Silicon Nanofluidic Membrane for Electrostatic Control of Drugs and Analytes Elution
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanofluidic Membrane Fabrication
2.2. Assessment of Membrane Structure
2.3. Electrode Connection
2.4. Electrochemical Characterization
2.5. In Vitro Release Modulation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Nanofluidic Membrane
3.2. Solid–Liquid Interface, SiO2 vs SiC
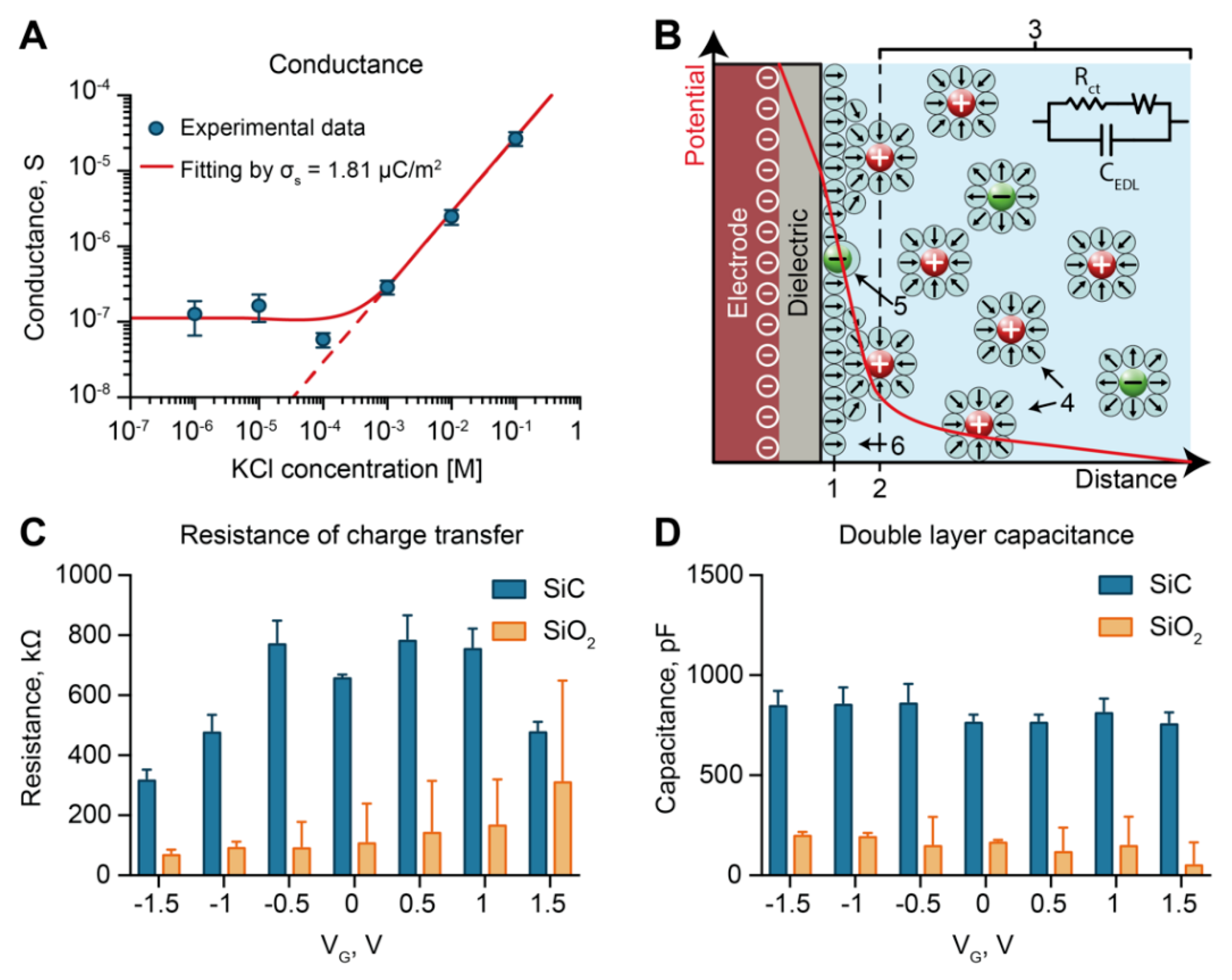
3.3. Electrochemical Characterization: Conductance
3.4. Electrochemical Characterization: Electrochemical Impedance Spectroscopy
3.5. Mechanism of Analyte Flow Control through Electrostatic Gating
3.6. In Vitro Release Modulation of Methotrexate
3.7. In Vitro Controlled Release of Quantum Dots
3.8. Considerations on Electrostatic Gating Performance
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Yach, D.; Leeder, S.R.; Bell, J.; Kistnasamy, B. Global chronic diseases. AAAS. 2005, 307, 317. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; WHO Press: Geneva, Switzerland, 2014. [Google Scholar]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respi. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lemstra, M.; Nwankwo, C.; Bird, Y.; Moraros, J. Primary nonadherence to chronic disease medications: A meta-analysis. Patient Prefer. Adherence 2018, 12, 721. [Google Scholar] [CrossRef]
- García-Lizana, F.; Sarría-Santamera, A. New technologies for chronic disease management and control: A systematic review. J. Telemed. Telecare. 2007, 13, 62–68. [Google Scholar] [CrossRef]
- Desai, T.A.; Hansford, D.J.; Ferrari, M. Micromachined interfaces: New approaches in cell immunoisolation and biomolecular separation. Biomol. Eng. 2000, 17, 23–36. [Google Scholar] [CrossRef]
- Peng, L.; Mendelsohn, A.D.; LaTempa, T.J.; Yoriya, S.; Grimes, C.A.; Desai, T.A. Long-term small molecule and protein elution from TiO2 nanotubes. Nano Lett 2009, 9, 1932–1936. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 2019, 21, 47. [Google Scholar] [CrossRef]
- Chua, C.Y.X.; Jain, P.; Ballerini, A.; Bruno, G.; Hood, R.L.; Gupte, M.; Gao, S.; Di Trani, N.; Susnjar, A.; Shelton, K.; et al. Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis. J. Controlled Release 2018, 286, 315–325. [Google Scholar] [CrossRef]
- Ballerini, A.; Chua, C.Y.X.; Rhudy, J.; Susnjar, A.; Di Trani, N.; Jain, P.R.; Laue, G.; Lubicka, D.; Shirazi-Fard, Y.; Ferrari, M. Counteracting Muscle Atrophy on Earth and in Space via Nanofluidics Delivery of Formoterol. Adv. Ther. 2020, 3, 2000014. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Smolensky, M.H.; Mojón, A.; Fernández, J.R.; Crespo, J.J.; Moyá, A.; Rios, M.T.; Portaluppi, F. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat. Rev. Nephrol. 