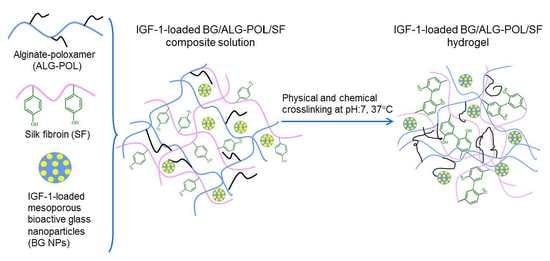
Controlled Delivery of Insulin-like Growth Factor-1 from Bioactive Glass-Incorporated Alginate-Poloxamer/Silk Fibroin Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
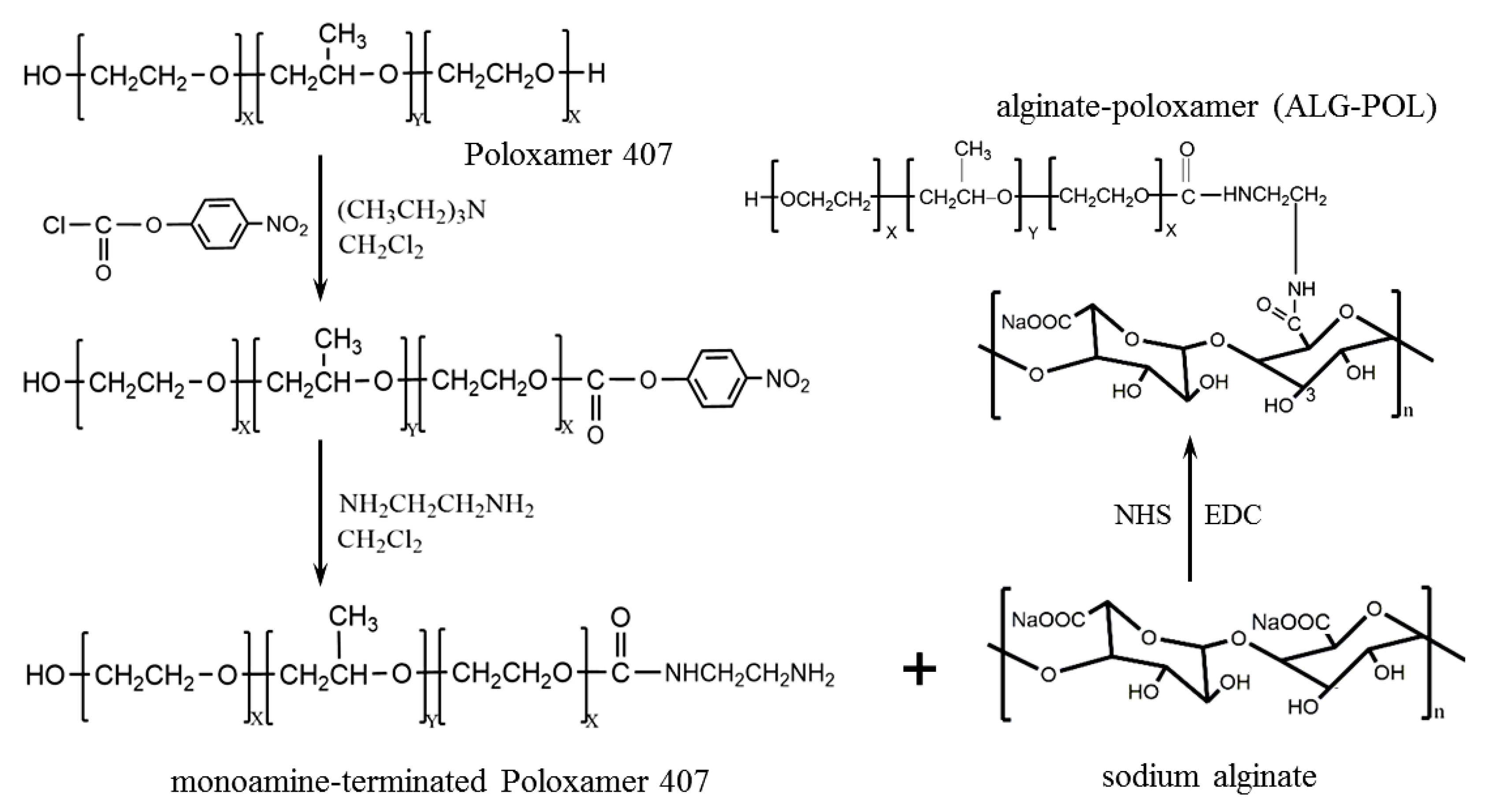
2.2. Synthesis of Alginate-Poloxamer Copolymers
2.3. Preparation of Bioactive Mesoporous Glasses
2.4. Preparation of Hydrogels
2.5. Characterization
2.6. Rheological Analysis
2.7. Release of IGF-1
2.8. Bioactivity of Released IGF-1
2.9. Statistical Analysis
3. Results and Discussions
3.1. Analysis of ALG–POL
3.2. Parameters for Mesoporous BG Nanoparticles
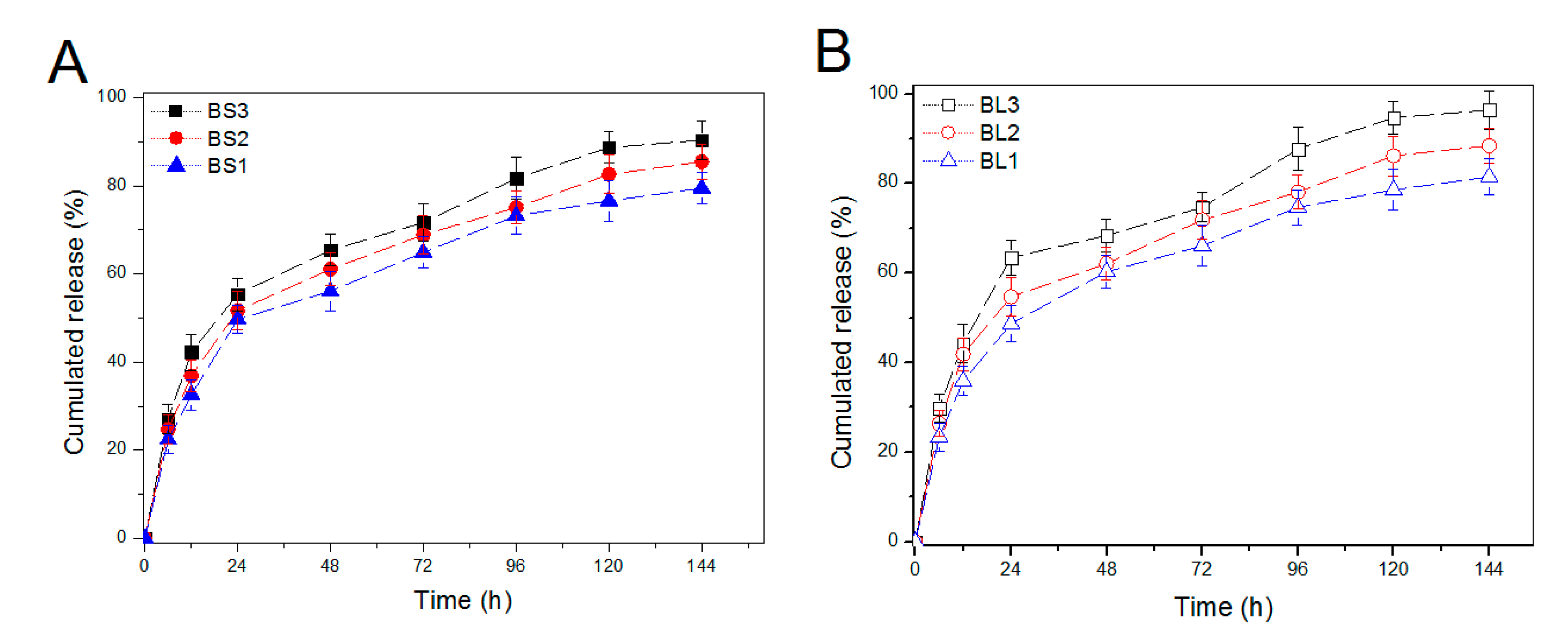
3.3. IGF-1 Release from BG Nanoparticles
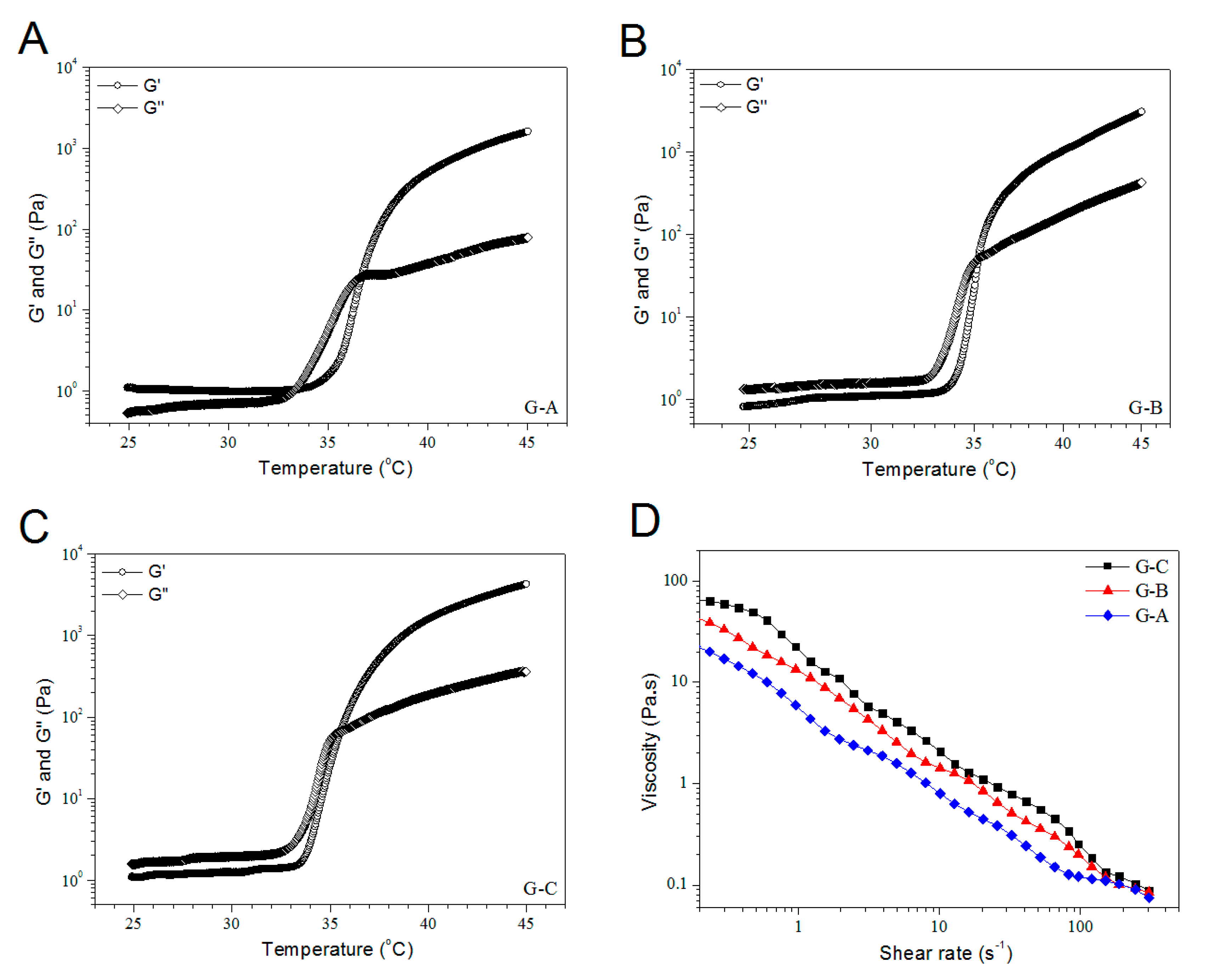
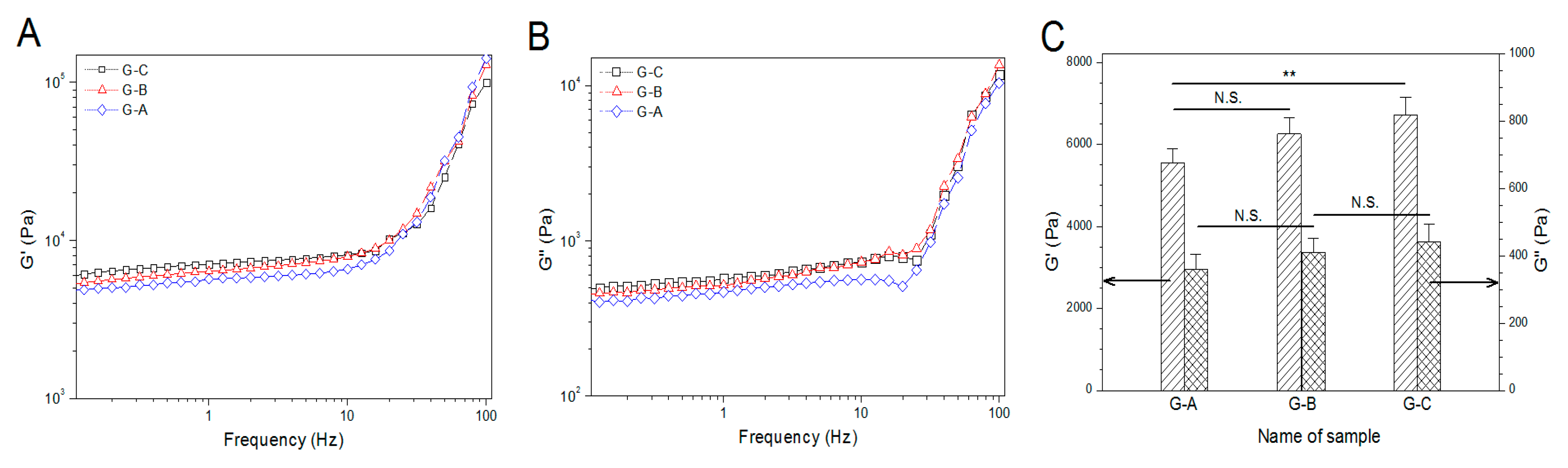
3.4. Rheological Properties of BG/ALG–POL/SF Gels without IGF-1 Load
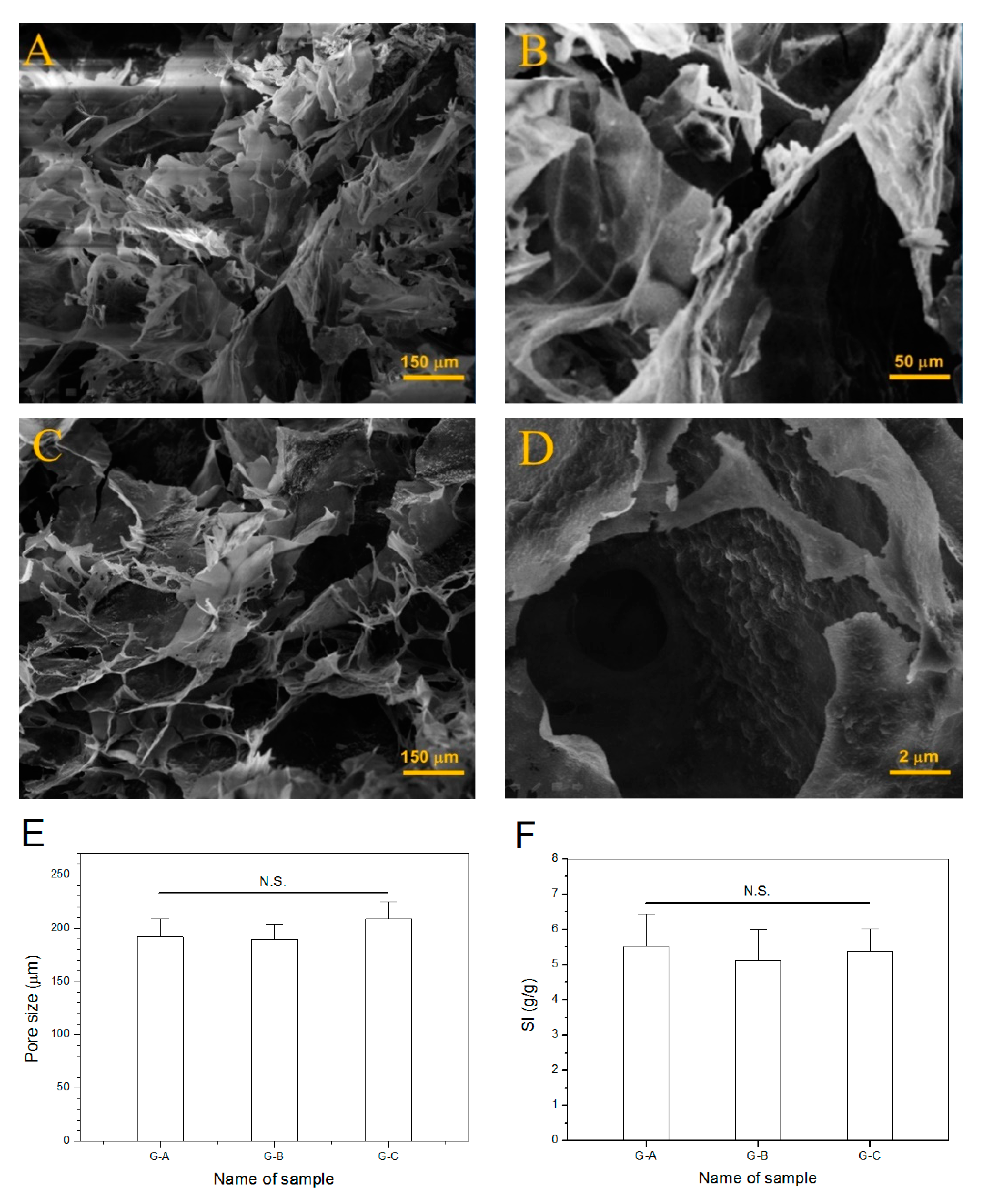
3.5. Morphological Analysis of Dry Gels
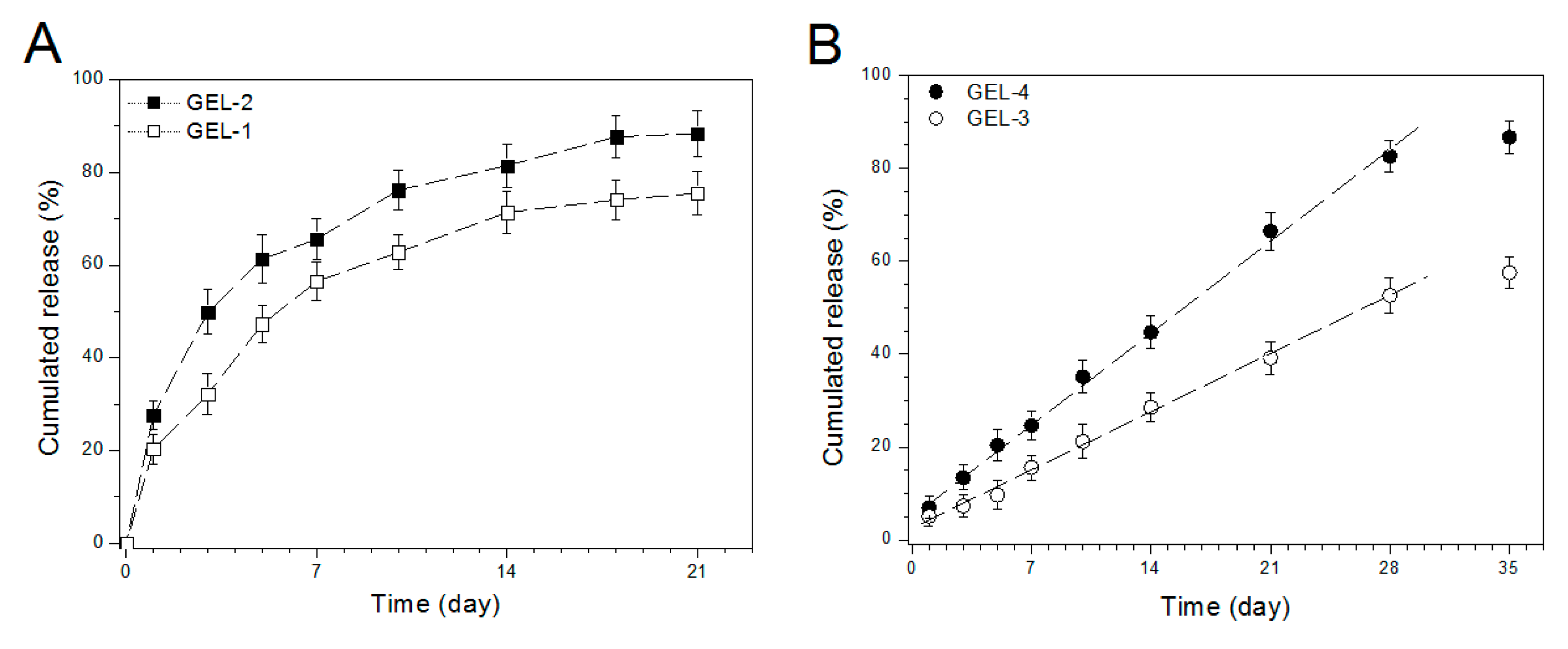
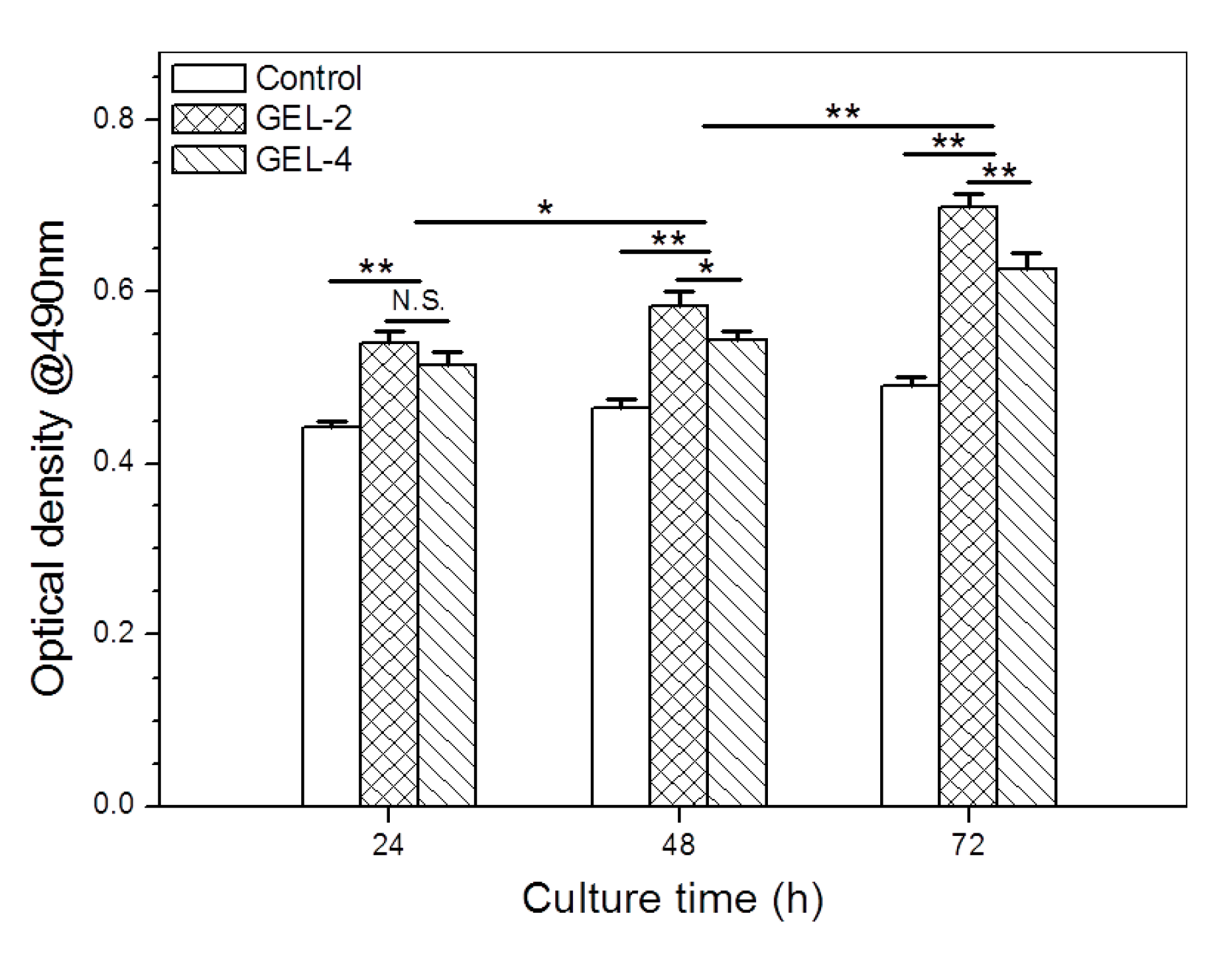
3.6. IGF-1 Release of Gels
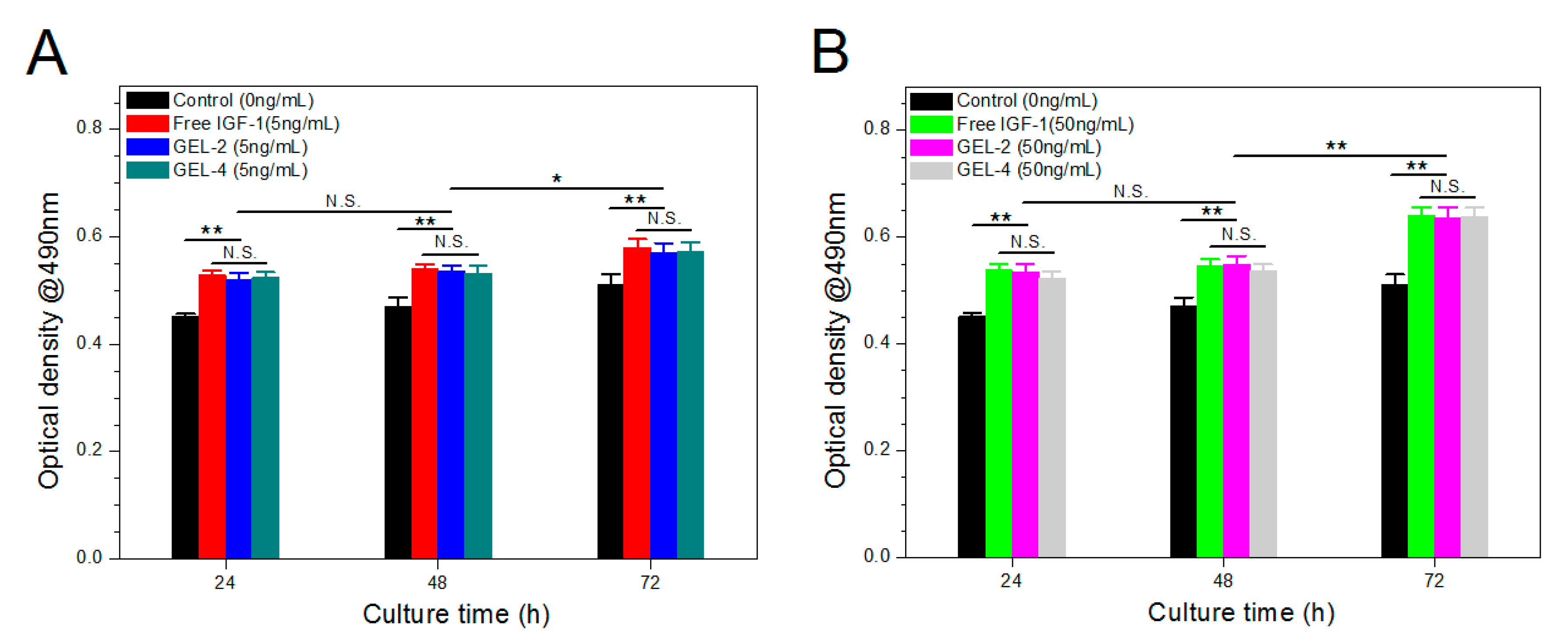
3.7. Bioactivity Assessment of IGF-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jarvelainen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Varshney, R.R.; Wang, D.A. Therapeutic cell delivery and fate control in hydrogels and hydrogel hybrids. Adv. Drug Delivery Rev. 2010, 62, 699–710. [Google Scholar] [CrossRef]
