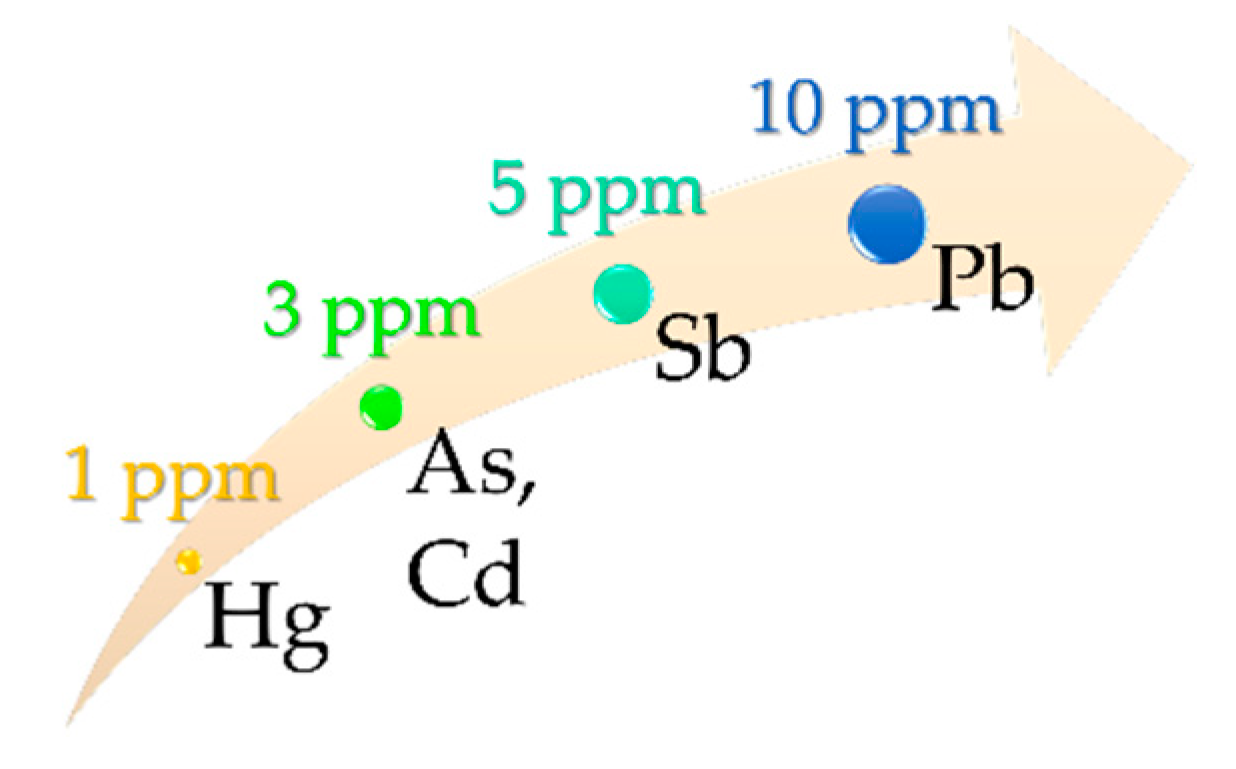
4.1. In Vitro Release of Elements: Safety Concerns and Doses
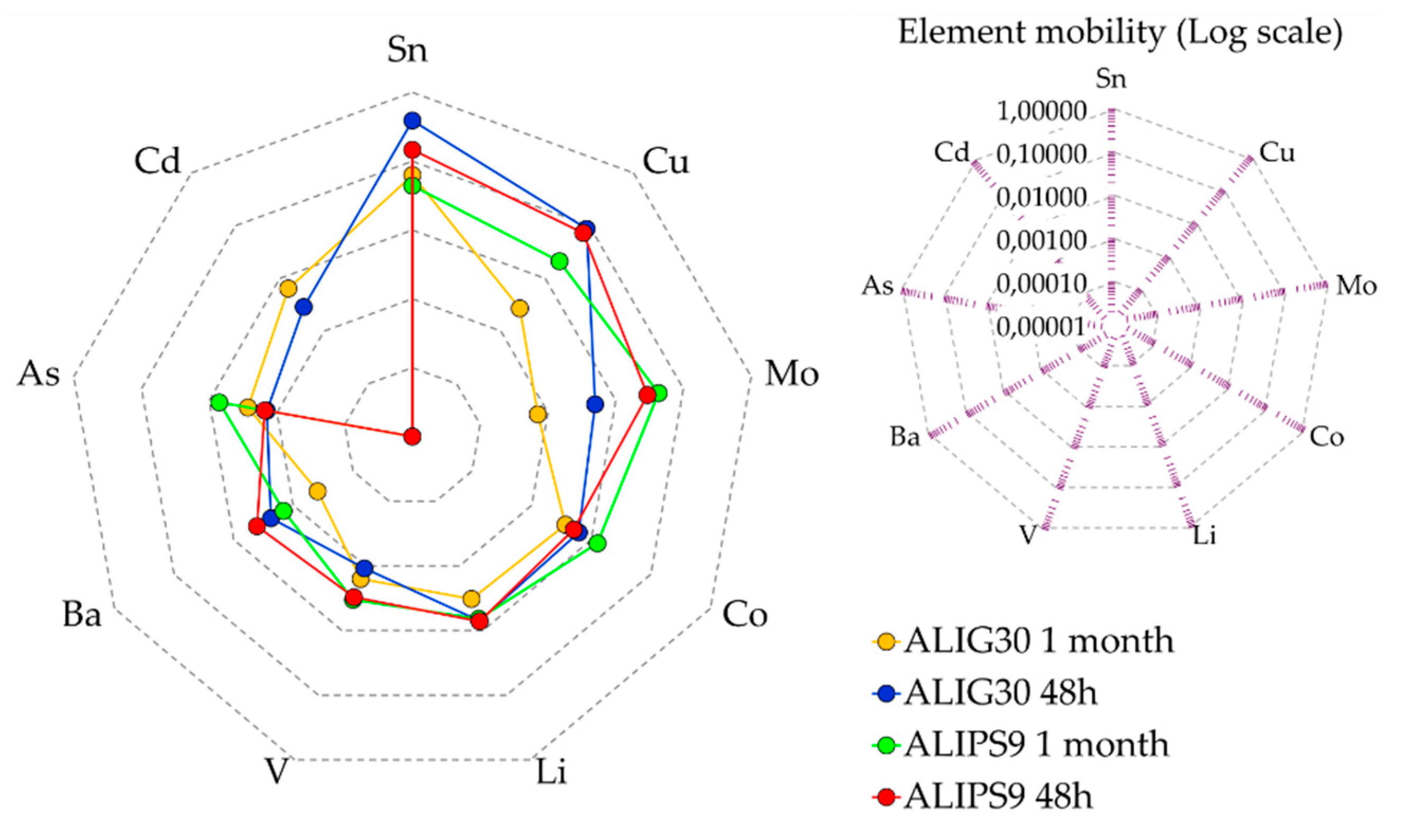
4.2. Mobility of Hazardous Elements
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Government of Canada Guidance on Heavy Metal Impurities in Cosmetics—Canada. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/industry-professionals/guidance-heavy-metal-impurities-cosmetics.html#a32 (accessed on 3 May 2018).
- FDA Prohibited and Restricted Ingredients in Cosmetics. Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/prohibited-restricted-ingredients-cosmetics (accessed on 12 December 2019).
- EU. Regulation (EC) No 1223/2009 on Cosmetic Products; European Union: Brussels, Belgium, 2009; pp. 1–151. [Google Scholar]
- EU. Manual of the Working Group on Cosmetic Products (Sub-Group on Borderline Products) on the Scope of Application of the Cosmetics Regulation (EC) No 1223/2009 (Art. 2(1)(A)); European Union: Brussels, Belgium, 2017; pp. 1–33. [Google Scholar]
- Annual Growth of the Global Cosmetics Market from 2004 to 2018; L’Oréal: Clichy/Paris, France, 2019; Available online: https://www.statista.com/statistics/297070/growth-rate-of-the-global-cosmetics-market/ (accessed on 1 July 2020).
- Directive 2003/15/EC of the European Parliament and of the Council Amending Council Directive 76/768/EEC on the Approximation of the Laws of the Member States Relating to Cosmetic Products 2003, 26–35. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:EN:PDF (accessed on 22 November 2019).
- United States Code—Federal Food, Drug, and Cosmetic Act; Office of the Law Revision Counsel: Washington, DC, USA, 2006. Available online: https://uscode.house.gov/browse/prelim@title21/chapter9/subchapter2&edition=prelim (accessed on 1 July 2020).