2013, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Kawashima, Y. Current status and approaches to developing press-coated chronodelivery drug systems. J. Controlled Release 2012, 157, 331–353. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, C.; Aoki, R.; Arai, T.; Kumari, P. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fifel, K.; Videnovic, A. Chronotherapies for Parkinson’s disease. Prog. Neurobiol. 2019, 174, 16–27. [Google Scholar] [CrossRef]
- Kaur, G.; Phillips, C.; Wong, K.; Saini, B. Timing is important in medication administration: A timely review of chronotherapy research. Int J. Clin. Pharm. 2013, 35, 344–358. [Google Scholar] [CrossRef]
- Sprintz, M.; Tasciotti, E.; Allegri, M.; Grattoni, A.; Driver Larry, C.; Ferrari, M. Nanomedicine: Ushering in a new era of pain management. Eur. J. Pain Suppl. 2012, 5, 317–322. [Google Scholar] [CrossRef]
- Celler, B.G.; Lovell, N.H.; Basilakis, J. Using information technology to improve the management of chronic disease. Med. J. Aust. 2003, 179, 242–246. [Google Scholar] [CrossRef]
- Milani, R.V.; Bober, R.M.; Lavie, C.J. The role of technology in chronic disease care. Prog. Cardiovasc. Dis. 2016, 58, 579–583. [Google Scholar] [CrossRef]
- Coye, M.J.; Haselkorn, A.; DeMello, S. Remote patient management: Technology-enabled innovation and evolving business models for chronic disease care. Health Aff. 2009, 28, 126–135. [Google Scholar] [CrossRef]
- Hoare, T.; Timko, B.P.; Santamaria, J.; Goya, G.F.; Irusta, S.; Lau, S.; Stefanescu, C.F.; Lin, D.; Langer, R.; Kohane, D.S. Magnetically triggered nanocomposite membranes: A versatile platform for triggered drug release. Nano Lett. 2011, 11, 1395–1400. [Google Scholar] [CrossRef]
- Timko, B.P.; Arruebo, M.; Shankarappa, S.A.; McAlvin, J.B.; Okonkwo, O.S.; Mizrahi, B.; Stefanescu, C.F.; Gomez, L.; Zhu, J.; Zhu, A.; et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc. Natl. Acad. Sci. USA 2014, 111, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jo, M.-C.; Jeong, S.; Palanikumar, L.; Rotello, V.M.; Ryu, J.-H.; Park, M.-H. Externally controlled drug release using a gold nanorod contained composite membrane. Nanoscale 2016, 8, 11949–11955. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Yu, J.; Alsawat, M.; Kurkuri, M.D.; Santos, A.; Abell, A.D.; Losic, D. Photoswitchable Membranes Based on Peptide-Modified Nanoporous Anodic Alumina: Toward Smart Membranes for On-Demand Molecular Transport. Adv. Mater. 2015, 27, 3019–3024. [Google Scholar] [CrossRef]
- Ferrara, K.W. Driving delivery vehicles with ultrasound. Adv. Drug Deliver Rev. 2008, 60, 1097–1102. [Google Scholar] [CrossRef]
