Novel Polymeric Formulation for Removal of Gastrointestinal Polyps by Digestive Endoscopy
Abstract
1. Introduction
2. Material and Methods
2.1. Formulation Design
2.2. Physicochemical Characterization
2.2.1. Appearance
2.2.2. pH Measurement
2.2.3. Rheological Behavior
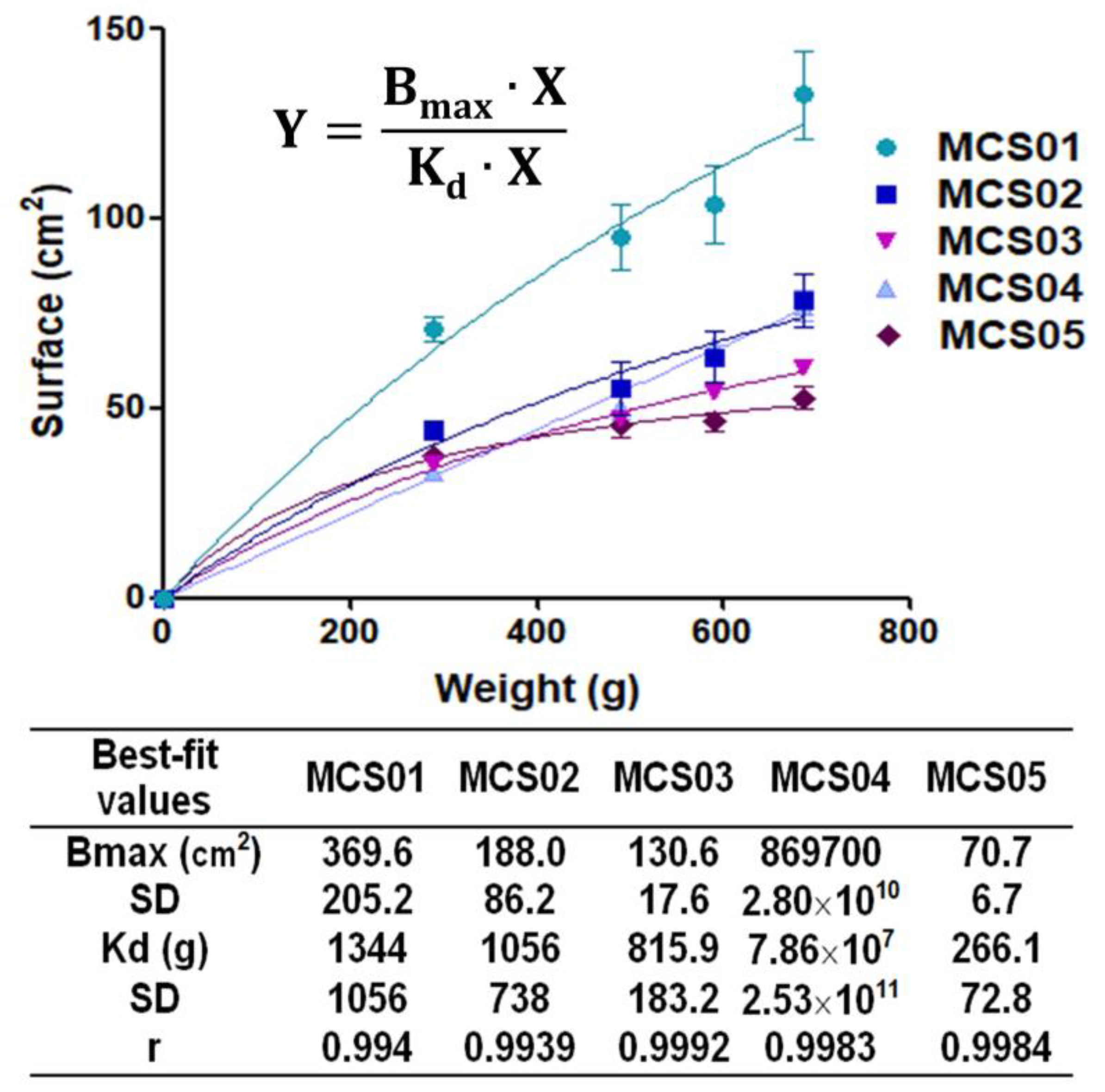
2.2.4. Extensibility
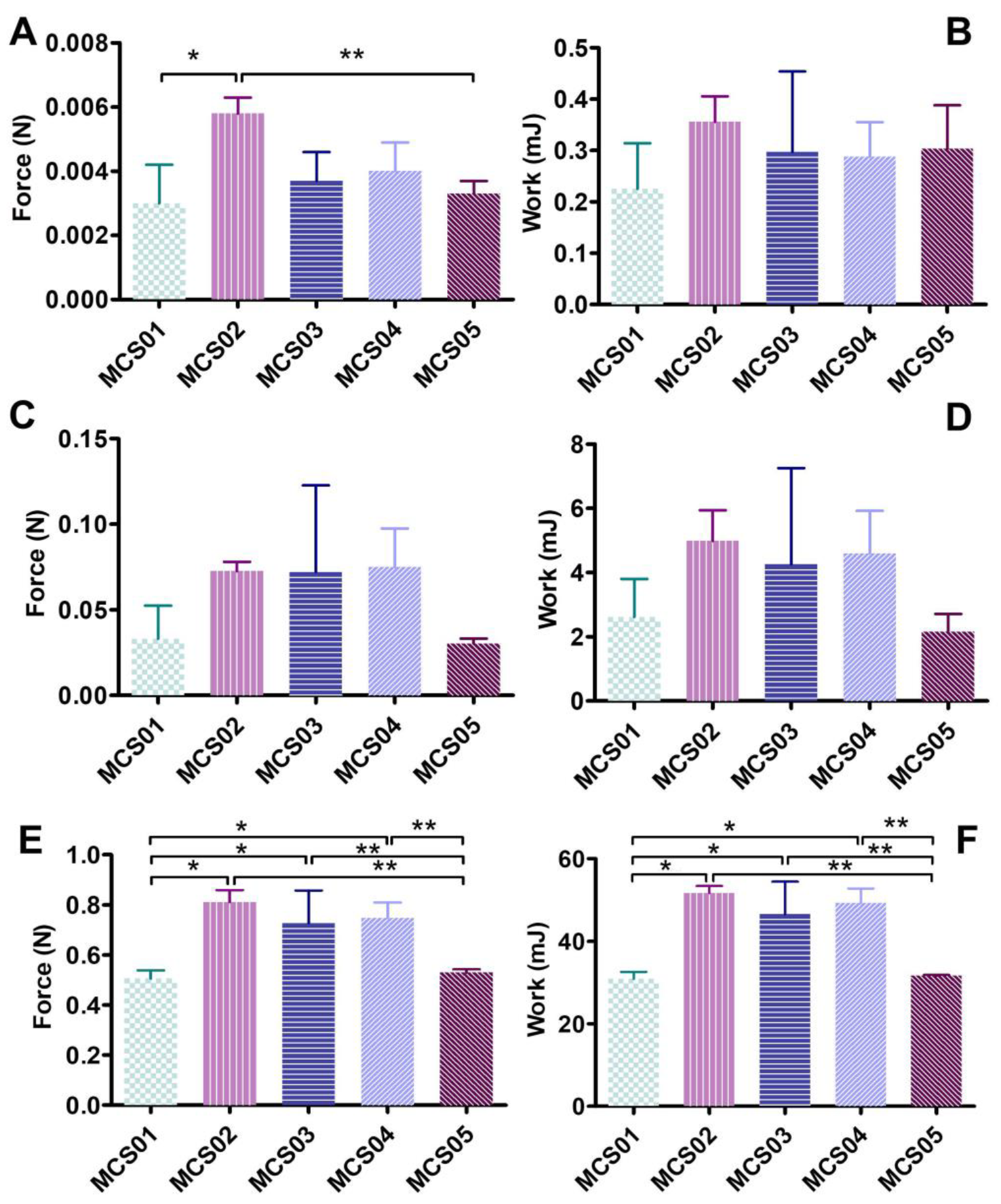
2.2.5. Syringeability
2.3. Stability of Polymeric Solutions
2.4. Microbiological Analysis
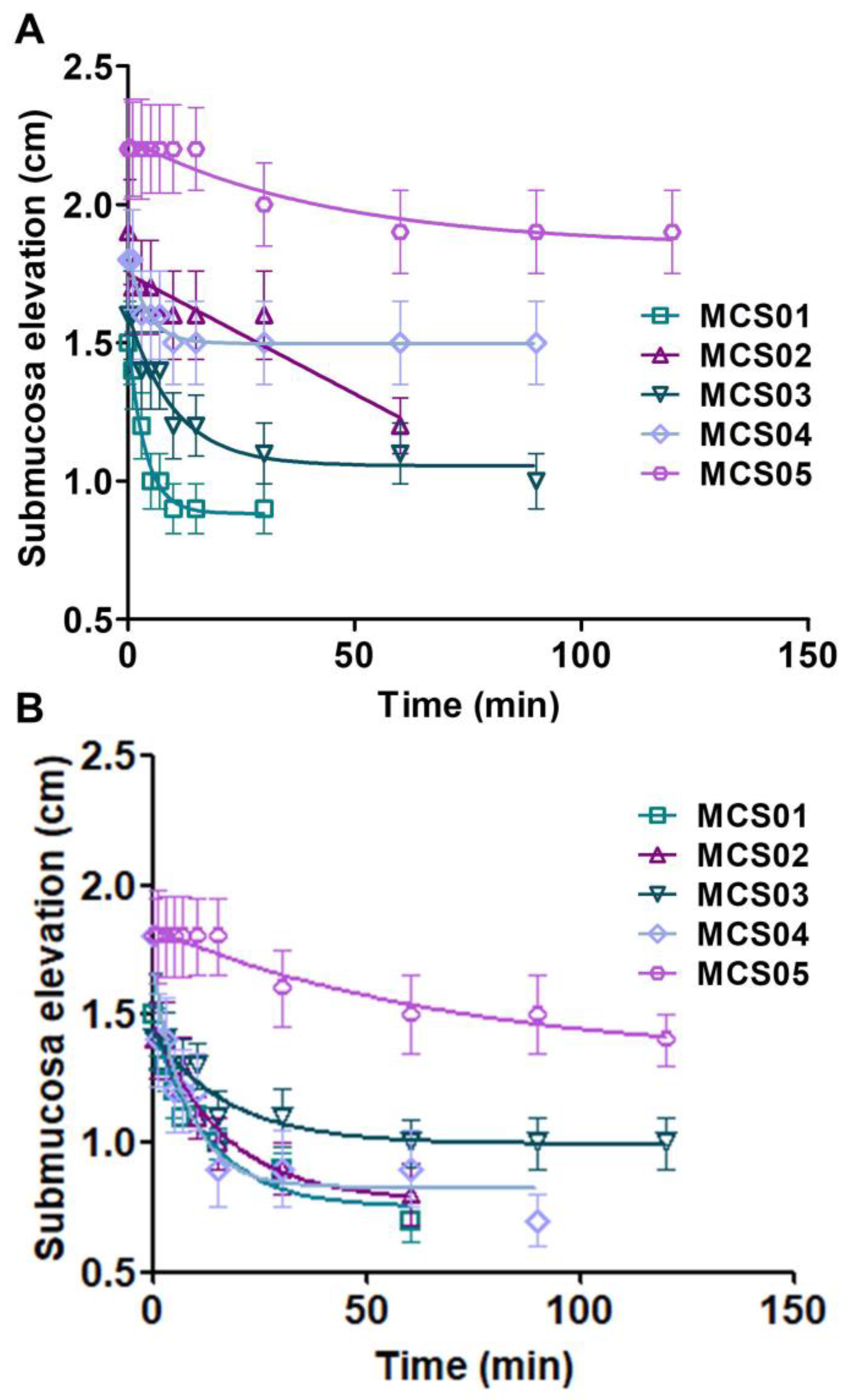
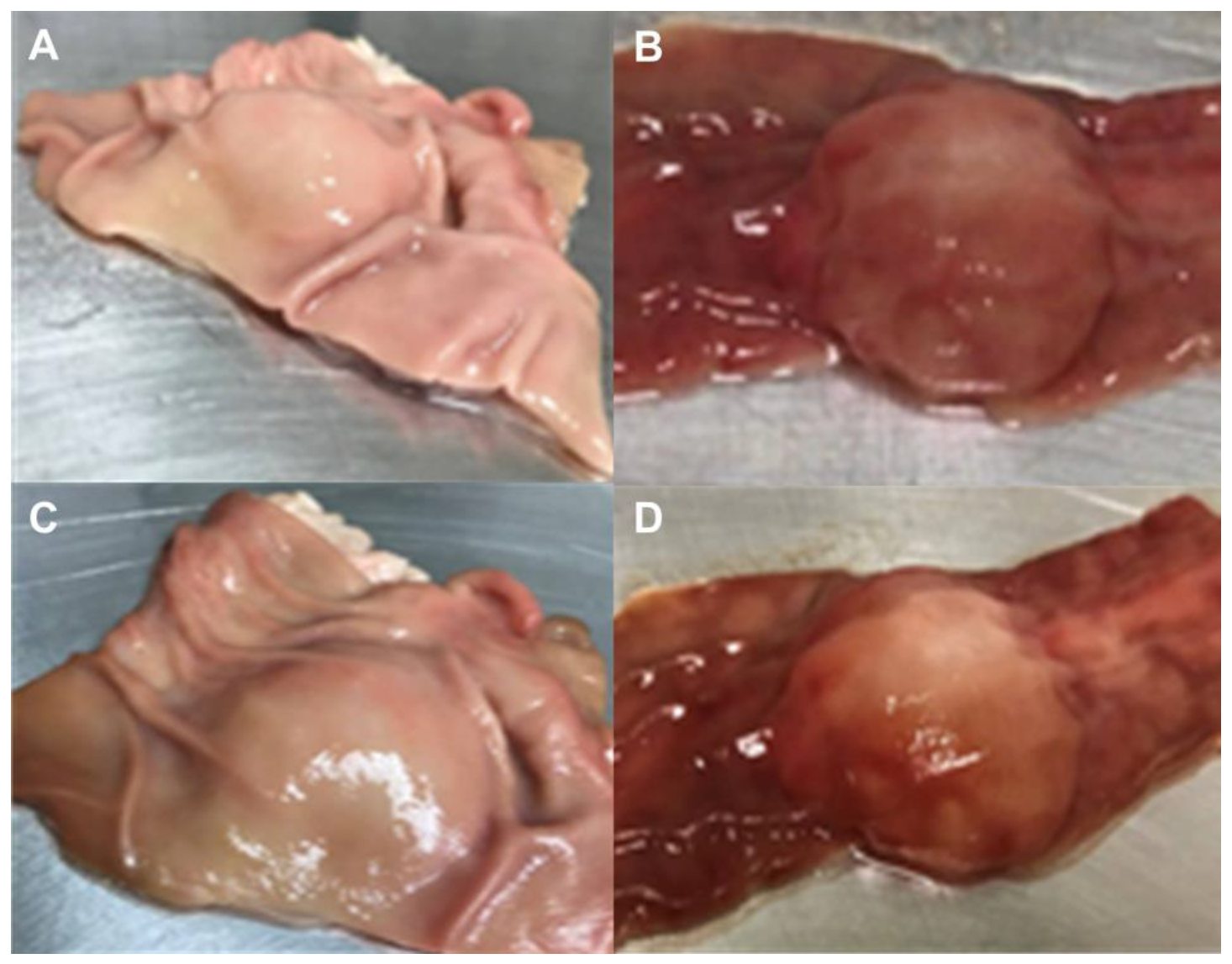
2.5. Ex Vivo Submucosal Elevation Studies
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
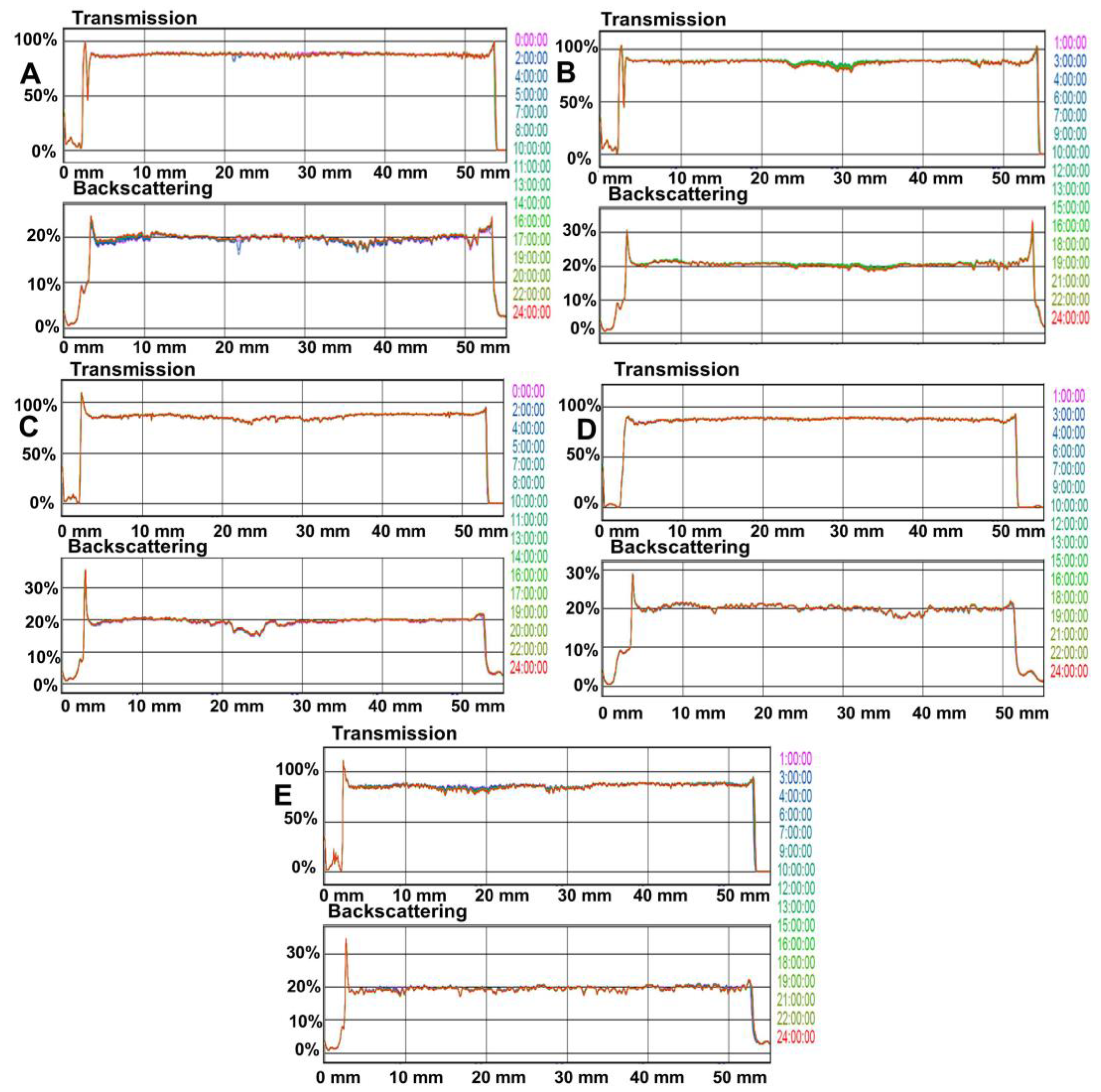
3.2. Optical and Microbiological Stability
3.3. Ex Vivo Submucosal Elevation Study
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kruszewski, W.J. Endoscopic methods in the treatment of early-stage esophageal cancer. Wideochir. Inne. Tech. Maloinwazyjne 2014, 9, 125–130. [Google Scholar] [CrossRef] [PubMed]
- De Ceglie, A.; Hassan, C.; Mangiavillano, B.; Matsuda, T.; Saito, Y.; Ridola, L.; Bhandari, P.; Boeri, F.; Conio, M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit. Rev. Oncol. Hematol. 2016, 104, 138–155. [Google Scholar] [CrossRef] [PubMed]
- Gotoda, T.; Yamamoto, H.; Soetikno, M.R. Endoscopic submucosal dissection of early gastric cancer. J. Gastroenterol. 2006, 41, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.; Bourke, M.J.; Williams, S.J.; Hourigan, L.F.; Brown, G.; Tam, W.; Singh, R.; Zanati, S.; Chen, R.Y.; Byth, K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011, 140, 1909–1918. [Google Scholar] [CrossRef]