- Yang, J.A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Hermansson, E.; Schuster, E.; Lindgren, L.; Altskar, A.; Strom, A. Impact of solvent quality on the network strength and structure of alginate gels. Carbohydr. Polym. 2016, 144, 289–296. [Google Scholar] [CrossRef]
- Chen, C.C.; Fang, C.L.; Al-Suwayeh, S.A.; Leu, Y.L.; Fang, J.Y. Transdermal delivery of selegiline from alginate-pluronic composite thermogels. Int. J. Pharm. 2011, 415, 119–128. [Google Scholar] [CrossRef]
- Lin, H.R.; Sung, K.C.; Vong, W.J. In situ gelling of alginate/pluronic solutions for ophthalmic delivery of pilocarpine. Biomacromolecules 2004, 5, 2358–2365. [Google Scholar] [CrossRef]
- Naseri, N.; Deepa, B.; Mathew, A.P.; Oksman, K.; Girandon, L. Nanocellulose-based interpenetrating polymer network (IPN) hydrogels for cartilage applications. Biomacromolecules 2016, 17, 3714–3723. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Pei, B.; Wang, Z.; Hu, Q. Construction of ordered structure in polysaccharide hydrogel: A review. Carbohydr. Polym. 2019, 205, 225–235. [Google Scholar] [CrossRef]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L.; et al. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Laporte, L.D.; Tortelli, F.; Spedden, E.; Staii, C.; Atherton, T.J.; Hubbell, J.A.; Kaplan, D.L. Silk hydrogels as soft substrates for neural tissue engineering. Adv. Funct. Mater. 2013, 23, 5140–5149. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Li, M.; Li, J.; Wan, Y. Enhanced dual network hydrogels consisting of thiolated chitosan and silk fibroin for cartilage tissue engineering. Carbohydr. Polym. 2020, 227, 115335. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering—Part II: Challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng. Part B 2013, 19, 327–352. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocrine Rev. 2008, 29, 535–559. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Jayasuriya, A.C. IGF-1 release kinetics from chitosan microparticles fabricated using environmentally benign conditions. Mater. Sci. Eng. C 2014, 42, 506–516. [Google Scholar] [CrossRef]
- Massicotte, F.; Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P.; Lajeunesse, D. Modulation of insulin-like growth factor 1 levels in human osteoarthritic subchondral bone osteoblasts. Bone 2006, 38, 333–341. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Min, Q.; Yu, X.; Liu, J.; Wu, J.; Wan, Y. Chitosan-based hydrogels embedded with hyaluronic acid complex nanoparticles for controlled delivery of bone morphogenetic protein-2. Mar. Drugs 2019, 17, 365. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Pontremoli, C.; Fiorilli, S.; Laurano, R.; Ciardelli, G.; Vitale-Brovarone, C. Injectable thermosensitive formulation based on polyurethane hydrogel/mesoporous glasses for sustained co-delivery of functional ions and drugs. Pharmaceutics 2019, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Chung, T.W.; Kim, B.C.; Kim, M.K.; Lee, J.H.; Wee, W.R.; Cho, C.S. Release of ciprofloxacin from poloxamer-graft-hyaluronic acid hydrogels in vitro. Int. J. Pharm. 2003, 260, 83–91. [Google Scholar] [CrossRef]
- Hsu, S.H.; Leu, Y.L.; Hu, J.W.; Fang, J.Y. Physicochemical characterization and drug release of thermosensitive hydrogels composed of a hyaluronic acid/pluronic F127 graft. Chem. Pharm. Bull. 2009, 57, 453–458. [Google Scholar] [CrossRef]
- Kim, T.H.; Singh, R.K.; Kang, M.S.; Kim, J.H.; Kim, H.W. Gene delivery nanocarriers of bioactive glass with unique potential to load BMP2 plasmid DNA and to internalize into mesenchymal stem cells for osteogenesis and bone regeneration. Nanoscale 2016, 8, 8300–8311. [Google Scholar] [CrossRef]