- Borowska, S.; Brzóska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef] [PubMed]
- Tateo, F.; Ravaglioli, A.; Andreoli, C.; Bonina, F.; Coiro, V.; Degetto, S.; Giaretta, A.; Menconi Orsini, A.; Puglia, C.; Summa, V. The in-vitro percutaneous migration of chemical elements from a thermal mud for healing use. Appl. Clay Sci. 2009, 44, 83–94. [Google Scholar] [CrossRef]
- Hostynek, J.J. Factors determining percutaneous metal absorption. Food Chem. Toxicol. 2003, 41, 327–345. [Google Scholar] [CrossRef]
- Sánchez-Espejo, R.; Aguzzi, C.; Salcedo, I.; Cerezo, P.; Viseras, C. Clays in complementary and alternative medicine. Mater. Technol. 2014, 29, B78–B81. [Google Scholar] [CrossRef]
- Natural Health Products Ingredients Database. Available online: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do (accessed on 18 December 2019).
- López-Galindo, A.; Viseras, C. Pharmaceutical and Cosmetic Application of Clays. In Clay Surfaces: Fundamentals and Applications; Wypyc, F., Satyanarayana, K.G., Eds.; Elsevier Ltd.: Berlin, Germany, 2004; pp. 267–289. Available online: https://www.sciencedirect.com/science/article/pii/B978044453607500013X (accessed on 1 July 2020).
- Viseras, C.; López-Galindo, A. Pharmaceutical applications of some spanish clays (sepiolite, palygorskite, bentonite): Some preformulation studies. Appl. Clay Sci. 1999, 14, 69–82. [Google Scholar] [CrossRef]
- Viseras, C.; Lopez-Galindo, A.; Yebra, A. Characteristics of pharmaceutical grade phyllosilicate compacts. Pharm Dev. Technol. 2000, 5, 53–58. [Google Scholar] [CrossRef]
- López-Galindo, A.; Viseras, C.; Aguzzi, C.; Cerezo, P. Pharmaceutical and cosmetic uses of fibrous clays. In Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier Ltd.: Berlin, Germany, 2011; Volume 3, pp. 299–324. ISBN 9780444536075. [Google Scholar]
- López-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Potpara, Z.; Duborija-Kovacevic, N. Effects of the peloid cream from the Montenegrin Adriatic coast on skin humidity, transepidermal water loss and erythema index, examined with skin bioengeneering in vivo methods. Farmacia 2012, 60, 524–534. [Google Scholar]
- Centini, M.; Tredici, M.R.; Biondi, N.; Buonocore, A.; Maffei Facino, R.; Anselmi, C. Thermal mud maturation: Organic matter and biological activity. Int. J. Cosmet. Sci. 2015, 37, 339–347. [Google Scholar] [CrossRef]
- Khiari, I.; Sánchez-Espejo, R.; García-Villén, F.; Cerezo, P.; Aguzzi, C.; López-Galindo, A.; Jamoussi, F.; Viseras, C. Rheology and cation release of tunisian medina mud-packs intended for topical applications. Appl. Clay Sci. 2019, 171, 110–117. [Google Scholar] [CrossRef]
- Elkayam, O.; Ophir, J.; Brener, S.; Paran, D.; Wigler, I.; Efron, D.; Even-Paz, Z.; Politi, Y.; Yaron, M. Immediate and delayed effects of treatment at the Dead Sea in patients with psoriatic arthritis. Rheumatol. Int. 2000, 19, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Delfino, M.; Russo, N.; Migliaccio, G.; Carraturo, N. Experimental study on efficacy of thermal muds of Ischia Island combined with balneotherapy in the treatment of psoriasis vulgaris with plaques. Clin. Ter. 2003, 154, 167–171. [Google Scholar] [PubMed]
