A Spray-Dried, Co-Processed Rice Starch as a Multifunctional Excipient for Direct Compression
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of a Rice Starch-Based, Co-Processed Excipient (CSs) Using a Spray Drying Technique
2.2.1. Selection of an Optimal Spray Drying Condition
2.2.2. CSs Preparation
2.3. Physicochemical Evaluation
2.3.1. FT-IR Spectroscopy
2.3.2. X-Ray Diffraction (XRD)
2.3.3. Solubility, Swelling Property, and pH
2.3.4. Moisture Content
2.3.5. Scanning Electron Micrograph (SEM)
2.3.6. Powder Characteristics
2.4. Pharmaceutical Properties Evaluation
2.4.1. Flow Property
2.4.2. Compression Behavior
Tablet Preparation
Tablet Tensile Strength and Porosity
Plastic Deformation Property
2.4.3. Disintegration Property
2.5. Statistics
3. Results and Discussion
3.1. Selection of an Optimal Spray Drying Condition and Preparation of CSs
3.2. FT-IR
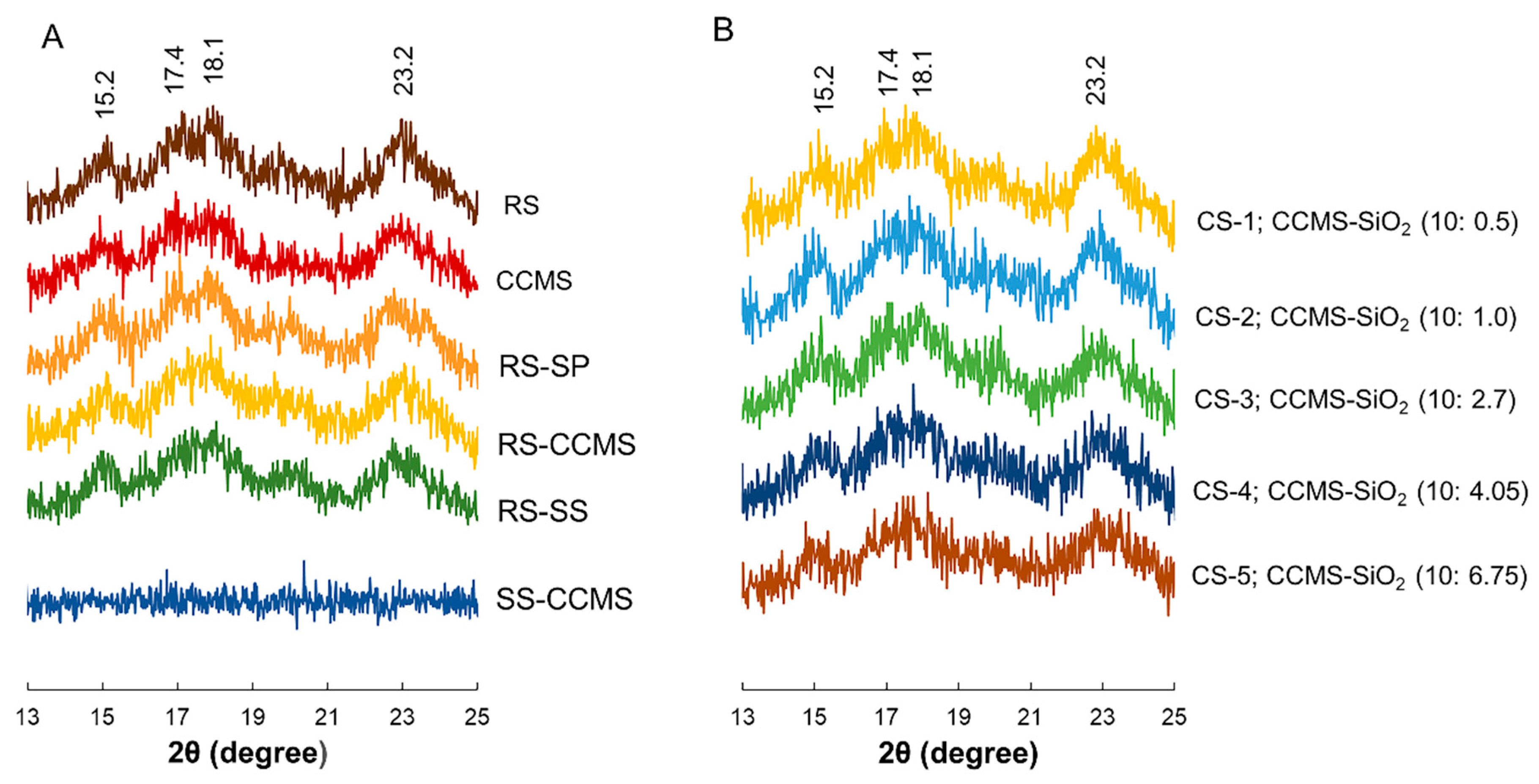
3.3. XRD
3.4. Solubility, Swelling Property, and pH
3.5. Moisture Content
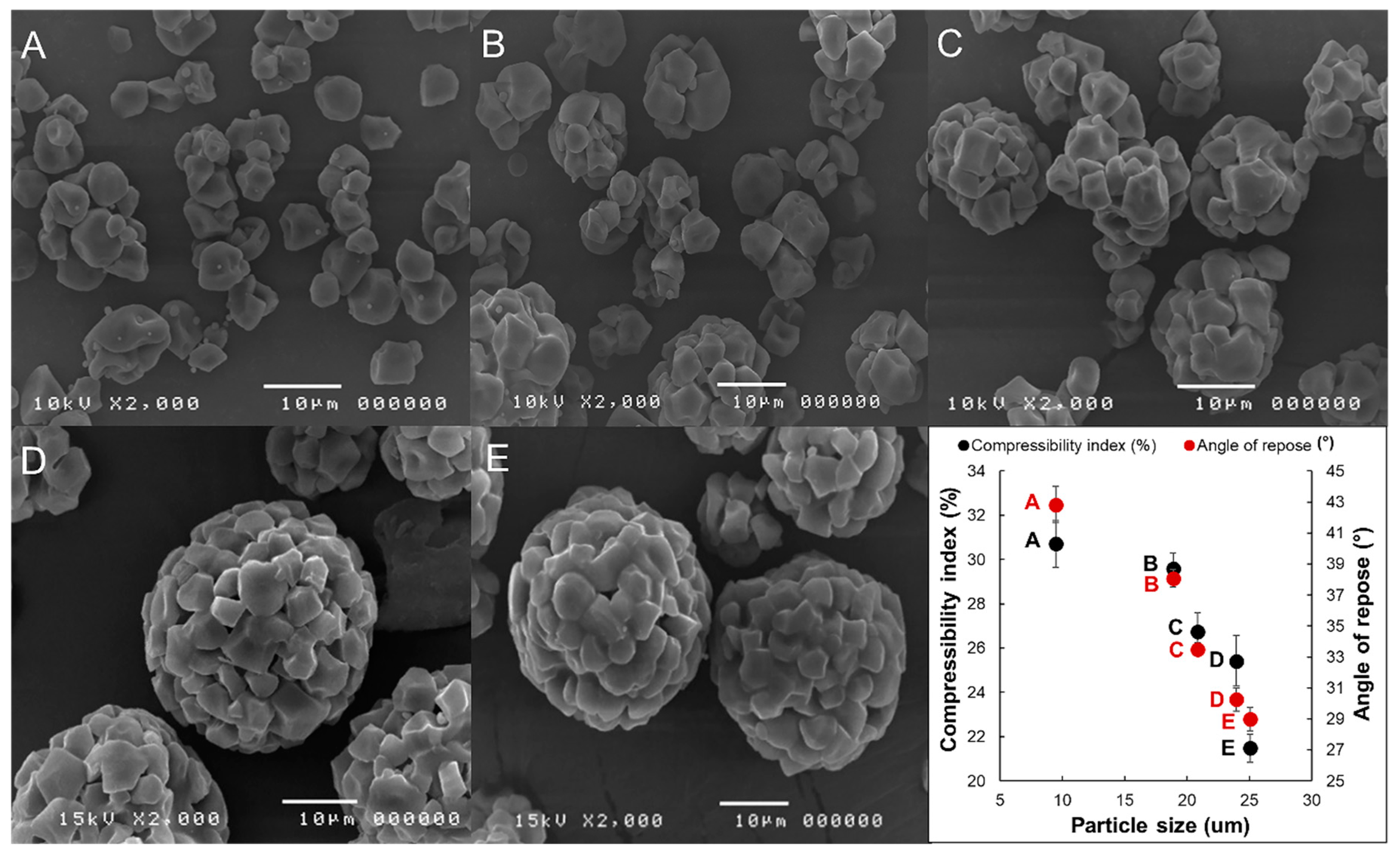
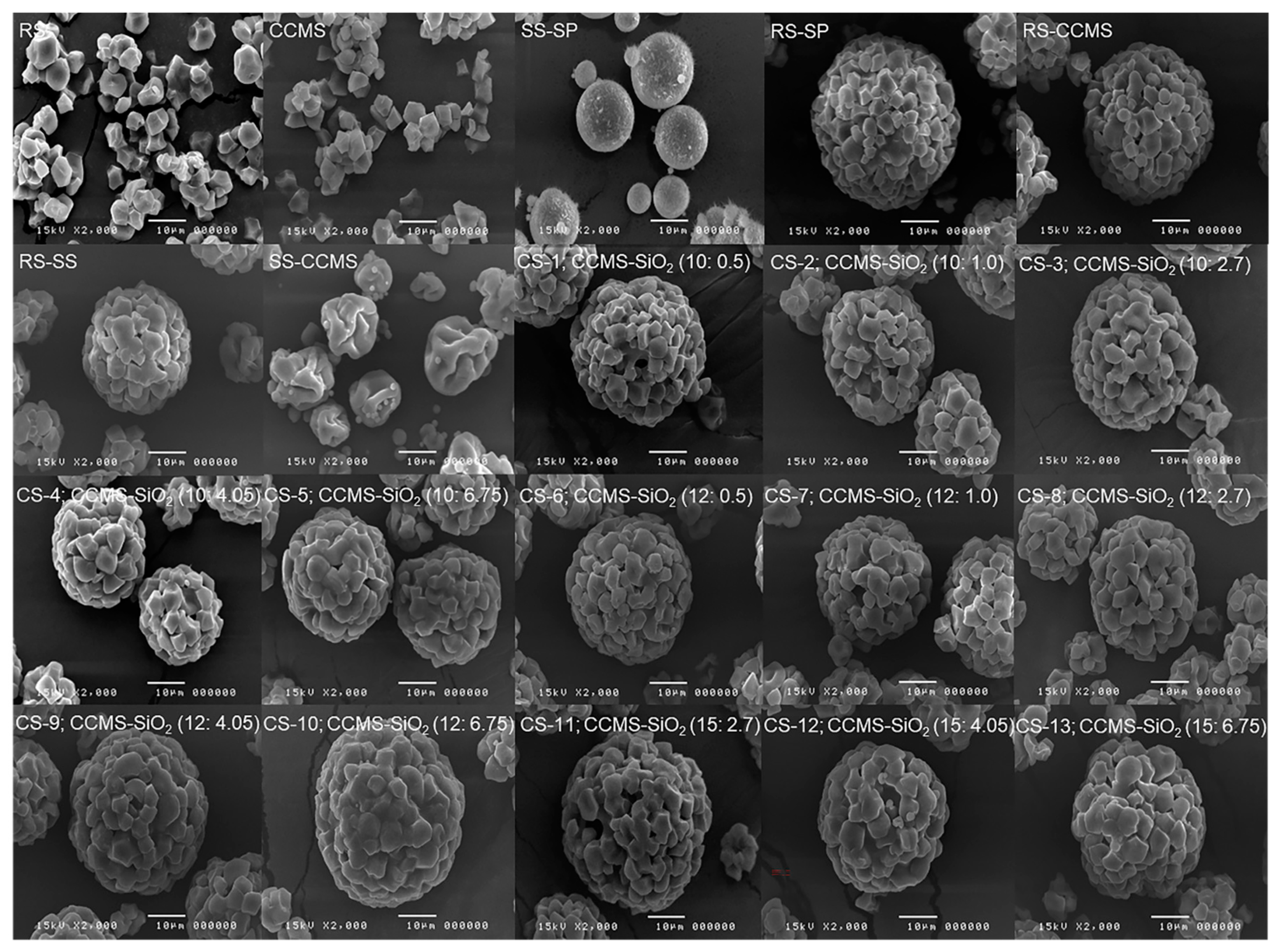
3.6. Scanning Electron Micrograph (SEM)
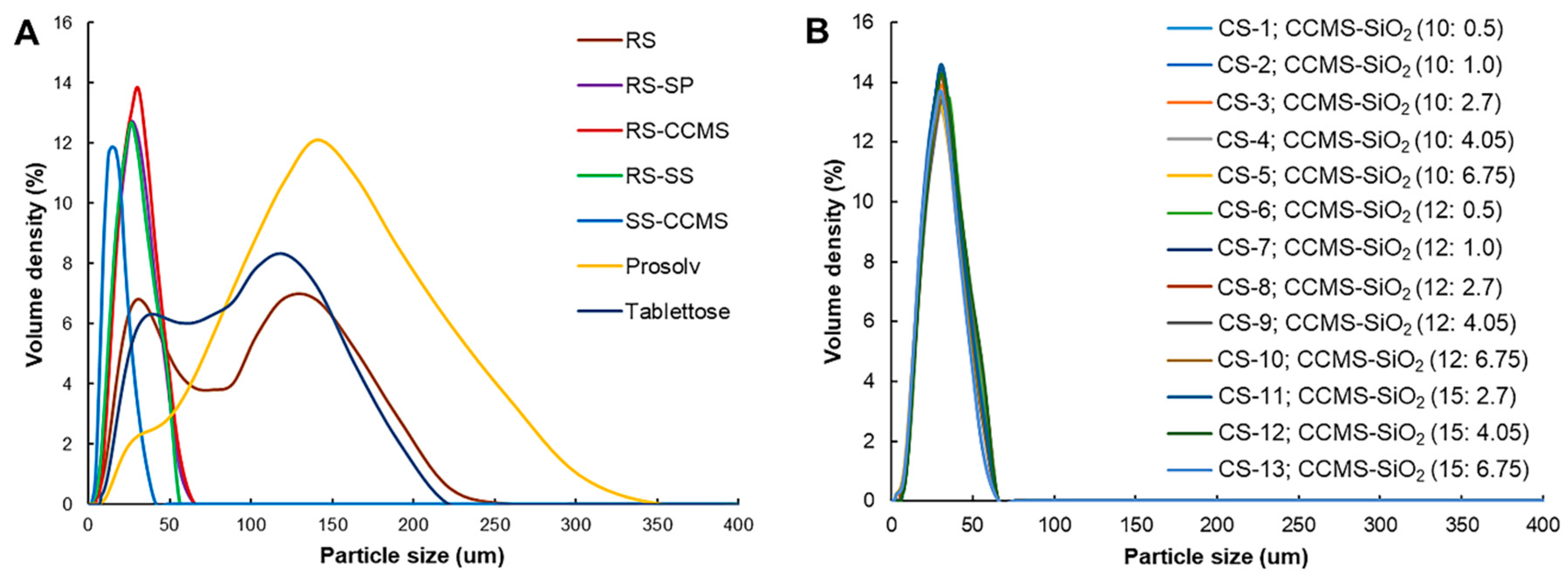
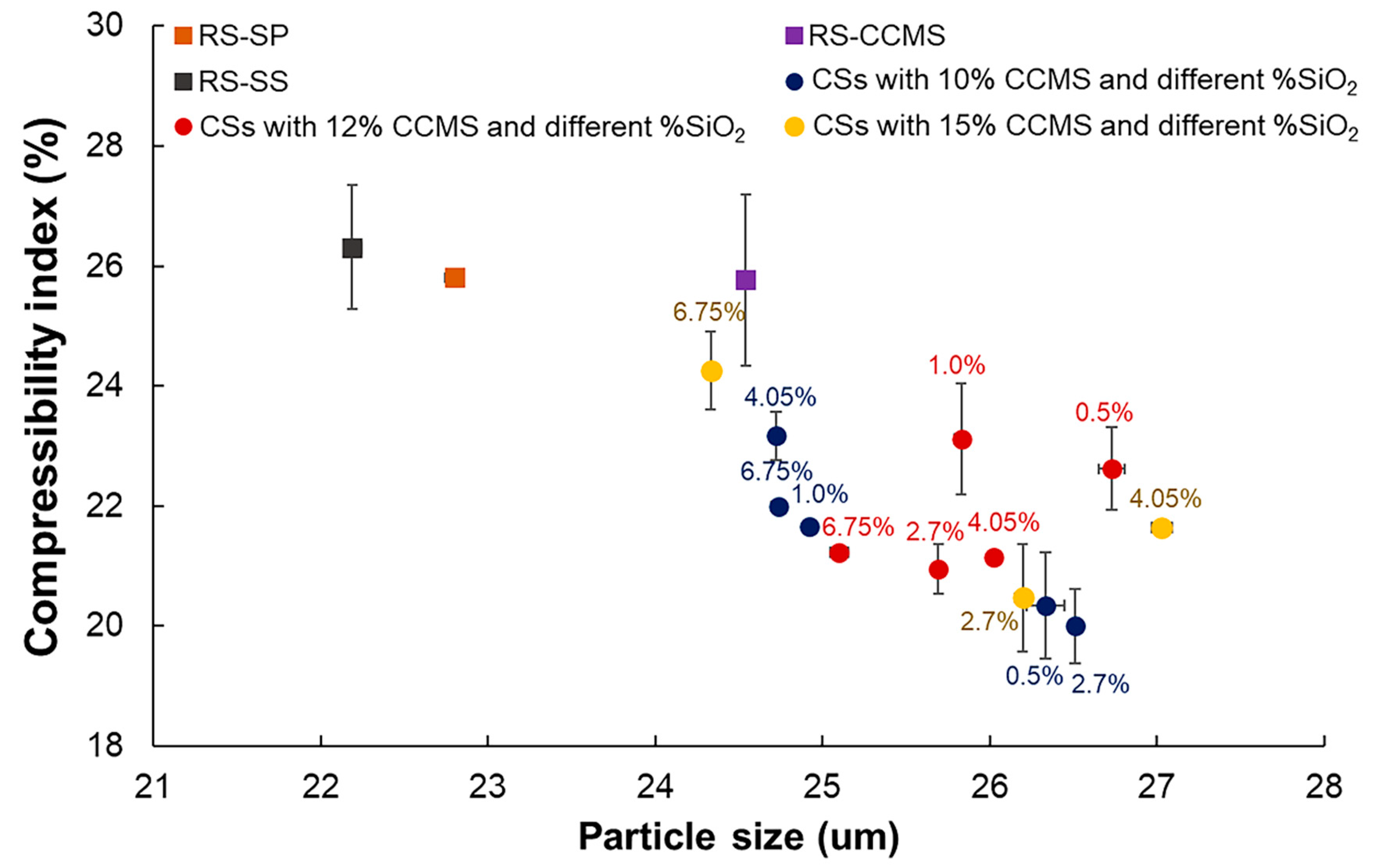
3.7. Powder Characteristics (Particle Size, and Powder Density)
3.8. Flow Property
3.9. Compression Behavior
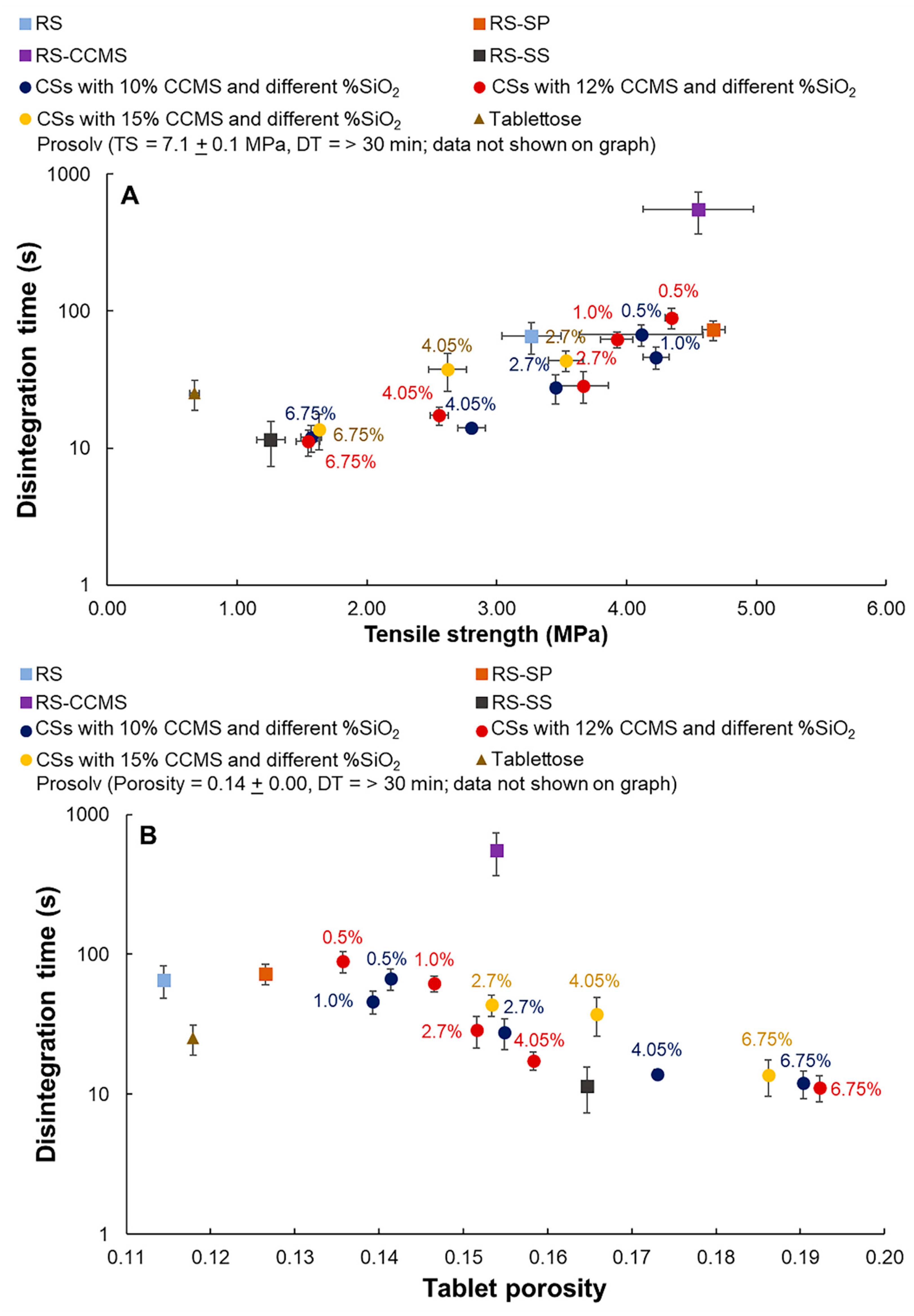
3.9.1. Tensile Strength
3.9.2. Plastic Deformation Property
3.10. Disintegration Property
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | angle of repose |
| CCMS | cross-linked carboxymethyl starch |
| CI | compressibility Index |
| CS | co-processed, rice starch-based excipient |
| DC | direct compression |
| PCS | preliminary co-processed rice starch |
| RS | native rice starch |
| RS-CCMS | rice starch co-processed with CCMS |
| RS-SP | spray dried rice starch |
| SS-CCMS | silicon dioxide co-processed with CCMS |
| SS-SP | spray-dried silicon dioxide |
References
- Rojas, J.; Buckner, I.; Kumar, V. Co-proccessed excipients with enhanced direct compression functionality for improved tableting performance. Drug Dev. Ind. Pharm. 2012, 38, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Gohel, M.C.; Jogani, P.D. A review of co-processed directly compressible excipients. J. Pharm. Pharm. Sci. 2005, 8, 76–93. [Google Scholar] [PubMed]