- Lee, S.H.; Piao, H.; Cho, Y.C.; Kim, S.N.; Choi, G.; Kim, C.R.; Ji, H.B.; Park, C.G.; Lee, C.; Shin, C.I.; et al. Implantable multireservoir device with stimulus-responsive membrane for on-demand and pulsatile delivery of growth hormone. Proc. Natl. Acad. Sci. USA 2019, 116, 11664–11672. [Google Scholar] [CrossRef] [PubMed]
- Farina, M. Remote magnetic switch off microgate for nanofluidic drug delivery implants. Biomed. Microdevices 2017, 19, 42. [Google Scholar] [CrossRef]
- Kim, S.; Ozalp, E.I.; Darwish, M.; Weldon, J.A. Electrically gated nanoporous membranes for smart molecular flow control. Nanoscale 2018, 10, 20740–20747. [Google Scholar] [CrossRef]
- Jeon, G.; Yang, S.Y.; Byun, J.; Kim, J.K. Electrically actuatable smart nanoporous membrane for pulsatile drug release. Nano Lett. 2011, 11, 1284–1288. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Xie, Z.; Diao, X.; Liu, Z.; Zhai, J. Highly Efficient Gating of Electrically Actuated Nanochannels for Pulsatile Drug Delivery Stemming from a Reversible Wettability Switch. Adv. Mater. 2018, 30, 1703323. [Google Scholar] [CrossRef]
- Kostaras, C.; Dellis, S.; Christoulaki, A.; Anastassopoulos, D.L.; Spiliopoulos, N.; Vradis, A.; Toprakcioglu, C.; Priftis, G.D. Flow through polydisperse pores in an anodic alumina membrane: A new method to measure the mean pore diameter. J. Appl. Phys. 2018, 124, 204307. [Google Scholar] [CrossRef]
- Grattoni, A.; Liu, X.; Ferrari, M. Gated Nanofluidic Valve For Active And Passive Electrosteric Control Of Molecular Transport, And Methods Of Fabrication. U.S. Patent 62/961,437, 15 January 2020. [Google Scholar]
- Lopez-Olivo, M.A.; Siddhanamatha, H.R.; Shea, B.; Tugwell, P.; Wells, G.A.; Suarez-Almazor, M.E. Methotrexate for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef]
- Cheki, M.; Moslehi, M.; Assadi, M. Marvelous applications of quantum dots. Eur Rev. Med. Pharmacol. Sci. 2013, 17, 1141–1148. [Google Scholar] [PubMed]
- Napoli, M.; Eijkel, J.C.; Pennathur, S. Nanofluidic technology for biomolecule applications: A critical review. Lab Chip 2010, 10, 957–985. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, T.; Lamanda, A.C.; Sin, M.L.; Gau, V.; Liao, J.C.; Wong, P.K. AC electrokinetics of physiological fluids for biomedical applications. J. Lab. Autom. 2015, 20, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Riahi, R.; Sin, M.L.; Zhang, S.; Wong, P.K. Electrokinetic focusing and separation of mammalian cells in conductive biological fluids. Analyst 2012, 137, 5215–5221. [Google Scholar] [CrossRef]
- Di Trani, N.; Silvestri, A.; Sizovs, A.; Wang, Y.; Erm, D.R.; Demarchi, D.; Liu, X.; Grattoni, A. Electrostatically gated nanofluidic membrane for ultra-low power controlled drug delivery. Lab Chip 2020, 20, 1562–1576. [Google Scholar] [CrossRef]
