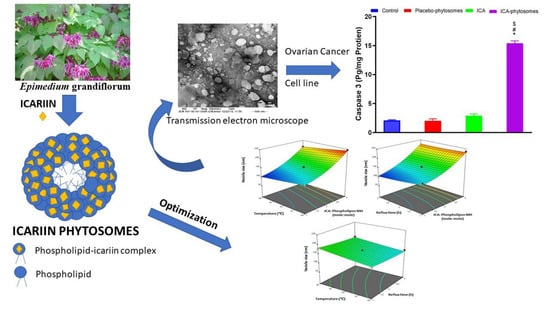
RETRACTED: Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Optimization
2.3. Preparation of Icariin (ICA) Phytosomes
2.4. Vesicle Size Measurement
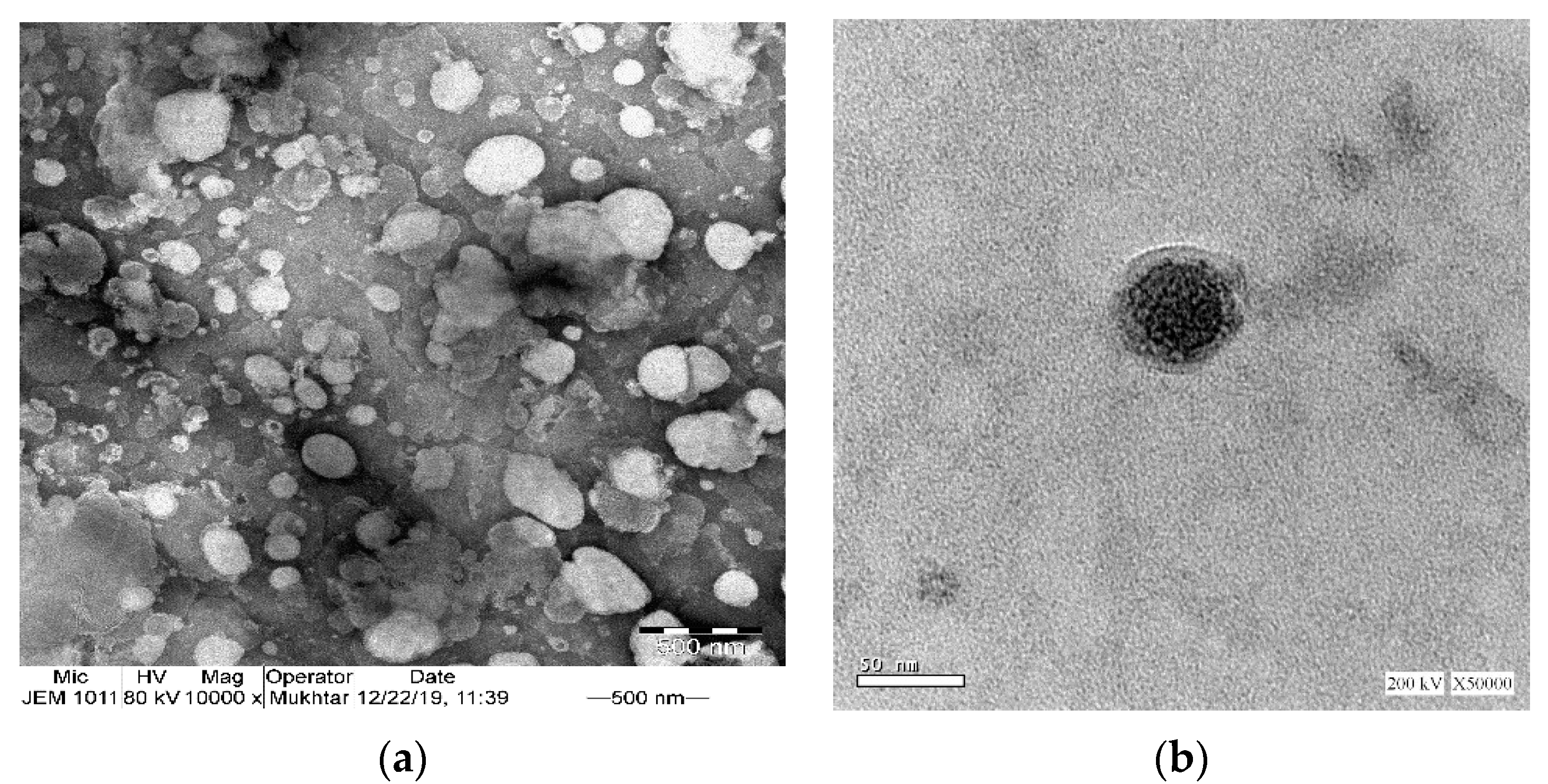
2.5. Transmission Electron Microscopy
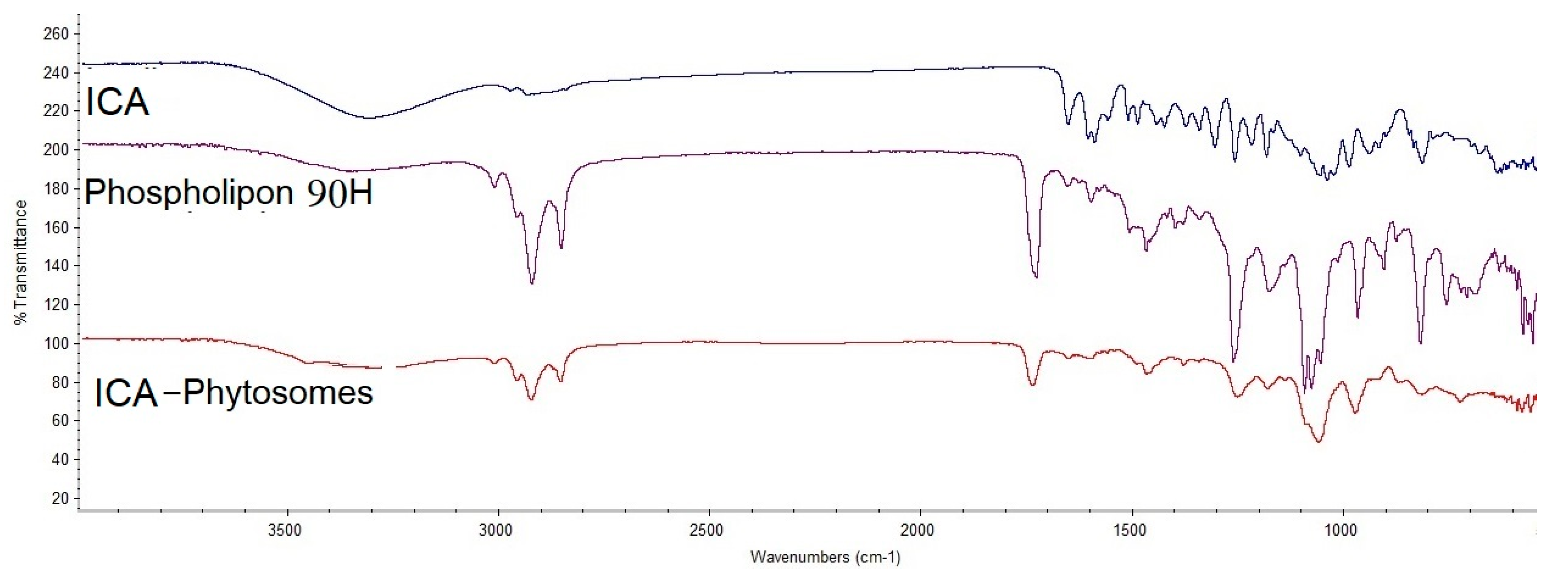
2.6. Fourier-Transform Infrared (FTIR) Spectroscopy Study
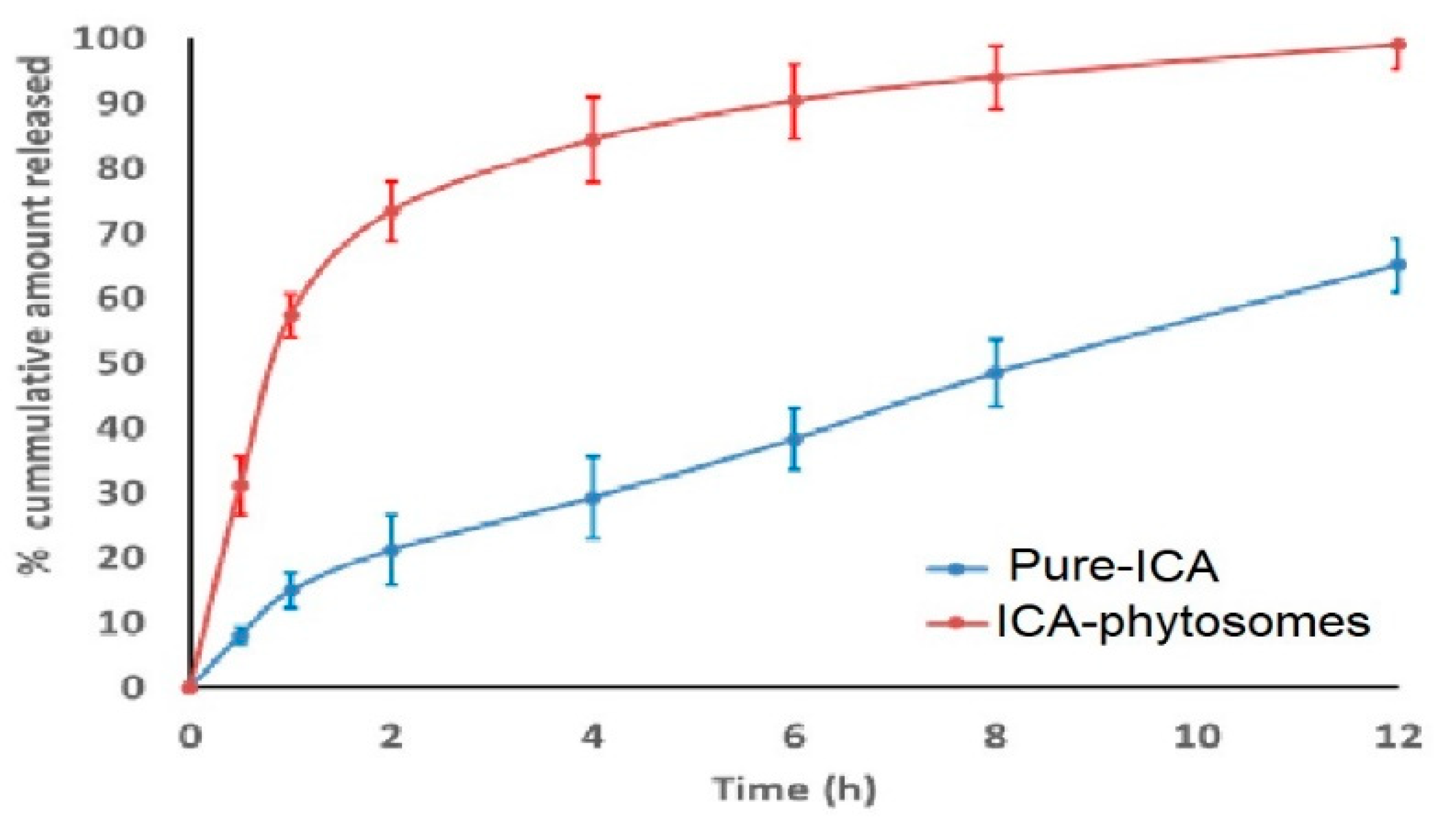
2.7. In Vitro Release
2.8. Cytotoxic Effects of Optimized ICA Phytosomes on Ovarian Cancer Cells
2.8.1. Cell Culture and Determination of IC50 using MTT Assay
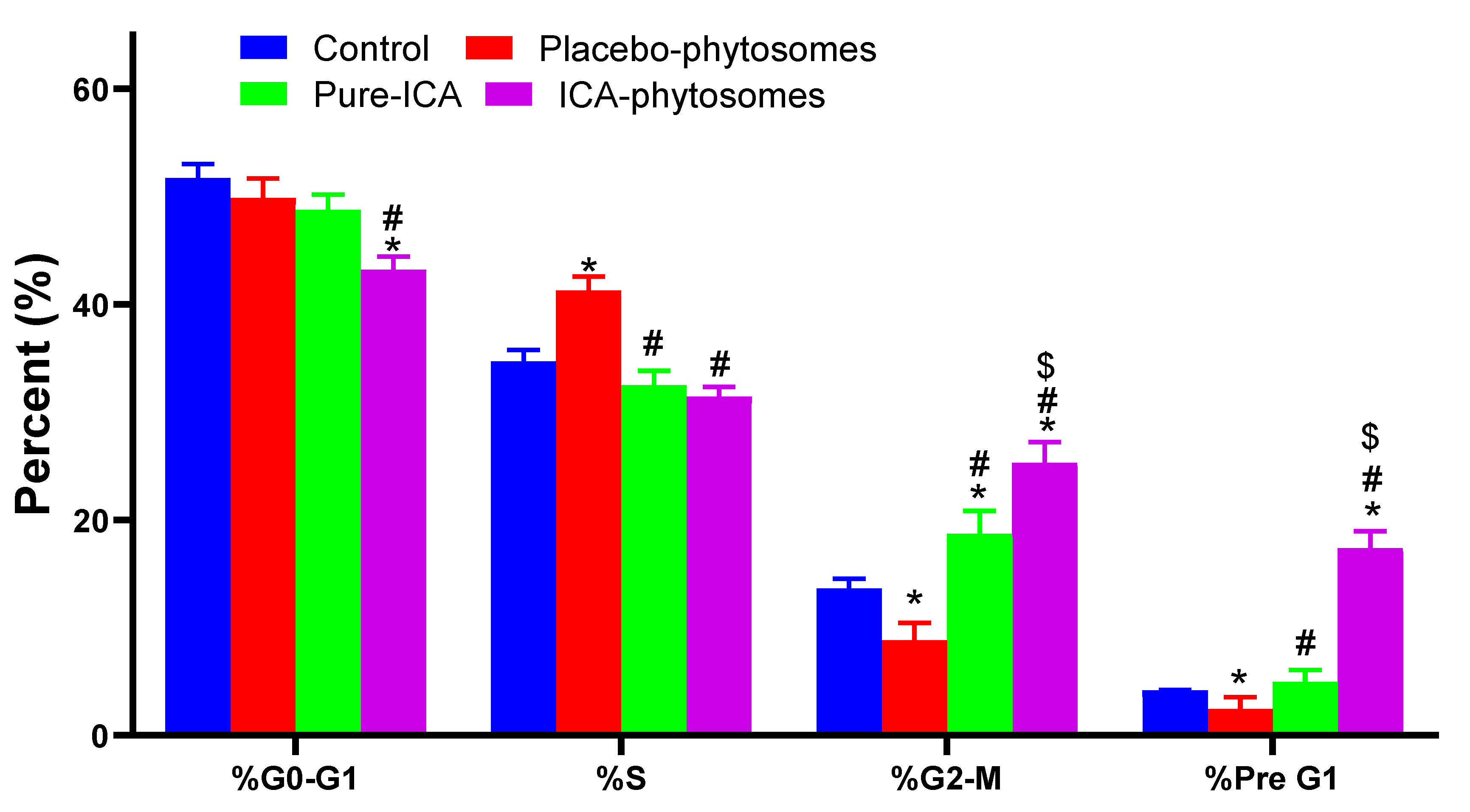
2.8.2. Cell Cycle Analysis
2.8.3. Annexin V Staining
2.8.4. Mitochondrial Membrane Potential
2.8.5. Caspase-3 Assay
2.8.6. Reactive Oxygen Species (ROS) Determination
2.8.7. Statistical Analysis
3. Results
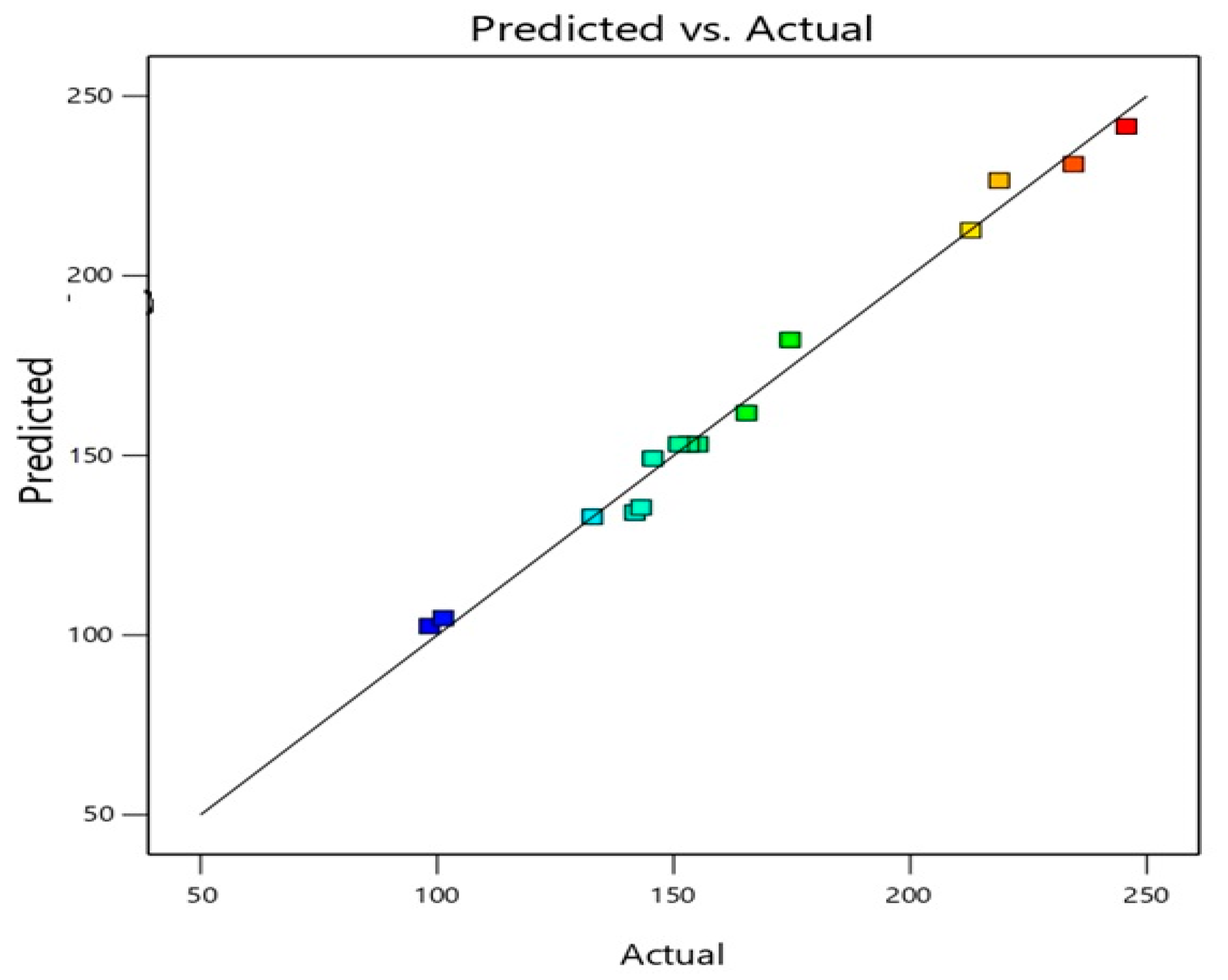
3.1. ICA Phytosomes Optimization
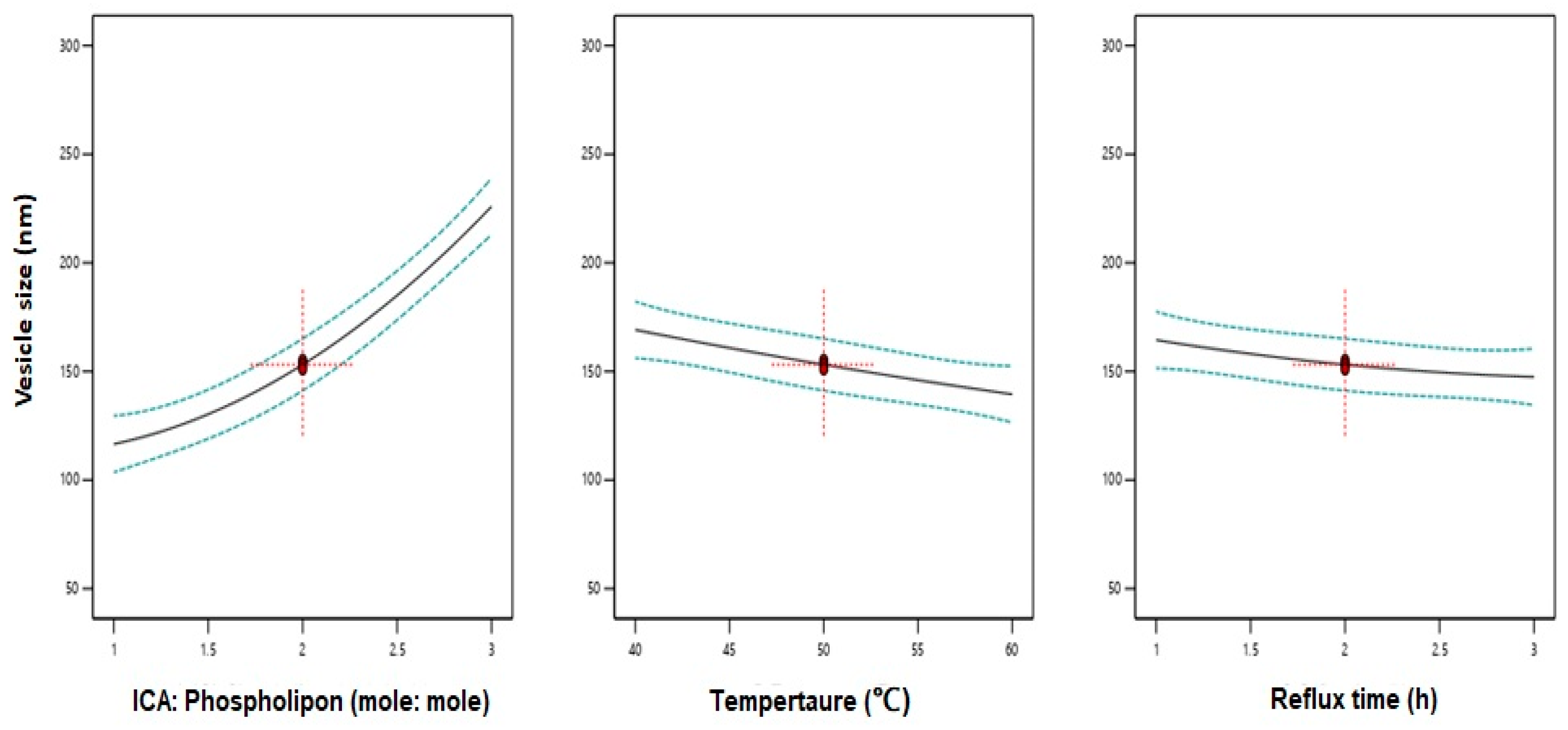
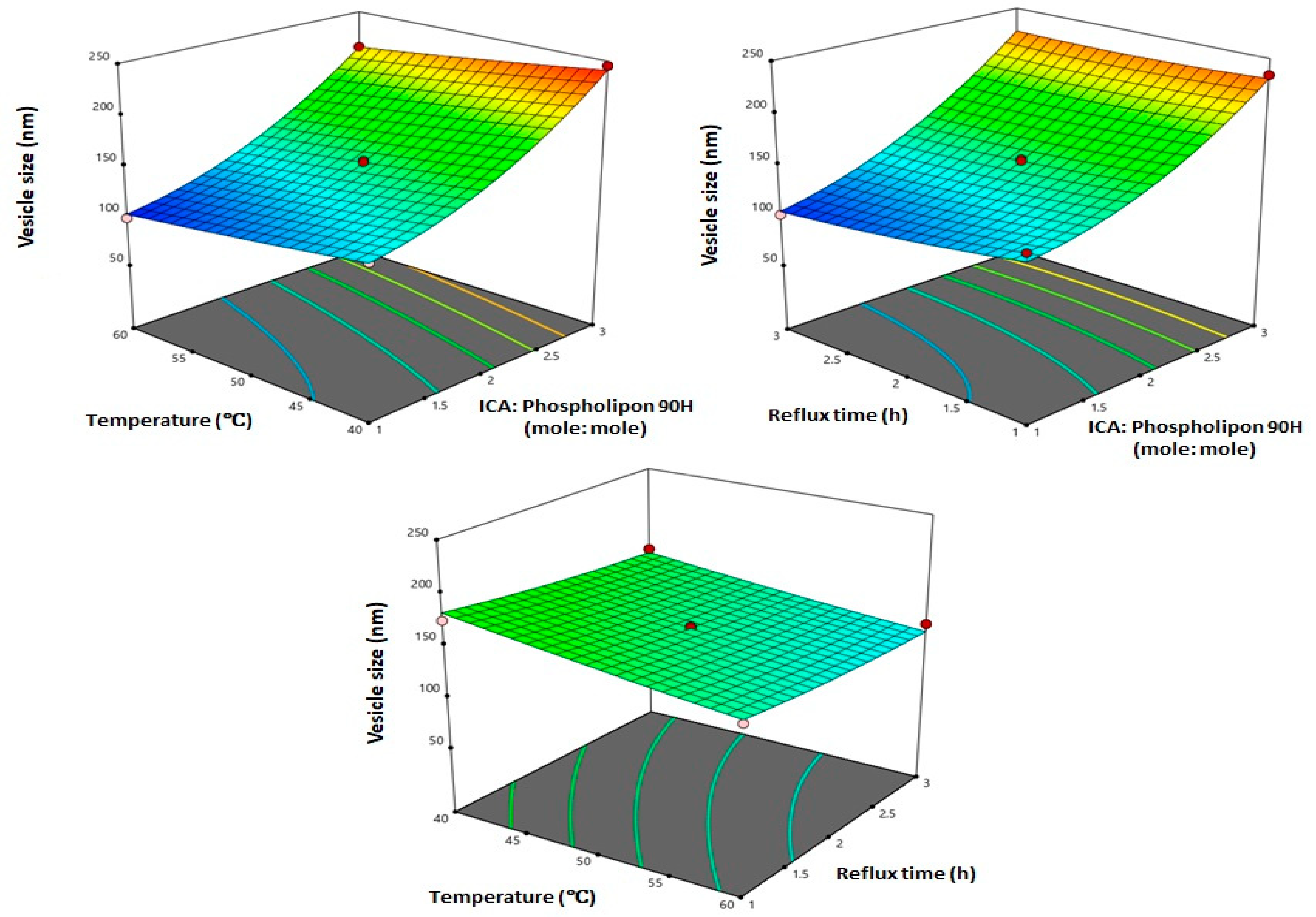
3.2. Influence of Variables on Vesicle Size
3.3. Optimization of ICA Phytosomes
3.4. In Vitro Characterization of Optimized ICA Phytosomes
3.4.1. Transmission Electron Microscopy
3.4.2. Fourier-Transform Infrared (FTIR) Spectroscopy
3.4.3. In Vitro Release
3.5. Cytotoxic Activity
3.5.1. Determination of IC50 Values
3.5.2. Cell Cycle Analysis
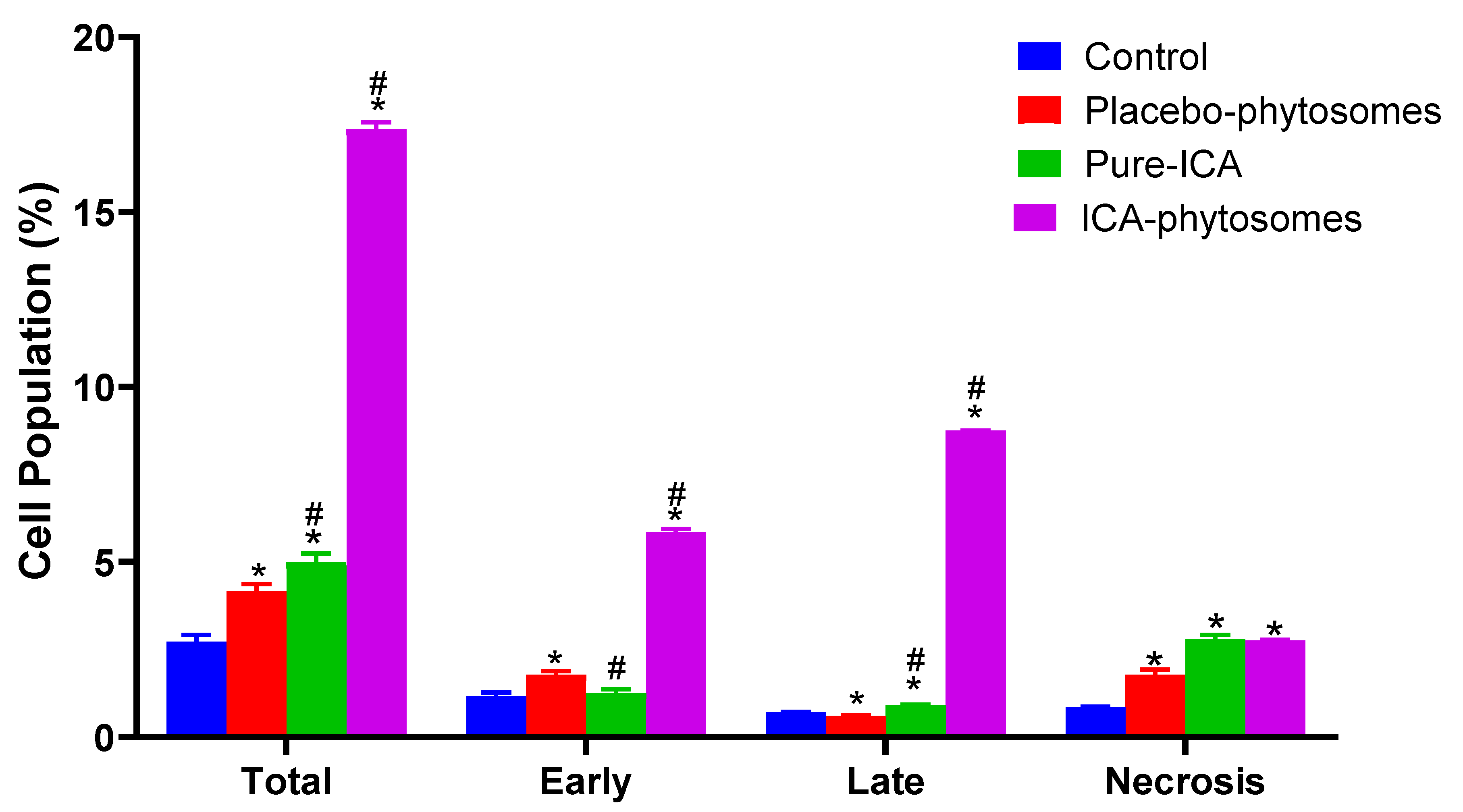
3.5.3. Annexin V staining
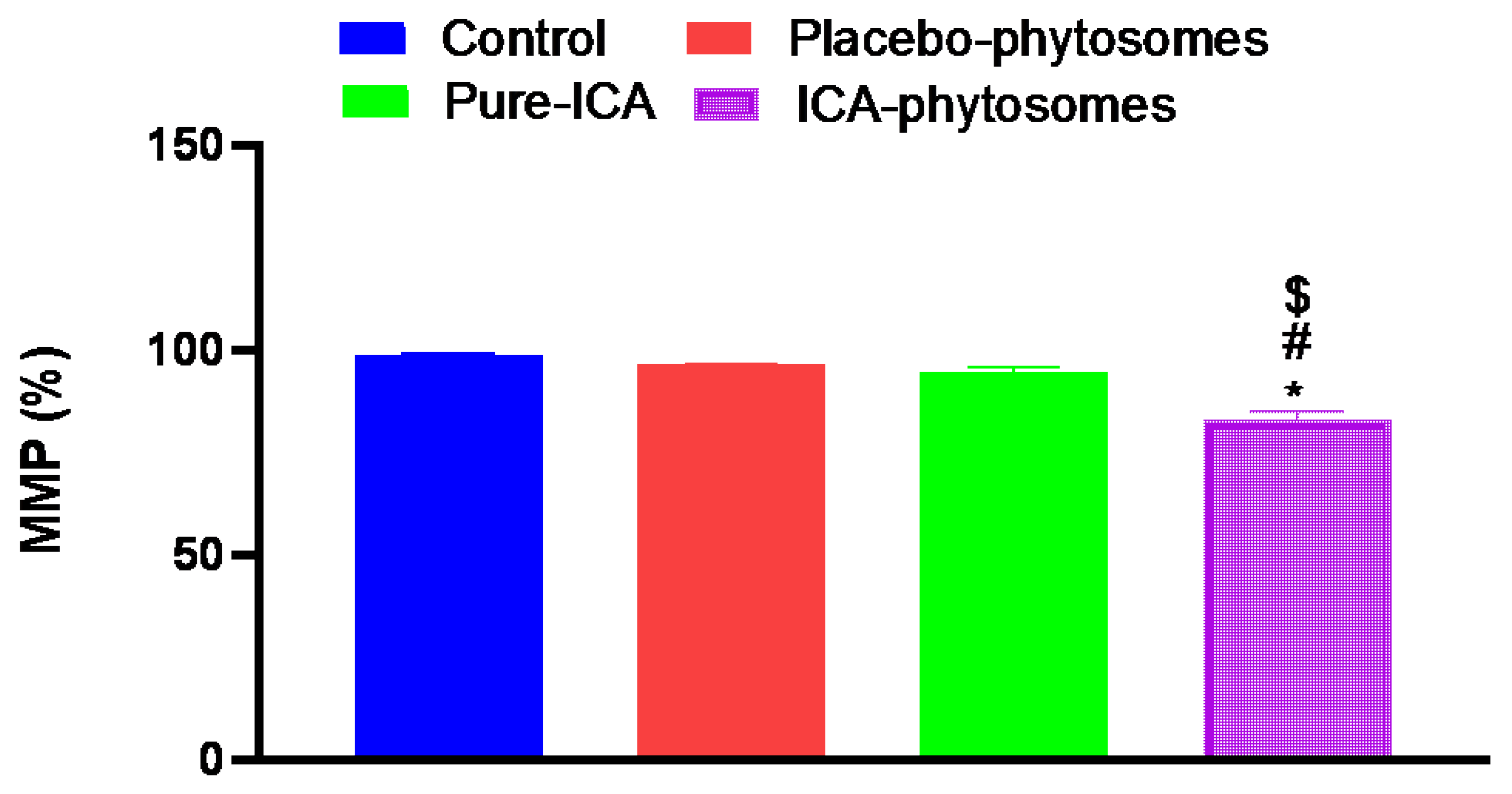
3.5.4. Mitochondrial Membrane Potential (MMP)
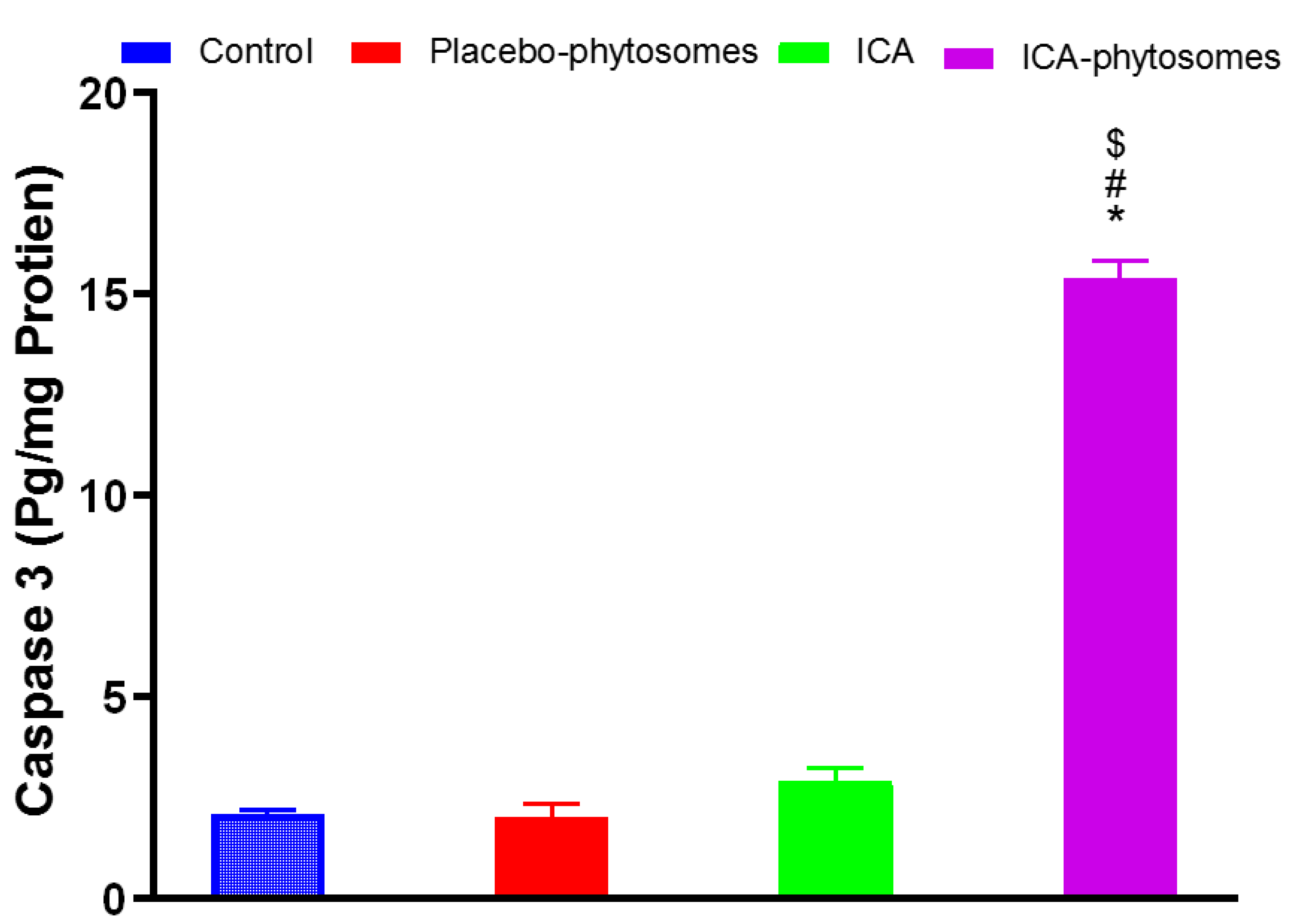
3.5.5. Caspase-3 Assay
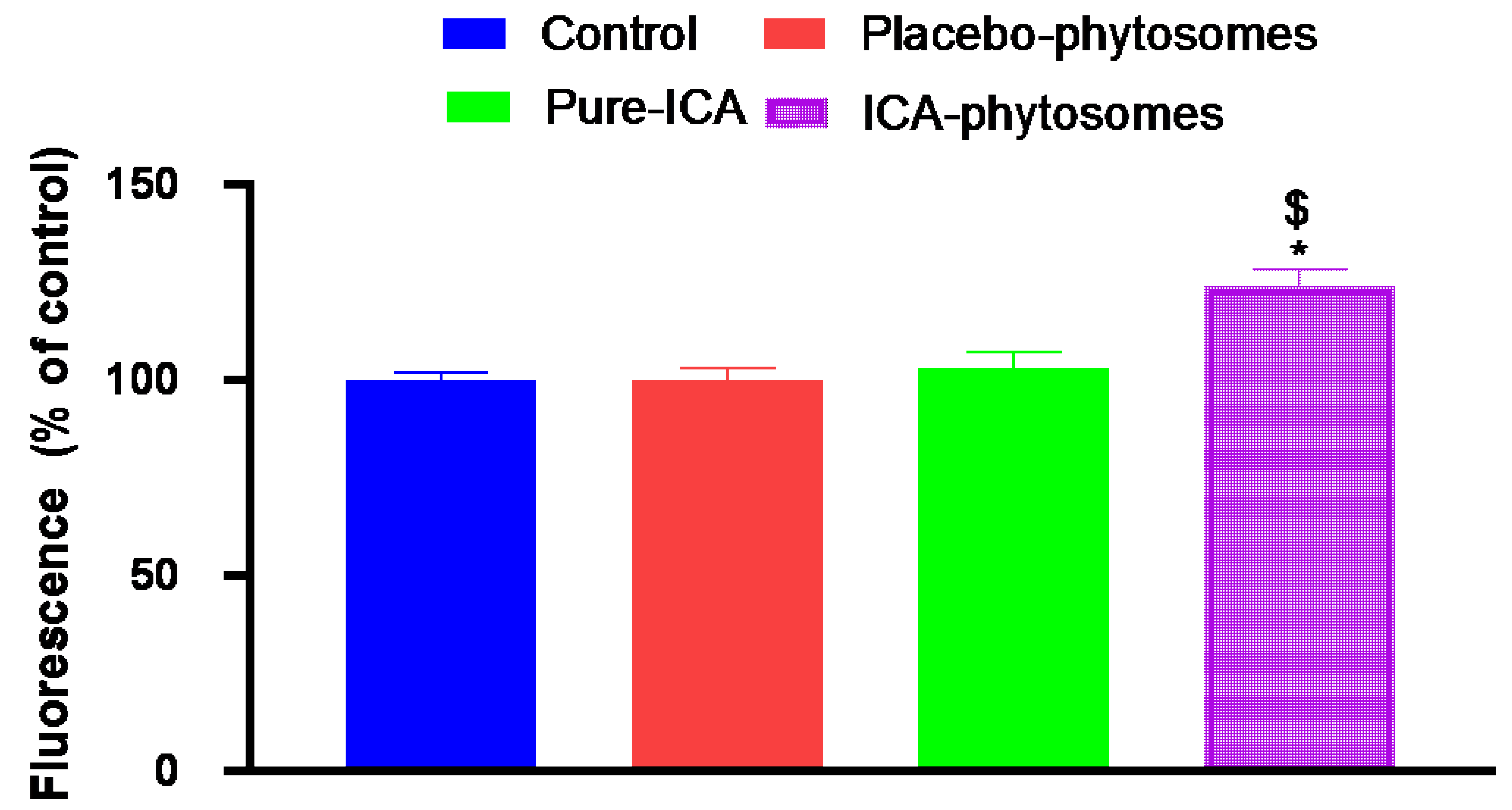
3.5.6. ROS Determination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dong, X.; Men, X.; Zhang, W.; Lei, P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J. Cancer 2014, 51, 72. [Google Scholar]
- Chobanian, N.; Dietrich, C.S. Ovarian Cancer. Surg. Clin. North Am. 2008, 88, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, H.; Hua, X.; Chen, D.; Huang, C.; Chen, Z. An outline for the pharmacological effect of icariin in the nervous system. Eur. J. Pharmacol. 2019, 842, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-M.; Low, W.Y.; Ng, C.J.; Chen, K.