Development and Evaluation of Docetaxel-Phospholipid Complex Loaded Self-Microemulsifying Drug Delivery System: Optimization and In Vitro/Ex Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. HPLC Analysis
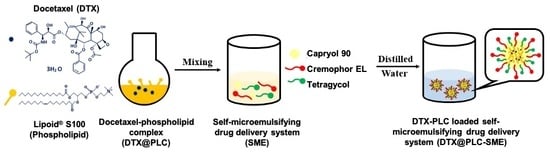
2.3. Preparation of DTX@PLC
2.4. Preparation of DTX@PLC-SME and DTX-SME
2.5. Screening of Excipients for DTX@PLC-SME
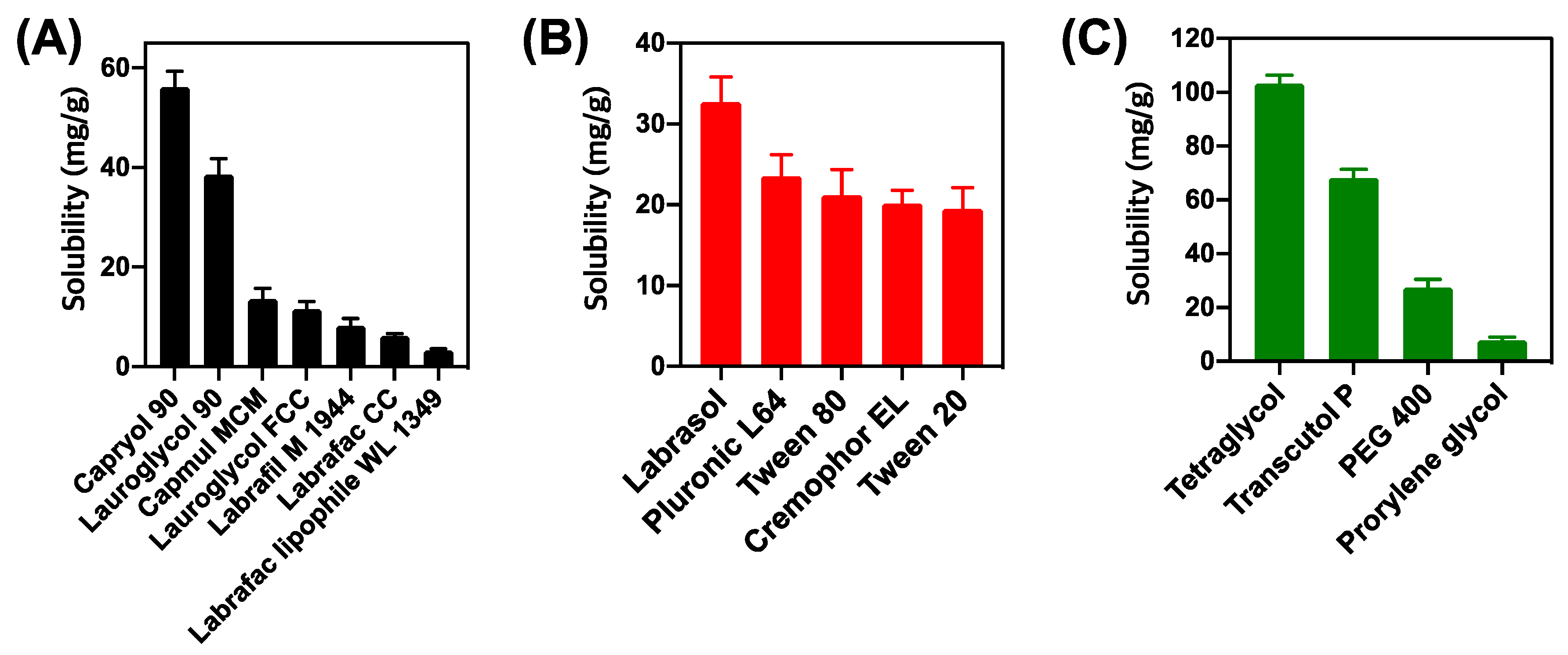
2.5.1. Solubility Test
2.5.2. Emulsification Study
2.5.3. Pseudo-Ternary Phase Diagram
2.6. Experimental Optimization of DTX@PLC-SME
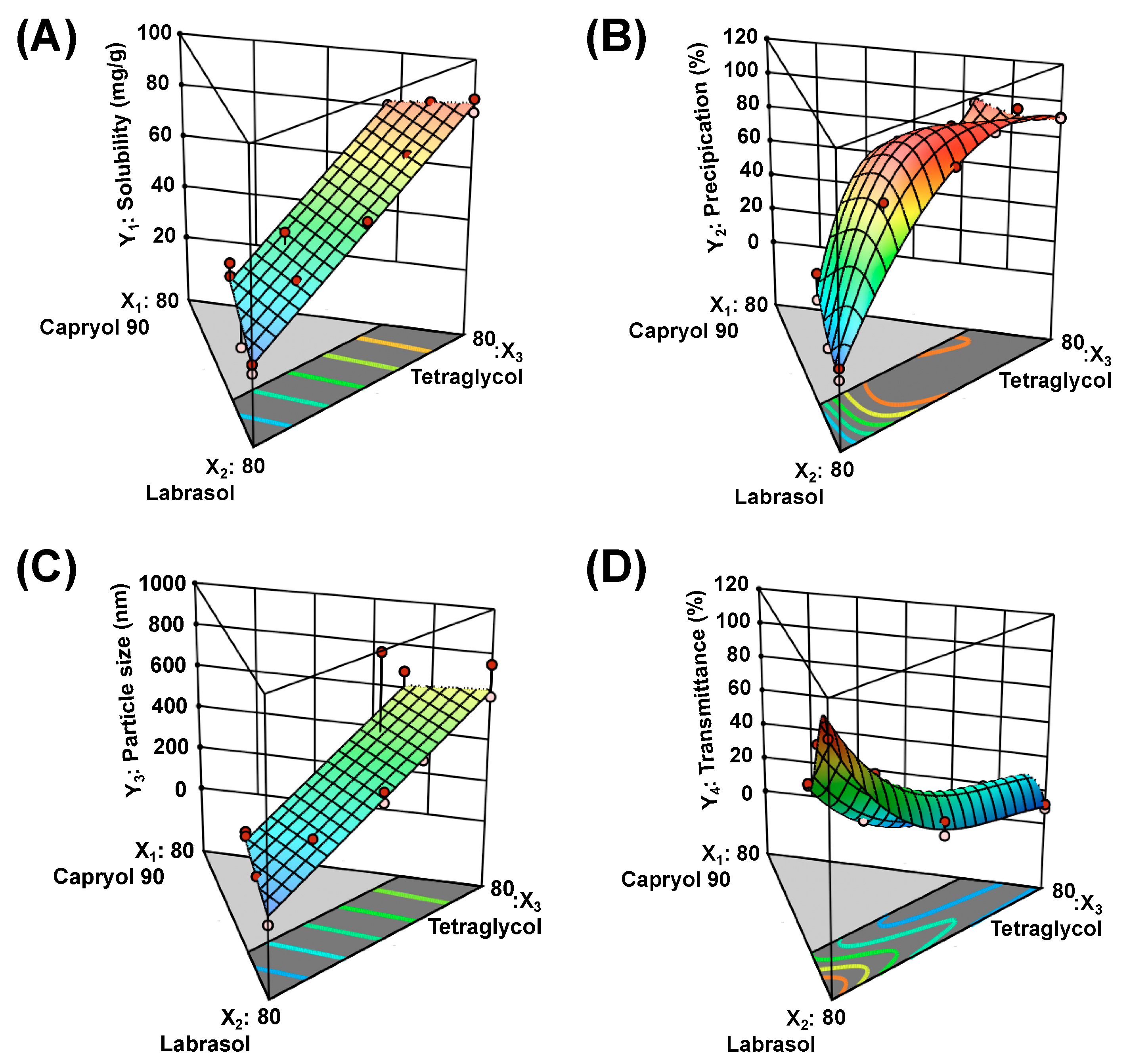
2.6.1. Solubility of DTX@PLC-SME in SME (Y1)
2.6.2. Precipitation (Y2)
2.6.3. Droplet Size (Y3)
2.6.4. Transmittance (Y4)
2.6.5. Transmission Electron Microscope
2.7. Dissolution Test
2.8. Cell Studies
2.8.1. Cell Culture
2.8.2. Cytotoxicity Study
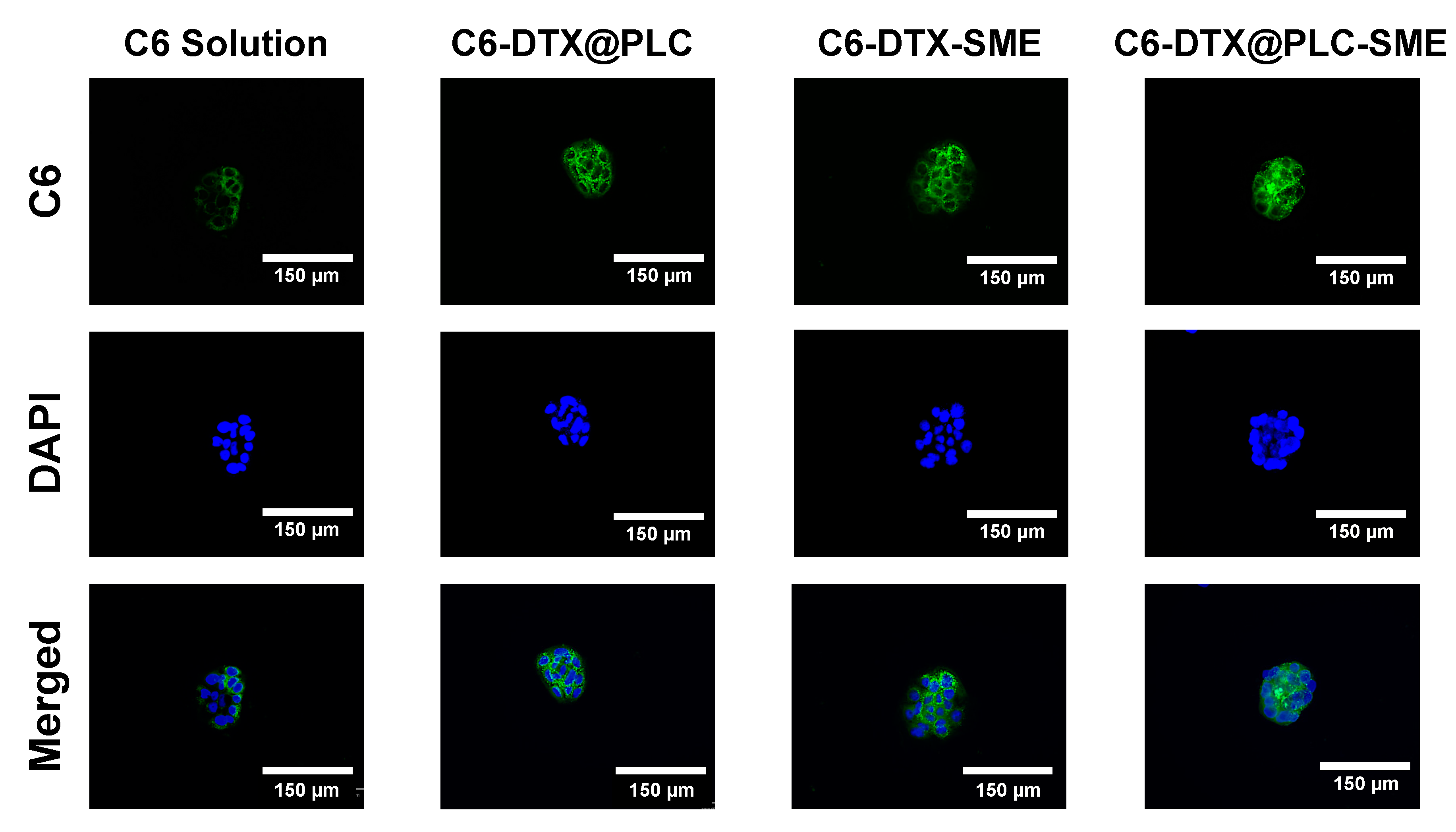
2.8.3. Cellular Uptake Study
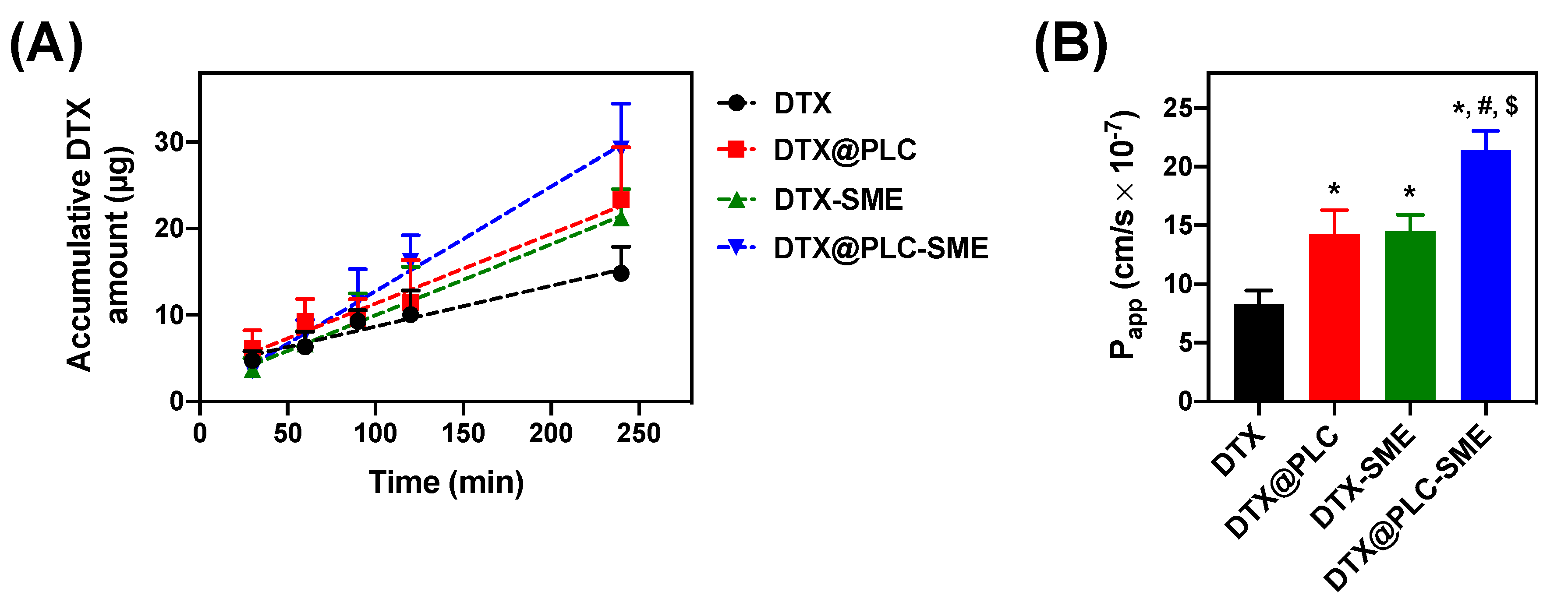
2.9. Ex Vivo Intestinal Permeability Study
2.9.1. Animals
2.9.2. Non-Everted Gut Sac Model
2.9.3. LC-MS/MS Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Screening of Excipients for DTX@PLC
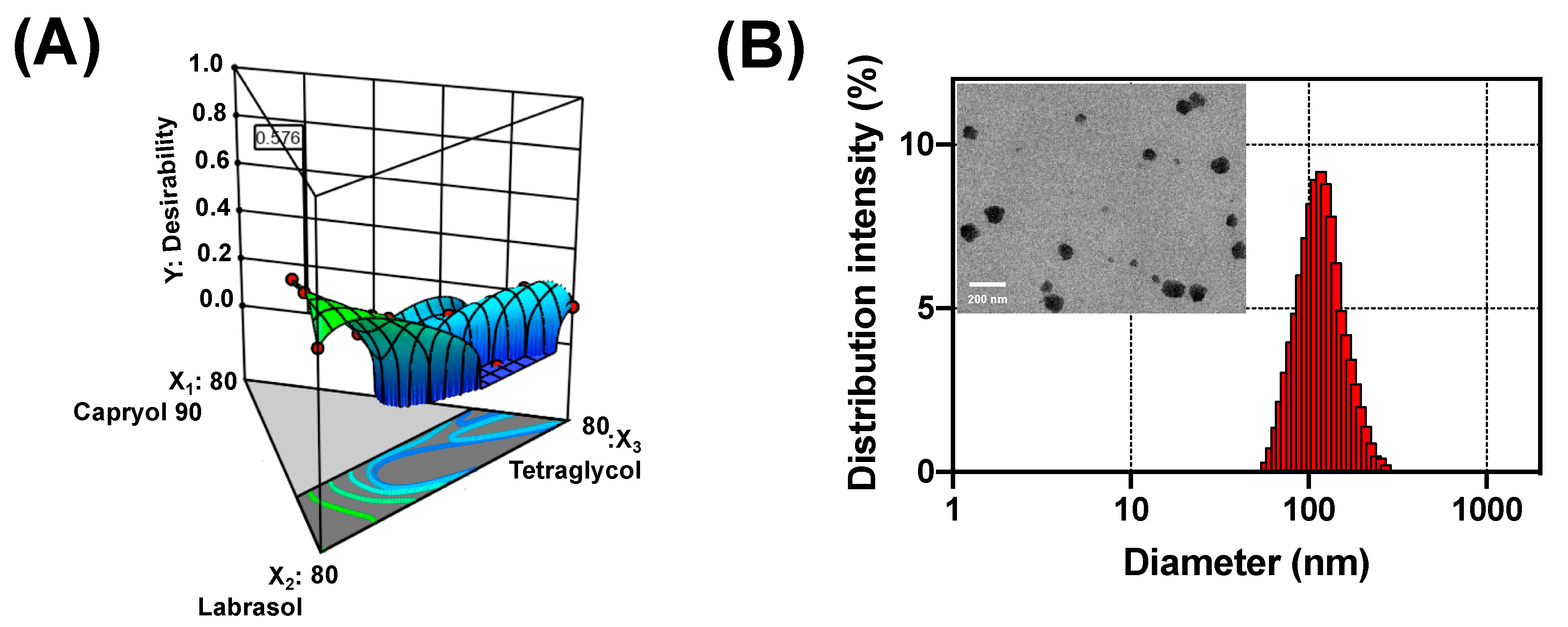
3.2. Optimization of DTX@PLC-SME
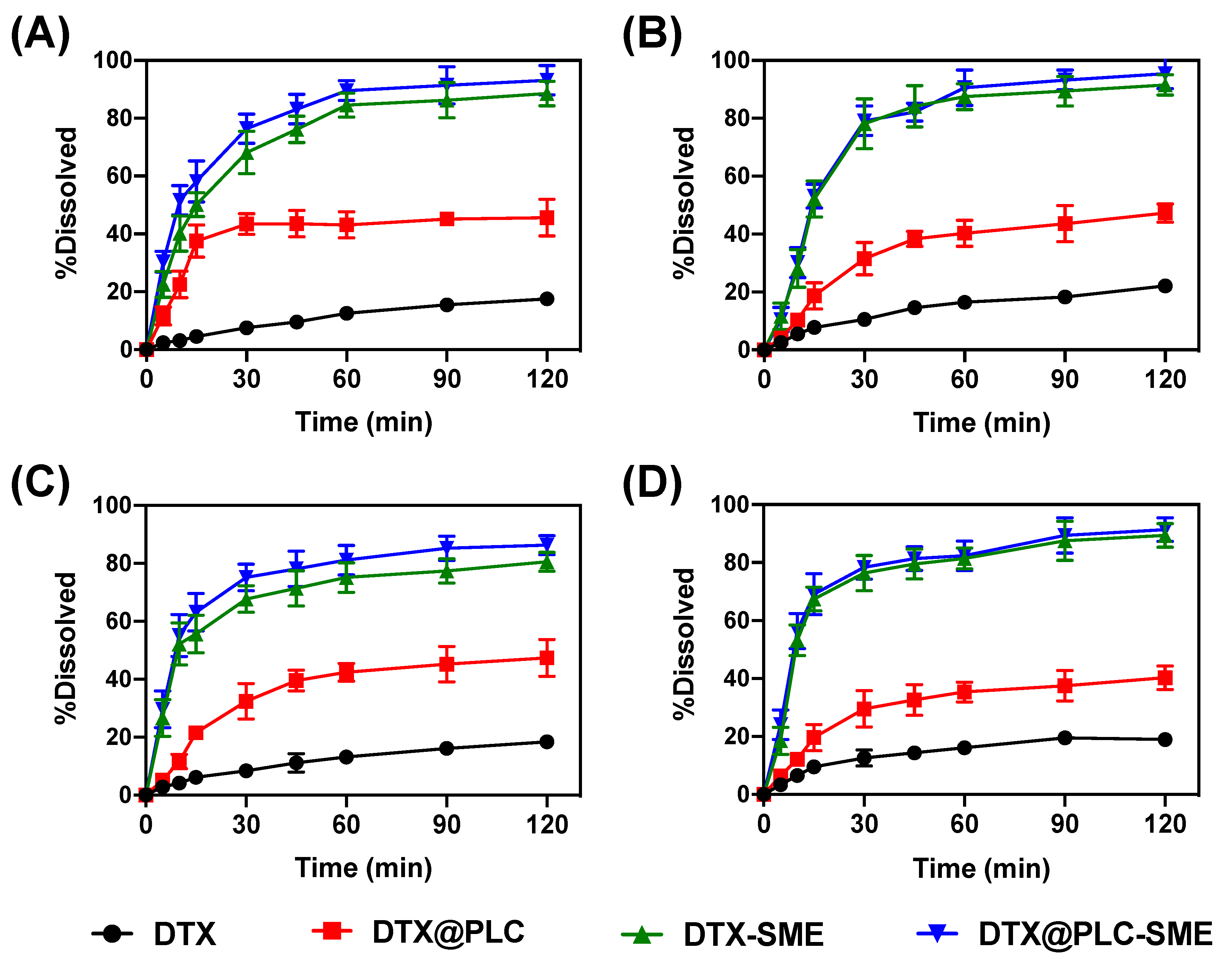
3.3. Dissolution Test
3.4. Cell Studies
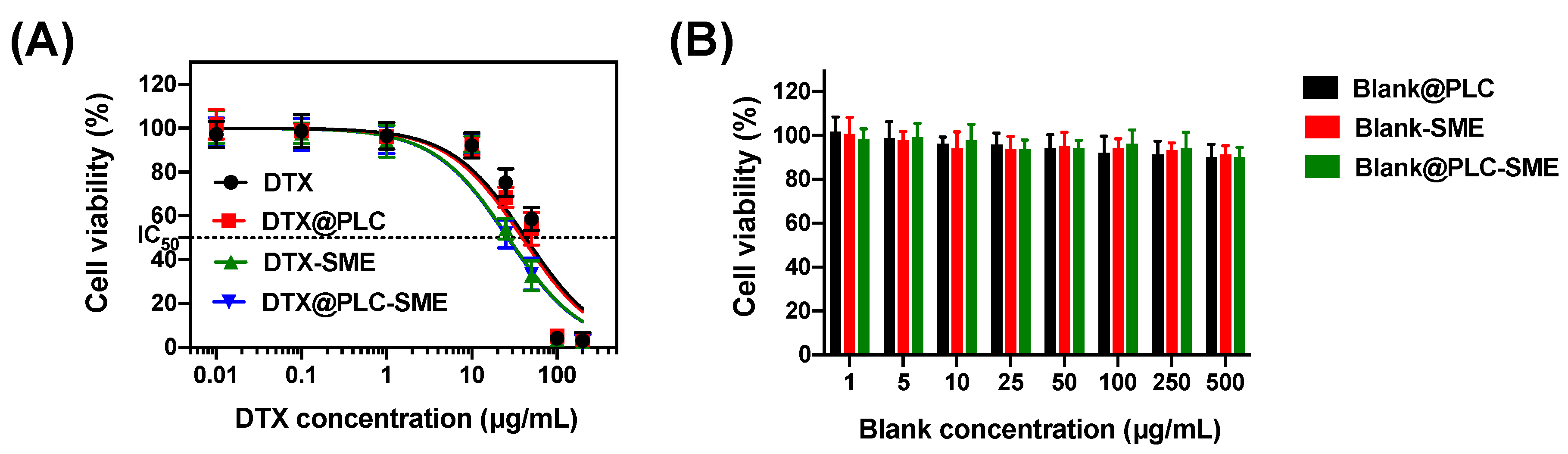
3.4.1. Cytotoxicity Study
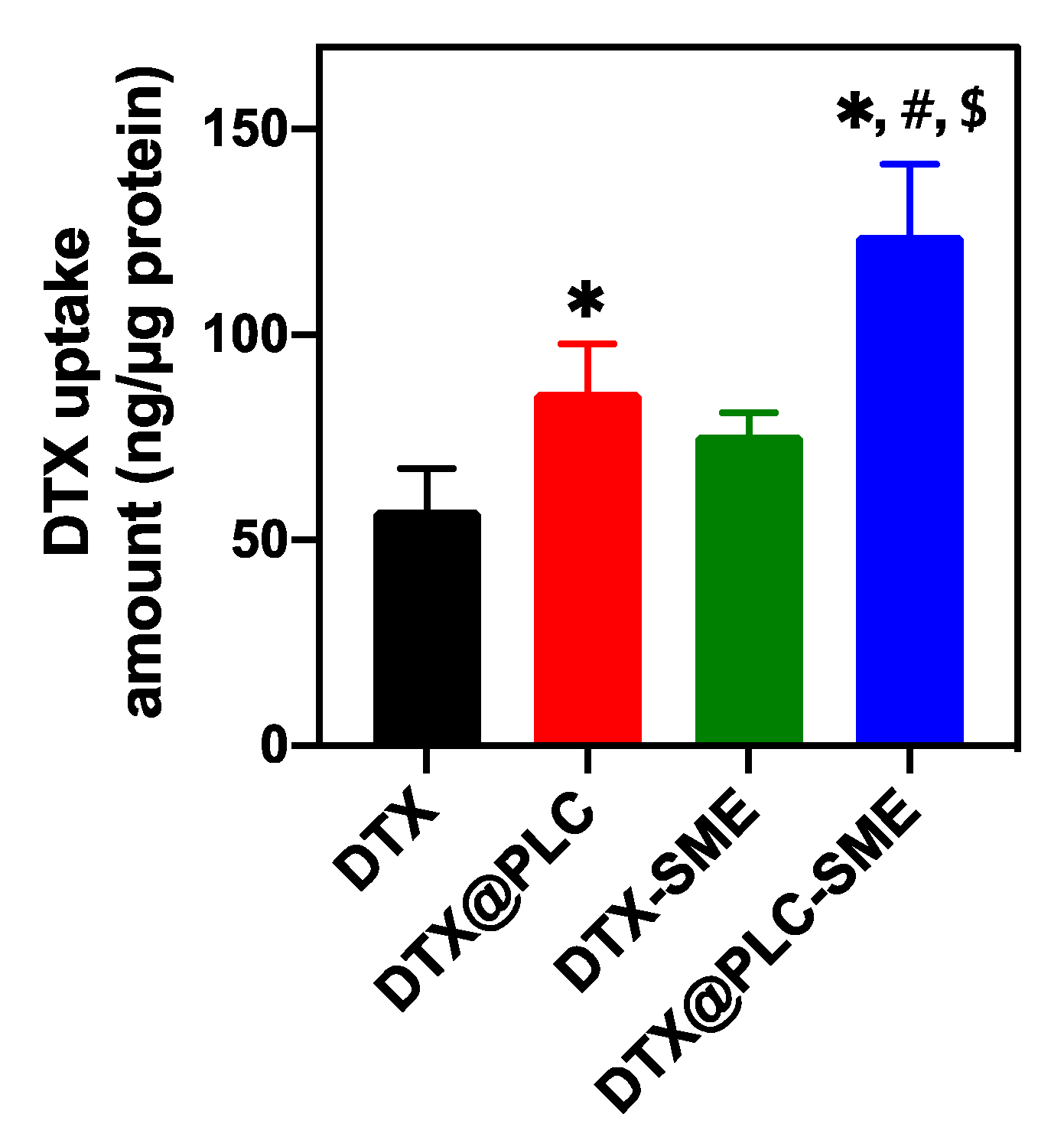
3.4.2. Cellular Uptake Study
3.5. Ex Vivo Intestinal Permeability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zhang, N. How nanotechnology can enhance docetaxel therapy. Int. J. Nanomed. 2013, 8, 2927–2941. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I.; Lichtenthal, B.; Lee, S.; Wang, C.; Wang, X. Taxane anticancer agents: A patent perspective. Expert Opin. Ther. Pat. 2016, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.L.; Gray, H.; Payne, C.M.; Gillies, R.J. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol. Cancer Ther. 2005, 4, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.; Wheeler, R. The immunopharmacology of paclitaxel (Taxol®), docetaxel (Taxotere®), and related agents. Int. Immunopharmacol. 2003, 3, 1699–1714. [Google Scholar] [CrossRef]