- Builders, P.F.; Arhewoh, M.I. Pharmaceutical applications of native starch in conventional drug delivery. Starch Stärke 2016, 68, 864–873. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice Starch Diversity: Effects on Structural, Morphological, Thermal, and Physicochemical Properties—A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Bos, C.E.; Bolhuis, G.K.; Doorne, H.V.; Lerk, C.F. Native starch in tablet formulations: Properties on compaction. Pharm. Weekbl. 1987, 9, 274–282. [Google Scholar] [CrossRef]
- Rashid, I.; Al Omari, M.M.H.; Badwan, A.A. From native to multifunctional starch-based excipients designed for direct compression formulation. Starch Stärke 2013, 65, 552–571. [Google Scholar] [CrossRef]
- Gupta, P.; Nachaegari, S.K.; Bansal, A.K. Improved Excipient Functionality by Coprocessing. In Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems; Katdare, A., Chaubal, M.V., Eds.; Informa Healthcare USA, Inc.: New York, NY, USA, 2006; pp. 109–126. [Google Scholar]
- Saha, S.; Shahiwala, A.F. Multifunctional coprocessed excipients for improved tabletting performance. Expert. Opin. Drug Deliv. 2009, 6, 197–208. [Google Scholar] [CrossRef]
- Rojas, J. Excipient design by co-processing for direct compression applications. In Excipient Applications in Formulation Design and Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 589–612. [Google Scholar]
- Garg, N.; Pandey, P.; Kaushik, D.; Dureja, H. Development of novel multifunction directly compressible co-processed excipient by melt granulation technique. Int. J. Pharm. Investig. 2015, 5, 266–274. [Google Scholar]
- Tye, C.K.; Sun, C.C.; Amidon, G.E. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J. Pharm. Sci. 2005, 94, 465–472. [Google Scholar] [CrossRef]
- Bolhuis, G.K.; Armstrong, N.A. Excipients for direct compaction—An update. Pharm. Dev. Technol. 2006, 11, 111–124. [Google Scholar] [CrossRef]
- Edge, S.; Steele, D.F.; Chen, A.; Staniforth, J.N. The mechanical properties of compacts of microcrystalline cellulose and silicified microcrystalline cellulose. Int. J. Pharm. 2000, 200, 67–72. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Morin, G.; Briens, L. The effect of lubricants on powder flowability for pharmaceutical application. Aaps Pharmscitech 2013, 14, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, B.; Bolhuis, G.K.; Wu, Y.S.; Zuurman, K.; Frijlink, H.W. Compaction mechanism and tablet strength of unlubricated and lubricated (silicified) microcrystalline cellulose. Eur. J. Pharm. Biopharm. 2005, 59, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mitrevej, A.; Sinchaipanid, N.; Faroongsarng, D. Spray-Dried Rice Starch: Comparative Evaluation of Direct Compression Fillers. Drug Dev. Ind. Pharm. 1996, 22, 587–594. [Google Scholar] [CrossRef]
