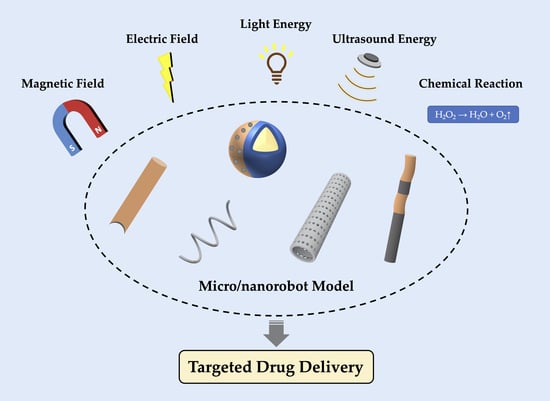
Micro/Nanorobot: A Promising Targeted Drug Delivery System
Abstract
1. Introduction
2. Micro/Nanorobots with Autonomous Movement Ability
2.1. Exogenous Power Driven Micro/Nanorobots
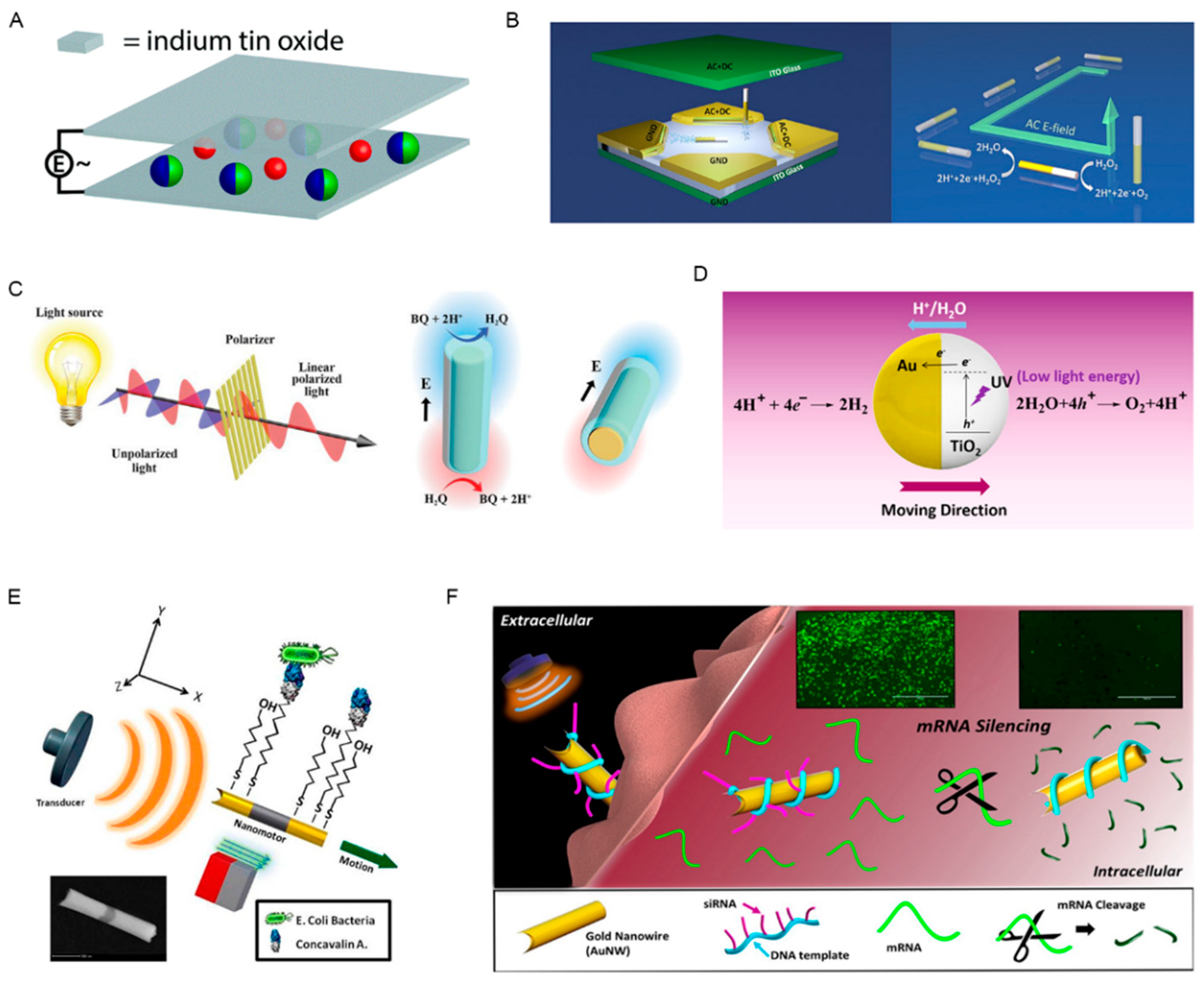
2.1.1. Magnetic Field Propelled Micro/Nanorobots
2.1.2. Electric Field Propelled Micro/Nanorobots
2.1.3. Light Energy Propelled Micro/Nanorobots
2.1.4. Ultrasound Energy Propelled Micro/Nanorobots
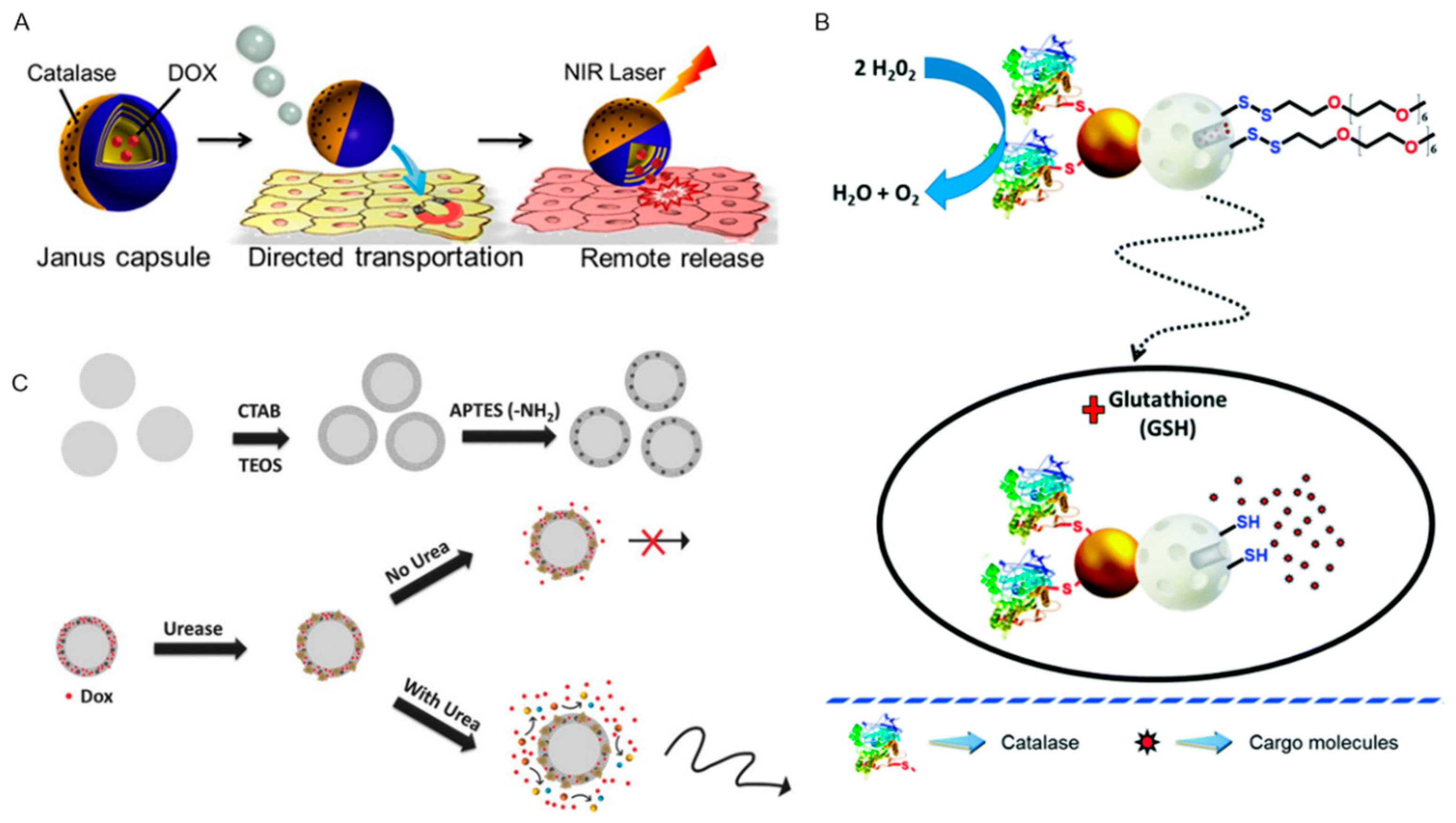
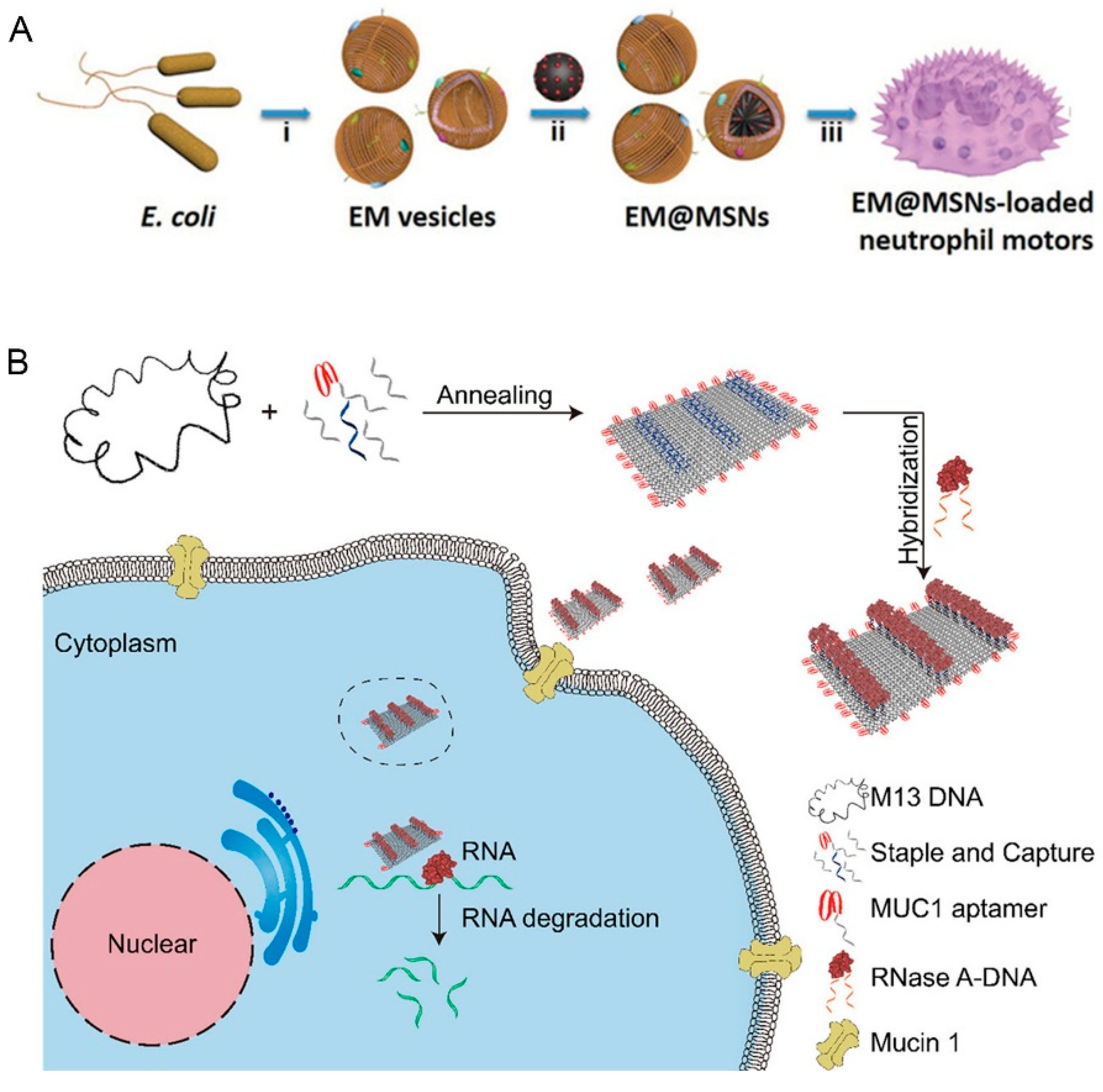
2.2. Endogenous Power Driven Micro/Nanorobots
3. Other Types of Micro/Nanorobots
4. Application of Drug-Loaded Micro/Nanorobots In Vivo
5. Summary and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Klochkov, S.G.; Neganova, M.E.; Nikolenko, V.N.; Chen, K.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Implications of nanotechnology for the treatment of cancer: Recent advances. Semin. Cancer Boil. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Pandey, C.; Sharma, N.; Kamal, M.A.; Sayeed, U.; Akhtar, S. Cancer Nanotechnology-An Excursion on Drug Delivery Systems. Anti-Cancer Agents Med. Chem. 2019, 18, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shi, Y.; Wang, L.; Kong, S.; Du, J.; Lin, G.; Feng, Y. Advances in mathematical models of the active targeting of tumor cells by functional nanoparticles. Comput. Methods Programs Biomed. 2020, 184, 105106. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, M. New Avenues for Nanoparticle-Related Therapies. Nanoscale Res. Lett. 2018, 13, 136. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconj. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Nair, P. Delivering Combination Chemotherapies and Targeting Oncogenic Pathways via Polymeric Drug Delivery Systems. Polymers 2019, 11, 630. [Google Scholar] [CrossRef]
- Nogueira-Librelotto, D.R.; Codevilla, C.F.; Farooqi, A.; Rolim, C.M.B. Transferrin-Conjugated Nanocarriers as Active-Targeted Drug Delivery Platforms for Cancer Therapy. Curr. Pharm. Des. 2017, 23, 454–466. [Google Scholar] [CrossRef]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Goushbolagh, N.A. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Ruenraroengsak, P.; Cook, J.M.; Florence, A.T. Nanosystem drug targeting: Facing up to complex realities. J. Control. Release 2010, 141, 265–276. [Google Scholar] [CrossRef]
- Kwon, I.K.; Lee, S.C.; Han, B.; Park, K. Analysis on the current status of targeted drug delivery to tumors. J. Control. Release 2012, 164, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P.; Cooper, S.R.; Hardee, G.E. Folate-Liposome-Mediated Antisense Oligodeoxynucleotide Targeting to Cancer Cells: Evaluation in Vitro and in Vivo. Bioconj. Chem. 2003, 14, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody Targeting of Long-Circulating Lipidic Nanoparticles Does Not Increase Tumor Localization but Does Increase Internalization in Animal Models. Cancer Res. 2006, 66, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; De Avila, B.E.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s plenty of room at the bottom. Resonance 2011, 16, 890–905. [Google Scholar] [CrossRef]