- Lee, S.W.; Yun, M.H.; Jeong, S.W.; In, C.H.; Kim, J.Y.; Seo, M.H.; Pai, C.M.; Kim, S.O. Development of docetaxel-loaded intravenous formulation, Nanoxel-PM™ using polymer-based delivery system. J. Control. Release 2011, 155, 262–271. [Google Scholar] [CrossRef]
- Sohail, M.F.; Rehman, M.; Sarwar, H.S.; Naveed, S.; Salman, O.; Bukhari, N.I.; Hussain, I.; Webster, T.J.; Shahnaz, G. Advancements in the oral delivery of docetaxel: Challenges, current state-of-the-art and future trends. Int. J. Nanomed. 2018, 13, 3145–3161. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Y.; Qiu, L. Loading docetaxel in β-cyclodextrin-based micelles for enhanced oral chemotherapy through inhibition of P-glycoprotein mediated efflux transport. RSC Adv. 2017, 7, 26161–26169. [Google Scholar] [CrossRef]
- Guo, B.; Liu, H.; Li, Y.; Zhao, J.; Yang, D.; Wang, X.; Zhang, T. Application of phospholipid complex technique to improve the dissolution and pharmacokinetic of probucol by solvent-evaporation and co-grinding methods. Int. J. Pharm. 2014, 474, 50–56. [Google Scholar] [CrossRef]
- Gnananath, K.; Nataraj, K.S.; Rao, B.G. Phospholipid complex technique for superior bioavailability of phytoconstituents. Adv. Pharm. Bulll. 2017, 7, 35–42. [Google Scholar] [CrossRef]
- Yadav, D.K.; Pawar, H.; Wankhade, S.; Suresh, S. Development of novel docetaxel phospholipid nanoparticles for intravenous administration: Quality by design approach. AAPS PharmSciTech 2015, 16, 855–864. [Google Scholar] [CrossRef]
- Kuche, K.; Bhargavi, N.; Dora, C.P.; Jain, S. Drug-phospholipid complex—A go through strategy for enhanced oral bioavailability. AAPS PharmSciTech 2019, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, P.X.; Rogers, M.A.; Wettig, S.D. Investigating the phospholipid effect on the bioaccessibility of rosmarinic acid-phospholipid complex through a dynamic gastrointestinal in vitro model. Pharmaceutics 2019, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.G.; Byeon, J.J.; Kim, M.K.; Han, M.G.; Cho, C.W.; Baek, J.S.; Lee, H.K.; Shin, Y.G. Pharmacokinetic/pharmacodynamic modeling to predict the antiplatelet effect of the ticagrelor-loaded self-microemulsifying drug delivery system in rats. Mol. Pharm. 