-K.; Sugita, M.; Ishii, N.; Marumo, K.; Lee, S.W.; Fisher, W.; Sand, M. Prevalence and correlates of erectile dysfunction (ED) and treatment seeking for ED in Asian Men: The Asian Men’s Attitudes to Life Events and Sexuality (MALES) study. J. Sex. Med. 2007, 4, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and Its Metabolites as Potential Protective Phytochemicals Against Alzheimer’s Disease. Front. Pharm. 2019, 10, 271. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y. Icariin, an Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed. Front. Pharmacol. 2017, 8, 734. [Google Scholar] [CrossRef]
- Jia, G.; Zhang, Y.; Li, W.; Dai, H. Neuroprotective role of icariin in experimental spinal cord injury via its antioxidant, anti-neuroinflammatory and anti-apoptotic properties. Mol. Med. Rep. 2019, 20, 3433–3439. [Google Scholar] [CrossRef]
- Kong, L.; Liu, J.; Wang, J.; Luo, Q.; Zhang, H.; Liu, B.; Xu, F.; Pang, Q.; Liu, Y.; Dong, J. Icariin inhibits TNF-α/IFN-γ induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int. Immunopharmacol. 2015, 29, 401–407. [Google Scholar] [CrossRef]
- Zhou, H.; Yuan, Y.; Liu, Y.; Deng, W.; Zong, J.; Bian, Z.-Y.; Dai, J.; Tang, Q.-Z. Icariin attenuates angiotensin II-induced hypertrophy and apoptosis in H9c2 cardiomyocytes by inhibiting reactive oxygen species-dependent JNK and p38 pathways. Exp. Ther. Med. 2014, 7, 1116–1122. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Breikaa, R.M.; Eid, B.G.; Neamatallah, T.A.; Abdel-Naim, A.B.; Ashour, O.M. Icariin protects against thioacetamide-induced liver fibrosis in rats: Implication of anti-angiogenic and anti-autophagic properties. Pharmacol. Rep. 2017, 69, 616–624. [Google Scholar] [CrossRef]
- Wong, M.S.; Mok, S.K.; Chen, W.F.; Lai, W.P.; Leung, P.C.; Wang, X.L.; Yao, X.S. Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor-dependent osteoblastic functions in UMR 106 cells. Br. J. Pharmacol. 2010, 159, 939–949. [Google Scholar]