- Rojas, J.; Kumar, V. Coprocessing of cellulose II with amorphous silicon dioxide: Effect of silicification on the powder and tableting properties. Drug Dev. Ind. Pharm. 2012, 38, 209–226. [Google Scholar] [CrossRef]
- Trisopon, K.; Kittipongpatana, O.S. Development of a Direct Compression Excipient from Epichlorohydrin-Crosslinked Carboxymethyl Rice Starch with Sodium Silicate Using a Coprocessing Technique. Starch Stärke 2019, 71, 1800220. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Pujchakarn, T.; Jitrakbumrung, S.; Chaiwat, W.; Fuongfuchat, A.; Varavinit, S.; Dangtip, S.; Suphantharika, M. Effect of cross-linking on physicochemical properties of tapioca starch and its application in soup product. Carbohydr. Polym. 2014, 101, 656–665. [Google Scholar] [CrossRef]
- United State Pharmacopeia. USP 42, Powder Flow; United States Pharmacopeia Convention: Rockville, MD, USA, 2019; pp. 7871–7875. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- RW, H. An analysis of powder compaction phenomena. Trans. AIME 1961, 221, 1001–1008. [Google Scholar]
- Nicklasson, F.; Alderborn, G.R. Analysis of the Compression Mechanics of Pharmaceutical Agglomerates of Different Porosity and Composition Using the Adams and Kawakita Equations. Pharm. Res. 2000, 17, 949–954. [Google Scholar] [CrossRef] [PubMed]
- United State Pharmacopeia. USP 42, Disintegration; United States Pharmacopeial Convention: Rockville, MD, USA, 2019; pp. 6866–6868. [Google Scholar]
- Spychaj, T.; Wilpiszewska, K.; Zdanowicz, M. Medium and high substituted carboxymethyl starch: Synthesis, characterization and application. Starch Stärke 2013, 65, 22–33. [Google Scholar] [CrossRef]
- Kalita, D.; Kaushik, N.; Mahanta, C.L. Physicochemical, morphological, thermal and IR spectral changes in the properties of waxy rice starch modified with vinyl acetate. J. Food Sci. Technol. 2014, 51, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, E.F.; Komarevskaya, A.S. IR Spectroscopic Study of the Phase Composition for Sodium Silicate Synthesized in Aqueous Medium. Glass Ceram. 2007, 64, 7–11. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Zheng, G.; Jiang, X. A TiO2/crosslinked carboxymethyl starch composite for high-efficiency adsorption and photodegradation of cationic golden yellow X-GL dye. Environ. Sci. Pollut. Res. Int. 2019, 26. [Google Scholar] [CrossRef]