2020, 17, 1079–1089. [Google Scholar] [CrossRef]
- Na, Y.G.; Byeon, J.J.; Wang, M.; Huh, H.W.; Kim, M.K.; Bang, K.H.; Han, M.G.; Lee, H.K.; Cho, C.W. Statistical approach for solidifying ticagrelor loaded self-microemulsifying drug delivery system with enhanced dissolution and oral bioavailability. Mater. Sci. Eng. C 2019, 104, 109980. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.M.; Cui, F.D.; Mu, C.F.; Choi, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Docetaxel microemulsion for enhanced oral bioavailability: Preparation and in vitro and in vivo evaluation. J. Control. Release 2009, 140, 86–94. [Google Scholar] [CrossRef]
- Ruan, J.; Liu, J.; Zhu, D.; Gong, T.; Yang, F.; Hao, X.; Zhang, Z. Preparation and evaluation of self-nanoemulsified drug delivery systems (SNEDDSs) of matrine based on drug–phospholipid complex technique. Int. J. Pharm. 2010, 386, 282–290. [Google Scholar] [CrossRef]
- Huang, J.; Gong, W.; Chen, Z.; Huang, J.; Chen, Q.; Huang, H.; Zhao, C. Emodin self-emulsifying platform ameliorates the expression of FN, ICAM-1 and TGF-β1 in AGEs-induced glomerular mesangial cells by promoting absorption. Eur. J. Pharm. Sci. 2017, 99, 128–136. [Google Scholar] [CrossRef]
- Bang, K.H.; Na, Y.G.; Huh, H.W.; Hwang, S.J.; Kim, M.S.; Kim, M.; Lee, H.K.; Cho, C.W. The delivery strategy of paclitaxel nanostructured lipid carrier coated with platelet membrane. Cancers 2019, 11, 807. [Google Scholar] [CrossRef]
- Yeom, D.W.; Song, Y.S.; Kim, S.R.; Lee, S.G.; Kang, M.H.; Lee, S.; Choi, Y.W. Development and optimization of a self-microemulsifying drug delivery system for ator vastatin calcium by using D-optimal mixture design. Int. J. Nanomed. 2015, 10, 3865–3877. [Google Scholar]
- Son, H.Y.; Chae, B.R.; Choi, J.Y.; Shin, D.J.; Goo, Y.T.; Lee, E.S.; Kang, T.H.; Kim, C.H.; Yoon, H.Y.; Choi, Y.W. Optimization of self-microemulsifying drug delivery system for phospholipid complex of telmisartan using D-optimal mixture design. PLoS ONE 2018, 13, e0208339. [Google Scholar] [CrossRef]
- Na, Y.G.; Huh, H.W.; Kim, M.K.; Byeon, J.J.; Han, M.G.; Lee, H.K.; Cho, C.W. Development and evaluation of a film-forming system hybridized with econazole-loaded nanostructured lipid carriers for enhanced antifungal activity against dermatophytes. Acta Biomater. 2020, 101, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.G.; Jeon, S.H.; Byeon, J.J.; Kim, M.K.; Lee, H.K.; Cho, C.W. Application of statistical design on the early development of sustained-release tablet containing ivy leaf extract. J. Drug Deliv. Sci. Technol. 2019, 54, 101319. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Q.; Li, H.; Sun, Y.; Cheng, G.; Zou, M.; Piao, H. Increased bioavailability of efonidipine hydrochloride nanosuspensions by the wet-milling method. Eur. J. Pharm. Biopharm. 2018, 130, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.G.; Pham, T.M.A.; Byeon, J.J.; Kim, M.K.; Han, M.G.; Baek, J.S.; Lee, H.K.; Cho, C.W. Development and evaluation of TPGS/PVA-based nanosuspension for enhancing dissolution and oral bioavailability of ticagrelor. Int. J. Pharm. 2020, 581, 119287. [Google Scholar] [CrossRef]
- Gurram, A.; Deshpande, P.B.; Kar, S.S.; Nayak, U.Y.; Udupa, N.; Reddy, M. Role of components in the formation of self-microemulsifying drug delivery systems. Indian J. Pharm. Sci. 2015, 77, 249–257. [Google Scholar]
- Hall, S.; Pacek, A.W.; Kowalski, A.J.; Cooke, M.; Rothman, D. The effect of scale and interfacial tension on liquid–liquid dispersion in in-line silverson rotor–stator mixers. Chem. Eng. Res. Des. 2013, 91, 2156–2168. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef]
