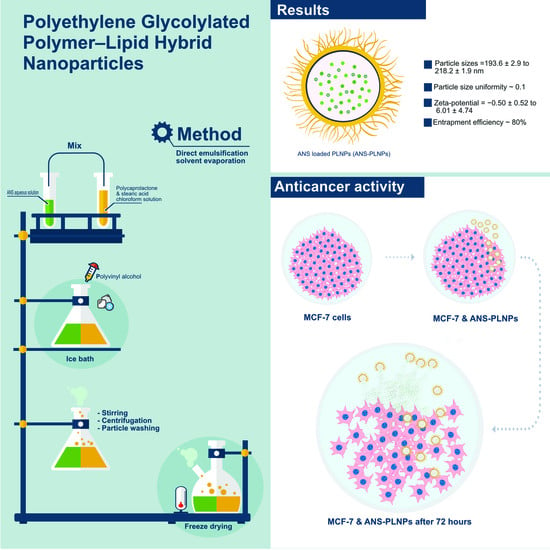
Optimized Polyethylene Glycolylated Polymer–Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
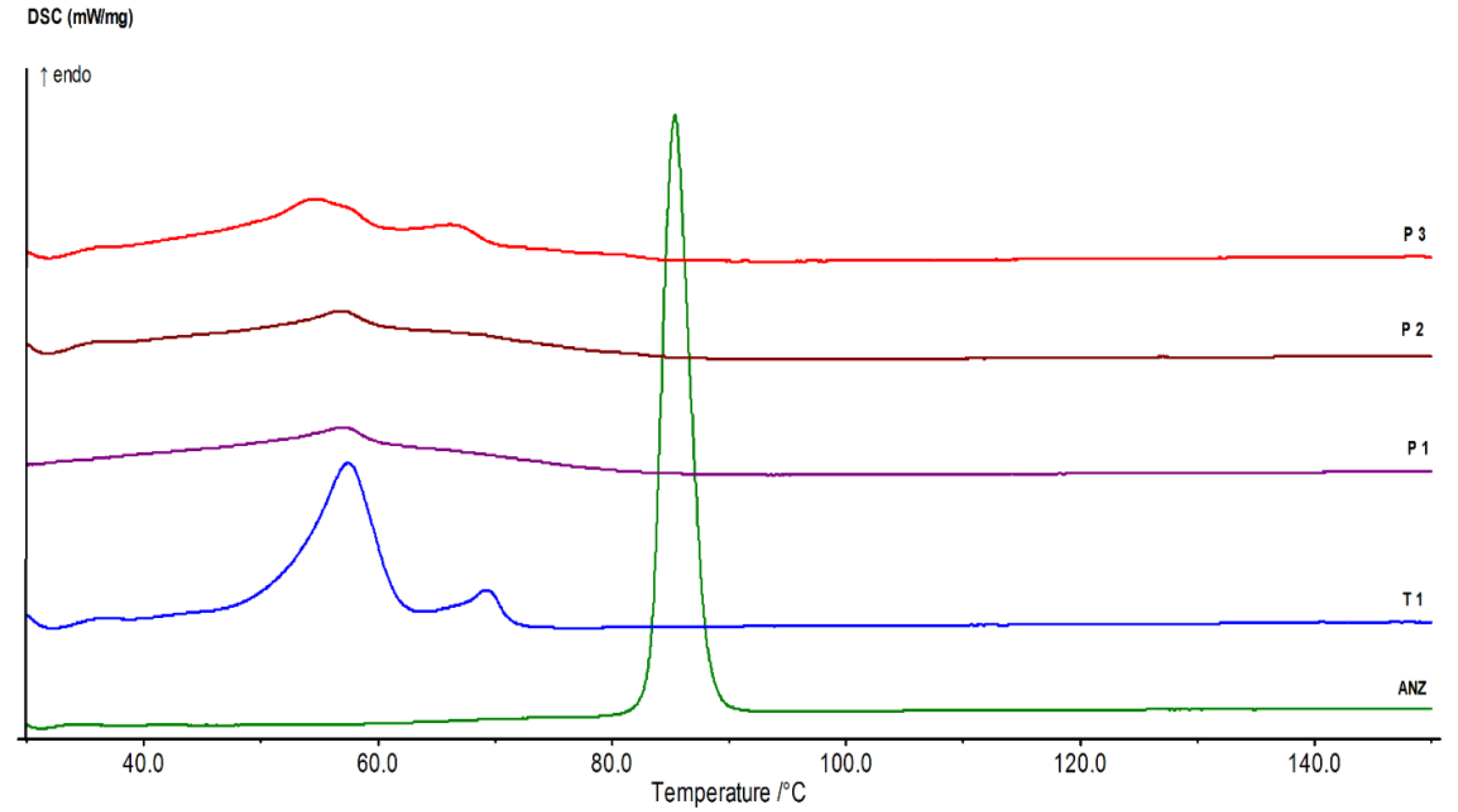
2.2. Identification of ANS Pure Drug
2.3. Preparation of ANS Polymeric Nanoparticles
2.4. Drug Analysis by Ultraviolet (UV) Spectrophotometry Method
2.5. Evaluation of the Prepared ANS Nanoparticle Formulations
2.5.1. Measurement of Particle Size and Polydispersity Index
2.5.2. Measurement of Zeta-Potential
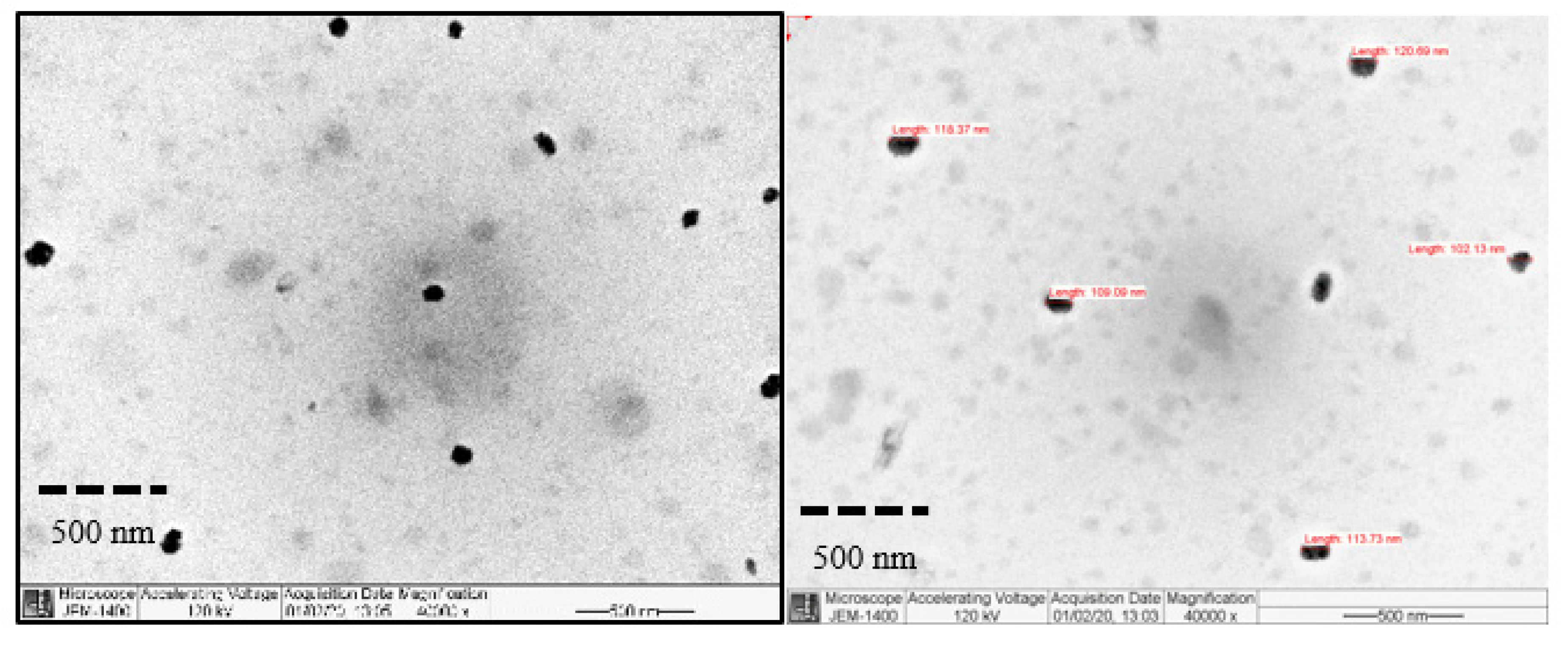
2.5.3. Particles Morphology
2.5.4. Measurement of Drug Entrapment Efficiency and Drug Loading
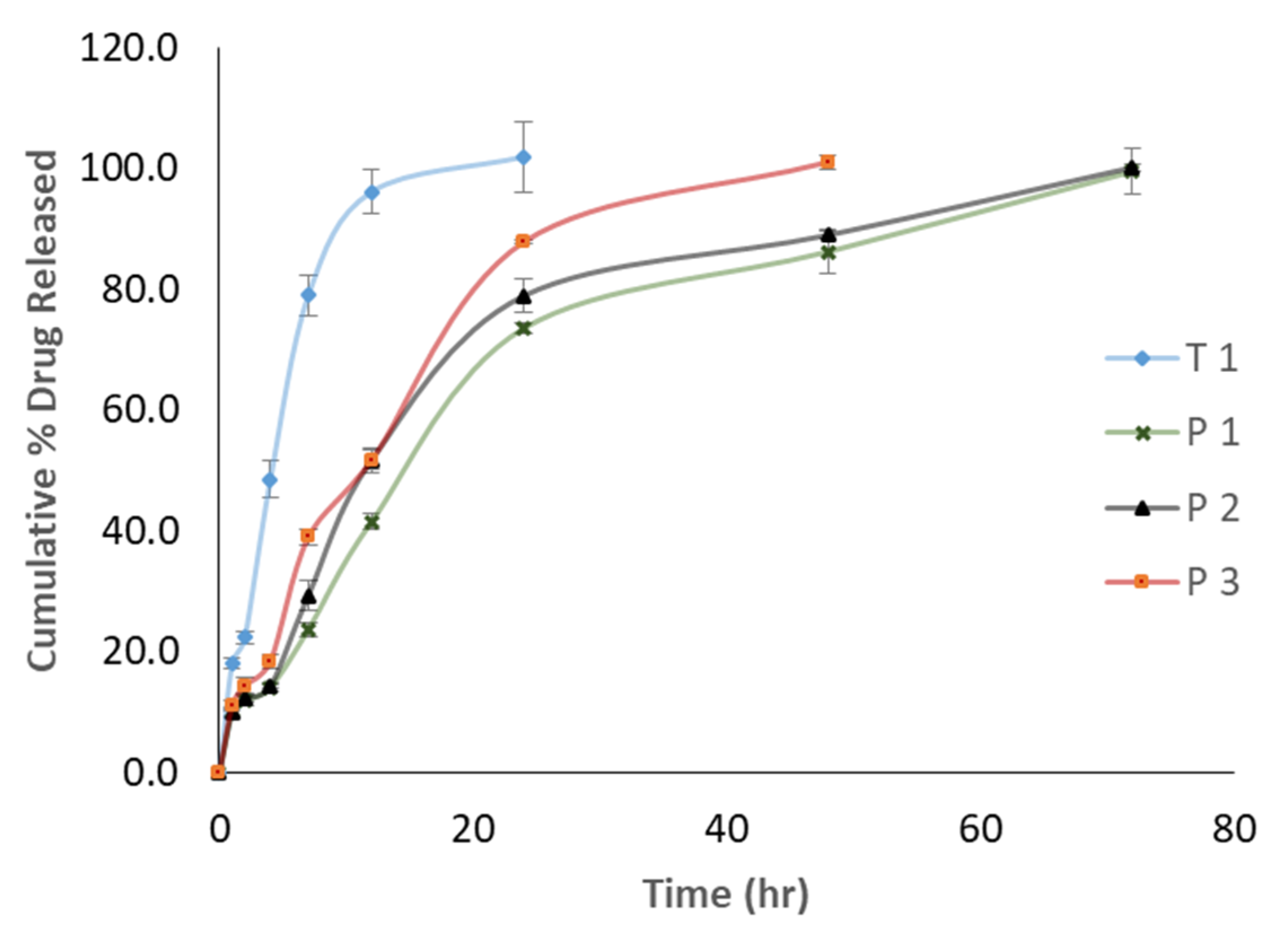
2.6. ANS Release Study
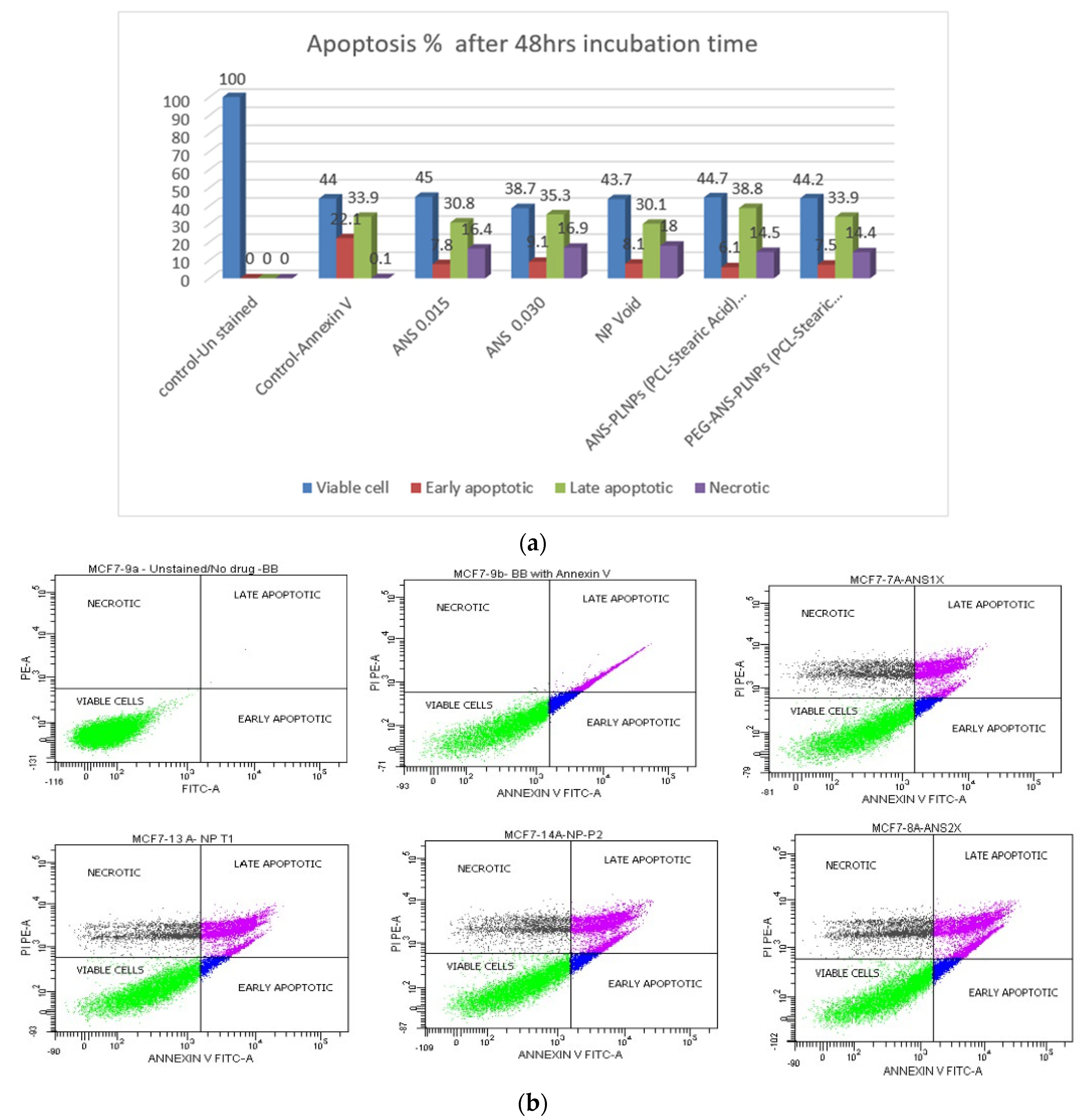
2.7. Evaluation of the Anticancer Activity Using Flow Cytometry
2.8. Fluorescence High Content Imaging
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saggu, S.; Rehman, H.; Abbas, Z.K.; Ansari, A.A. Recent incidence and descriptive epidemiological survey of breast cancer in Saudi Arabia. Saudi Med. J. 2015, 36, 1176–1180. [Google Scholar] [CrossRef]
- Dhingra, K. Antiestrogens—Tamoxifen, SERMs and beyond. Investig. New Drugs 1999, 17, 285–311. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F. Chemoprevention for High-Risk Women: Tamoxifen and Beyond. Breast J. 2001, 7, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Mikelman, S.; Mardirossian, N.; Gnegy, M.E. Tamoxifen and amphetamine abuse: Are there therapeutic possibilities? J. Chem. Neuroanat. 2017, 83–84, 50–58. [Google Scholar] [CrossRef]