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metabolism 2017, 69, S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Grifantini, K. The State of Nanorobotics in Medicine. IEEE Pulse 2019, 10, 13–17. [Google Scholar] [CrossRef]
- Chen, X.Z.; Jang, B.; Ahmed, D.; Hu, C.; De Marco, C.; Hoop, M.; Mushtaq, F.; Nelson, B.J.; Pané, S. Small-Scale Machines Driven by External Power Sources. Adv. Mater. 2018, 30, e1705061. [Google Scholar] [CrossRef]
- Ma, X.; Sánchez, S. Self-propelling micro-nanorobots: Challenges and future perspectives in nanomedicine. Nanomedicine 2017, 12, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Z.; Hoop, M.; Mushtaq, F.; Siringil, E.; Hu, C.; Nelson, B.J.; Pane, S. Recent developments in magnetically driven micro- and nanorobots. Appl. Mater. Today 2017, 9, 37–48. [Google Scholar] [CrossRef]
- Guo, J.; Gallegos, J.J.; Tom, A.R.; Fan, D.E. Electric-Field-Guided Precision Manipulation of Catalytic Nanomotors for Cargo Delivery and Powering Nanoelectromechanical Devices. ACS Nano 2018, 12, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, Z.; Zheng, J.; Zhan, X.; Tang, J. Light-Driven Micro/Nanomotor for Promising Biomedical Tools: Principle, Challenge, and Prospect. Accounts Chem. Res. 2018, 51, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shen, H.; Zhao, K.; Wang, Z.; Peng, H.; Liu, W. Micro-/Nanomachines Driven by Ultrasonic Power Sources. Chem. Asian J. 2019, 14, 2406–2416. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Iwata, K.; Ishihara, Y.; Sugita, K.; Takato, M.; Uchikoba, F. Miniaturized Rotary Actuators Using Shape Memory Alloy for Insect-Type MEMS Microrobot. Micromachines 2016, 7, 58. [Google Scholar] [CrossRef]
- Alcanzare, M.M.; Karttunen, M.; Ala-Nissilä, T.; Alcanzare, M. Propulsion and controlled steering of magnetic nanohelices. Soft Matter 2019, 15, 1684–1691. [Google Scholar] [CrossRef]
- Qiu, F.; Mhanna, R.; Zhang, L.; Ding, Y.; Fujita, S.; Nelson, B.J. Artificial bacterial flagella functionalized with temperature-sensitive liposomes for controlled release. Sens. Actuators B Chem. 2014, 196, 676–681. [Google Scholar] [CrossRef]
- Qiu, F.; Fujita, S.; Mhanna, R.; Zhang, L.; Simona, B.R.; Nelson, B.J. Magnetic Helical Microswimmers Functionalized with Lipoplexes for Targeted Gene Delivery. Adv. Funct. Mater. 2015, 25, 1666–1671. [Google Scholar] [CrossRef]
- Pal, M.; Somalwar, N.; Singh, A.; Bhat, R.; Eswarappa, S.M.; Saini, D.K.; Ghosh, A. Maneuverability of Magnetic Nanomotors Inside Living Cells. Adv. Mater. 2018, 30, 1800429. [Google Scholar] [CrossRef]
- Ali, J.; Cheang, U.K.; Martindale, J.D.; Jabbarzadeh, M.; Fu, H.C.; Kim, M.J. Bacteria-inspired nanorobots with flagellar polymorphic transformations and bundling. Sci. Rep. 2017, 7, 14098. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Gutman, E.; Stucki, N.; Seitz, B.F.; Wendel-García, P.D.; Newton, T.; Pokki, J.; Ergeneman, O.; Pane, S.; Or, Y.; et al. Undulatory Locomotion of Magnetic Multilink Nanoswimmers. Nano Lett. 2015, 15, 4829–4833. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sattayasamitsathit, S.; Manesh, K.M.; Weihs, D.; Wang, J. Magnetically Powered Flexible Metal Nanowire Motors. J. Am. Chem. Soc. 2010, 132, 14403–14405. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, J.; Zhang, H.; Chang, X.; Song, W.; Hu, Y.; Shao, G.; Sandraz, E.; Zhang, G.; Li, L.; et al. Magnetically Propelled Fish-Like Nanoswimmers. Small 2016, 12, 6098–6105. [Google Scholar] [CrossRef]