- Zhang, X.; Sun, H.; Su, Q.; Lin, T.; Zhang, H.; Zhang, J.; Dang, S.; Zhu, Z. Antidepressant-like activity of icariin mediated by group I mGluRs in prenatally stressed offspring. Brain Dev. 2017, 39, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Saokaew, S.; Duangjai, A.; Lee, L.-H.; Goh, B.-H. Anti-Cancer Properties of the Naturally Occurring Aphrodisiacs: Icariin and Its Derivatives. Front. Pharmacol. 2016, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Lee, J.H.; Nam, D.; Narula, A.S.; Namjoshi, O.A.; Blough, B.E.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Anti-myeloma Effects of Icariin Are Mediated Through the Attenuation of JAK/STAT3-Dependent Signaling Cascade. Front. Pharmacol. 2018, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, Z.; Shi, J. Pharmacological effects of icariin. Adv. Pharmacol. 2020, 87, 179–203. [Google Scholar]
- Li, S.; Dong, P.; Wang, J.; Zhang, J.; Gu, J.; Wu, X.; Wu, W.; Fei, X.; Zhang, Z.; Wang, Y.; et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett. 2010, 298, 222–230. [Google Scholar] [CrossRef]
- Da, Z. Suppressive Effect of Icaritin on Angiogenesis and Its Mechanisms. J. Int. Transl. Med. 2014, 2, 385–388. [Google Scholar]
- Di, S.; Fan, C.; Yang, Y.; Jiang, S.; Liang, M.; Wu, G.; Wang, B.; Xin, Z.; Hu, W.; Zhu, Y.; et al. Activation of endoplasmic reticulum stress is involved in the activity of icariin against human lung adenocarcinoma cells. Apoptosis 2015, 20, 1229–1241. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef]
- Jiang, S.; Chang, H.; Deng, S.; Fan, D. Icariin enhances the chemosensitivity of cisplatin-resistant ovarian cancer cells by suppressing autophagy via activation of the AKT/mTOR/ATG5 pathway. Int. J. Oncol. 2019, 54, 1933–1942. [Google Scholar] [CrossRef]
- Jiang, H.L.; Zhu, K.J. Bioadhesive fluorescent microspheres as visible carriers for local delivery of drugs. I: Preparation and characterization of insulin-loaded PCEFB/PLGA microspheres. J. Microencapsul. 2002, 19, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Mishra, G.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Effect of P-glycoprotein inhibitor, verapamil, on oral bioavailability and pharmacokinetics of irinotecan in rats. Eur. J. Pharm. Sci. 2009, 36, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Q.; Mo, J.; Dai, H. Drug-Loaded Polymeric Nanoparticles for Cancer Stem Cell Targeting. Front. Pharmacol. 