- Shagufta, N.; Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Margolese, R.G.; Cecchini, R.S.; Julian, T.B.; Ganz, P.A.; Costantino, J.P.; Vallow, L.A.; Albain, K.S.; Whitworth, P.W.; Cianfrocca, M.E.; Brufsky, A.M.; et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): A randomised, double-blind, phase 3 clinical trial. Lancet 2016, 387, 849–856. [Google Scholar] [CrossRef] [Green Version]
- Forbes, J.F.; Sestak, I.; Howell, A.; Bonanni, B.; Bundred, N.; Levy, C.; von Minckwitz, G.; Eiermann, W.; Neven, P.; Stierer, M.; et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): A double-blind, randomised controlled trial. Lancet 2016, 387, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Buzdar, A.U. Anastrozole (ArimidexTM)—An aromatase inhibitor for the adjuvant setting? Br. J. Cancer 2001, 85, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.S.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A.; Hussain, M.D.; Khan, M.A.; Habib, M.J. Formulation of anastrozole microparticles as biodegradable anticancer drug carriers. Aaps Pharmscitech 2006, 7, E38–E46. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, K.; Yang, H. Encapsulation and Extended Release of Anti-Cancer Anastrozole by Stealth Nanoparticles. Drug Deliv. 2008, 15, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Sumer, B.; Gao, J. Theranostic nanomedicine for cancer. Nanomedicine 2008, 3, 137–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabanov, A.V.; Gendelman, H.E. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog. Polym. Sci. 2007, 32, 1054–1082. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Au, J.L.-S.; Jang, S.H.; Zheng, J.; Chen, C.-T.; Song, S.; Hu, L.; Wientjes, M.G. Determinants of drug delivery and transport to solid tumors. J. Control. Release 2001, 74, 31–46. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Cattel, L.; Ceruti, M.; Dosio, F. From conventional to stealth liposomes: A new frontier in cancer chemotherapy. Tumori 2003, 89, 237–249. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef] [Green Version]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied. Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, E.; Barattin, M.; Bersani, S.; Shubber, S.; Uddin, S.; van der Walle, C.F.; Caliceti, P.; Salmaso, S. A novel combined strategy for the physical PEGylation of polypeptides. J. Control. Release 2016, 226, 35–46. [Google Scholar] [CrossRef]
- Alyafee, Y.A.; Alaamery, M.; Bawazeer, S.; Almutairi, M.S.; Alghamdi, B.; Alomran, N.; Sheereen, A.; Daghestani, M.; Massadeh, S. Preparation of anastrozole loaded PEG-PLA nanoparticles: Evaluation of apoptotic response of breast cancer cell lines. Int. J. Nanomed. 2017, 13, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Luciani, A.; Coccoli, V.; Orsi, S.; Ambrosio, L.; Netti, P.A. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials 2008, 29, 4800–4807. [Google Scholar] [CrossRef]
- Huang, H.; Oizumi, S.; Kojima, N.; Niino, T.; Sakai, Y. Avidin-biotin binding-based cell seeding and perfusion culture of liver-derived cells in a porous scaffold with a three-dimensional interconnected flow-channel network. Biomaterials 2007, 28, 3815–3823. [Google Scholar] [CrossRef]
- Massadeh, S.; Alaamery, M.; Al-Qatanani, S.; Alarifi, S.; Bawazeer, S.; Alyafee, Y. Synthesis of protein-coated biocompatible methotrexate-loaded PLA-PEG-PLA nanoparticles for breast cancer treatment. Nano Rev. Exp. 2016, 7, 31996. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Yang, C.; Liu, J.; Wang, W.; Guo, S.; Li, J.; Sun, Y.; Dong, H.; Deng, L.; Zhang, J.; et al. Improving the oral delivery efficiency of anticancer drugs by chitosan coated polycaprolactone-grafted hyaluronic acid nanoparticles. J. Mater. Chem. B 2014, 2, 4021–4033. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.E.; Badran, M.M.; Kumar, A.; Rishi, A.K.; Yassin, A.E. Di-Block PLCL and Tri-Block PLCLG Matrix Polymeric Nanoparticles Enhanced the Anticancer Activity of Loaded 5-Fluorouracil. IEEE Trans. Nanobioscience 2016, 15, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.M.; Alomrani, A.H.; Harisa, G.I.; Ashour, A.E.; Kumar, A.; Yassin, A.E. Novel docetaxel chitosan-coated PLGA/PCL nanoparticles with magnified cytotoxicity and bioavailability. Biomed. Pharmacother. 2018, 106, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.E.; Badran, M.; Kumar, A.; Hussain, T.; Alsarra, I.A.; Yassin, A.E.B. Physical PEGylation Enhances the Cytotoxicity of 5-Fluorouracil-Loaded PLGA and PCL Nanoparticles. Available online: https://www.dovepress.com/physical-pegylation-enhances-the-cytotoxicity-of-5-fluorouracil-loaded-peer-reviewed-article-IJN (accessed on 13 April 2020).
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine 2013, 9, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-Assembled Lipid−Polymer Hybrid Nanoparticles: A Robust Drug Delivery Platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.; Cheng, J.; Shuhendler, A.; Rauth, A.; Wu, X.Y. A novel nanoparticle formulation overcomes multiple types of membrane efflux pumps in human breast cancer cells. Drug Deliv. Transl. Res. 2012, 2, 95–105. [Google Scholar] [CrossRef]