- Cozzi, F.; Raffeiner, B.; Beltrame, V.; Ciprian, L.; Coran, A.; Botsios, C.; Perissinotto, E.; Grisan, E.; Ramonda, R.; Oliviero, F.; et al. Effects of mud-bath therapy in psoriatic arthritis patients treated with TNF inhibitors. Clinical evaluation and assessment of synovial inflammation by contrast-enhanced ultrasound (CEUS). Jt. Bone Spine 2015, 82, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Riyaz, N.; Arakkal, F. Spa therapy in dermatology. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 128. [Google Scholar] [CrossRef]
- Harari, M. Beauty is not only skin deep: The Dead Sea features and cosmetics. Anales de Hidrología Médica 2012, 5, 75–88. [Google Scholar]
- Williams, L.B.; Haydel, S.E.; Giese, R.F., Jr.; Eberl, D.D. Chemical and mineralogical characteristics of french green clays used for healing. Clays Clay Miner. 2008, 56, 437–452. [Google Scholar] [CrossRef]
- Comacchi, C.; Hercogova, J. A single mud treatment induces normalization of stratum corneum hydration, transepidermal water loss, skin surface pH and sebum content in patients with seborrhoeic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 372–374. [Google Scholar] [CrossRef]
- Argenziano, G.; Delfino, M.; Russo, N. Mud and baththerapy in the acne cure. Clin. Ter. 2004, 155, 125. [Google Scholar]
- Masiero, S.; Maccarone, M.C.; Magro, G. Balneotherapy and human immune function in the era of COVID-19. Int. J. Biometeorol. 2020, 1, 1–2. [Google Scholar] [CrossRef]
- Guideline for Elemental Impurities Q3D(R1); European Medicines Agency: Amsterdam, The Netherlands, 2019.
- Bocca, B.; Pino, A.; Alimonti, A.; Forte, G. Toxic metals contained in cosmetics: A status report. Regul. Toxicol. Pharmacol. 2014, 68, 447–467. [Google Scholar] [CrossRef]
- García-Villén, F.; Sánchez-Espejo, R.; López-Galindo, A.; Cerezo, P.; Viseras, C. Design and characterization of spring water hydrogels with natural inorganic excipients. Appl. Clay Sci. 2020, 197, 105772. [Google Scholar] [CrossRef]
- United States Pharmacopeia and National Formulary, 2019; United States Pharmacopeial Convention: Rockville, MD, USA, 2016.
- Ph. Eur. 9th. Magnesium trisilicate monograph. In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2018.
- Ph. Eur. 9th. Magnesium Aluminium silicate monograph. In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2018. [Google Scholar]
- Diputación Provincial de Granada; Instituto Tecnológico Geominero de España Atlas Hidrogeológico de la Provincia de Granada. Available online: http://aguas.igme.es/igme/publica/libro75/lib_75.htm (accessed on 10 September 2018).
- Maraver Eyzaguirre, F.; Armijo de Castro, F. Vademécum II de Aguas Mineromedicinales Españolas; Maraver Eyzaguirre, F., Armijo Castro, F., Eds.; Editorial Complutense: Madrid, Spain, 2010; ISBN 9788474919981. [Google Scholar]
- The European Cosmetic and Perfumeryn Association. Guidelines for Percutaneous Absorption/Penetration; COLIPA: Brussels, Belgium, 1997; pp. 1–36. [Google Scholar]
- Quality of Natural Health Products Guide; Natural and Non-Prescription Health Products Directorate: Ottawa, ON, Canada, 2015.