- Grattoni, A.; Gill, J.; Zabre, E.; Fine, D.; Hussain, F.; Ferrari, M. Device for rapid and agile measurement of diffusivity in micro- and nanochannels. Anal. Chem. 2011, 83, 3096–3103. [Google Scholar] [CrossRef]
- Voráčová, I.; Klepárník, K.; Lišková, M.; Foret, F. Determination of ζ-potential, charge, and number of organic ligands on the surface of water soluble quantum dots by capillary electrophoresis. Electrophoresis 2015, 36, 867–874. [Google Scholar] [CrossRef]
- Swain, M. Chemicalize.org. J. Chem. Inf. Model. 2012, 52, 613–615. [Google Scholar] [CrossRef]
- Haro-González, P.; Martínez-Maestro, L.; Martín, I.; García-Solé, J.; Jaque, D. High-Sensitivity Fluorescence Lifetime Thermal Sensing Based on CdTe Quantum Dots. Small 2012, 8, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Geninatti, T.; Small, E.; Grattoni, A. Robotic UV-Vis apparatus for long-term characterization of drug release from nanochannels. Meas. Sci. Technol. 2014, 25. [Google Scholar] [CrossRef]
- Scorrano, G.; Bruno, G.; Di Trani, N.; Ferrari, M.; Pimpinelli, A.; Grattoni, A. Gas Flow at the Ultra-nanoscale: Universal Predictive Model and Validation in Nanochannels of Ångstrom-Level Resolution. ACS Appl. Mater. Interfaces 2018, 10, 32233–32238. [Google Scholar] [CrossRef] [PubMed]
- Kotzar, G.; Freas, M.; Abel, P.; Fleischman, A.; Roy, S.; Zorman, C.; Moran, J.M.; Melzak, J. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials 2002, 23, 2737–2750. [Google Scholar] [CrossRef]
- Voskerician, G.; Shive, M.S.; Shawgo, R.S.; Von Recum, H.; Anderson, J.M.; Cima, M.J.; Langer, R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials 2003, 24, 1959–1967. [Google Scholar] [CrossRef]
- Oliveros, A.; Guiseppi-Elie, A.; Saddow, S.E. Silicon carbide: A versatile material for biosensor applications. Biomed. Microdevices 2013, 15, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Ghazanfari, L. Physics and Technology of Silicon Carbide Devices; Hijikata, Y., Ed.; InTech: Vienna, Austria, 2012. [Google Scholar]
- Cogan, S.F.; Edell, D.J.; Guzelian, A.A.; Ping Liu, Y.; Edell, R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J. Biomed. Mater. Res. 2003, 67, 856–867. [Google Scholar] [CrossRef]
- Zorman, C.A.; Eldridge, A.; Du, J.G.; Johnston, M.; Dubnisheva, A.; Manley, S.; Fissell, W.; Fleischman, A.; Roy, S. Amorphous Silicon Carbide as a Non-Biofouling Structural Material for Biomedical Microdevices; Materials Science Forum, Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2012; pp. 537–540. [Google Scholar] [CrossRef]
- Takami, Y.; Yamane, S.; Makinouchi, K.; Otsuka, G.; Glueck, J.; Benkowski, R.; Nosé, Y. Protein adsorption onto ceramic surfaces. J. Biomed. Mater. Res. 1998, 40, 24–30. [Google Scholar] [CrossRef]
- Ferrati, S.; Nicolov, E.; Zabre, E.; Geninatti, T.; Shirkey, B.A.; Hudson, L.; Hosali, S.; Crawley, M.; Khera, M.; Palapattu, G.; et al. The Nanochannel Delivery System for Constant Testosterone Replacement Therapy. J. Sex. Med. 2015, 12, 1375–1380. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Sizovs, A.; Shelton, K.A.; Momin, Z.; Bushman, L.R.; Chua, C.Y.X.; Nichols, J.E.; Hawkins, T.; Rooney, J.F.; Marzinke, M.A. Preventive efficacy of a tenofovir alafenamide fumarate nanofluidic implant in SHIV-challenged nonhuman primates. BioRxiv. 2020. [Google Scholar] [CrossRef]
- Bruno, G.; Canavese, G.; Liu, X.; Filgueira, C.S.; Sacco, A.; Demarchi, D.; Ferrari, M.; Grattoni, A. The active modulation of drug release by an ionic field effect transistor for an ultra-low power implantable nanofluidic system. Nanoscale 2016, 8, 18718–18725. [Google Scholar] [CrossRef] [PubMed]
- Ferrati, S.; Fine, D.; You, J.; De Rosa, E.; Hudson, L.; Zabre, E.; Hosali, S.; Zhang, L.; Hickman, C.; Sunder Bansal, S.; et al. Leveraging nanochannels for universal, zero-order drug delivery in vivo. J. Controlled Release 2013, 172, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Di Trani, N.; Jain, P.; Chua, C.Y.X.; Ho, J.S.; Bruno, G.; Susnjar, A.; Pons-Faudoa, F.P.; Sizovs, A.; Hood, R.L.; Smith, Z.W.; et al. Nanofluidic microsystem for sustained intraocular delivery of therapeutics. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.; Grattoni, A.; Hosali, S.; Ziemys, A.; De Rosa, E.; Gill, J.; Medema, R.; Hudson, L.; Kojic, M.; Milosevic, M.; et al. A robust nanofluidic membrane with tunable zero-order release for implantable dose specific drug delivery. Lab Chip 2010, 10, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Di Trani, N.; Silvestri, A.; Bruno, G.; Geninatti, T.; Chua, C.Y.X.; Gilbert, A.; Rizzo, G.; Filgueira, C.S.; Demarchi, D.; Grattoni, A. Remotely controlled nanofluidic implantable platform for tunable drug delivery. Lab.Chip 2019, 19, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.; Grattoni, A.; Zabre, E.; Hussein, F.; Ferrari, M.; Liu, X. A low-voltage electrokinetic nanochannel drug delivery system. Lab Chip 2011, 11, 2526–2534. [Google Scholar] [CrossRef]
- Prakash, S.; Conlisk, A.T. Field effect nanofluidics. Lab Chip 2016, 16, 3855–3865. [Google Scholar] [CrossRef]
- Plecis, A.; Tazid, J.; Pallandre, A.; Martinhon, P.; Deslouis, C.; Chen, Y.; Haghiri-Gosnet, A. Flow field effect transistors with polarisable interface for EOF tunable microfluidic separation devices. Lab Chip 2010, 10, 1245–1253. [Google Scholar] [CrossRef]
- Robertson, J. High dielectric constant gate oxides for metal oxide Si transistors. Rep. Prog. Phys. 2005, 69, 327–396. [Google Scholar] [CrossRef]
- Padovani, A.; Gao, D.Z.; Shluger, A.L.; Larcher, L. A microscopic mechanism of dielectric breakdown in SiO2 films: An insight from multi-scale modeling. J. Appl. Phys. 2017, 121, 155101. [Google Scholar] [CrossRef]