- Alam, F.; Hasnain, A. Studies on swelling and solubility of modified starch from Taro (Colocasia esculenta): Effect of pH and temperature. Agric. Conspec. Sci. 2009, 74, 45–50. [Google Scholar]
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Wang, J.; Trinkle, D.; Derbin, G.; Timmins; Desai, D. Moisture adsorption and desorption properties of colloidal silicon dioxide and its impact on layer adhesion of a bilayer tablet formulation. J. Excip. Food Chem. 2014, 5, 21–31. [Google Scholar]
- Kittipongpatana, O.S.; Kittipongpatana, N. Physicochemical, in vitro digestibility and functional properties of carboxymethyl rice starch cross-linked with epichlorohydrin. Food Chem. 2013, 141, 1438–1444. [Google Scholar] [CrossRef]
- United State Pharmacopeia. USP 42, Rice Starch; United States Pharmacopeia Convention: Rockville, MD, USA, 2019; pp. 6002–6004. [Google Scholar]
- United State Pharmacopeia. USP 42, Silicified Microcrystalline Cellulose; United States Pharmacopeia Convention: Rockville, MD, USA, 2019; pp. 5652–5654. [Google Scholar]
- United State Pharmacopeia. USP 42, Lactose Monohydrate; United States Pharmacopeia Convention: Rockville, MD, USA, 2019; pp. 5796–5797. [Google Scholar]
- Chauhan, S.I.; Nathwani, S.V.; Soniwala, M.M.; Chavda, J.R. Development and characterization of multifunctional directly compressible co-processed excipient by spray drying method. Aaps Pharmscitech 2017, 18, 1293–1301. [Google Scholar] [CrossRef]
- Sun, Z.; Ya, N.; Adams, R.C.; Fang, F.S. Particle Size Specifications for Solid Oral Dosage Forms: A Regulatory Perspective. Am. Pharm. Rev. 2010, 13. [Google Scholar]
- Rashid, I.; Al-Remawi, M.; Eftaiha, A.; Badwan, A. Chitin-silicon dioxide coprecipitate as a novel superdisintegrant. J. Pharm. Sci. 2008, 97, 4955–4969. [Google Scholar] [CrossRef] [PubMed]
- Abu Fara, D.; Al-Hmoud, L.; Rashid, I.; Chowdhry, B.Z.; Badwan, A. Understanding the Performance of a Novel Direct Compression Excipient Comprising Roller Compacted Chitin. Mar. Drugs 2020, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Hoag, S.W.; Dave, V.S.; Moolchandani, V. Pharmaceutical Dosage Form: Tablets; Informa Healthcare USA, Inc.: New York, NY, USA, 2008; Volume 1, pp. 562–563. [Google Scholar]