- Blume, G.; Cevc, G. Liposomes for the sustained drug release in vivo. Biochim. Et Biophys. Acta Biomembr. 1990, 1029, 91–97. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Srirangarajan, S.; Agnihotri, S.A.; Patil, S.A.; Ravindra, S.; Setty, S.B.; Aminabhavi, T.M. Development and evaluation of novel biodegradable microspheres based on poly(d,l-lactide-co-glycolide) and poly(epsilon-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: In vitro and in vivo studies. J. Control. Release 2007, 119, 59–68. [Google Scholar] [CrossRef]
- Carrio, A.; Schwach, G.; Coudane, J.; Vert, M. Preparation and degradation of surfactant-free PLAGA microspheres. J. Control. Release 1995, 37, 113–121. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Cho, C.-S.; Kim, S.-H.; Ko, K.-S.; Kim, S.-I.; Shim, Y.-H.; Nah, J.-W. Preparation of poly(DL-lactide-co-glycolide) nanoparticles without surfactant. J. Appl. Polym. Sci. 2001, 80, 2228–2236. [Google Scholar] [CrossRef]
- Yang, M.; Lai, S.K.; Yu, T.; Wang, Y.-Y.; Happe, C.; Zhong, W.; Zhang, M.; Anonuevo, A.; Fridley, C.; Hung, A.; et al. Nanoparticle penetration of human cervicovaginal mucus: The effect of polyvinyl alcohol. J. Control. Release 2014, 192, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-Gómez, G.; Piñón-Segundo, E.; Mendoza-Muñoz, N.; Zambrano-Zaragoza, M.L.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Approaches in Polymeric Nanoparticles for Vaginal Drug Delivery: A Review of the State of the Art. Int. J. Mol. Sci. 2018, 19, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miteva, M.; Kirkbride, K.C.; Kilchrist, K.V.; Werfel, T.A.; Li, H.; Nelson, C.E.; Gupta, M.K.; Giorgio, T.D.; Duvall, C.L. Tuning PEGylation of mixed micelles to overcome intracellular and systemic siRNA delivery barriers. Biomaterials 2015, 38, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado Cruz, R.; Santos-Martinez, M.J.; Tajber, L. Impact of polyethylene glycol polymers on the physicochemical properties and mucoadhesivity of itraconazole nanoparticles. Eur. J. Pharm. Biopharm. 