- Na, Y.G.; Byeon, J.J.; Wang, M.; Huh, H.W.; Son, G.H.; Jeon, S.H.; Bang, K.H.; Kim, S.J.; Lee, H.J.; Lee, H.K.; et al. Strategic approach to developing a self-microemulsifying drug delivery system to enhance antiplatelet activity and bioavailability of ticagrelor. Int. J. Nanomed. 2019, 14, 1193–1212. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 2007, 82, 591–605. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Rajput, P.V.; Belgamwar, V.S.; Tekade, A.R.; Surana, S.J. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: Application of factorial design approach. Drug Deliv. 2013, 20, 47–56. [Google Scholar] [CrossRef]
- Shekhawat, P.; Pokharkar, V. Risk assessment and QbD based optimization of an eprosartan mesylate nanosuspension: In-vitro characterization, PAMPA and in-vivo assessment. Int. J. Pharm. 2019, 567, 118415. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Feng, S.S. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol®). J. Control. Release 2002, 80, 129–144. [Google Scholar] [CrossRef]
- Tummons, E.N.; Tarabara, V.V.; Chew, J.W.; Fane, A.G. Behavior of oil droplets at the membrane surface during crossflow microfiltration of oil–water emulsions. J. Membr. Sci. 2016, 500, 211–224. [Google Scholar] [CrossRef]
- Son, G.H.; Lee, H.J.; Na, Y.G.; Lee, H.K.; Kim, S.J.; Huh, H.W.; Kim, K.T.; Kang, J.S.; Kim, Y.H.; Myung, C.S.; et al. Formulation and statistical analysis of an herbal medicine tablet containing Morus alba leaf extracts. J. Pharm. Investig. 2019, 49, 625–634. [Google Scholar] [CrossRef]
- Mishra, B.; Sahoo, J.; Dixit, P.K. Formulation and process optimization of naproxen nanosuspensions stabilized by hydroxy propyl methyl cellulose. Carbohydr. Polym. 2015, 127, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Son, G.H.; Na, Y.G.; Huh, H.W.; Wang, M.; Kim, M.K.; Han, M.G.; Byeon, J.J.; Lee, H.K.; Cho, C.W. Systemic design and evaluation of ticagrelor-loaded nanostructured lipid carriers for enhancing bioavailability and antiplatelet activity. Pharmaceutics 2019, 11, 222. [Google Scholar] [CrossRef]
- Liu, W.; Pan, H.; Zhang, C.; Zhao, L.; Zhao, R.; Zhu, Y.; Pan, W. Developments in methods for measuring the intestinal absorption of nanoparticle-bound drugs. Int. J. Mol. Sci. 2016, 17, 1171. [Google Scholar] [CrossRef]
- Collnot, E.M.; Baldes, C.; Wempe, M.F.; Kappl, R.; Hüttermann, J.; Hyatt, J.A.; Edgar, K.J.; Schaefer, U.F.; Lehr, C.M. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: Influence on ATPase activity and membrane fluidity. Mol. Pharm. 2007, 4, 465–474. [Google Scholar] [CrossRef]
- Yun, Y.; Cho, Y.W.; Park, K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 2013, 65, 822–832. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of drugs: Effect on dissolution rate. Res. Pharm. Sci. 2015, 10, 95–108. [Google Scholar] [PubMed]
- Gigliobianco, M.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of poorly soluble drugs: Drug bioavailability and physicochemical stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
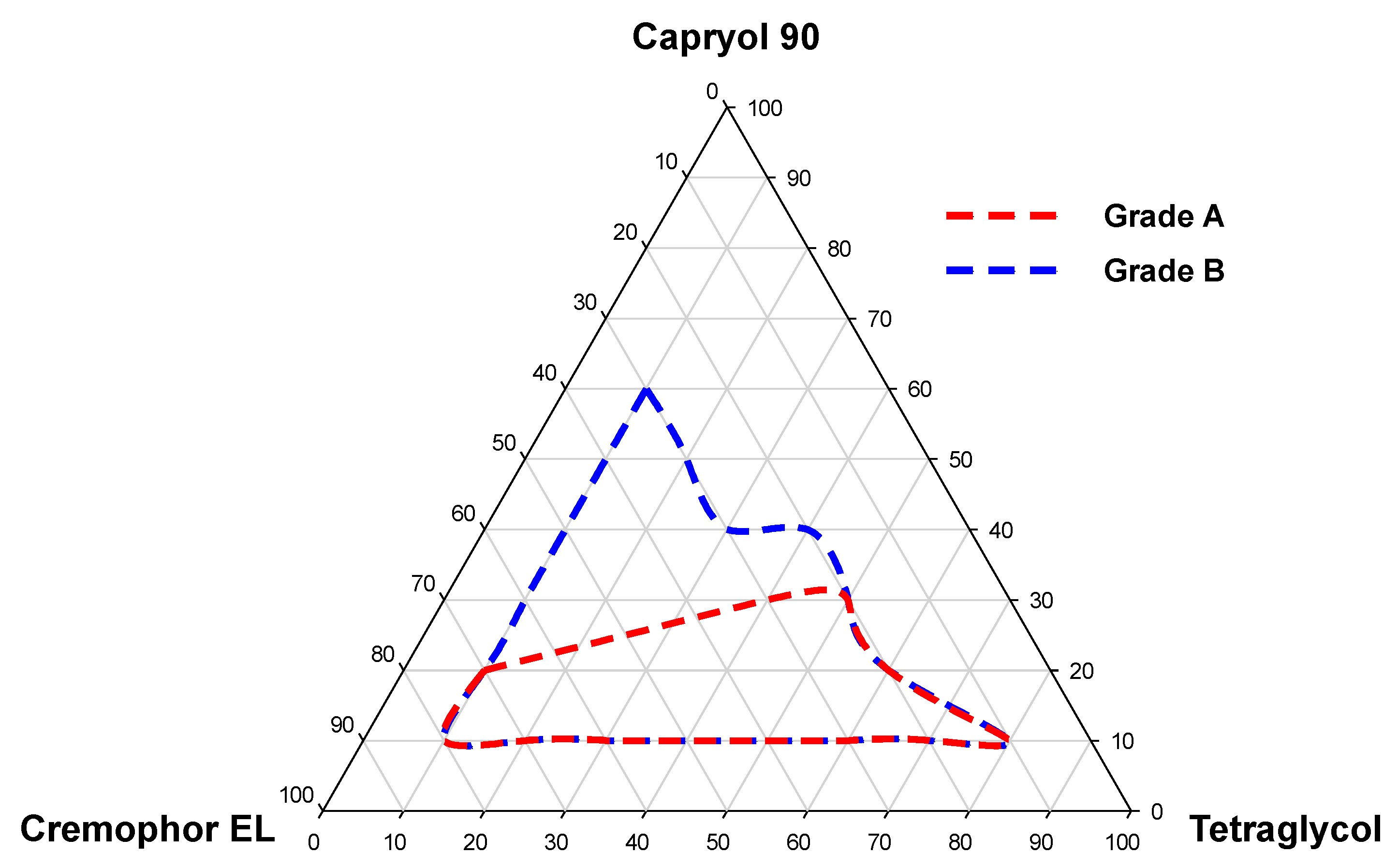
| Grade | Emulsification Capacity | Droplet Size (nm) | Transmittance (%) | Appearance |
|---|---|---|---|---|
| A | Excellent | <100 | >95 | Clear and transparent |
| B | Good | 100–300 | 85–95 | Slightly less clear |
| C | Fair | 300–1000 | 50–85 | Milky or grayish white |
| D | Poor | >1000 | <50 | No homogeneity |
| Factors | Low Limit (w/w%) | High Limit (w/w%) |
|---|---|---|
| X1: Capryol 90 | 10 | 30 |
| X2: Cremophor EL | 10 | 80 |
| X3: Tetraglycol | 10 | 80 |
| Responses | Goal | |
| Y1: Solubility (mg/g) | Maximize | |
| Y2: Precipitation (%) | Minimize | |
| Y3: Droplet size (nm) | Minimize | |
| Y4: Transmittance (%) | Maximize | |
| 2:4:4 (w/w%) | Media | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oil | Surfactant | Cosurfactant | Water | pH 1.2 | pH 4.0 | pH 6.8 | ||||
| Grade | Phase Separation | Grade | Phase Separation | Grade | Phase Separation | Grade | Phase Separation | |||
| Capryol 90 | Tween 20 | Transcutol P | A/B | X a | B | X | B | X | B | X |
| Tetraglycol | A/B | X | B | X | B | X | B | X | ||
| Tween 80 | Transcutol P | B | X | B/C | X | B | X | B/C | X | |
| Tetraglycol | A/B | X | B | X | B | X | B | X | ||
| Labrasol | Transcutol P | C/D | X | C/D | O | C/D | O | C/D | O | |
| Tetraglycol | C/D | X | C/D | O | C/D | O | C/D | O | ||
| Pluronic L64 | Transcutol P | C/D | O b | D | O | D | O | D | O | |
| Tetraglycol | D | O | D | O | C/D | O | D | O | ||
| Cremophor EL | Transcutol P | A/B | X | B | X | B | X | B | X | |
| Tetraglycol | A/B | X | A | X | A | X | A/B | X | ||
| Responses | Suggested Model | Model p-Value | Lack of Fit p-Value | R2 | Adjusted R2 | Adequate Precision |
|---|---|---|---|---|---|---|
| Y1: Solubility | Linear | <0.0001 | 0.0789 | 0.9694 | 0.9650 | 34.5639 |
| Y2: Precipitation | Special quadratic | 0.0057 | 0.2571 | 0.9872 | 0.9744 | 22.0896 |
| Y3: Droplet size | Linear | <0.0001 | 0.2788 | 0.7361 | 0.6984 | 10.2590 |
| Y4: Transmittance | Cubic | 0.0005 | 0.7260 | 0.9973 | 0.9938 | 47.3457 |
| Run | Factors | Responses | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 | |
| Capryol 90 | Cremophor EL | Tetraglycol | Solubility | Precipitation | Droplet size | Transmittance | |
| (w/w%) | (w/w%) | (w/w%) | (mg/g) | (%) | (nm) | (%) | |
| 1 | 30 | 60 | 10 | 45.7 ± 0.8 | 35.2 ± 19.9 | 219.4 ± 19.7 | 55.5 ± 0.2 |
| 2 | 25 | 50 | 25 | 52.7 ± 0.6 | 84.7 ± 3.2 | 241.5 ± 28.5 | 29.7 ± 0.6 |
| 3 | 30 | 10 | 60 | 80.4 ± 1.1 | 94.8 ± 1.5 | 371.0 ± 83.1 | 4.9 ± 0.2 |
| 4 | 15 | 25 | 60 | 70.7 ± 1.5 | 91.1 ± 0.4 | 378.4 ± 53.7 | 32.7 ± 0.4 |
| 5 | 20 | 10 | 70 | 83.1 ± 1.1 | 93.6 ± 0.2 | 529.6 ± 192.2 | 18.9 ± 0.8 |
| 6 | 15 | 60 | 25 | 42.2 ± 1.0 | 77.5 ± 6.2 | 243.9 ± 7.1 | 66.1 ± 0.5 |
| 7 | 10 | 45 | 45 | 56.8 ± 0.5 | 87.1 ± 1.1 | 319.7 ± 23.3 | 21.5 ± 0.3 |
| 8 | 10 | 80 | 10 | 24.2 ± 0.5 | 6.7 ± 0.7 | 11.9 ± 1.1 | 99.9 ± 0.1 |
| 9 | 30 | 60 | 10 | 40.8 ± 0.5 | 20.5 ± 11.2 | 239.6 ± 7.5 | 56.5 ± 0.4 |
| 10 | 10 | 45 | 45 | 56.5 ± 0.4 | 87.1 ± 0.8 | 370.4 ± 25.9 | 29.6 ± 0.1 |
| 11 | 25 | 25 | 50 | 67.9 ± 1.9 | 92.3 ± 0.5 | 855.4 ± 137.3 | 10.5 ± 0.3 |
| 12 | 20 | 70 | 10 | 22.1 ± 0.2 | 5.5 ± 2.3 | 131.0 ± 1.3 | 87.5 ± 0.1 |
| 13 | 30 | 10 | 60 | 80.6 ± 2.9 | 94.5 ± 0.1 | 678.6 ± 54.5 | 7.7 ± 0.2 |
| 14 | 10 | 10 | 80 | 80.7 ± 0.8 | 90.6 ± 0.6 | 748.8 ± 39.2 | 9.1 ± 6.8 |
| 15 | 30 | 35 | 35 | 54.7 ± 1.3 | 89.4 ± 0.3 | 324.2 ± 2.5 | 20.1 ± 0.1 |
| 16 | 10 | 10 | 80 | 85.7 ± 1.6 | 91.2 ± 0.2 | 599.5 ± 35.0 | 11.6 ± 0.5 |
| 17 | 10 | 80 | 10 | 20.8 ± 0.7 | 0.1 ± 3.4 | 12.4 ± 1.0 | 99.3 ± 0.1 |
| Optimal Factors | Responses | 95% CI a Low Predicted Value | Predicted Value | 95% CI High Predicted Value | Observed Value | Error Percentage (%) |
|---|---|---|---|---|---|---|
| X1: 18.8% | Y1: Solubility (mg/g) | 27.1 | 30.5 | 34.0 | 33.0 ± 0.2 | 8.2 |
| X2: 71.2% | Y2: Precipitation (%) | –1.7 | 8.7 | 19.0 | 8.9 ± 2.6 | 2.3 |
| X3: 10.0% | Y3: Droplet size (nm) | 4.8 | 116.1 | 227.4 | 117.1 ± 6.7 | 0.9 |
| Y4: Transmittance (%) | 87.2 | 92.7 | 98.3 | 96.0 ± 0.3 | 3.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; You, S.-K.; Lee, H.-K.; Han, M.-G.; Lee, H.-M.; Pham, T.M.A.; Na, Y.-G.; Cho, C.-W. Development and Evaluation of Docetaxel-Phospholipid Complex Loaded Self-Microemulsifying Drug Delivery System: Optimization and In Vitro/Ex Vivo Studies. Pharmaceutics 2020, 12, 544. https://doi.org/10.3390/pharmaceutics12060544
Wang M, You S-K, Lee H-K, Han M-G, Lee H-M, Pham TMA, Na Y-G, Cho C-W. Development and Evaluation of Docetaxel-Phospholipid Complex Loaded Self-Microemulsifying Drug Delivery System: Optimization and In Vitro/Ex Vivo Studies. Pharmaceutics. 2020; 12(6):544. https://doi.org/10.3390/pharmaceutics12060544
Chicago/Turabian StyleWang, Miao, Sung-Kyun You, Hong-Ki Lee, Min-Gu Han, Hyeon-Min Lee, Thi Mai Anh Pham, Young-Guk Na, and Cheong-Weon Cho. 2020. "Development and Evaluation of Docetaxel-Phospholipid Complex Loaded Self-Microemulsifying Drug Delivery System: Optimization and In Vitro/Ex Vivo Studies" Pharmaceutics 12, no. 6: 544. https://doi.org/10.3390/pharmaceutics12060544
APA StyleWang, M., You, S.-K., Lee, H.-K., Han, M.-G., Lee, H.-M., Pham, T. M. A., Na, Y.-G., & Cho, C.-W. (2020). Development and Evaluation of Docetaxel-Phospholipid Complex Loaded Self-Microemulsifying Drug Delivery System: Optimization and In Vitro/Ex Vivo Studies. Pharmaceutics, 12(6), 544. https://doi.org/10.3390/pharmaceutics12060544