- Mirani, A.G.; Patankar, S.P.; Borole, V.S.; Pawar, A.S.; Kadam, V.J. Direct Compression High Functionality Excipient Using Coprocessing Technique: A Brief Review. Curr. Drug Deliv. 2011, 8, 426–435. [Google Scholar] [CrossRef]
- Jonat, S.; Albers, P.; Gray, A.; Schmidt, P.C. Investigation of the glidant properties of compacted colloidal silicon dioxide by angle of repose and X-ray photoelectron spectroscopy. Eur. J. Pharm. Biopharm. 2006, 63, 356–359. [Google Scholar] [CrossRef]
- Fu, X.; Huck, D.; Makein, L.; Armstrong, B.; Willen, U.; Freeman, T. Effect of particle shape and size on flow properties of lactose powders. Particuology 2012, 10, 203–208. [Google Scholar] [CrossRef]
- Rojas, J.; Kumar, V. Comparative evaluation of silicified microcrystalline cellulose II as a direct compression vehicle. Int. J. Pharm. 2011, 416, 120–128. [Google Scholar] [CrossRef]
- Byrn, S.R.; Zografi, G.; Chen, X. Mechanical properties of pharmaceutical materials. In Solid-State Properties of Pharmaceutical Materials; John Wiley & Sons, Inc.: New Delhi, India, 2017; pp. 231–248. [Google Scholar]
- Rojas, J.; Aristizabal, J.; Henao, M. Screening of several excipients for direct compression of tablets: A new perspective based on functional properties. J. Basic Appl. Pharm. Sci. 2013, 34, 17–23. [Google Scholar]
- Wunsch, I.; Finke, J.H.; John, E.; Juhnke, M.; Kwade, A. A Mathematical Approach to Consider Solid Compressibility in the Compression of Pharmaceutical Powders. Pharmaceutics 2019, 11, 121. [Google Scholar] [CrossRef]
- Abdel-Hamid, S.; Betz, G. Study of radial die-wall pressure changes during pharmaceutical powder compaction. Drug Dev. Ind. Pharm. 2011, 37, 387–395. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.Y.; Pan, X.; Wu, C. On identification of critical material attributes for compression behaviour of pharmaceutical diluent powders. Materials 2017, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, N.A.; Chu, K.R.; Jung, Y.J.; Yoon, J.-H.; Jeong, S.H. Material Properties and Compressibility Using Heckel and Kawakita Equation with Commonly Used Pharmaceutical Excipients. J. Pharm. Investig. 2010, 40, 237–244. [Google Scholar]
- Skelbaek-Pedersen, A.; Vilhelmsen, T.; Wallaert, V.; Rantanen, J. Quantification of fragmentation of pharmaceutical materials after tableting. J. Pharm. Sci. 2019, 108, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kou, X.; Hou, H.H.; Huang, Y.B.; Strong, J.C.; Zhang, G.G.Z.; Sun, C.C. Mechanical properties and tableting behavior of amorphous solid dispersions. J. Pharm. Sci. 2017, 106, 217–223. [Google Scholar] [CrossRef]