2019, 144, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, M.G.; Sun, X.M.; Snowden, R.T.; Davies, R.; Fearnhead, H.; Cohen, G.M. Increased membrane permeability of apoptotic thymocytes: A flow cytometric study. Cytometry 1993, 14, 595–602. [Google Scholar] [CrossRef]
- Andreau, K.; Castedo, M.; Perfettini, J.-L.; Roumier, T.; Pichart, E.; Souquere, S.; Vivet, S.; Larochette, N.; Kroemer, G. Preapoptotic Chromatin Condensation Upstream of the Mitochondrial Checkpoint. J. Biol. Chem. 2004, 279, 55937–55945. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Vargas, J.M.; Ruiz-Magaña, M.J.; Ruiz-Ruiz, C.; Majuelos-Melguizo, J.; Peralta-Leal, A.; Rodríguez, M.I.; Muñoz-Gámez, J.A.; de Almodóvar, M.R.; Siles, E.; Rivas, A.L.; et al. ROS-induced DNA damage and PARP-1 are required for optimal induction of starvation-induced autophagy. Cell Res. 2012, 22, 1181–1198. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Li, C.; Qi, W.; Meng, X.; Tian, R.; Qi, Y.; Yang, W.; Li, J. Synthesis, cytotoxicity and antitumour mechanism investigations of polyoxometalate doped silica nanospheres on breast cancer MCF-7 cells. PLoS ONE 2017, 12, e0181018. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Formulation | PEG 6000 (mg) | PCL (mg) | SA (mg) | ANS (mg) |
|---|---|---|---|---|
| T1 | - | 20 | 5 | 1 |
| P1 | 10 | 40 | 5 | 1 |
| P2 | 7.5 | 30 | 5 | 1 |
| P3 | 5 | 20 | 5 | 1 |
| Formulation ID | Mean Particle Size (nm) | Polydispersity Index | Zeta-Potential | %EE |