- Park, Y.M.; Cho, E.; Kang, H.Y.; Kim, J.M. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: A systematic review and metaanalysis. Surg. Endosc. 2011, 25, 2666–2677. [Google Scholar] [CrossRef]
- Lian, J.; Chen, S.; Zhang, Y.; Qiu, F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest. Endosc. 2012, 76, 763–770. [Google Scholar] [CrossRef]
- Uraoka, T.; Saito, Y.; Yamamoto, K.; Fujii, T. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug. Des. Devel. Ther. 2008, 2, 131–138. [Google Scholar] [CrossRef]
- Uemura, N.; Oda, I.; Saito, Y.; Ono, H.; Fujisaki, J.; Matsuhashi, N.; Ohata, K.; Yahagi, N.; Yada, T.; Satoh, M.; et al. Efficacy and safety of 0.6% sodium alginate solution in endoscopic submucosal dissection for esophageal and gastric neoplastic lesion: A randomized controlled study. Dig. Endosc. 2019, 31, 396–404. [Google Scholar] [CrossRef]
- Alonci, G.; Fiorini, F.; Riva, P.; Monroy, F.; López-Montero, I.; Perretta, S.; De Cola, L. Injectable hybrid hydrogels, with cell-responsive degradation, for tumor resection. ACS Appl. Bio Mater. 2018, 1, 1301–1310. [Google Scholar] [CrossRef]
- Ferlitsch, M.; Moss, A.; Hassan, C.; Bhandari, P.; Dumonceau, J.M.; Paspatis, G.; Jover, R.; Langner, C.; Bronzwaer, M.; Nalankilli, K.; et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017, 49, 270–297. [Google Scholar] [CrossRef]
- Bon, I.; Bartolí, R.; Cano-Sarabia, M.; de la Ossa, N.; de Vega, V.M.; Marín, I.; Boix, J.; Lorenzo-Zúñiga, V. Comparative study of electrical and rheological properties of different solutions used in endoscopic mucosal resection. Dig. Endosc. 