Prevalence of Potential Drug–Drug Interaction Risk among Chronic Kidney Disease Patients in a Spanish Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Clinical and Demographic and Characteristics of Patients
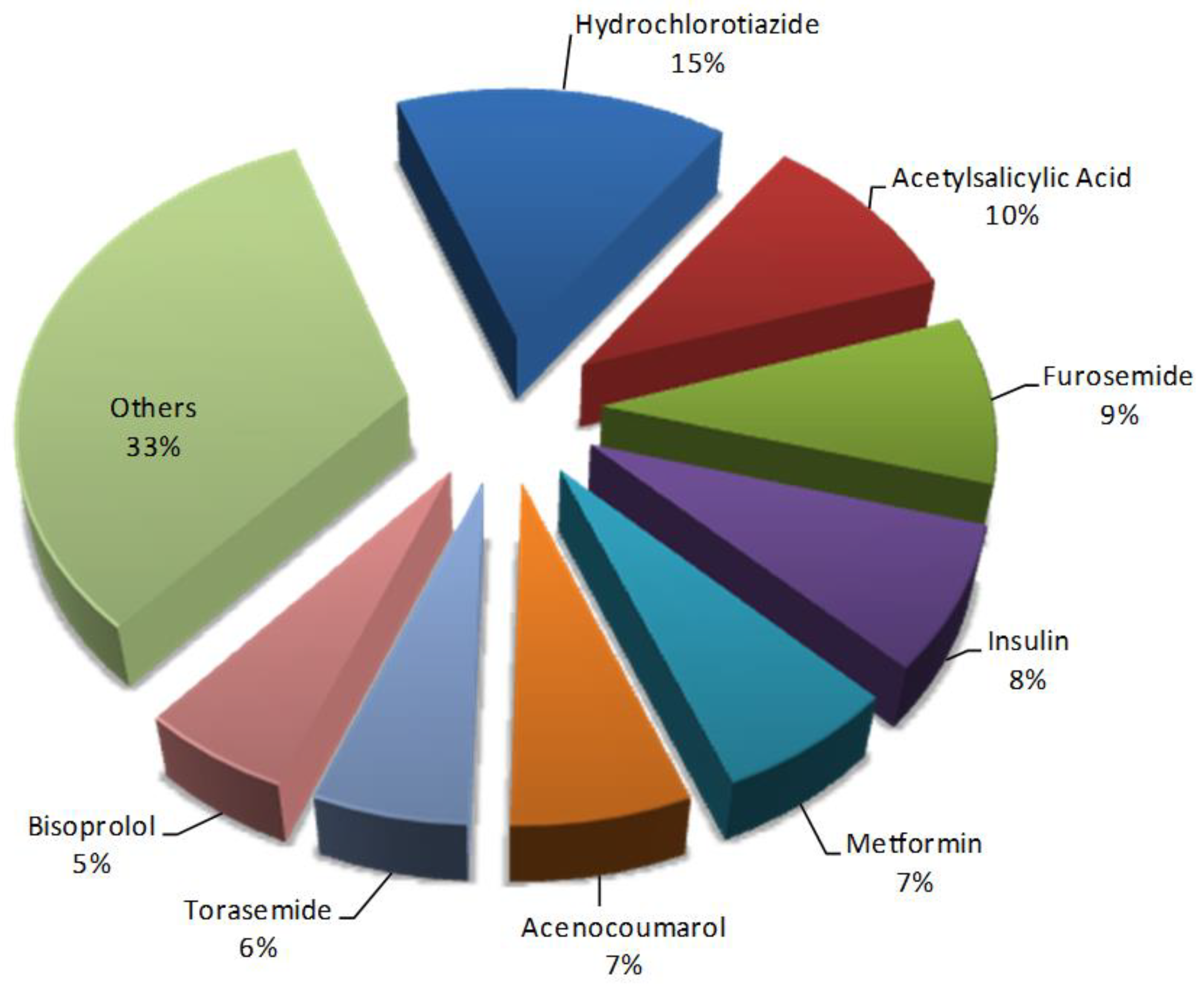
3.2. Prevalence and Pattern of Potential Drug–Drug Interactions
3.3. Factors Associated with Potential Drug–Drug Interactions on CKD Patients
4. Discussion
4.1. Frequency and Severity of Potential Drug–Drug Interactions
4.2. Factors Associated with Potential Drug–Drug Interactions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Gorostidi, M.; Sánchez-Martínez, M.; Ruilope, L.M.; Graciani, A.; de la Cruz, J.J.; Santamaría, R.; del Pino, M.; Guallar-Castillón, P.; de Álvaro, F.; Rodríguez-Artalejo, F.; et al. Prevalencia de enfermedad renal crónica en España: Impacto de la acumulación de factores de riesgo cardiovascular. Nefrologia 2018, 38, 606–615. [Google Scholar] [CrossRef]
- Webster, A.; Nagler, E.; Morton, R.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Kearney, P.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Major, R.; Cheng, M.; Grant, R.; Shantikumar, S.; Xu, G.; Oozeerally, I.; Brunskill, N.; Gray, L. Cardiovascular disease risk factors in chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0192895. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Hajjar, E. Polypharmacy, Adverse Drug Reactions, and Geriatric Syndromes. Clin. Geriatr. Med. 2012, 28, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Marquito, A.; Fernandes, N.; Colugnati, F.; Paula, R. Identifying potential drug interactions in chronic kidney disease patients. J. Bras. Nefrol. 2014, 36, 26–34. [Google Scholar] [CrossRef]
- Sgnaolin, V.; Sgnaolin, V.; Engroff, P.; De Carli, G.; Prado Lima Figueiredo, A. Avaliação dos medicamentos utilizados e possíveis interações medicamentosas em doentes renais crônicos. Sci. Med. (Porto Alegre) 2014, 24, 329–335. [Google Scholar] [CrossRef]
- Rama, M.; Viswanathan, G.; Acharya, L.; Attur, R.; Reddy, P.; Raghavan, S. Assessment of drug-drug interactions among renal failure patients of nephrology ward in a south Indian tertiary care hospital. Indian J. Pharm. Sci. 2012, 74, 63–68. [Google Scholar] [PubMed]
- Hegde, S.; Udaykumar, P.; Manjuprasad, M.S. Potential drug interactions in chronic kidney disease patients-A cross sectional study. Int. J. Recent Trends Sci. Technol. 2015, 16, 56–60. [Google Scholar]