- Mahapatra, C.; Singh, R.K.; Kim, J.J.; Patel, K.D.; Perez, R.A.; Jang, J.H.; Kim, H.W. Osteopromoting reservoir of stem cells: Bioactive mesoporous nanocarrier/collagen gel through slow-releasing FGF18 and the activated bmp signaling. ACS Appl. Mater. Interfaces 2016, 8, 27573–27584. [Google Scholar] [CrossRef]
- Kim, S.K.; Kwon, J.Y.; Nam, T.J. Involvement of ligand occupancy in Insulin-like growth factor-I (IGF-I) induced cell growth in osteoblast like MC3T3-E1 cells. BioFactors 2007, 29, 187–202. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Kassem, M.; Moller, M.K.; Eriksen, E.F. The effects of IGF-I and IGF-II on proliferation and differentiation of human osteoblasts and interactions with growth hormone. Eur. J. Clin. Invest. 1998, 28, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Machwate, M.; Zerath, E.; Holy, X.; Pastoureau, P.; Marie, P.J. Insulin-like growth factor-I increases trabecular bone formation and osteoblastic cell proliferation in unloaded rats. Endocrinology 1994, 134, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Hsu, S.H.; Leu, Y.L.; Hu, J.W. Delivery of cisplatin from pluronic co-polymer systems: Liposome inclusion and alginate coupling. J. Biomater. Sci. Polym. Ed. 2009, 20, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Shavlakadze, T.; Winn, N.; Rosenthal, N.; Grounds, M.D. Reconciling data from transgenic mice that overexpress IGF-I specifically in skeletal muscle. Growth Horm. IGF Res. 2005, 15, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Rinderknecht, E.; Humbel, R.E. The amino acid sequence of human insulin like growth factor I and its structural homology, with proinsulin. J. Biol. Chem. 1978, 253, 2769–2776. [Google Scholar] [PubMed]
- Clark, A.H.; Ross-Murphy, S.B. Structural and mechanical properties of biopolymer gel. Adv. Polym. Sci. 1987, 83, 60–192. [Google Scholar]
- Yoshida, R.; Kaneko, Y.; Sakai, K.; Okano, T.; Sakurai, Y.; Bae, Y.H.; Kim, S.W. Positive thermo sensitive pulsatile drug release using negative thermosensitive hydrogels. J. Control. Release 1994, 32, 97–102. [Google Scholar] [CrossRef]
- Dang, J.M.; Sun, D.D.N.; Shin-Ya, Y.; Sieber, A.N.; Kostuik, J.P.; Leong, K.W. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials 2006, 27, 406–418. [Google Scholar] [CrossRef]
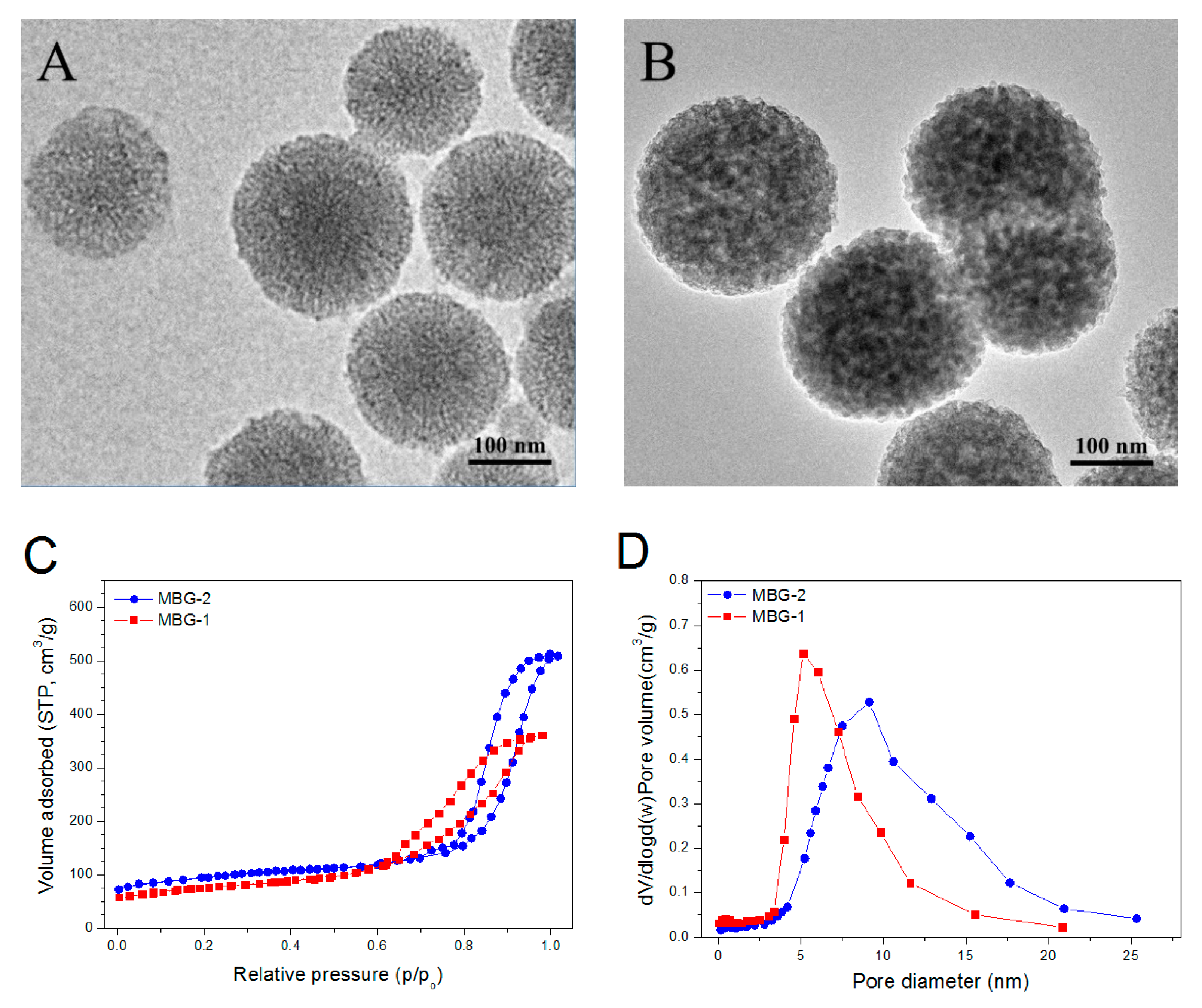
| Sample Name | Surface Area (m2/g) | Pore Volume (mL/g) | Pore Size (nm) | ζ-Potential (mV) | Particle Size (nm) | PDI |
|---|---|---|---|---|---|---|
| MBG-1 | 562.4 ± 10.2 | 0.49 ± 0.02 | 6.1 ± 0.13 | −14.9 ± 0.5 | 227.2 ± 10.3 | 0.11 |