2019, 31, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.O.; Moleiro, J.; Torres, J.; Dinis-Ribeiro, M. Solutions for submucosal injection in endoscopic resection: A systematic review and meta-analysis. Endosc. Int. Open 2016, 4, E1–E16. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.; Bourke, M.J.; Kwan, V.; Tran, K.; Godfrey, C.; McKay, G.; Hopper, A.D. Succinylated gelatin substantially increases en bloc resection size in colonic EMR: A randomized, blinded trial in a porcine model. Gastrointest. Endosc. 2010, 71, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Polymeros, D.; Kotsalidis, G.; Triantafyllou, K.; Karamanolis, G.; Panagiotides, J.G.; Ladas, S.D. Comparative performance of novel solutions for submucosal injection in porcine stomachs: An ex vivo stud. Dig. Liver Dis. 2010, 42, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Yandrapu, H.; Desai, M.; Siddique, S.; Vennalganti, P.; Vennalaganti, S.; Parasa, S.; Rai, T.; Kanakadandi, V.; Bansal, A.; Titi, M.; et al. Normal saline solution versus other viscous solutions for submucosal injection during endoscopic mucosal resection: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.; Clares, B.; Mallandrich, M.; Suñer-Carbó, J.; Montes, M.J.; Calpena, A.C. Development of a mucoadhesive delivery system for control release of doxepin with application in vaginal pain relief associated with gynecological surgery. Int. J. Pharm. 2018, 535, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Matsumoto, K.; Nagahara, A.; Takeda, T.; Matsumoto, K.; Ueyama, H.; Shimada, Y.; Asaoka, D.; Hojo, M.; Watanabe, S. Evaluation of the optimal injection solution in hybrid endoscopic submucosal dissection (ESD) for various organs in an ex vivo porcine model. J. Gastrointest. Dig. Syst. 2015, 5, 366. [Google Scholar] [CrossRef]