- Gao, W.; Kagan, D.; Pak, O.S.; Clawson, C.; Campuzano, S.; Chuluun-Erdene, E.; Shipton, E.; Fullerton, E.E.; Zhang, L.; Lauga, E.; et al. Cargo-Towing Fuel-Free Magnetic Nanoswimmers for Targeted Drug Delivery. Small 2012, 8, 460–467. [Google Scholar] [CrossRef]
- Gutman, E.; Or, Y. Optimizing an undulating magnetic microswimmer for cargo towing. Phys. Rev. E 2016, 93, 063105. [Google Scholar] [CrossRef]
- Liu, M.; Pan, L.; Piao, H.; Sun, H.; Huang, X.; Peng, C.; Liu, Y. Magnetically Actuated Wormlike Nanomotors for Controlled Cargo Release. ACS Appl. Mater. Interfaces 2015, 7, 26017–26021. [Google Scholar] [CrossRef]
- Zhang, L.; Petit, T.; Lu, Y.; Kratochvil, B.E.; Peyer, K.E.; Pei, R.; Lou, J.; Nelson, B.J. Controlled Propulsion and Cargo Transport of Rotating Nickel Nanowires near a Patterned Solid Surface. ACS Nano 2010, 4, 6228–6234. [Google Scholar] [CrossRef]
- Tasci, T.O.; Herson, P.S.; Neeves, K.B.; Marr, D.W.M. Surface-enabled propulsion and control of colloidal microwheels. Nat. Commun. 2016, 7, 10225. [Google Scholar] [CrossRef]
- Sun, M.; Fan, X.; Meng, X.; Song, J.; Chen, W.; Sun, L.; Xie, H. Magnetic biohybrid micromotors with high maneuverability for efficient drug loading and targeted drug delivery. Nanoscale 2019, 11, 18382–18392. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W.; Ke, W.; Wu, S. Nickel-based (Ni–Cr and Ni–Cr–Be) alloys used in dental restorations may be a potential cause for immune-mediated hypersensitivity. Med. Hypotheses 2009, 73, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, T.; Wang, T.; Ji, Y.; Shen, Y.; Chen, J.; Xu, H.; Wu, X. In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine. Int. J. Mol. Sci. 2020, 21, 384. [Google Scholar] [CrossRef] [PubMed]
- Demirörs, A.F.; Akan, M.T.; Poloni, E.; Studart, A.R. Active cargo transport with Janus colloidal shuttles using electric and magnetic fields. Soft Matter 2018, 14, 4741–4749. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Chowdhury, M.M.; Alam, K. Rotating-Electric-Field-Induced Carbon-Nanotube-Based Nanomotor in Water: A Molecular Dynamics Study. Small 2017, 13, 1603978. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Teal, D.; Fan, D.E. Light programmable micro/nanomotors with optically tunable in-phase electric polarization. Nat. Commun. 2019, 10, 5275. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zheng, J.; Zhao, Y.; Zhu, B.; Cheng, R.; Wang, J.; Liu, J.; Tang, J.; Tang, J. From Strong Dichroic Nanomotor to Polarotactic Microswimmer. Adv. Mater. 2019, 31, e1903329. [Google Scholar] [CrossRef]
- Kong, L.; Mayorga-Martinez, C.C.; Guan, J.; Pumera, M. Photocatalytic Micromotors Activated by UV to Visible Light for Environmental Remediation, Micropumps, Reversible Assembly, Transportation, and Biomimicry. Small 2019, e1903179. [Google Scholar] [CrossRef]