- Sánchez-Espejo, R.M. Suspensions of Special Clays in Mineromedicinal Waters to Be Used in Therapeutics; Andalusian Institute of Earth Science and University of Granada: Granada, Spain, 2014. [Google Scholar]
- Meijide Faílde, R.; Mourelle Mosqueira, L.; Vela Anero, Á.; Muíños López, E.; Fernández Burguera, E.; Gómez Pérez, C.P. Aplicación a pacientes: Peloterapia en patologías dermatológicas. In Peloterapia: Aplicaciones Médicas y Cosméticas de Fangos Termales; Fundación BILBILIS para la Investigación e Innovación en Hidrología Médica y Balneaoterapia: Madrid, Spain, 2013; pp. 169–183. [Google Scholar]
- Gomes, C.; Silva, J.; Gomes, J. Natural peloids versus designed and engineered peloids. Bol. Soc. Española Hidrol. Medica 2015, 30, 15–36. [Google Scholar] [CrossRef]
- Post, J.L.; Crawford, S. Varied forms of palygorskite and sepiolite from different geologic systems. Appl. Clay Sci. 2007, 36, 232–244. [Google Scholar] [CrossRef]
- Ababneh, F.A.; Abu-Sbeih, K.A.; Al-Momani, I.F. Evaluation of allergenic metals and other trace elements in personal care products. Jordan J. Chem. 2013, 8, 179–190. [Google Scholar] [CrossRef]
- Yuan, J.; Hou, Q.; Chen, D.; Zhong, L.; Dai, X.; Zhu, Z.; Li, M.; Fu, X. Chitosan/LiCl composite scaffolds promote skin regeneration in full-thickness loss. Sci. China Life Sci. 2019, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leeming, J.P. Use of topical lithium succinate in the treatment of seborrhoeic dermatitis. Dermatology 1993, 187, 149. [Google Scholar] [CrossRef]
- Dreno, B.; Moyse, D. Lithium gluconate in the treatment of seborrhoeic dermatitis: A multicenter, randomised, double-blind study versus placebo. Eur. J. Dermatol. 2002, 12, 549–552. [Google Scholar]
- Dreno, B.; Chosidow, O.; Revuz, J.; Moyse, D. Lithium gluconate 8% vs. ketoconazole 2% in the treatment of seborrhoeic dermatitis: A multicentre, randomized study. Br. J. Dermatol. 2003, 148, 1230–1236. [Google Scholar] [CrossRef]
- Gupta, A.K.; Versteeg, S.G. Topical treatment of facial seborrheic dermatitis: A systematic review. Am. J. Clin. Dermatol. 2017, 18, 193–213. [Google Scholar] [CrossRef]
- McKnight, R.; Adida, M.; Budge, K.; Stockton, S.; Goodwin, G.; Gedded, J. Lithium toxicity profile: A systematic review and meta-analysis. Lancet 2012, 379, 721–728. [Google Scholar] [CrossRef]
- Grandjean, E.; Aubry, J. Lithium: Updated human knowledge using an evidence-based approach. Part II: Clinical pharmacology and therapeutic monitoring. CNS Drugs 2009, 23, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Granjeiro, J.M.; Cruz, R.; Leite, P.E.; Gemini-Piperni, S.; Boldrini, L.C.; Ribeiro, A.R. Health and environment perspective of tin nanocompounds: A safety approach. In Tin Oxide Materials. Synthesis, Properties, and Applications; Ornaghi-Orlandi, M., Ed.; Elsevier: Berlin, Germany, 2020; pp. 133–162. [Google Scholar]
- Bai, D.; Li, Q.; Xiong, Y.; Zhao, J.; Bai, L.; Shen, P.; Yuan, L. Editor’s highlight: Effects of intraperitoneal injection of SnS2 flowers on mouse testicle. Toxicol. Sci. 2018, 161, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Tabei, Y.; Sonoda, A.; Nakajima, Y.; Biju, V.; Makita, Y.; Yoshida, Y.; Horie, M. In vitro evaluation of the cellular effect of indium tin oxide nanoparticles using the human lung adenocarcinoma A549 cells. Metallomics 2015, 7, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Roopan, S.M.; Kumar, S.H.S.; Madhumitha, G.; Suthindhiran, K. Biogenic-production of SnO2 nanoparticles and its cytotoxic effect against hepatocellular carcinoma cell line (HepG2). Appl. Biochem. Biotechnol. 2014, 175, 1567–1575. [Google Scholar] [CrossRef]
- Boogaard, P.J.; Boisset, M.; Blunden, S.; Davies, S.; Ong, T.J.; Taverne, J.P. Comparative assessment of gastrointestinal irritant potency in man of tin (II) chloride and tin migrated from packaging. Food Chem. Toxicol. 2003, 41, 1663–1670. [Google Scholar] [CrossRef]
- García-Villén, F.; Faccendini, A.; Miele, D.; Ruggeri, M.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Rossi, S.; Viseras, C.; Sandri, G. Wound healing activity of nanoclay/spring water hydrogels. Pharmaceutics 2020, 12, 467. [Google Scholar] [CrossRef]
- Hepp, N.M.; Mindak, W.R.; Gasper, J.W.; Thompson, C.B.; Barrows, J.N. Survey of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. J. Cosmet. Sci. 2014, 65, 125–145. [Google Scholar]
- Zulaikha, S.; Syed Ismail, S.N.; Praveena, S.M. Hazardous ingredients in cosmetics and personal care products and health concern: A review. Public Health Res. 2015, 5, 7–15. [Google Scholar]