- Córdova, H.; Cuatrecasas, M.; García-Rodríguez, A.; Montenegro, A.; Melo, J.; Rodríguez-de Miguel, C.; Martínez-Pallí, G.; Garcés-Durán, R.; Llach, J.; Fernández-Esparrach, G. Successful outcomes of a new combined solution of hyaluronic acid, chondroitin sulfate and poloxamer 407 for submucosal injection: Animal survival study. Endosc. Int. Open 2019, 7, E576–E582. [Google Scholar] [CrossRef]
- Hikichi, T.; Yamasaki, M.; Watanabe, K.; Nakamura, J.; Sato, M.; Takagi, T.; Suzuki, R.; Sugimoto, M.; Kikuchi, H.; Konno, N. Gastric endoscopic submucosal dissection using sodium carboxymethylcellulose as a new injection substance. Fukushima J. Med. Sci. 2016, 62, 43–50. [Google Scholar] [CrossRef][Green Version]
- Fujishiro, M.; Yahagi, N.; Nakamura, M.; Kakushima, N.; Kodashima, S.; Ono, S.; Kobayashi, K.; Hashimoto, T.; Yamamichi, N.; Tateishi, A.; et al. Successful outcomes of a novel endoscopic treatment for GI tumors: Endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest. Endosc. 2006, 63, 243–249. [Google Scholar] [CrossRef]
- Barollo, M.; Medici, V.; D’Incà, R.; Banerjee, A.; Ingravallo, G.; Scarpa, M.; Patak, S.; Ruffolo, C.; Cardin, R.; Sturniolo, G.C. Antioxidative potential of a combined therapy of anti TNFα and Zn acetate in experimental colitis. World J. Gastroenterol. 2011, 17, 4099–4103. [Google Scholar] [CrossRef]