- Yao, J.; Zhong, L.; Natelson, D.; Tour, J.M. In situ imaging of the conducting filament in a silicon oxide resistive switch. Sci. Rep. 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.H.; Pey, K.L.; Tang, L.J.; Radhakrishnan, M.K.; Lin, W.H.; Palumbo, F.; Lombardo, S. Percolation path and dielectric-breakdown-induced-epitaxy evolution during ultrathin gate dielectric breakdown transient. Appl. Phys. Lett. 2003, 83, 2223–2225. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Sun, G.; Ma, X.; Gao, J.; Wu, W. Resistive switching characteristic of electrolyte-oxide-semiconductor structures. J. Semicond. 2017, 38, 8. [Google Scholar] [CrossRef]
- Daiguji, H.; Yang, P.; Majumdar, A. Ion transport in nanofluidic channels. Nano Lett. 2004, 4, 137–142. [Google Scholar] [CrossRef]
- Yossifon, G.; Mushenheim, P.; Chang, Y.-C.; Chang, H.-C. Nonlinear current-voltage characteristics of nanochannels. Phys. Rev. E 2009. [Google Scholar] [CrossRef]
- Grosjean, A.; Rezrazi, M.; Tachez, M. Study of the surface charge of silicon carbide (SIC) particles for electroless composite deposits: Nickel-SiC. Surf. Coat. Technol. 1997, 96, 300–304. [Google Scholar] [CrossRef]
- Karnik, R.; Fan, R.; Yue, M.; Li, D.; Yang, P.; Majumdar, A. Electrostatic control of ions and molecules in nanofluidic transistors. Nano lett. 2005, 5, 943–948. [Google Scholar] [CrossRef]
- Jiang, Z.; Stein, D. Electrofluidic Gating of a Chemically Reactive Surface. Langmuir 2010, 26, 8161–8173. [Google Scholar] [CrossRef]
- Schoch, R.B.; Han, J.; Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys. 2008, 80, 839–883. [Google Scholar] [CrossRef]
- Herbowski, L.; Gurgul, H.; Staron, W. Experimental determination of the Stern layer thickness at the interface of the human arachnoid membrane and the cerebrospinal fluid. Z. Med. Phys. 2009, 19, 189–192. [Google Scholar] [CrossRef]
- Lu, P.; Dai, Q.; Wu, L.; Liu, X. Structure and Capacitance of Electrical Double Layers at the Graphene–Ionic Liquid Interface. Appl. Sci. 2017, 7, 939. [Google Scholar] [CrossRef]
- Bruno, G.; Di Trani, N.; Hood, R.L.; Zabre, E.; Filgueira, C.S.; Canavese, G.; Jain, P.; Smith, Z.; Demarchi, D.; Hosali, S. Unexpected behaviors in molecular transport through size-controlled nanochannels down to the ultra-nanoscale. Nat. Commun. 2018, 9, 1682. [Google Scholar] [CrossRef] [PubMed]
- Di Trani, N.; Pimpinelli, A.; Grattoni, A. Finite-Size Charged Species Diffusion and pH Change in Nanochannels. ACS Appl. Mater. Interfaces 2020, 12, 12246–12255. [Google Scholar] [CrossRef] [PubMed]