- Schaumlöffel, D. Nickel species: Analysis and toxic effects. J. Trace Elem. Med. Biol. 2012, 26, 1–6. [Google Scholar] [CrossRef]
- Román-Razo, E.A.; O’Farrill, P.M.; Cambray, C.; Herrera, A.; Mendoza-Revilla, D.A.; Aguirre, D. Allergic contact dermatitis to cobalt and nickel in a metal industry worker. Case report and literature review. Rev. Alerg. Mex. 2019, 66, 371–374. [Google Scholar]
- Chou, T.C.; Wang, P.C.; De Wu, J.; Sheu, S.C. Chromium-induced skin damage among Taiwanese cement workers. Toxicol. Ind. Health 2016, 32, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Pathania, Y.S.; Budania, A. Chrome Ulcers: An occupational hazard. J. Eur. Acad. Dermatol. Venereol. 2019, 34, e180–e182. [Google Scholar] [CrossRef] [PubMed]
- Al Hossain, M.M.A.; Yajima, I.; Tazaki, A.; Xu, H.; Saheduzzaman, M.; Ohgami, N.; Ahsan, N.; Akhand, A.A.; Kato, M. Chromium-mediated hyperpigmentation of skin in male tannery workers in Bangladesh. Chemosphere 2019, 229, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Maina, G.; Adami, G.; Venier, M.; Coceani, N.; Bussani, R.; Massiccio, M.; Barbieri, P.; Spinelli, P. In vitro percutaneous absorption of cobalt. Int. Arch. Occup. Environ. Health 2004, 77, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Filon, F.L.; D’Agostin, F.; Crosera, M.; Adami, G.; Bovenzi, M.; Maina, G. In vitro absorption of metal powders through intact and damaged human skin. Toxicol. In Vitro 2009, 23, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—An element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar] [CrossRef]
- Pisano, M.; Arru, C.; Serra, M.; Galleri, G.; Sanna, D.; Garribba, E.; Palmieri, G.; Rozzo, C. Antiproliferative activity of vanadium compounds: Effects on the major malignant melanoma molecular pathways. Metallomics 2019, 11, 1687–1699. [Google Scholar] [CrossRef]
- EU. Regulation (EC) No 1272/2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006; Official Journal of the European Union, 2008; pp. 1–1355. Available online: https://osha.europa.eu/es/legislation/directives/regulation-ec-no-1272-2008-classification-labelling-and-packaging-of-substances-and-mixtures (accessed on 10 August 2020).
- US Environmental Protection Agency. Technical Fact Sheet: Final Rule for Arsenic in Drinking Water; Office of Water; United States. 2001. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/20001XXE.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2000+Thru+2005&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C00thru05%5CTxt%5C00000001%5C20001XXE.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 10 August 2020).
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- Shirvani, M.; Kalbasi, M.; Shariatmadari, H.; Nourbakhsh, F.; Najafi, B. Sorption-desorption of cadmium in aqueous palygorskite, sepiolite, and calcite suspensions: Isotherm hysteresis. Chemosphere 2006, 65, 2178–2184. [Google Scholar] [CrossRef]
- Tucovic, D.; Popov Aleksandrov, A.; Mirkov, I.; Ninkov, M.; Kulas, J.; Zolotarevski, L.; Vukojevic, V.; Mutic, J.; Tatalovic, N.; Kataranovski, M. Oral cadmium exposure affects skin immune reactivity in rats. Ecotoxicol. Environ. Saf. 2018, 164, 12–20. [Google Scholar] [CrossRef]
- Wester, R.C.; Maibach, H.I.; Sedik, L.; Melendres, J.; DiZio, S.; Wade, M. In vitro percutaneous absorption of cadmium from water and soil into human skin. Fundam. Appl. Toxicol. 1992, 19, 1–5. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B.; Geckeis, H.; Rabung, T. Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 2: Surface complexation modelling. Geochim. Cosmochim. Acta 2005, 69, 5403–5412. [Google Scholar] [CrossRef]
- Elzinga, E.J.; Rouff, A.A.; Reeder, R.J. The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: An X-ray absorption spectroscopy study. Geochim. Cosmochim. Acta 2006, 70, 2715–2725. [Google Scholar] [CrossRef]
- Altmann, S.; Tournassat, C.; Goutelard, F.; Parneix, J.C.; Gimmi, T.; Maes, N. Diffusion-driven transport in clayrock formations. Appl. Geochemistry 2012, 27, 463–478. [Google Scholar] [CrossRef]
- Guo, N.; Wang, J.S.; Li, J.; Teng, Y.G.; Zhai, Y.Z. Dynamic adsorption of Cd2+ onto acid-modified attapulgite from aqueous solution. Clays Clay Miner. 2014, 62, 415–424. [Google Scholar] [CrossRef]
- Cheng, Q.; Ye, R. A Kind of Hydrogel Expanded Material for Handling Heavy Metal Containing Sewage and Preparation Method Thereof. 2015. Available online: https://patents.google.com/patent/CN105542095B/en (accessed on 1 July 2020).