- Dong, R.; Hu, Y.; Wu, Y.; Gao, W.; Ren, B.; Wang, Q.; Cai, Y.-P. Visible-Light-Driven BiOI-Based Janus Micromotor in Pure Water. J. Am. Chem. Soc. 2017, 139, 1722–1725. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, R.-F.; Wang, C.; Xu, S.; Chen, D.; Liang, Y.; Ren, B.; Gao, W.; Cai, Y.-P. Glucose-Fueled Micromotors with Highly Efficient Visible-Light Photocatalytic Propulsion. ACS Appl. Mater. Interfaces 2019, 11, 6201–6207. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, Q.; Gao, W.; Pei, A.; Ren, B. Highly Efficient Light-Driven TiO2–Au Janus Micromotors. ACS Nano 2016, 10, 839–844. [Google Scholar] [CrossRef]
- Villa, K.; Pumera, M. Fuel-free light-driven micro/nanomachines: Artificial active matter mimicking nature. Chem. Soc. Rev. 2019, 48, 4966–4978. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Si, T.; Gao, W.; Lin, X.; Wang, J.; He, Q. Superfast Near-Infrared Light-Driven Polymer Multilayer Rockets. Small 2015, 12, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Gao, C.; Wang, W.; Dai, L.; He, Q. Self-Propelled Nanomotors for Thermomechanically Percolating Cell Membranes. Angew. Chem. Int. Ed. 2018, 57, 12463–12467. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, T.; Xu, T.; Kiristi, M.; Liu, W.; Wu, Z.; Wang, J. Magneto–Acoustic Hybrid Nanomotor. Nano Lett. 2015, 15, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gradilla, V.; Orozco, J.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Pourazary, A.; Katzenberg, A.; Gao, W.; Shen, Y.; Wang, J. Functionalized Ultrasound-Propelled Magnetically Guided Nanomotors: Toward Practical Biomedical Applications. ACS Nano 2013, 7, 9232–9240. [Google Scholar] [CrossRef]
- Garcia-Gradilla, V.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Yardımcı, C.; Wiitala, D.; Galarnyk, M.; Wang, J.; Yardimci, C. Ultrasound-Propelled Nanoporous Gold Wire for Efficient Drug Loading and Release. Small 2014, 10, 4154–4159. [Google Scholar] [CrossRef]
- De Ávila, B.E.-F.; Angell, C.; Soto, F.; Lopez-Ramirez, M.A.; Baez, D.F.; Xie, S.; Wang, J.; Chen, Y. Acoustically Propelled Nanomotors for Intracellular siRNA Delivery. ACS Nano 2016, 10, 4997–5005. [Google Scholar] [CrossRef]
- Basta, G.; Venneri, L.; Lazzerini, G.; Pasanisi, E.; Pianelli, M.; Vesentini, N.; Del Turco, S.; Kusmic, C.; Picano, E. In vitro modulation of intracellular oxidative stress of endothelial cells by diagnostic cardiac ultrasound. Cardiovasc. Res. 2003, 58, 156–161. [Google Scholar] [CrossRef]
- Sokolov, I.L.; Cherkasov, V.R.; Tregubov, A.A.; Buiucli, S.R.; Nikitin, M.P. Smart materials on the way to theranostic nanorobots: Molecular machines and nanomotors, advanced biosensors, and intelligent vehicles for drug delivery. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1530–1544. [Google Scholar] [CrossRef]