2017, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Ahmed, O.A.A.; Aldawsari, H.M.; Alfaifi, M.Y.; Eid, B.G.; Abdel-Naim, A.B.; Fahmy, U.A. Encapsulation of Lovastatin in Zein Nanoparticles Exhibits Enhanced Apoptotic Activity in HepG2 Cells. Int. J. Mol. Sci. 2019, 20, 5788. [Google Scholar] [CrossRef]
- Algandaby, M.M.; Al-Sawahli, M.M.; Ahmed, O.A.A.; Fahmy, U.A.; Abdallah, H.M.; Hattori, M.; Ashour, O.M.; Abdel-Naim, A.B. Curcumin-zein nanospheres improve liver targeting and antifibrotic activity of curcumin in carbon tetrachloride-induced mice liver fibrosis. J. Biomed. Nanotechnol. 2016, 12, 1746–1757. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Fahmy, U.A.; Al-Ghamdi, A.S.; Aljaeid, B.M.; Aldawsari, H.; Fahmy, O.; Sarhan, H.A.; Khairul Asri, M.G. Finasteride-loaded biodegradable nanoparticles: Near-infrared quantification of plasma and prostate levels. J. Bioact. Compat. Polym. 2017, 32, 557–567. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2019, 14, 265–274. [Google Scholar] [CrossRef]
- Gnananath, K.; Sri Nataraj, K.; Ganga Rao, B. Phospholipid Complex Technique for Superior Bioavailability of Phytoconstituents. Adv. Pharm. Bull. 2017, 7, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Damle, M.; Mallya, R. Development and Evaluation of a Novel Delivery System Containing Phytophospholipid Complex for Skin Aging. AAPS PharmSciTech 2016, 17, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Azeez, N.A.; Deepa, V.S.; Sivapriya, V. Phytosomes: Emergent promising nano vesicular drug delivery system for targeted tumor therapy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 33001. [Google Scholar] [CrossRef]
- Alfaifi, M.Y.; Shati, A.A.; Elbehairi, S.E.I.; Fahmy, U.A.; Alhakamy, N.A.; Md, S. Anti-tumor effect of PEG-coated PLGA nanoparticles of febuxostat on A549 non-small cell lung cancer cells. 3 Biotech 2020, 10, 133. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, L.; Yan, Z.; Jiang, S.; Yang, S.; Zhang, Y.; Xu, K.; He, C.; Tao, X.; Zhang, Q. mPEG-icariin nanoparticles for treating myocardial ischaemia. Artif. Cells Nanomed. Biotechnol. 2019, 47, 799–809. [Google Scholar] [CrossRef]