- Behrens, S.H.; Grier, D.G. The charge of glass and silica surfaces. J Chem. Phys. 2001, 115, 6716–6721. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Liu, D.Y.; Lon, H.K.; Wang, Y.L.; DuBois, D.C.; Almon, R.R.; Jusko, W.J. Pharmacokinetics, pharmacodynamics and toxicities of methotrexate in healthy and collagen-induced arthritic rats. Biopharm. Drug Dispos. 2013, 34, 203–214. [Google Scholar] [CrossRef]
- Yasin, M.N.; Svirskis, D.; Seyfoddin, A.; Rupenthal, I.D. Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. J. Controlled Release 2014, 196, 208–221. [Google Scholar] [CrossRef]
- Langer, R.D. Efficacy, Safety, and Tolerability of Low-Dose Hormone Therapy in Managing Menopausal Symptoms. J Am. Board Fam. Med. 2009, 22, 563. [Google Scholar] [CrossRef]
- Charles, N.C.; Steiner, G.C. Ganciclovir intraocular implant. A clinicopathologic study. Ophthalmology 1996, 103, 416–421. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.-J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst 2013, 138, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-P.; Leong, K.W. Quantum dot-based theranostics. Nanoscale 2010, 2, 60–68. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.H.; Jin, Y.; Park, H.-J.; Yoon, H.; Jeon, S.; Cho, S.-W. Multiphoton luminescent graphene quantum dots for in vivo tracking of human adipose-derived stem cells. Nanoscale 2016, 8, 8512–8519. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, N.; Mehra, N.K.; Jain, K.; Jain, N.K. Pharmaceutical and biomedical applications of quantum dots. Artif. Cells Nanomed. Biotechnol. 2016, 44, 758–768. [Google Scholar] [CrossRef]
- Hsu, J.-M.; Tathireddy, P.; Rieth, L.; Normann, A.R.; Solzbacher, F. Characterization of a-SiCx: H thin films as an encapsulation material for integrated silicon based neural interface devices. Thin Solid Films 2007, 516, 34–41. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Trani, N.; Silvestri, A.; Wang, Y.; Demarchi, D.; Liu, X.; Grattoni, A. Silicon Nanofluidic Membrane for Electrostatic Control of Drugs and Analytes Elution. Pharmaceutics 2020, 12, 679. https://doi.org/10.3390/pharmaceutics12070679
Di Trani N, Silvestri A, Wang Y, Demarchi D, Liu X, Grattoni A. Silicon Nanofluidic Membrane for Electrostatic Control of Drugs and Analytes Elution. Pharmaceutics. 2020; 12(7):679. https://doi.org/10.3390/pharmaceutics12070679
Chicago/Turabian StyleDi Trani, Nicola, Antonia Silvestri, Yu Wang, Danilo Demarchi, Xuewu Liu, and Alessandro Grattoni. 2020. "Silicon Nanofluidic Membrane for Electrostatic Control of Drugs and Analytes Elution" Pharmaceutics 12, no. 7: 679. https://doi.org/10.3390/pharmaceutics12070679
APA StyleDi Trani, N., Silvestri, A., Wang, Y., Demarchi, D., Liu, X., & Grattoni, A. (2020). Silicon Nanofluidic Membrane for Electrostatic Control of Drugs and Analytes Elution. Pharmaceutics, 12(7), 679. https://doi.org/10.3390/pharmaceutics12070679