- Kagan, D.; Laocharoensuk, R.; Zimmerman, M.; Clawson, C.; Balasubramanian, S.; Kang, D.; Bishop, D.; Sattayasamitsathit, S.; Zhang, L.; Wang, J. Rapid Delivery of Drug Carriers Propelled and Navigated by Catalytic Nanoshuttles. Small 2010, 6, 2741–2747. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, X.; Wu, Z.; Möhwald, H.; He, Q.; Möhwald, H. Self-Propelled Polymer Multilayer Janus Capsules for Effective Drug Delivery and Light-Triggered Release. ACS Appl. Mater. Interfaces 2014, 6, 10476–10481. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; André, A.A.M.; Men, Y.; Srinivas, M.; Wilson, D.A. Biodegradable Hybrid Stomatocyte Nanomotors for Drug Delivery. ACS Nano 2017, 11, 1957–1963. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; White, P.B.; Wilson, D.A. Redox-Sensitive Stomatocyte Nanomotors: Destruction and Drug Release in the Presence of Glutathione. Angew. Chem. Int. Ed. 2017, 56, 7620–7624. [Google Scholar] [CrossRef] [PubMed]
- Llopis-Lorente, A.; Garcia-Fernandez, A.; Lucena-Sánchez, E.; Diez, P.; Sancenón, F.; Villalonga, R.; Wilson, D.A.; Martínez-Máñez, R.; Lucena, E. Stimulus-responsive nanomotors based on gated enzyme-powered Janus Au–mesoporous silica nanoparticles for enhanced cargo delivery. Chem. Commun. 2019, 55, 13164–13167. [Google Scholar] [CrossRef] [PubMed]
- Medina-Sánchez, M.; Xu, H.; Schmidt, O.G. Micro- and nano-motors: The new generation of drug carriers. Ther. Deliv. 2018, 9, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Mou, F.; Chen, C.; Ma, H.; Yin, Y.; Wu, Q.; Guan, J. Self-Propelled Micromotors Driven by the Magnesium-Water Reaction and Their Hemolytic Properties. Angew. Chem. Int. Ed. 2013, 52, 7208–7212. [Google Scholar] [CrossRef]
- Patiño, T.; Arqué, X.; Mestre, R.; Palacios, L.; Sánchez, S. Fundamental Aspects of Enzyme-Powered Micro- and Nanoswimmers. Acc. Chem. Res. 2018, 51, 2662–2671. [Google Scholar] [CrossRef]
- Hortelão, A.C.; Patiňo, T.; Perez-Jiménez, A.; Blanco, À.; Sanchez, S. Enzyme-Powered Nanobots Enhance Anticancer Drug Delivery. Adv. Funct. Mater. 2018, 28, 1705086. [Google Scholar] [CrossRef]
- Shao, J.; Xuan, M.; Zhang, H.; Lin, X.; Wu, Z.; He, Q. Chemotaxis-Guided Hybrid Neutrophil Micromotors for Targeted Drug Transport. Angew. Chem. Int. Ed. 2017, 56, 12935–12939. [Google Scholar] [CrossRef]
- Wu, Z.; De Avila, B.E.-F.; Martin, A.; Christianson, C.; Gao, W.; Thamphiwatana, S.K.; Escarpa, A.; He, Q.; Zhang, L.; Wang, J. RBC micromotors carrying multiple cargos towards potential theranostic applications. Nanoscale 2015, 7, 13680–13686. [Google Scholar] [CrossRef]
- Felfoul, O.; Mohammadi, M.; Taherkhani, S.; De Lanauze, D.; Xu, Y.Z.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; LaFleur, M.; et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 2016, 11, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, J.; Wu, X.; Ding, B. Multifunctional DNA Origami Nanoplatforms for Drug Delivery. Chem. Asian J. 2019, 14, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, S.; Hadla, M.; Spena, C.R.; Bayda, S.; Kumar, V.; Re, F.L.; Adeel, M.; Caligiuri, I.; Romano, F.; Corona, G.; et al. Proof-of-Concept Multistage Biomimetic Liposomal DNA Origami Nanosystem for the Remote Loading of Doxorubicin. ACS Med. Chem. Lett. 2019, 10, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, S.; Hadla, M.; Spena, C.R.; Caligiuri, I.; Rotondo, R.; Adeel, M.; Kumar, V.; Corona, G.; Canzonieri, V.; Toffoli, G.; et al. An Effective Multi-Stage Liposomal DNA Origami Nanosystem for In Vivo Cancer Therapy. Cancers 2019, 11, 1997. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Li, H.; Shi, X.; Zheng, P. A Programming 20–30nm Rectangular DNA Origami for Loading Doxorubicin to Penetrate Ovarian Cancer Cells. IEEE Trans. NanoBiosci. 2020, 19, 152–157. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Zhao, S.; Duan, F.; Liu, S.; Wu, T.; Shang, Y.; Tian, R.; Liu, J.; Wang, Z.-G.; Jiang, Q.; Ding, B. Efficient Intracellular Delivery of RNase A Using DNA Origami Carriers. ACS Appl. Mater. Interfaces 2019, 11, 11112–11118. [Google Scholar] [CrossRef]
- De Ávila, B.E.-F.; Angsantikul, P.; Li, J.; Lopez-Ramirez, M.A.; Ramírez-Herrera, D.E.; Thamphiwatana, S.; Chen, C.; Delezuk, J.; Samakapiruk, R.; Ramez, V.; et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Gao, W.; Dong, R.; Thamphiwatana, S.; Li, J.; Gao, W.; Zhang, L.; Wang, J. Artificial Micromotors in the Mouse’s Stomach: A Step toward in Vivo Use of Synthetic Motors. ACS Nano 2015, 9, 117–123. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Yang, Y.; Hu, P.; Li, Y.; Yang, S.-Y.; Wang, L.V.; Gao, W. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 2019, 4, eaax0613. [Google Scholar] [CrossRef] [PubMed]
- Baylis, J.R.; Yeon, J.H.; Thomson, M.H.; Kazerooni, A.; Wang, X.; John, A.E.S.; Lim, E.B.; Chien, D.; Lee, A.; Zhang, J.Q.; et al. Self-propelled particles that transport cargo through flowing blood and halt hemorrhage. Sci. Adv. 2015, 1, e1500379. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Medina-Sánchez, M.; Maitz, M.F.; Werner, C.; Schmidt, O.G. Sperm Micromotors for Cargo Delivery through Flowing Blood. ACS Nano 2020, 14, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Alapan, Y.; Bozuyuk, U.; Erkoc, P.; Karacakol, A.C.; Sitti, M. Multifunctional surface microrollers for targeted cargo delivery in physiological blood flow. Sci. Robot. 2020, 5, eaba5726. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Kwon, S.; Sung, Y.J.; Song, W.K.; Park, S. Bilayer Hydrogel Sheet-Type Intraocular Microrobot for Drug Delivery and Magnetic Nanoparticles Retrieval. Adv. Health Mater. 2020, 9, e2000118. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Luo, T.; Wang, R.; Liu, C.; Chen, S.; Li, D.; Yue, J.; Cheng, S.H.; Sun, D. Development of a magnetic microrobot for carrying and delivering targeted cells. Sci. Robot. 2018, 3, eaat8829. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, S.; Ha, S.; Lee, S.; Kim, E.; Kim, S.Y.; Park, S.H.; Jeon, J.H.; Kim, S.W.; Moon, C.; et al. Magnetically actuated microrobots as a platform for stem cell transplantation. Sci. Robot. 2019, 4, eaav4317. [Google Scholar] [CrossRef]
- Go, G.; Jeong, S.-G.; Yoo, A.; Han, J.; Kang, B.; Kim, S.; Nguyen, K.T.; Jin, Z.; Kim, C.-S.; Seo, Y.R.; et al. Human adipose–derived mesenchymal stem cell–based medical microrobot system for knee cartilage regeneration in vivo. Sci. Robot. 2020, 5, eaay6626. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Schmidt, O.G. Medical microbots need better imaging and control. Nature 2017, 545, 406–408. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Y.; Mukasa, D.; Pak, O.S.; Gao, W. Medical micro/nanorobots in complex media. Chem. Soc. Rev. 2020. [Google Scholar] [CrossRef]