- Deng, G.; Wang, H.; Yao, T. Simultaneous determination of four saponins and icariin in “Qihuotongqiao” tablets by a high performance liquid chromatography - DAD method. Asian J. Pharm. Sci. 2009, 4, 115–120. [Google Scholar]
- Anwar, M.M.; Abd El-Karim, S.S.; Mahmoud, A.H.; Amr, A.E.-G.E.; Al-Omar, M.A. A Comparative Study of the Anticancer Activity and PARP-1 Inhibiting Effect of Benzofuran-Pyrazole Scaffold and Its Nano-Sized Particles in Human Breast Cancer Cells. Molecules 2019, 24, 2413. [Google Scholar] [CrossRef]
- Hu, G.; Cun, X.; Ruan, S.; Shi, K.; Wang, Y.; Kuang, Q.; Hu, C.; Xiao, W.; He, Q.; Gao, H. Utilizing G2/M retention effect to enhance tumor accumulation of active targeting nanoparticles. Sci. Rep. 2016, 6, 27669. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Wu, Y.-J.; Liu, Z.N.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Anticancer Effects of Sinulariolide-Conjugated Hyaluronan Nanoparticles on Lung Adenocarcinoma Cells. Molecules 2016, 21, 297. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, Q.; Ding, T.; Sun, J. SiO₂ nanoparticle-induced impairment of mitochondrial energy metabolism in hepatocytes directly and through a Kupffer cell-mediated pathway in vitro. Int. J. Nanomed. 2014, 9, 2891–2903. [Google Scholar]
- Hussain, S. Measurement of Nanoparticle-Induced Mitochondrial Membrane Potential Alterations BT. In Nanotoxicity: Methods and Protocols; Zhang, Q., Ed.; Springer New York: New York, NY, USA, 2019; pp. 123–131. ISBN 978-1-4939-8916-4. [Google Scholar]
- Jiang, Y.; Yu, X.; Su, C.; Zhao, L.; Shi, Y. Chitosan nanoparticles induced the antitumor effect in hepatocellular carcinoma cells by regulating ROS-mediated mitochondrial damage and endoplasmic reticulum stress. Artif. Cells Nanomed. Biotechnol. 2019, 47, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K. Passive targeting with liposomal drug carriers. Drug Deliv. Syst. 1999, 14, 433–447. [Google Scholar] [CrossRef]
- Angelico, R.; Ceglie, A.; Sacco, P.; Colafemmina, G.; Ripoli, M.; Mangia, A. Phyto-liposomes as nanoshuttles for water-insoluble silybin–phospholipid complex. Int. J. Pharm. 2014, 471, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Mishra, D.; Dutta, T.; Nahar, M.; Saraf, D.K.; Jain, N.K. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J. Control. Release 2007, 123, 148–154. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Badr-Eldin, S.M. Development of an optimized avanafil-loaded invasomal transdermal film: Ex vivo skin permeation and in vivo evaluation. Int. J. Pharm. 2019, 570, 118657. [Google Scholar] [CrossRef]
- Saoji, S.D.; Raut, N.A.; Dhore, P.W.; Borkar, C.D.; Popielarczyk, M.; Dave, V.S. Preparation and Evaluation of Phospholipid-Based Complex of Standardized Centella Extract (SCE) for the Enhanced Delivery of Phytoconstituents. AAPS J. 2016, 18, 102–114. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement BT. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. ISBN 978-1-60327-198-1. [Google Scholar]
- Mazumder, A.; Dwivedi, A.; du Preez, J.L.; du Plessis, J. In vitro wound healing and cytotoxic effects of sinigrin–phytosome complex. Int. J. Pharm. 2016, 498, 283–293. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. WIREs Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Freag, M.S.; Saleh, W.M.; Abdallah, O.Y. Self-assembled phospholipid-based phytosomal nanocarriers as promising platforms for improving oral bioavailability of the anticancer celastrol. Int. J. Pharm. 2018, 535, 18–26. [Google Scholar] [CrossRef]
- Rupp, C.; Steckel, H.; Müller, B.W. Solubilization of poorly water-soluble drugs by mixed micelles based on hydrogenated phosphatidylcholine. Int. J. Pharm. 2010, 395, 272–280. [Google Scholar] [CrossRef]
- Xu, L.; Xu, D.; Li, Z.; Gao, Y.; Chen, H. Synthesis and potent cytotoxic activity of a novel diosgenin derivative and its phytosomes against lung cancer cells. Beilstein J. Nanotechnol. 2019, 10, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Brophy, A.; Gorey, B.; Casey, A. Liposomal encapsulation of silver nanoparticles enhances cytotoxicity and causes induction of reactive oxygen species-independent apoptosis. J. Appl. Toxicol. 2018, 38, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Sakamuru, S.; Attene-Ramos, M.S.; Xia, M. Mitochondrial Membrane Potential Assay. Methods Mol. Biol. 2016, 1473, 17–22. [Google Scholar] [PubMed]
- Costantini, P.; Jacotot, E.; Decaudin, D.; Kroemer, G. Mitochondrion as a Novel Target of Anticancer Chemotherapy. J. Natl. Cancer Inst. 2000, 92, 1042–1053. [Google Scholar] [CrossRef]
- Yassemi, A.; Kashanian, S.; Zhaleh, H. Folic acid receptor-targeted solid lipid nanoparticles to enhance cytotoxicity of letrozole through induction of caspase-3 dependent-apoptosis for breast cancer treatment. Pharm. Dev. Technol. 2020, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Castro-Aceituno, V.; Abbai, R.; Lee, H.A.; Simu, S.Y.; Han, Y.; Hurh, J.; Kim, Y.-J.; Yang, D.C. Caspase-3/MAPK pathways as main regulators of the apoptotic effect of the phyto-mediated synthesized silver nanoparticle from dried stem of Eleutherococcus senticosus in human cancer cells. Biomed. Pharmacother. 2018, 99, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
| Independent Variable | Levels | ||
|---|---|---|---|
| (0) | (−1) | (+1) | |
| X1: ICA:Phospholipon® 90H molar ratio | 1:1 | 1:2 | 1:3 |
| X2: Temperature (°C) | 40 | 50 | 60 |
| X3: Reflux time (h) | 1 | 2 | 3 |
| Response | Desirability constraints | ||
| Y: Vesicle size (nm) | Minimize | ||
| Run # | Independent Variables | Vesicle Size (nm) | ||
|---|---|---|---|---|
| ICA:Phospholipon® 90H Molar Ratio | Temperature (°C) | Reflux Time (h) | ||
| 1 | 1:3 | 40 | 2 | 245.7 ± 9.8 |
| 2 | 1:1 | 60 | 2 | 98.4 ± 3.2 |
| 3 | 1:2 | 50 | 2 | 151.1 ± 6.5 |
| 4 | 1:2 | 60 | 3 | 143.1 ± 4.9 |
| 5 | 1:3 | 60 | 2 | 212.8 ± 10.9 |
| 6 | 1:1 | 50 | 3 | 101.3 ± 3.8 |
| 7 | 1:2 | 60 | 1 | 145.6 ± 4.1 |
| 8 | 1:1 | 40 | 2 | 132.8 ± 4.7 |
| 9 | 1:3 | 50 | 3 | 218.8 ± 6.9 |
| 10 | 1:1 | 50 | 1 | 141.8 ± 2.9 |
| 11 | 1:3 | 50 | 1 | 234.5 ± 8.7 |
| 12 | 1:2 | 40 | 3 | 165.4 ± 6.5 |
| 13 | 1:2 | 50 | 2 | 153.2 ± 7.4 |
| 14 | 1:2 | 50 | 2 | 155.1 ± 6.1 |
| 15 | 1:2 | 40 | 1 | 174.6 ± 8.3 |
| R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Sequential p-Value | Lack of Fit p-Value |
|---|---|---|---|---|---|
| 0.9884 | 0.9676 | 0.8185 | 21.1491 | 0.0379 | 0.0368 |
| p-value of significant terms | X1 | X2 | X3 | X12 | |
| <0.0001 | 0.0034 | 0.0307 | 0.0076 |
| Variables | X1: ICA:Phospholipon® 90H Molar Ratio | X2: Temperature (°C) | X3: Reflux Time (h) |
|---|---|---|---|
| Optimum values | 1:1.07 | 58.70 °C | 2.67 h |
| Vesicle size (nm) | Predicted value | Observed value | Error % |
| 98.25 | 104.15 | 6.01% |
| Sample | IC50 Value (µM) |
|---|---|
| Placebo-phytosomes | 94.85 ± 3.12 |
| Pure-ICA | 13.1 ± 4.0 * |
| ICA-phytosomes | 6.31 ± 0.21 *# |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhakamy, N.A.; A. Fahmy, U.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Asfour, H.Z.; Aldawsari, H.M.; Algandaby, M.M.; Eid, B.G.; Abdel-Naim, A.B.; Awan, Z.A.; et al. RETRACTED: Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells. Pharmaceutics 2020, 12, 346. https://doi.org/10.3390/pharmaceutics12040346
Alhakamy NA, A. Fahmy U, Badr-Eldin SM, Ahmed OAA, Asfour HZ, Aldawsari HM, Algandaby MM, Eid BG, Abdel-Naim AB, Awan ZA, et al. RETRACTED: Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells. Pharmaceutics. 2020; 12(4):346. https://doi.org/10.3390/pharmaceutics12040346
Chicago/Turabian StyleAlhakamy, Nabil A., Usama A. Fahmy, Shaimaa M. Badr-Eldin, Osama A. A. Ahmed, Hani Z. Asfour, Hibah M. Aldawsari, Mardi M. Algandaby, Basma G. Eid, Ashraf B. Abdel-Naim, Zuhier A. Awan, and et al. 2020. "RETRACTED: Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells" Pharmaceutics 12, no. 4: 346. https://doi.org/10.3390/pharmaceutics12040346
APA StyleAlhakamy, N. A., A. Fahmy, U., Badr-Eldin, S. M., Ahmed, O. A. A., Asfour, H. Z., Aldawsari, H. M., Algandaby, M. M., Eid, B. G., Abdel-Naim, A. B., Awan, Z. A., Alruwaili, N. K., & Mohamed, A. I. (2020). RETRACTED: Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells. Pharmaceutics, 12(4), 346. https://doi.org/10.3390/pharmaceutics12040346