| MBG-2 | 498.3 ± 9.4 | 0.61 ± 0.03 | 10.3 ± 0.18 | −17.1 ± 0.7 | 264.7 ± 12.1 | 0.13 |
| Sample Name | Feed Amount of IGF-1 (ng/mL) | Particle Size (nm) | PDI | ζ-Potential (mV) | LE (%) |
|---|---|---|---|---|---|
| BS1 (a) | 50 | 229.1 ± 11.4 | 0.14 | −10.3 ± 0.5 | 38.6 ± 1.7 |
| BS2 | 100 | 230.4 ± 12.6 | 0.18 | −9.1 ± 0.6 | 46.1 ± 2.1 |
| BS3 | 150 | 232.7 ± 10.8 | 0.19 | −8.6 ± 0.3 | 48.5 ± 2.5 |
| BL1 (b) | 50 | 265.3 ± 11.35 | 0.17 | −10.5 ± 0.5 | 45.9 ± 2.4 |
| BL2 | 100 | 268.2 ± 12.46 | 0.16 | −8.1 ± 0.4 | 57.2 ± 1.9 |
| BL3 | 150 | 267.9 ± 13.14 | 0.18 | −7.2 ± 0.6 | 61.4 ± 2.3 |
| Sample Name | SF (wt%) | ALG–POL (wt%) | Blank MBG-2 NPs (wt%) (b) | H2O2 (µL) (c) | HRP (µL) (d) | pH | Gelation Time (min) (d) | Ti (°C) |
|---|---|---|---|---|---|---|---|---|
| G-A | 7.0 | 5.0 | − | 10 | 10 | 7.06 ± 0.09 | 11.75 ± 0.96 | 36.1 ± 0.42 |
| G-B | 7.0 | 5.0 | 1.0 | 10 | 10 | 7.13 ± 0.06 | 10.5 ± 1.29 | 35.2 ± 0.57 |
| G-C | 7.0 | 5.0 | 2.0 | 10 | 10 | 7.11 ± 0.07 | 9.75 ± 0.96 | 34.6 ± 0.49 |
| Sample Name | SF (wt%) | ALG–POL (wt%) | IGF-1-Loaded MBG-2 NPs (wt%) (b) | H2O2 (µL) (c) | HRP (µL) (d) | IGF-1 Content in Gel (ng/mL) |
|---|---|---|---|---|---|---|
| GEL-1 (a) | 7.0 | 5.0 | − | 10 | 10 | 154.2 ± 11.27 |