- Burger, K.; Illés, J.; Gyurcsik, B.; Gazdag, M.; Forrai, E.; Dékány, I.; Mihályfi, K. Metal ion coordination of macromolecular bioligands: Formation of zinc (II) complex of hyaluronic acid. Carbohydr. Res. 2001, 332, 197–207. [Google Scholar] [CrossRef]
- Wu, D.; Ensinas, A.; Verrier, B.; Primard, C.; Cuvillier, A.; Champier, G.; Paul, S.; Delair, T. Zinc-stabilized chitosan-chondroitin sulfate nanocomplexes for HIV-1 infection inhibition application. Mol. Pharm. 2016, 13, 3279–3291. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, J.; Moussa, Z.L.; Collins, J.E.; McDonnell, S.; Hayward, A.M.; Jajoo, K.; Langer, R.; Traverso, G. Endoscopically injectable shear-thinning hydrogels facilitating polyp removal. Adv. Sci. 2019, 6, 1901041. [Google Scholar] [CrossRef] [PubMed]
- Allmendinger, A.; Fischer, S.; Huwyler, J.; Mahler, H.C.; Schwarb, E.; Zarraga, I.E.; Mueller, R. Rheological characterization and injection forces of concentrated protein formulations: An alternative predictive model for non-Newtonian solutions. Eur. J. Pharm. Biopharm. 2014, 87, 318–328. [Google Scholar] [CrossRef]
- Hirose, R.; Nakaya, T.; Naito, Y.; Daidoji, T.; Yasuda, H.; Konishi, H.; Itoh, Y. Development of a new ex vivo model for evaluation of endoscopic submucosal injection materials performance. J. Mech. Behav. Biomed. Mater. 2018, 79, 219–225. [Google Scholar] [CrossRef]
- Hirose, R.; Yoshida, N.; Naito, Y.; Yoshida, T.; Bandou, R.; Daidoji, T.; Inoue, K.; Dohi, O.; Konishi, H.; Nakaya, T.; et al. Development of sodium polyacrylate-based high-performance submucosal injection material with pseudoplastic fluid characteristics. ACS Biomater. Sci. Eng. 2019, 5, 6794–6800. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kume, K.; Yoshikawa, I.; Otsuki, M. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: An animal preliminary study. Gastrointest. Endosc. 2006, 64, 958–965. [Google Scholar] [CrossRef]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião, M.P.D.; de Freitas, O.; Lara, E.H.G. Semisolid systems containing propolis for the treatment of periodontal disease: In vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J. Pharm. Sci. 2007, 96, 2074–2089. [Google Scholar] [CrossRef]
| Ingredients | MCS01 | MCS02 | MCS03 | MCS04 | MCS05 |
|---|---|---|---|---|---|
| Na-CMC (% w/v) | - | 0.2 | 0.2 | 0.1 | 0.1 |
| HA (% v/v) | 0.03 | - | 0.03 | 0.12 | 0.12 |
| Fructose (% w/v) | 17 | 17 | 17 | 17 | 17 |
| Citric acid (% v/v) | - | - | - | - | 0.02 |
| Zinc (% w/v) | - | - | - | - | 0.02 |
| Methylene blue (% v/v) | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 |
| PBS (mL) | q.s. 100 | q.s. 100 | q.s. 100 | q.s. 100 | q.s. 100 |
| Formulation | pH (t0) | pH (t6) | ||
|---|---|---|---|---|
| 8 °C | 25 °C | 8 °C | 25 °C | |
| MCS01 | 5.50 ± 0.01 | 5.63 ± 0.01 | 5.49 ± 0.02 | 5.42 ± 0.08 |
| MCS02 | 5.88 ± 0.05 | 5.56 ± 0.02 | 5.77 ± 0.05 | 5.38 ± 0.05 |
| MCS03 | 5.82 ± 0.09 | 5.75 ± 0.01 | 5.29 ± 0.07 | 5.89 ± 0.02 |
| MCS04 | 6.19 ± 0.03 | 6.23 ± 0.02 | 6.07 ± 0.05 | 6.18 ± 0.04 |
| MCS05 | 4.63 ± 0.02 | 4.58 ± 0.03 | 4.61 ± 0.01 | 4.46 ± 0.02 |
| Formulation | Measurement Temperature (°C) | Storage Temperature (°C) | Mathematical Model Fitting | Rheological Behavior | Viscosity (mPa·s) | |
|---|---|---|---|---|---|---|
| Ramp-Up Stretch | Ramp-Down Stretch | |||||
| MCS01 | 8 | 8 | OdW 1 r = 0.9996 | OdW r = 0.9998 | Newtonian | 2.23 ± 0.04 |
| 25 | Newton r = 0.9978 | Newton r = 0.9975 | Newtonian | 1.48 ± 0.03 | ||
| 37 | 8 | Newton r = 0.9995 | Newton r = 0.9957 | Newtonian | 1.16 ± 0.03 | |
| 25 | Newton r = 0.9995 | Newton r = 0.9941 | Newtonian | 1.10 ± 0.03 | ||
| MCS02 | 8 | 8 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 25.35 ± 0.05 |
| 25 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 5.35 ± 0.03 | ||
| 37 | 8 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 13.14 ± 0.05 | |
| 25 | OdW r = 0.9994 | OdW r = 0.9995 | Pseudoplastic | 3.25 ± 0.04 | ||
| MCS03 | 8 | 8 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 21.27 ± 0.08 |
| 25 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 13.25 ± 0.05 | ||
| 37 | 8 | OdW r = 0.9999 | OdW r = 0.9999 | Pseudoplastic | 13.62 ± 0.04 | |
| 25 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 13.70 ± 0.04 | ||
| MCS04 | 8 | 8 | OdW r = 0.9996 | OdW r = 0.9997 | Pseudoplastic | 37.98 ± 0.05 |
| 25 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 21.94 ± 0.01 | ||
| 37 | 8 | Newton r = 0.995 | Newton r = 0.9925 | Newtonian | 17.28 ± 0.03 | |
| 25 | Newton r = 0.9934 | Newton r = 0.9955 | Newtonian | 17.32 ± 0.03 | ||
| MCS05 | 8 | 8 | Newton r = 0.9983 | Newton r = 0.9993 | Newtonian | 8.47 ± 0.01 |
| 25 | Newton r = 0.9995 | Newton r = 0.9997 | Newtonian | 4.42 ± 0.01 | ||
| 37 | 8 | Newton r = 0.9999 | Newton r = 0.9999 | Newtonian | 3.50 ± 0.02 | |
| 25 | Newton r = 0.9997 | Newton r = 0.9989 | Newtonian | 3.47 ± 0.03 | ||
| Formulation | Measurement Temperature (°C) | Storage Temperature (°C) | Mathematical Model Fitting | Rheological Behavior | Viscosity (mPa·s) | |