- El-Barghouthi, M.; Eftaiha, A.; Rashid, I.; Al-Remawi, M.; Badwan, A. A novel superdisintegrating agent made from physically modified chitosan with silicon dioxide. Drug Dev. Ind. Pharm. 2008, 34, 373–383. [Google Scholar] [CrossRef]
- Markl, D.; Sauerwein, J.; Goodwin, D.J.; Van den Ban, S.; Zeitler, J.A. Non-destructive determination of disintegration time and dissolution in immediate release tablets by terahertz transmission measurements. Pharm. Res. 2017, 34, 1012–1022. [Google Scholar] [CrossRef]
- Vodackova, P.; Vranikova, B.; Svacinova, P.; Franc, A.; Elbl, J.; Muselik, J.; Kubalak, R.; Solny, T. Evaluation and Comparison of Three Types of Spray Dried Coprocessed Excipient Avicel® for Direct Compression. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef]


| Samples | Solubility (%) n = 3 | Swellability (%) n = 3 | pH n = 3 |
|---|---|---|---|
| CS-1; CCMS-SiO2 (10:0.5) | 0.86 ± 0.16 a | 5.80 ± 0.41 b,c | 7.0 ± 0.0 a |
| CS-2; CCMS-SiO2 (10:1.0) | 1.86 ± 0.61 a | 5.57 ± 0.71 b,c | 7.1 ± 0.3 a |
| CS-3; CCMS-SiO2 (10:2.7) | 3.39 ± 0.42 a,b | 5.37 ± 0.71 b,c | 7.2 ± 0.1 a |
| CS-4; CCMS-SiO2 (10:4.05) | 5.14 ± 0.12 b,c | 5.32 ± 0.38 b,c | 10.1 ± 0.1 b |
| CS-5; CCMS-SiO2 (10:6.75) | 7.70 ± 0.64 c | 5.35 ± 0.52 b,c | 10.0 ± 0.1 b |
| CS-6; CCMS-SiO2 (12:0.5) | 2.28 ± 0.09 a,b | 5.14 ± 0.29 b,c | 7.2 ± 0.3 a |
| CS-7; CCMS-SiO2 (12:1.0) | 2.83 ± 0.12 a,b | 5.32 ± 0.38 b,c | 7.0 ± 0.0 a |
| CS-8; CCMS-SiO2 (12:2.7) | 4.90 ± 0.46 b,c | 5.24 ± 0.32 b,c | 7.1 ± 0.1 a |
| CS-9; CCMS-SiO2 (12:4.05) | 5.61 ± 0.29 b,c | 5.51 ± 0.11 b,c | 10.0 ± 0.1 b |
| CS-10; CCMS-SiO2 (12:6.75) | 8.08 ± 0.31 c | 5.24 ± 0.08 b,c | 10.1 ± 0.1 b |
| CS-11; CCMS-SiO2 (15:2.7) | 4.30 ± 0.18 b | 6.33 ± 0.34 c | 7.0 ± 0.1 a |
| CS-12; CCMS-SiO2 (15:4.05) | 5.66 ± 0.16 b,c | 5.67 ± 0.38 b,c | 10.0 ± 0.1 b |
| CS-13; CCMS-SiO2 (15:6.75) | 8.12 ± 0.15 c | 6.01 ± 0.37 b,c | 10.1 ± 0.0 b |
| RS | 1.12 ± 0.41 a | 3.49 ± 1.23 a | 7.4 ± 0.2 a |
| RS-SP | 1.26 ± 0.25 a | 3.13 ± 0.20 a | 7.2 ± 0.1 a |
| RS-CCMS | 1.53 ± 0.12 a | 4.97 ± 0.58 b | 7.0 ± 0.1 a |
| RS-SS | 4.36 ± 0.19 b | 3.73 ± 0.11 a | 7.0 ± 0.0 a |
| SS-CCMS | 35.26 ± 0.36 d | 14.89 ± 0.42 d | 10.0 ± 0.0 b |
| Materials | Density | Moisture Content n = 3 | Particle Size (um) n = 3 | ||