- Saleem, A.; Masood, I.; Khan, T. Clinical relevancy and determinants of potential drug-drug interactions in chronic kidney disease patients: Results from a retrospective analysis. Integr. Pharm. Res. Pract. 2017, 6, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Al-Ramahi, R.; Raddad, A.; Rashed, A.; Bsharat, A.; Abu-Ghazaleh, D.; Yasin, E.; Shehab, O. Evaluation of potential drug-drug interactions among Palestinian hemodialysis patients. BMC Nephrol. 2016, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Adibe, M.O.; Ewelum, P.C.; Amorha, K.C. Evaluation of drug-drug interactions among patients with chronic kidney disease in a South-Eastern Nigeria tertiary hospital: A retrospective study. Pan Afr. Med. J. 2017, 28, 199. [Google Scholar] [CrossRef]
- Fasipe, O.J.; Akhideno, P.E.; Nwaiwu, O.; Adelosoye, A.A. Assessment of prescribed medications and pattern of distribution for potential drug–drug interactions among chronic kidney disease patients attending the Nephrology Clinic of Lagos University Teaching Hospital in Sub-Saharan West Africa. Clin. Pharmacol. 2017, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Olumuyiwa, J.F.; Akinwumi, A.A.; Ademola, O.A.; Oluwole, B.A.; Ibiene, E.O. Prevalence and pattern of potential drug-drug interactions among chronic kidney disease patients in south-western Nigeria. Niger. Postgrad. Med. J. 2017, 24, 88–92. [Google Scholar]
- Okoro, R.; Farate, V. Evaluation of potential drug–drug interactions among patients with chronic kidney disease in northeastern Nigeria. Afr. J. Nephrol. 2019, 22, 77–81. [Google Scholar]
- Roblek, T.; Vaupotic, T.; Mrhar, A.; Lainscak, M. Drug-drug interaction software in clinical practice: A systematic review. Eur. J. Clin. Pharmacol. 2015, 1, 131–142. [Google Scholar] [CrossRef]
- Kheshti, R.; Aalipour, M.; Namazi, S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J. Res. Pharm. Pract. 2016, 5, 257–263. [Google Scholar] [PubMed]
- Patel, R.I.; Beckett, R.D. Evaluation of resources for analyzing drug interactions. J. Med. Libr. Assoc. 2016, 104, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.; Allen, E. Polypharmacy in the elderly: A literature review. J. Am. Acad. Nurse Pract. 2005, 17, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, B.; Makubate, B.; Hernandez-Santiago, V.; Dreischulte, T. The rising tide of polypharmacy and drug-drug interactions: Population database analysis 1995–2010. BMC Med. 2015, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.I.; Hwang, S.J.; Larson, M.G.; Levy, D.; Fox, C.S. Chronic kidney disease as a predictor of cardiovascular disease (from the Framingham Heart Study). Am. J. Cardiol. 2008, 102, 47–53. [Google Scholar] [CrossRef][Green Version]
| Characteristics | N (%) or Mean ± SD |
|---|---|
| Female | 69 (61.6) |
| Male | 43 (38.4) |
| Mean age (years) | 77.1 ± 10.4 |
| Age group (years) | |
| 30–60 | 11 (10.0) |
| 61–70 | 8 (7.1) |
| 71–80 | 44 (39.3) |
| >80 | 49 (43.7) |
| CKD stage | |
| 1 | 10 (8.9) |
| 2 | 15 (13.4) |
| 3a | 34 (30.3) |