- Zhang, J.; Jin, Y.; Wang, A. Rapid removal of Pb(II) from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite/sodium humate composite hydrogels. Environ. Technol. 2011, 32, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, A. Removal of Cd(II) from aqueous solution by a composite hydrogel based on attapulgite. Environ. Technol. 2010, 31, 745–753. [Google Scholar] [CrossRef]

| Type of Document | Region | Year | Ref |
|---|---|---|---|
| Regulation of the European Parliament and Council of the European Union on cosmetic products (EC 1223/2009) | EU | 2009 | [3] |
| Guidance on Heavy Metal Impurities in Cosmetics (HC-SC) | Canada | 2012 | [1] |
| Quality of Natural Health Products Guide - Natural and Non-prescription Health Products Directorate (NNPHD) | Canada | 2015 | [39] |
| Guideline for Elemental Impurities Q3D(R1) | EU | 2019 | [30] |
| Element | PS9 (ppm) | G30 (ppm) | ALI (ppb) | Comments |
|---|---|---|---|---|
| Ba | 56.2 | 144.8 | 18.8 | Class 3 in Q3D(R1) [30]; Not listed as element in EC 1223/2009 [3] |
| Cr | 14.0 | 391.8 | 4.3 | Class 3 in Q3D(R1) [30]; Not allowed in EC 1223/2009 [3] |
| Cu | 8.1 | 11.3 | 2.5 | Class 3 in Q3D(R1) [30]; Allowed in EC 1223/2009 [3] |
| Li | 149.0 | 30.4 | 244.2 | Class 3 in Q3D(R1) [30]; Not listed in EC 1223/2009 [3] |
| Mo | 0.2 | 0.2 | 3.8 | |
| Sb | 0.3 | 2.1 | 0.1 | Class 3 in Q3D(R1) [30]; Not allowed in EC 1223/2009 [3] |
| Sn | 10.8 | 3.3 | ND | Class 3 in Q3D(R1) [30]; Not listed in EC 1223/2009 [3] |
| Ag | 0.04 | 0.2 | 0.1 | Class 2B in Q3D(R1) [30]; Allowed in EC 1223/2009 [3] |
| Au | ND | ND | ND | |
| Ir | 0.2 | 0.9 | ND | Class 2B in Q3D(R1) [30]; Not listed in EC 1223/2009 [3] |
| Se | 0.9 | 1.5 | 2.3 | Class 2B in Q3D(R1) [30]; Not allowed in EC 1223/2009 [3] |
| Tl | 0.2 | 0.1 | 0.1 | |
| Co | 2.3 | 7.4 | 0.4 | Class 2A in Q3D(R1) [30]; Not listed in EC 1223/2009 [3] |
| Ni | 3.7 | 50.7 | 9.4 | Class 2A in Q3D(R1) [30]; Not allowed in EC 1223/2009 [3] |
| V | 24.9 | 249.1 | ND | Class 2A in Q3D(R1) [30]; Not listed in EC 1223/2009 [3] |
| As | 2.0 | 1.3 | 0.2 | Class 1 in Q3D(R1) [30]; Not allowed in EC 1223/2009 [3] |
| Cd | 0.02 | 1.5 | ND | |
| Hg | ND | ND | ND | |
| Pb | 3.2 | 4.1 | ND | |
| P | 0.3 | 8.5 | 0.1 | Not allowed in EC 1223/2009 [3] |
| Be | 1.8 | 3.6 | 0.01 | |
| Zr | 21.5 | 50.3 | 0.2 | |
| Te | ND | ND | ND | |
| Nd | 8.0 | 30.2 | ND | |
| Ta | 0.9 | 0.7 | 0.005 |
| Elements | ALIPS9 | ALIG30 | ||
|---|---|---|---|---|
| 48h | 1 Month | 48h | 1 Month | |