|---|---|---|---|---|---|
| Bulk (n = 3) | Tapped (n = 3) | True (n = 3) | |||
| CS-1; CCMS-SiO2 (10:0.5) | 0.4 ± 0.0 c | 0.6 ± 0.0 c | 1.5320 ± 0.0004 b | 7.61 ± 0.12 e | 26.33 ± 0.11 e |
| CS-2; CCMS-SiO2 (10:1.0) | 0.4 ± 0.0 c | 0.6 ± 0.0 c | 1.5383 ± 0.0003 c | 7.13 ± 0.39 d,e | 24.92 ± 0.02 c |
| CS-3; CCMS-SiO2 (10:2.7) | 0.5 ± 0.0 d | 0.6 ± 0.0 d | 1.5598 ± 0.0004 d | 6.91 ± 0.10 d,e | 26.51 ± 0.03 e,f |
| CS-4; CCMS-SiO2 (10:4.05) | 0.5 ± 0.0 d | 0.6 ± 0.0 e | 1.5749 ± 0.0002 e | 7.19 ± 0.54 d,e | 24.72 ± 0.03 c |
| CS-5; CCMS-SiO2 (10:6.75) | 0.5 ± 0.0 e | 0.7 ± 0.0 f | 1.5901 ± 0.0003 f | 5.45 ± 0.14 c | 24.73 ± 0.04 c |
| CS-6; CCMS-SiO2 (12:0.5) | 0.4 ± 0.0 c | 0.6 ± 0.0 c | 1.5279 ± 0.0002 b | 6.16 ± 0.44 c,d | 26.73 ± 0.08 f |
| CS-7; CCMS-SiO2 (12:1.0) | 0.4 ± 0.0 c | 0.6 ± 0.0 c | 1.5334 ± 0.0004 b | 6.69 ± 0.26 d | 25.83 ± 0.04 d |
| CS-8; CCMS-SiO2 (12:2.7) | 0.5 ± 0.0 d | 0.6 ± 0.0 d | 1.5572 ± 0.0002 d | 7.13 ± 0.10 d,e | 25.69 ± 0.02 d |
| CS-9; CCMS-SiO2 (12:4.05) | 0.5 ± 0.0 e | 0.7 ± 0.0 e,f | 1.5690 ± 0.0003 e | 6.99 ± 0.13 d,e | 26.02 ± 0.03 d,e |
| CS-10; CCMS-SiO2 (12:6.75) | 0.5 ± 0.0 e | 0.7 ± 0.0 f | 1.5888 ± 0.0009 f | 6.88 ± 0.21 d,e | 25.10 ± 0.05 c |
| CS-11; CCMS-SiO2 (15:2.7) | 0.50.0 e | 0.6 ± 0.0 d | 1.5589 ± 0.0004 d | 8.25 ± 0.23 e | 26.20 ± 0.05 d,e |
| CS-12; CCMS-SiO2 (15:4.05) | 0.5 ± 0.0 d | 0.6 ± 0.0 e | 1.5716 ± 0.0003 e | 6.88 ± 0.38 d,e | 27.03 ± 0.06 f |
| CS-13; CCMS-SiO2 (15:6.75) | 0.5 ± 0.0 d | 0.7 ± 0.0 f | 1.5928 ± 0.0005 f | 5.78 ± 0.23 c | 24.33 ± 0.01 c |
| RS | 0.3 ± 0.0 a | 0.5 ± 0.0 b | 1.5291 ± 0.0009 b | 10.75 ± 0.51 f | 60.42 ± 0.06 g |
| RS-SP | 0.4 ± 0.0 c | 0.6 ± 0.0 c,d | 1.5154 ± 0.0003 a | 5.77 ± 0.29 c | 22.80 ± 0.06 b |
| RS-CCMS | 0.4 ± 0.0 b | 0.5 ± 0.0 c | 1.5225 ± 0.0008 a | 6.24 ± 0.14 c,d | 24.54 ± 0.03 c |
| RS-SS | 0.5 ± 0.0 c,d | 0.6 ± 0.0 d,e | 1.5466 ± 0.0004 c | 5.28 ± 0.14 c | 22.19 ± 0.02 b |
| SS-CCMS | 0.6 ± 0.0 f | 1.5 ± 0.0 h | 1.7553 ± 0.0007 h | 3.11 ± 0.09 b | 13.55 ± 0.03 a |
| Prosolv® | 0.3 ± 0.0 a | 0.4 ± 0.0 a | 1.6023 ± 0.0009 g | 4.97 ± 0.24 c | 110.88 ± 0.50 i |
| Tablettose® | 0.6 ± 0.0 f | 0.7 ± 0.0 g | 1.5464 ± 0.0034 c | 0.46 ± 0.05 a | 66.49 ± 0.15 h |
| Samples | Heckel Constants | Kawakita Constants | ||||
|---|---|---|---|---|---|---|
| Py | A | r2 | a | 1/b | r2 | |
| CS-1; CCMS-SiO2 (10:0.5) | 200.00 | 1.3254 | 0.9342 | 0.69 | 4.86 | 0.9999 |
| CS-2; CCMS-SiO2 (10:1.0) | 204.08 | 1.4015 | 0.9622 | 0.69 | 3.94 | 0.9999 |
| CS-3; CCMS-SiO2 (10:2.7) | 188.68 | 1.2377 | 0.8898 | 0.68 | 6.66 | 0.9999 |
| CS-4; CCMS-SiO2 (10:4.05) | 175.44 | 1.1102 | 0.8999 | 0.68 | 8.74 | 0.9997 |
| CS-5; CCMS-SiO2 (10:6.75) | 151.52 | 0.9522 | 0.9640 | 0.66 | 13.91 | 0.9997 |
| CS-6; CCMS-SiO2 (12:0.5) | 196.08 | 1.3628 | 0.8636 | 0.70 | 4.44 | 0.9999 |
| CS-7; CCMS-SiO2 (12:1.0) | 196.08 | 1.3235 | 0.8774 | 0.71 | 5.12 | 0.9999 |
| CS-8; CCMS-SiO2 (12:2.7) | 161.29 | 1.1862 | 0.9424 | 0.69 | 7.31 | 0.9999 |
| CS-9; CCMS-SiO2 (12:4.05) | 169.49 | 1.1640 | 0.9284 | 0.66 | 8.78 | 0.9999 |
| CS-10; CCMS-SiO2 (12:6.75) | 161.29 | 0.9644 | 0.9569 | 0.65 | 13.87 | 0.9999 |
| CS-11; CCMS-SiO2 (15:2.7) | 151.52 | 1.1495 | 0.9596 | 0.69 | 7.45 | 0.9999 |
| CS-12; CCMS-SiO2 (15:4.05) | 163.93 | 1.1099 | 0.9379 | 0.67 | 9.09 | 0.9999 |
| CS-13; CCMS-SiO2 (15:6.75) | 163.93 | 1.0143 | 0.9677 | 0.67 | 11.00 | 0.9999 |
| RS | 196.08 | 1.5486 | 0.9519 | 0.74 | 5.45 | 0.9999 |
| RS-SP | 161.29 | 1.3240 | 0.9044 | 0.71 | 5.34 | 0.9999 |
| RS-CCMS | 175.44 | 1.2507 | 0.9819 | 0.74 | 5.45 | 0.9999 |
| RS-SS | 204.08 | 1.1767 | 0.8839 | 0.69 | 6.88 | 0.9999 |
| SS-CCMS | 212.77 | 1.0315 | 0.9933 | 0.69 | 6.88 | 0.9999 |
| Prosolv® | 156.25 | 1.3610 | 0.9692 | 0.79 | 11.39 | 0.9999 |
| Tablettose® | 243.90 | 1.7658 | 0.9915 | 0.60 | 4.16 | 0.9999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trisopon, K.; Kittipongpatana, N.; Kittipongpatana, O.S. A Spray-Dried, Co-Processed Rice Starch as a Multifunctional Excipient for Direct Compression. Pharmaceutics 2020, 12, 518. https://doi.org/10.3390/pharmaceutics12060518
Trisopon K, Kittipongpatana N, Kittipongpatana OS. A Spray-Dried, Co-Processed Rice Starch as a Multifunctional Excipient for Direct Compression. Pharmaceutics. 2020; 12(6):518. https://doi.org/10.3390/pharmaceutics12060518
Chicago/Turabian StyleTrisopon, Karnkamol, Nisit Kittipongpatana, and Ornanong Suwannapakul Kittipongpatana. 2020. "A Spray-Dried, Co-Processed Rice Starch as a Multifunctional Excipient for Direct Compression" Pharmaceutics 12, no. 6: 518. https://doi.org/10.3390/pharmaceutics12060518
APA StyleTrisopon, K., Kittipongpatana, N., & Kittipongpatana, O. S. (2020). A Spray-Dried, Co-Processed Rice Starch as a Multifunctional Excipient for Direct Compression. Pharmaceutics, 12(6), 518. https://doi.org/10.3390/pharmaceutics12060518