|---|---|---|---|---|---|---|
| Ramp-Up Stretch | Ramp-Down Stretch | |||||
| MCS01 | 8 | 8 | OdW 1 r = 0.9998 | OdW r = 0.9998 | Newtonian | 2.28 ± 0.04 |
| 25 | Newton r = 0.9945 | Newton r = 0.9925 | Newtonian | 1.48 ± 0.04 | ||
| 37 | 8 | Newton r = 0.9907 | Newton r = 0.9888 | Newtonian | 1.14 ± 0.04 | |
| 25 | Newton r = 0.9781 | Newton r = 0.9889 | Newtonian | 1.13 ± 0.04 | ||
| MCS02 | 8 | 8 | OdW r = 0.9998 | OdW r = 0.9999 | Pseudoplastic | 26.88 ± 0.06 |
| 25 | OdW r = 0.9997 | OdW r = 1.0000 | Pseudoplastic | 4.38 ± 0.05 | ||
| 37 | 8 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 13.07 ± 0.05 | |
| 25 | OdW r = 0.9962 | OdW r = 0.9961 | Pseudoplastic | 3.80 ± 0.05 | ||
| MCS03 | 8 | 8 | OdW r = 0.9998 | OdW r = 0.9999 | Pseudoplastic | 23.61 ± 0.03 |
| 25 | OdW r = 0.9997 | OdW r = 1.0000 | Pseudoplastic | 12.28 ± 0.02 | ||
| 37 | 8 | OdW r = 0.9999 | OdW r = 0.9998 | Pseudoplastic | 12.92 ± 0.05 | |
| 25 | OdW r = 0.9998 | OdW r = 0.9999 | Pseudoplastic | 12.03 ± 0.05 | ||
| MCS04 | 8 | 8 | OdW r = 0.9998 | OdW r = 0.9996 | Pseudoplastic | 38.53 ± 0.05 |
| 25 | OdW r = 0.9997 | OdW r = 0.9999 | Pseudoplastic | 22.46 ± 0.09 | ||
| 37 | 8 | Newton r = 0.9988 | Newton r = 0.9996 | Newtonian | 16.74 ± 0.06 | |
| 25 | Newton r = 0.9934 | Newton r = 0.9955 | Newtonian | 17.11 ± 0.03 | ||
| MCS05 | 8 | 8 | Newton r = 0.9935 | Newton r = 0.9990 | Newtonian | 8.55 ± 0.03 |
| 25 | Newton r = 0.9988 | Newton r = 0.9996 | Newtonian | 4.40 ± 0.02 | ||
| 37 | 8 | Newton r = 0.9990 | Newton r = 0.9989 | Newtonian | 3.25 ± 0.04 | |
| 25 | Newton r = 0.9998 | