HPLC-Based Analysis of Impurities in Sapropterin Branded and Generic Tablets
Abstract
1. Introduction
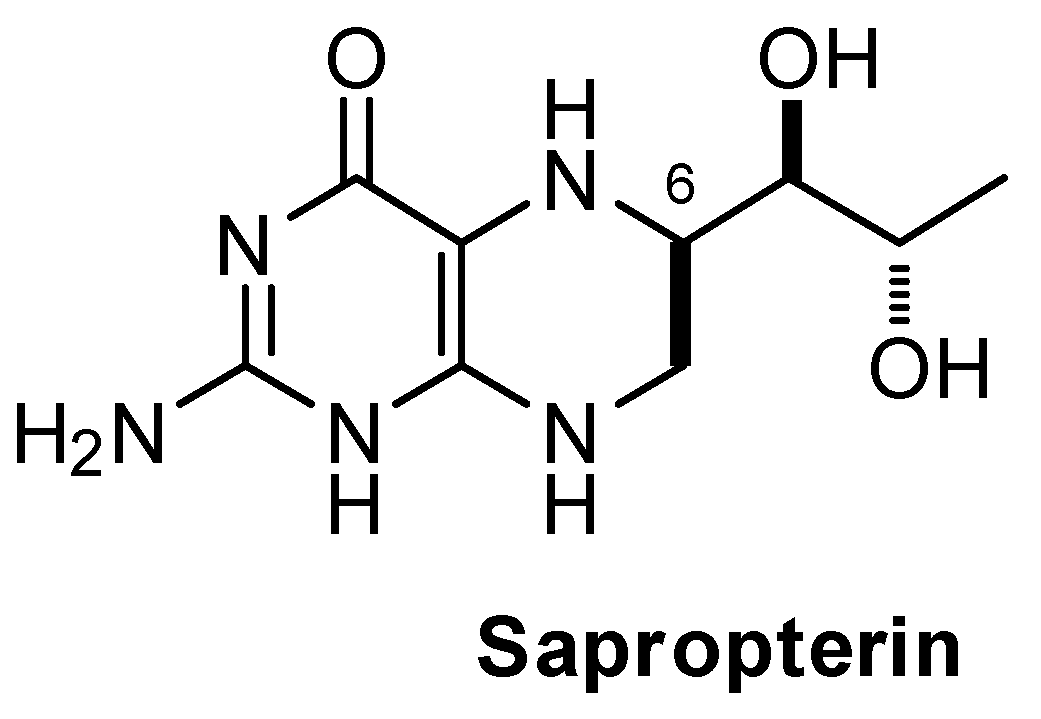
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. HPLC
2.2.2. Mass Spectrometry
3. Results
3.1. Individual Impurity Identification
3.2. Mass Spectrometry Analysis
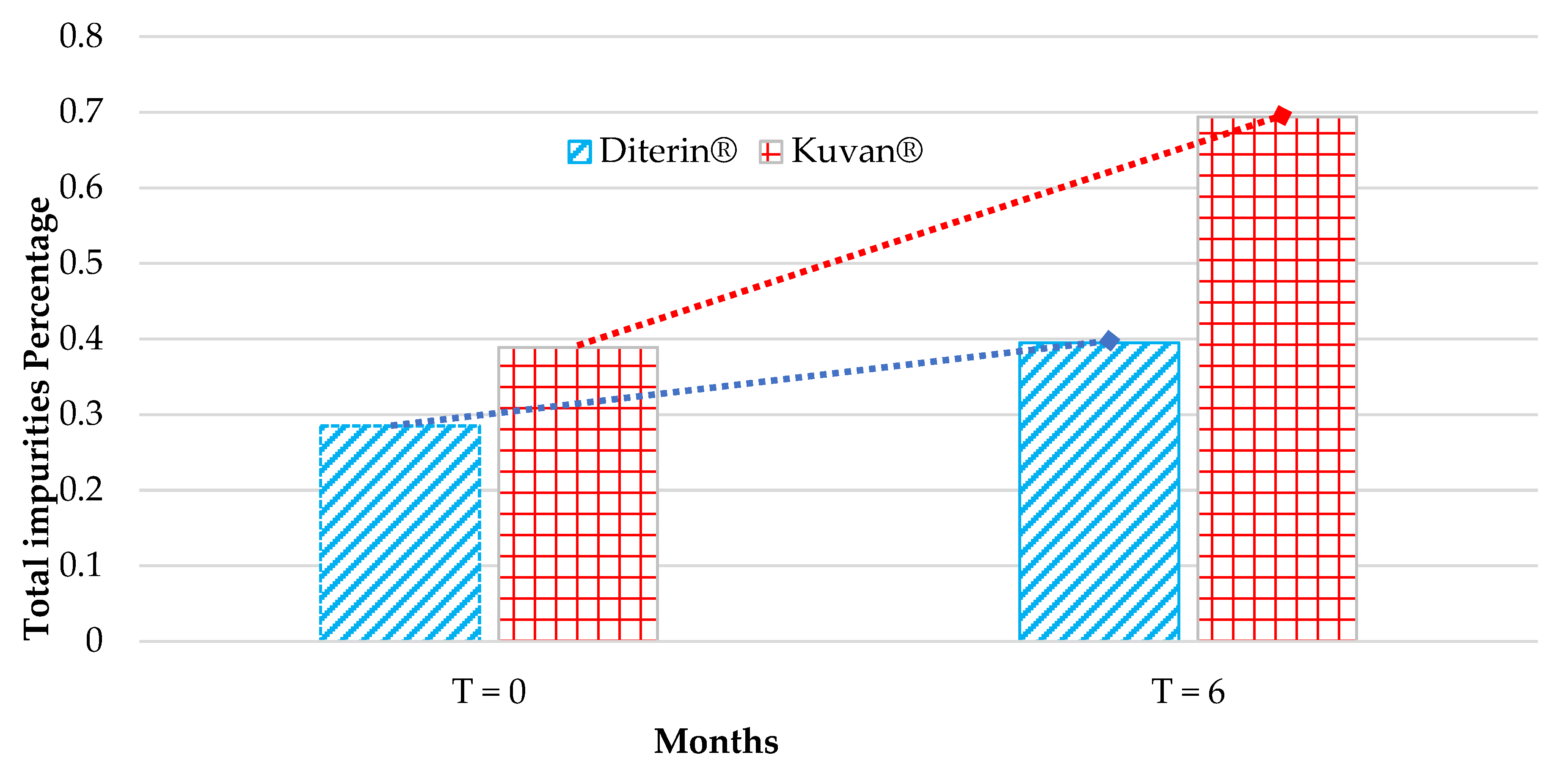
3.3. Comparative Analysis of Kuvan® and Diterin® Tablets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Muntau, A.; Röschinger, W.; Habich, M.; Demmelmair, H.; Hoffmann, B.; Sommerhoff, C.; Roscher, A. Tetrahydrobiopterin as an alternative treatment for mild phenylketonuria. N. Engl. J. Med. 2002, 347, 2122–2132. [Google Scholar] [CrossRef]
- Ogier de Baulny, H.; Abadie, V.; Feillet, F.; de Parscau, L. Management of phenylketonuria and hyperphenylalaninemia. J. Nutr. 2007, 242, 1561S–1563S. [Google Scholar] [CrossRef]
- Kuvan® Smpc, EMA Website. Available online: https://www.ema.europa.eu/en/documents/product-information/Kuvan®-epar-product-information.en.pdf (accessed on 1 February 2020).
- Mitchell, J.J.; Trakadis, Y.J.; Scriver, C.R. Phenylalanine hydroxylase deficiency. Genet. Med. 2011, 13, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Vial, J.; Cohen, M.; Sassiat, P.; Thiébaut, D. Pharmaceutical quality of docetaxel generics versus originator drug product: A comparative analysis. Curr. Med. Res. Opin. 2008, 24, 2019–2033. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug; News & Events/FDA Newsroom/Press Announcements; Gottlieb, S.; Woodcock, J. Available online: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-director-fdas-center-drug-evaluation-and-research-0 (accessed on 16 July 2019).
- Hyland, K. Estimation of tetrahydro, dihydro and fully oxidised pterins by high-performance liquid chromatography using sequential electrochemical and fluorometric detection. J. Chromatogr. B 1985, 343, 35–41. [Google Scholar] [CrossRef]
- Biondi, R.; Ambrosio, G.; De Pascali, F.; Tritto, I.; Capodicasa, E.; Druhan, L.J.; Hemann, C.; Zweier, J.L. HPLC analysis of tetrahydrobiopterin and its pteridine derivatives using sequential electrochemical and fluorimetric detection: Application to tetrahydrobiopterin autoxidation and chemical oxidation. Arch. Biochem. Biphys. 2012, 520, 7–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kappel, M.; Mengel, R.; Pfeiderer, W. Pteridines, LXXV. Synthesis and properties of biopterin and biopterin analogs. Liebigs Ann. Chem. 1984, 1815–1825. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Waring, P.; Paal, B. Pterins. VIII. The absolute configuration at C 6 of natural 2-Amino-6-[(1’R, 2’S)-1’, 2’-dihydroxypropyl]-5, 6, 7, 8-tetrahydropteridin-4 (3H)-one (L-erythro-5, 6, 7, 8-tetrahydrobiopterin). Aust. J. Chem. 1982, 35, 785–793. [Google Scholar] [CrossRef]
- Schmidt, H.; Tegederand, I.; Geisslinger, G. Determination of neopterin and biopterin by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) in rat and human plasma, cell extracts and tissue homogenates. Available online: https://protocolexchange.researchsquare.com/article/nprot-86/v1 (accessed on 1 February 2020). [CrossRef]
- Zhao, Y.; Cao, J.; Chen, Y.S.; Zhu, Y.; Patrick, C.; Chien, B.; Cheng, A.; Foehr, E.D. Detection of tetrahydrobiopterin by LC-MS/MS in plasma from multiple species. Bioanalysis 2009, 1, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Fismen, L.; Eide, T.; Djurhuus, R.; Svardal, A.M. Simultaneous quantification of tetrahydrobiopterin, dihydrobiopterin, and biopterin by liquid chromatography coupled electrospray tandem mass spectrometry. Anal. Biochem. 2012, 430, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.M.; Kaufman, S.; Milstien, S. The auto-oxidation of tetrahydrobiopterin. Eur. J. Biochem. 1988, 173, 345–351. [Google Scholar] [CrossRef] [PubMed]
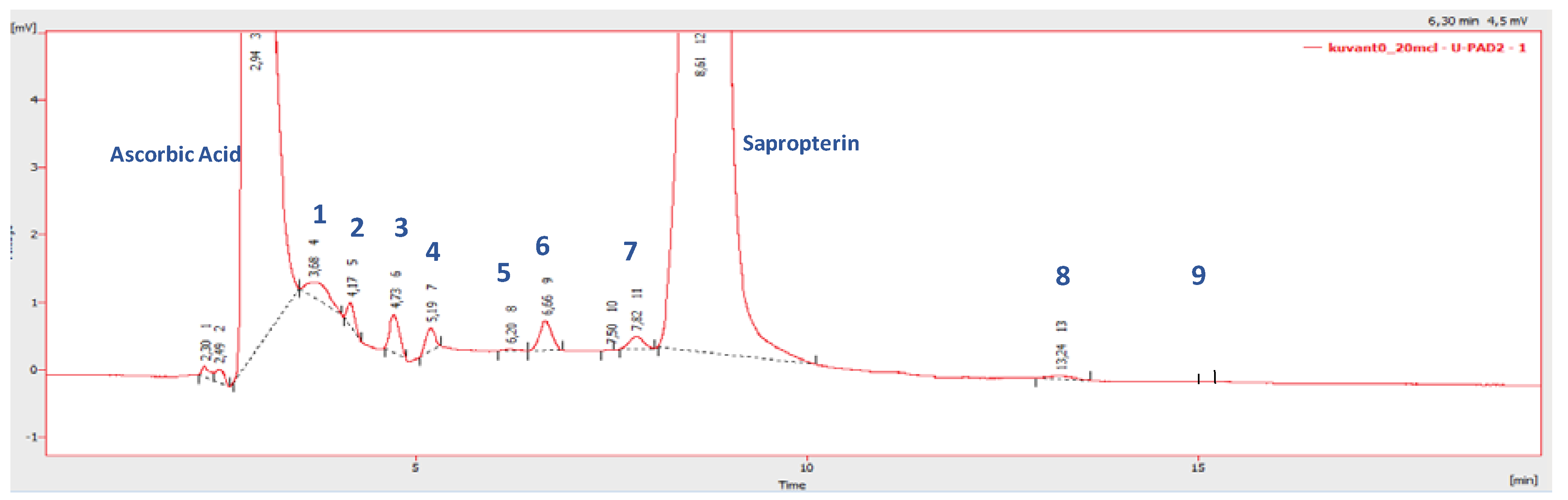
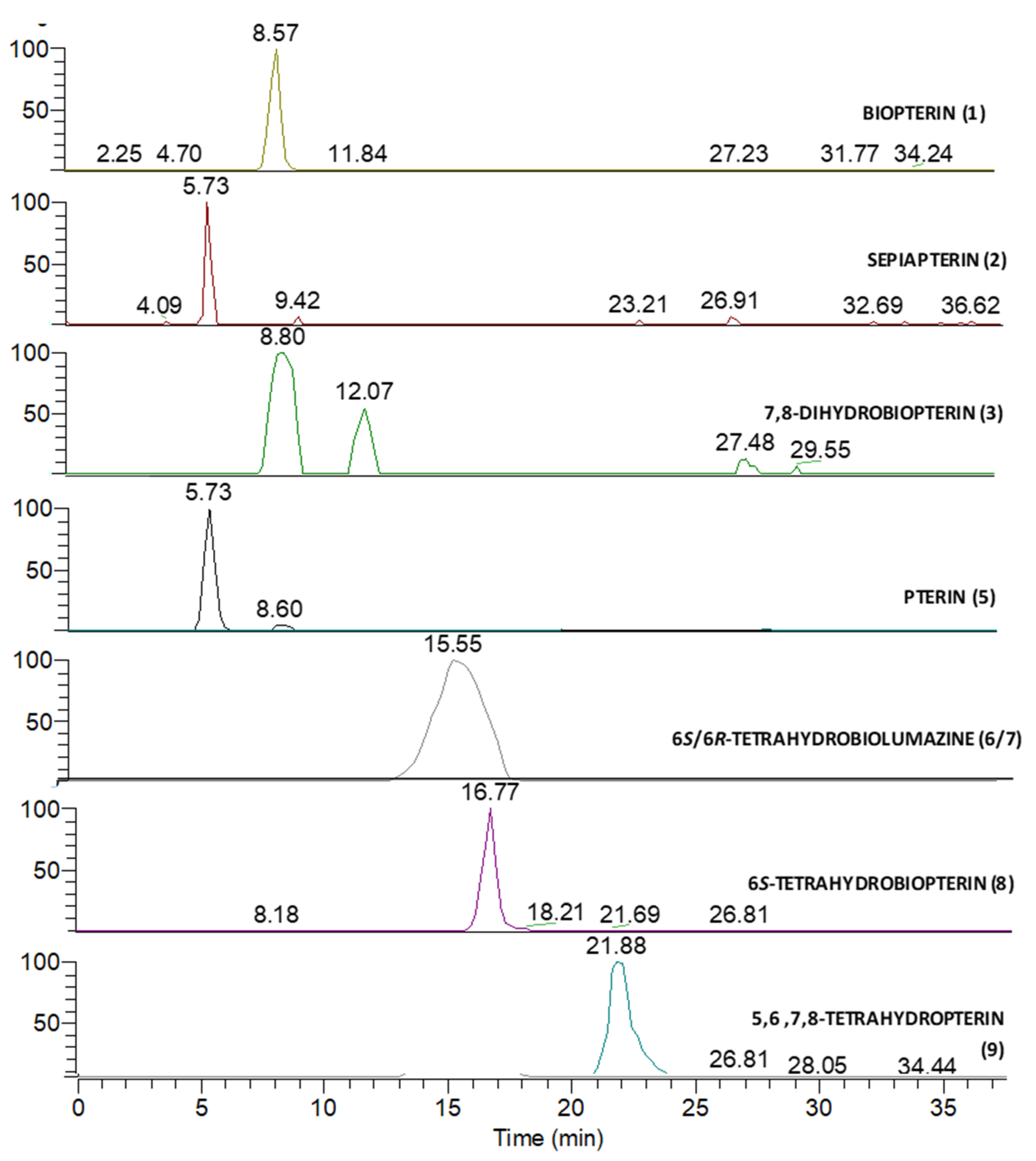
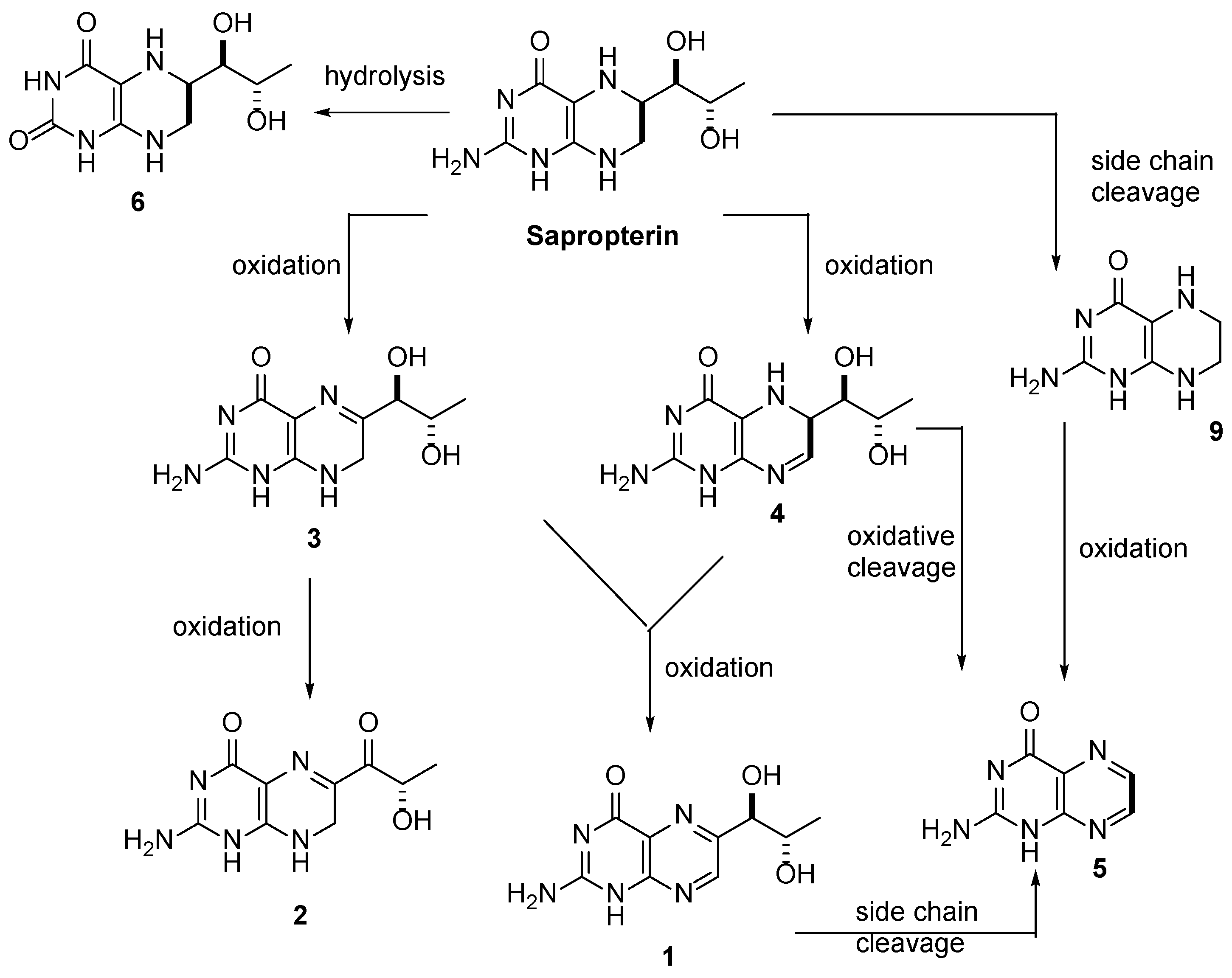
| Peak Number | Name | Chemical Structure | Standard RT (min) | Sample RT (min) |
|---|---|---|---|---|
| 1 | Biopterin |  | 3.65 | 3.68 |
| 2 | Sepiapterin | 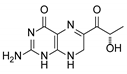 | 4.16 | 4.17 |
| 3 | 7,8-dihydrobiopterin | 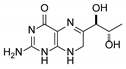 | 4.74 | 4.73 |
| 4 | 5,6-dihydrobiopterin | 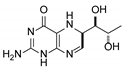 | 5.20 | 5.19 |
| 5 | Pterin | 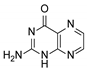 | 6.21 | 6.20 |
| 6 | 6R-tetrahydrobiolumazine |  | 6.63 | 6.66 |
| 7 | 6S-tetrahydrobiolumazine |  | 7.77 | 7.82 |
| 8 | 6S-tetrahydrobiopterin | 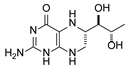 | 13.22 | 13.24 |
| 9 | 5,6,7,8-tetrahydropterin | 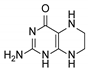 | 14.99 | 15.01 |
| Peak Number | Kuvan® T = 0 | Diterin® T = 0 | Kuvan® T = 6 | Diterin® T = 6 |
|---|---|---|---|---|
| 1 | 0.058 ± 0.008 | 0.031 ± 0.002 | 0.027 ± 0.002 | 0.030 ± 0.003 |
| 2 | 0.035 ± 0.006 | 0.037 ± 0.003 | 0.221 ± 0.012 | 0.169 ± 0.007 |
| 3 | 0.091 ± 0.004 | 0.101 ± 0.008 | 0.037 ± 0.004 | 0.031 ± 0.004 |
| 4 | 0.051 ± 0.002 | 0.032 ± 0.003 | 0.176 ± 0.009 | 0.050 ± 0.005 |
| 5 | 0.004 ± 0.001 | 0.003 ± 0.000 | 0.019 ± 0.002 | 0.004 ± 0.004 |
| 6 | 0.090 ± 0.007 | 0.011 ± 0.001 | 0.135 ± 0.009 | 0.010 ± 0.001 |
| 7 | 0.042 ± 0.001 | 0.009 ± 0.001 | 0.045 ± 0.003 | 0.006 ± 0.001 |
| 8 | 0.018 ± 0.001 | Not detectable | 0.026 ± 0.003 | 0.015 ± 0.003 |
| 9 | Not detectable | 0.061 ± 0.004 | 0.008 ± 0.001 | 0.080 ± 0.006 |
| Total | 0.389 ± 0.0046 | 0.285 ± 0.0039 | 0.694 ± 0.0066 | 0.395 ± 0.011 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scudellaro, E.; Tartaglione, L.; Varriale, F.; Dell’Aversano, C.; Taglialatela-Scafati, O. HPLC-Based Analysis of Impurities in Sapropterin Branded and Generic Tablets. Pharmaceutics 2020, 12, 323. https://doi.org/10.3390/pharmaceutics12040323
Scudellaro E, Tartaglione L, Varriale F, Dell’Aversano C, Taglialatela-Scafati O. HPLC-Based Analysis of Impurities in Sapropterin Branded and Generic Tablets. Pharmaceutics. 2020; 12(4):323. https://doi.org/10.3390/pharmaceutics12040323
Chicago/Turabian StyleScudellaro, Emanuela, Luciana Tartaglione, Fabio Varriale, Carmela Dell’Aversano, and Orazio Taglialatela-Scafati. 2020. "HPLC-Based Analysis of Impurities in Sapropterin Branded and Generic Tablets" Pharmaceutics 12, no. 4: 323. https://doi.org/10.3390/pharmaceutics12040323
APA StyleScudellaro, E., Tartaglione, L., Varriale, F., Dell’Aversano, C., & Taglialatela-Scafati, O. (2020). HPLC-Based Analysis of Impurities in Sapropterin Branded and Generic Tablets. Pharmaceutics, 12(4), 323. https://doi.org/10.3390/pharmaceutics12040323