| GEL-2 | 7.0 | 5.0 | − | 10 | 10 | 512.9 ± 16.35 |
| GEL-3 | 7.0 | 5.0 | 1.0 | 10 | 10 | 160.3 ± 10.84 |
| GEL-4 | 7.2 | 5.0 | 2.0 | 10 | 10 | 520.7 ± 19.52 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Q.; Yu, X.; Liu, J.; Zhang, Y.; Wan, Y.; Wu, J. Controlled Delivery of Insulin-like Growth Factor-1 from Bioactive Glass-Incorporated Alginate-Poloxamer/Silk Fibroin Hydrogels. Pharmaceutics 2020, 12, 574. https://doi.org/10.3390/pharmaceutics12060574
Min Q, Yu X, Liu J, Zhang Y, Wan Y, Wu J. Controlled Delivery of Insulin-like Growth Factor-1 from Bioactive Glass-Incorporated Alginate-Poloxamer/Silk Fibroin Hydrogels. Pharmaceutics. 2020; 12(6):574. https://doi.org/10.3390/pharmaceutics12060574
Chicago/Turabian StyleMin, Qing, Xiaofeng Yu, Jiaoyan Liu, Yuchen Zhang, Ying Wan, and Jiliang Wu. 2020. "Controlled Delivery of Insulin-like Growth Factor-1 from Bioactive Glass-Incorporated Alginate-Poloxamer/Silk Fibroin Hydrogels" Pharmaceutics 12, no. 6: 574. https://doi.org/10.3390/pharmaceutics12060574
APA StyleMin, Q., Yu, X., Liu, J., Zhang, Y., Wan, Y., & Wu, J. (2020). Controlled Delivery of Insulin-like Growth Factor-1 from Bioactive Glass-Incorporated Alginate-Poloxamer/Silk Fibroin Hydrogels. Pharmaceutics, 12(6), 574. https://doi.org/10.3390/pharmaceutics12060574