| 3b | 33 (29.5) |
| 4 | 15 (13.4) |
| 5 | 5 (4.5) |
| Comorbidities | 91 (81.2) |
| Hypertension | 52 (46.4) |
| Diabetes mellitus | 25 (22.3) |
| Dyslipidemia/hypercholesterolemia | 33 (29.5) |
| Anemia | 13 (11.6) |
| Hyperuricemia | 11 (9.8) |
| Mean prescribed drugs per patient | 8.6 ± 3.4 |
| Number of prescribed drugs | |
| ≤5 | 22 (19.6) |
| 6–10 | 59 (53.2) |
| 11–15 | 29 (25.9) |
| ≥16 | 2 (1.8) |
| Number of pDDIs | N | % |
|---|---|---|
| None | 10 | 9.0 |
| 1–5 | 40 | 36.0 |
| 6–10 | 27 | 24.3 |
| 11–15 | 15 | 13.5 |
| 16–20 | 11 | 9.9 |
| 21–25 | 4 | 3.6 |
| >25 | 4 | 3.6 |
| Severity of pDDIs | N | % |
|---|---|---|
| Type A (No known interaction) | 11 | 1.2 |
| Type B (mild severity) | 84 | 9.1 |
| Type C (moderate severity) | 717 | 77.3 |
| Type D (major severity) | 106 | 11.4 |
| Type X (avoid drug combination) | 10 | 1.1 |
| Severity of pDDI | N | PDDIs | Frequency (%) |
|---|---|---|---|
| Type B (mild severity) | 84 | Levothyroxine + Omeprazole | 10.7 |
| Levothyroxine + Furosemide | 9.5 | ||
| Acenocoumarol + Spironolactone | 7.1 | ||
| Type C (moderate severity) | 717 | Acenocoumarol + Omeprazole | 1.5 |
| Ferrous Sulfate + Omeprazole | 1.3 | ||
| Metformin + Acetylsalicylic Acid | 1.3 | ||
| Type D (major severity) | 106 | Acenocoumarol + Allopurinol | 7.5 |
| Levothyroxine + Ferrous Sulfate | 4.7 | ||
| Tramadol + Trazodone | 3.8 |
| Drug–Drug Interaction | Potential Adverse Effects | Severity | Reliability Rating |
|---|---|---|---|
| Amitriptyline + Aclidinium | Aclidinium may enhance the anticholinergic effect of Anticholinergic Agents | Major | Fair |
| Doxazosin + Dutasteride and Tamsulosin | Alpha1-Blockers may enhance the antihypertensive effect of other Alpha1-Blockers | Major | Fair |
| Dutasteride And Tamsulosin + Tamsulosin | Alpha1-Blockers may enhance the antihypertensive effect of other Alpha1-Blockers | Major | Fair |
| Levosulpiride + Solifenacin | Anticholinergic Agents may diminish the therapeutic effect of Levosulpiride | Major | Fair |
| Aclidinium and Formoterol + Propranolol | Beta-Blockers (Nonselective) may diminish the bronchodilatory effect of Beta2-Agonists | Major | Fair |
| Metamizole + Dexketoprofen | Dexketoprofen may enhance the adverse/toxic effect of Nonsteroidal Anti-Inflammatory Agents | Major | Fair |
| Tramadol and Acetaminophen + Buprenorphine | Opioids (Mixed Agonist/Antagonist) may diminish the analgesic effect of Opioid Agonists | Major | Good |
| Atenolol + Rivastigmine | Rivastigmine may enhance the bradycardic effect of Beta-Blockers | Moderate | Fair |
| Bisoprolol + Rivastigmine | Rivastigmine may enhance the bradycardic effect of Beta-Blockers | Moderate | Fair |
| Dexketoprofen + Acetylsalicylic Acid | Salicylates may enhance the adverse/toxic effect of Dexketoprofen. Dexketoprofen may diminish the therapeutic effect of Salicylates. Salicylates may decrease the serum concentration of Dexketoprofen | Major | Fair |
| Variable | Number | Median (25%–75% Percentile) | p–Value 1 |
|---|---|---|---|
| Gender | 0.5768 2 | ||
| Female | 68 | 7 (2–12.7) | |
| Male | 43 | 7 (2–11) | |
| Age | 0.0191 | ||
| 30–60 | 11 | 2 (0–5) | |
| 61–70 | 8 | 3.5 (0.5–7.7) | |
| 71–80 | 44 | 8 (3.2–16) | |
| >80 | 48 | 8 (3–11) | |
| CKD stage ** | 0.4930 | ||
| G1 | 10 | 3.5 (0.7–12.7) | |
| G2 | 15 | 8 (3–12) | |
| G3a | 34 | 5 (1–9.2) | |
| G3b | 33 | 8 (3–13) | |
| G4 | 14 | 4 (3–13) | |
| G5 | 5 | 11 (4–17.5) | |
| Concomitant drugs | <0.0001 | ||
| ≤5 | 21 | 1 (0–2) | |
| 6–10 | 59 | 6 (4–9) | |
| 11–15 | 29 | 16 (11–20) | |
| >15 | 2 | 26 (16–36) | |
| Chronic comorbid disease | 0.0611 | ||
| 0 | 20 | 6.5 (3.2–11) | |
| 1 | 21 | 7 (2–12.5) | |
| 2 | 20 | 6.5 (3–10.7) | |
| 3 | 10 | 12 (7.2–20.5) | |
| 4 | 17 | 7 (1–17.5) | |
| ≥5 | 23 | 4 (1–7) |
| Study | Number of Patients (% Female) | Years (Mean ± SD) | Country | CKD Patients on Stage 5 or Hemodialysis (%) | Software for Potential DDI | Number of Drugs per Patient (Mean ± SD) | Most Frequent Drug Combinations with Potential DDIs (%) | Number of Patients with Potential DDIs Contraindicated (%) |
|---|---|---|---|---|---|---|---|---|
| Rama et al. [12]. | 205 (25.8%) | 48.6 ± 16.2 | India | 68.5% | Micromedex | 12.1 ± 6.3 | Ascorbid Acid + Cyanocobalamine (12.4%) Clonidine + Metoprolol (3.8%) Amlodipine + Metoprolol (3.4%) Insulin + Metoprolol (2.9%) | 0 (0.0%) |
| Marquito et al. [10]. | 558 (45.3%) | n.s. | Brazil | 6.6% | Micromedex | 5.6 ± 3.2 | Furosemide + Aspirin (7.8%) Enalapril + Furosemide (5.9%) Captopril + Furosemide (5.1%) Enalapril + Losartan (3.7%) | 5 (0.9%) |
| Sgnaolin et al. [11]. | 65 (50.8%) | 59.1±14.7 | Brazil | 100% | Micromedex | 6.3 ± 3.1 | Calcium Carbonate + Atenolol (8.0%) Calcium Carbonate + Ferrous Sulfate (8.0%) Calcium Carbonate + Ticlopidine (6.3%) Enalapril + Eritropoietin (4.5%) | 2 (3.1%) |
| Hegde et al. [13]. | 120 (45%) | 58.5 ± 8.4 | India | n.s. | Medscape Drug interaction checker | 9.4 ± 3.9 | Sodium Bicarbonate + Ferrous Sulfate (8.9%) Calcium Carbonate + Ferrous Sulfate (5.5%) Aspirin + Carvedilol (5.5%) Sodium Bicarbonate + Allopurinol (5.5%) | 0 (0.0%) |
| Al-Ramahi et al. [15]. | 275 (45.1%) | 50.7 ± 15.9 | Palestina | 100% | LexiComp | 7.9 ± 2.4 | Calcium Carbonate + Amlodipine (12.3%) Calcium Carbonate + Aspirin (8.2%) Aspirin + Furosemide (7.9%) Aspirin + Enoxaparin (4.3%) | 2 (0.7%) |
| Olumuyiwa et al. [18]. | 123 (33.3%) | 53.8 ± 16.0 | Nigeria | 69.9% | Lexi-Interact database | 10.1 ± 4.0 | Calcium Carbonate + Ferrous Sulfate (8.4%) Folic Acid + Furosemide (3.4%) Calcium Carbonate + Calcidol (3.2%) Vitamin E + Ferrous Sulfate (3.0%) | 1 (0.8%) |
| Fasipe et al. [17]. | 123 (48.8%) | 53.8 ± 16.0 | Nigeria | 69.9% | Medscape Drug interaction checker | 10.3 ± 3.9 | Calcium Carbonate + Ferrous Sulfate (9.9%) Folic Acid + Furosemide (3.4%) Calcium Carbonate + Calcidol (3.2%) Vitamin E + Ferrous Sulfate (3.0%) | 1 (0.8%) |
| Saleem et al. [14]. | 209 (39.2%) | 38.3 ± 16.8 | Pakistan | 74.2% | Micromedex Drug-Reax | n.s. | Ferrous Sulfate + Omeprazole (5.8%) Calcium/Vitamin D + Ciprofloxacin (4.8%) Captopril + Furosemide (4.1%) Calcium Gluconate + Ceftriaxone (3.6%) | 28 (13.4) |
| Adibe et al. [16]. | 169 (52.1%) | 51.0 ± 14.9 | Nigeria | 28.4% | Medscape Drug interaction checker | 6.1 ± 2.0 | Lisinopril + Furosemide (9.1%) Furosemide + Calcium Carbonate (7.2%) Calcium Carbonate + Lisinipril (6.1%) Aspirin + Furosemide (4.6%) | 0 (0.0%) |
| Okoro and Farate [19]. | 201 (66%) | 49.5 ± 14.5 | Nigeria | 69.2% | Omnio drug interaction checker | 5.8 ± 1.5 | Calcium Carbonate + Ferrous Sulfate (45.8%) Lisinopril + Furosemide (7.7%) Captopril + Furosemide (6.6%) Captopril + Spironolactone (6.6%) | 5 (2.5%) |
| Present study | 111 (61.3%) | 77.1 ± 10.4 | Spain | 4.5% | LexiComp | 8.6 ± 3.4 | Acenocoumarol + Omeprazole (1.1%) Ferrous Sulfate + Omeprazole (1.0%) Metformin + Aspirin (1.0%) Levothyroxine + Omeprazole (1.0%) | 10 (9.0%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Díaz, G.; Pérez-Pico, A.M.; Suárez-Santisteban, M.Á.; García-Bernalt, V.; Mayordomo, R.; Dorado, P. Prevalence of Potential Drug–Drug Interaction Risk among Chronic Kidney Disease Patients in a Spanish Hospital. Pharmaceutics 2020, 12, 713. https://doi.org/10.3390/pharmaceutics12080713
Santos-Díaz G, Pérez-Pico AM, Suárez-Santisteban MÁ, García-Bernalt V, Mayordomo R, Dorado P. Prevalence of Potential Drug–Drug Interaction Risk among Chronic Kidney Disease Patients in a Spanish Hospital. Pharmaceutics. 2020; 12(8):713. https://doi.org/10.3390/pharmaceutics12080713
Chicago/Turabian StyleSantos-Díaz, Gracia, Ana María Pérez-Pico, Miguel Ángel Suárez-Santisteban, Vanesa García-Bernalt, Raquel Mayordomo, and Pedro Dorado. 2020. "Prevalence of Potential Drug–Drug Interaction Risk among Chronic Kidney Disease Patients in a Spanish Hospital" Pharmaceutics 12, no. 8: 713. https://doi.org/10.3390/pharmaceutics12080713
APA StyleSantos-Díaz, G., Pérez-Pico, A. M., Suárez-Santisteban, M. Á., García-Bernalt, V., Mayordomo, R., & Dorado, P. (2020). Prevalence of Potential Drug–Drug Interaction Risk among Chronic Kidney Disease Patients in a Spanish Hospital. Pharmaceutics, 12(8), 713. https://doi.org/10.3390/pharmaceutics12080713