- Peng, F.; Tu, Y.; Wilson, D.A. Micro/nanomotors towards in vivo application: Cell, tissue and biofluid. Chem. Soc. Rev. 2017, 46, 5289–5310. [Google Scholar] [CrossRef] [PubMed]
- Ibele, M.; Mallouk, T.E.; Sen, A. Schooling Behavior of Light-Powered Autonomous Micromotors in Water. Angew. Chem. Int. Ed. 2009, 48, 3308–3312. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Ibele, M.; Liu, R.; Sen, A. Motion analysis of light-powered autonomous silver chloride nanomotors. Eur. Phys. J. E 2012, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Type | Energy | Penetration | Move Ability | Persistence | Safety |
|---|---|---|---|---|---|
| Exogenous power | Magnetic fields | Good, can work under a relative weak magnetic field | Precise 3D-navigation in fluids under rotating magnetic fields | Good, micro/nanorobots can keep moving with the guidance of external forces | The magnetic field used is within safe range; metal materials will bring potential harm to human body |
| Electric energy | Relatively weak, need to increase the electric field intensity | Directional movement under the combination of electric energy and other energy | Strong electric field intensity may affect human body; metal materials will bring potential harm to human body | ||
| Light energy | The transmittance of different light (visible light, UV, NIR, etc.) is different | Usually function as a trigger for other reactions, can achieve directional movement | Ultraviolet light is harmful, other lights are basically safe. | ||
| Ultrasound energy | Good, with a strong penetration ability | Usually combined with magnetic field, can achieve directional movement | Ultrasound might cause oxidative stress in cells (affect normal cells); metal materials will bring potential harm to human body | ||
| Endogenous power | Chemical energy | Not applicable | With the movement ability, but still need to be positioned by external forces (such as magnetic attraction) | Not so good, chemical energy may be depleted; when the energy decreases gradually, the motion performance of the micro/nanorobots can not be guaranteed either. | The safety of fuel needs to be considered, H2O2 is noxious, glucose and urea are nontoxic fuels |
| Types of Micro/Nanorobots | Drug Delivery Method | Target Site | Safety | Ref 1 |
|---|---|---|---|---|
| Mg-based core–shell composite loaded with drug and driven by chemical energy | The positively charged chitosan outer coating adhered to the stomach wall and led to the drug release | Stomach of mice | No effect on the body weight, apparent alteration of gastrointestinal tract histopathology or observable inflammation in the mice orally administered with micromotors for 5 days | [79] |
| Zn-based microtube loaded with gold nanoparticles and driven by chemical energy | Microrobot gradually dissolved in the gastric acid, autonomously released their carried payloads | Stomach of mice | No gastric histopathologic change and toxicity in the mice orally administrated with micromotors | [80] |
| Mg-based micromotors covered by an enteric coating and driven by chemical energy | The capsule shell was destroyed by NIR, and the drug was released in the process of gradual dissolution of the microrobot | Intestine of mice | Materials (Mg, Au, gelatin, alginate, enteric polymer) were biocompatible; had no toxicity to mice taking two days continuously | [81] |
| Self-propelled particles loaded with drug and driven by chemical energy | Thrombin played a role in the process of particles being transported throughout blood | Vessels of tail-amputated mice, mice with liver incision; vessels of pigs with carotid artery perforation | All mice remained healthy during a single-dose test for toxicity lasted for 3 days; No signs of distress, tissue necrosis or increase in the infiltration of inflammatory cells in histological sections of the tail | [82] |
| Magneto-aerotactic bacteria loaded with drug-containing nanoliposomes and driven by magnetic field | The drug was released from liposomes after reaching the target site | Hypoxic regions of tumor in SCID Beige mice | No inflammation, blood counts changes, abnormal biochemical parameters in mice injected with MC-1 intravenously for 6, 24, 72 h | [71] |
| Bilayer hydrogel microrobot loaded with drug particles and driven by chemical energy | The therapeutic layer dissolved when heated by an alternating magnetic field, delivering drug particles to the lesion | Bovine vitreous | The remaining microrobots could be retrieved using a magnetic field | [85] |
| Burr-like porous spherical microrobots loaded with cells and driven by magnetic field | The carried cells were released from the microrobot and attached to the tissues after reaching the target | Dorsum of a nude mouse | Cell experiment for 1, 3 and 5 days confirmed the safety of the microrobot | [86] |
| Porous 3D microrobots loaded with stem cells and driven by magnetic field | After reaching the target, the cells adhered to and proliferated within the tissue | Intraperitoneal cavity of a nude mouse | The microrobot was biocompatible, but its safety in vivo was not mentioned | [87] |
| Porous 3D microrobots carried with stem cells and driven by magnetic field | Cells would adhere to the tissues when reaching the target | Knee cartilage of rabbit | Microrobots would degrade in 3 weeks without causing any inflammation in rabbits | [88] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Ge, X.; Chen, X.; Mao, W.; Qian, X.; Yuan, W.-E. Micro/Nanorobot: A Promising Targeted Drug Delivery System. Pharmaceutics 2020, 12, 665. https://doi.org/10.3390/pharmaceutics12070665
Hu M, Ge X, Chen X, Mao W, Qian X, Yuan W-E. Micro/Nanorobot: A Promising Targeted Drug Delivery System. Pharmaceutics. 2020; 12(7):665. https://doi.org/10.3390/pharmaceutics12070665
Chicago/Turabian StyleHu, Mengyi, Xuemei Ge, Xuan Chen, Wenwei Mao, Xiuping Qian, and Wei-En Yuan. 2020. "Micro/Nanorobot: A Promising Targeted Drug Delivery System" Pharmaceutics 12, no. 7: 665. https://doi.org/10.3390/pharmaceutics12070665
APA StyleHu, M., Ge, X., Chen, X., Mao, W., Qian, X., & Yuan, W.-E. (2020). Micro/Nanorobot: A Promising Targeted Drug Delivery System. Pharmaceutics, 12(7), 665. https://doi.org/10.3390/pharmaceutics12070665