|---|---|---|---|---|
| T1 | 218.16 ± 1.91 | 0.11 ± 0.02 | −0.50 ± 0.52 | 79.7 |
| P1 | 202.01 ± 2.02 | 0.10 ± 0.02 | 2.56 ± 6.78 | 80.1 |
| P2 | 205.96 ± 4.04 | 0.11 ± 0.02 | 1.69 ± 3.68 | 80.4 |
| P3 | 193.60 ± 2.89 | 0.12 ± 0.01 | 6.01 ± 4.74 | 81.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massadeh, S.; Omer, M.E.; Alterawi, A.; Ali, R.; Alanazi, F.H.; Almutairi, F.; Almotairi, W.; Alobaidi, F.F.; Alhelal, K.; Almutairi, M.S.; et al. Optimized Polyethylene Glycolylated Polymer–Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment. Pharmaceutics 2020, 12, 666. https://doi.org/10.3390/pharmaceutics12070666
Massadeh S, Omer ME, Alterawi A, Ali R, Alanazi FH, Almutairi F, Almotairi W, Alobaidi FF, Alhelal K, Almutairi MS, et al. Optimized Polyethylene Glycolylated Polymer–Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment. Pharmaceutics. 2020; 12(7):666. https://doi.org/10.3390/pharmaceutics12070666
Chicago/Turabian StyleMassadeh, Salam, Mustafa E Omer, Asmaa Alterawi, Rizwan Ali, Fayez H Alanazi, Fares Almutairi, Wejdan Almotairi, Faris F Alobaidi, Khulud Alhelal, Mansour S Almutairi, and et al. 2020. "Optimized Polyethylene Glycolylated Polymer–Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment" Pharmaceutics 12, no. 7: 666. https://doi.org/10.3390/pharmaceutics12070666
APA StyleMassadeh, S., Omer, M. E., Alterawi, A., Ali, R., Alanazi, F. H., Almutairi, F., Almotairi, W., Alobaidi, F. F., Alhelal, K., Almutairi, M. S., Almalik, A., Obaidat, A. A., Alaamery, M., & Yassin, A. E. (2020). Optimized Polyethylene Glycolylated Polymer–Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment. Pharmaceutics, 12(7), 666. https://doi.org/10.3390/pharmaceutics12070666