| Ba | 5.6 ± 1.55 | 1.8 ± 0.934 | 8.3 ± 0.944 | 1.2 ± 0.360 |
| Cr | ND | ND | ||
| Cu | 10.8 ± 3.293 | 3.6 ± 2.17 | 20.6 ± 3.725 | 0.91 ± 0.608 |
| Li | 20.5 ± 3.293 | 17.7 ± 3.214 | 4.3 ± 0.362 | 1.7 ± 0.379 |
| Mo | 0.7 ± 0.095 | 0.61 ± 0.125 | 1.8 ± 0.0572 | 0.21 ± 0.123 |
| Sb | ND | ND | ||
| Sn | 28.7 ± 8.232 | 10.4 ± 2.138 | 50.4 ± 4.866 | 6.5 ± 1.945 |
| Ag, Au, Ir, Se, Tl | ND | ND | ||
| Co | 0.26 ± 0.206 | 0.6 ± 0.093 | 1.1 ± 0.660 | 0.58 ± 0.237 |
| Ni | ND | ND | ||
| V | 1.7 ± 0.310 | 1.8 ± 0.492 | 5.9 ± 0.306 | 7.5 ± 0.315 |
| As | 0.1 ± 0.010 | 0.4 ± 0.039 | 0.08 ± 0.050 | 0.1 ± 0.063 |
| Cd | ND | ND | 0.1 ± 0.064 | 0.2 ± 0.0087 |
| Hg, Pb | ND | ND | ||
| P, Be, Zr, Te, Nd, Ta | ||||
| Element | PDEparent Limits [29] | Maximum Release Detected | Hydrogel Safe Dose/Day |
|---|---|---|---|
| Ba | 730 μg/day | 8.3 μg/100 g (ALIPS9–1 month) | ≤8.79 kg |
| Cu | 340 μg/day | 20.6 μg/100 g (ALIG30–48 h) | ≤1.65 kg |
| Li | 280 μg/day | 20.5 μg/100 g (ALIPS9–48 h) | ≤1.37 kg |
| Mo | 1700 μg/day | 1.8 μg/100 g (ALIG30–48 h) | ≤94.4 kg |
| Sn | 640 μg/day | 50.4 μg/100 g (ALIG30–48 h) | ≤1.27 kg |
| Co | 5 μg/day | 1.1 μg/100 g (ALIG30–48 h) | ≤454 g |
| V | 12 μg/day | 7.5 μg/100 g (ALIG30–1 month) | ≤160 g |
| As | 15 μg/day | 0.4 μg/100 g (ALIPS9–1 month) | ≤3.75 kg |
| Cd | 1.7 μg/day | 0.2 μg/100 g (ALIG30–1 month) | ≤850 g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Villén, F.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Perioli, L.; Viseras, C. Safety of Nanoclay/Spring Water Hydrogels: Assessment and Mobility of Hazardous Elements. Pharmaceutics 2020, 12, 764. https://doi.org/10.3390/pharmaceutics12080764
García-Villén F, Sánchez-Espejo R, Borrego-Sánchez A, Cerezo P, Perioli L, Viseras C. Safety of Nanoclay/Spring Water Hydrogels: Assessment and Mobility of Hazardous Elements. Pharmaceutics. 2020; 12(8):764. https://doi.org/10.3390/pharmaceutics12080764
Chicago/Turabian StyleGarcía-Villén, Fátima, Rita Sánchez-Espejo, Ana Borrego-Sánchez, Pilar Cerezo, Luana Perioli, and César Viseras. 2020. "Safety of Nanoclay/Spring Water Hydrogels: Assessment and Mobility of Hazardous Elements" Pharmaceutics 12, no. 8: 764. https://doi.org/10.3390/pharmaceutics12080764
APA StyleGarcía-Villén, F., Sánchez-Espejo, R., Borrego-Sánchez, A., Cerezo, P., Perioli, L., & Viseras, C. (2020). Safety of Nanoclay/Spring Water Hydrogels: Assessment and Mobility of Hazardous Elements. Pharmaceutics, 12(8), 764. https://doi.org/10.3390/pharmaceutics12080764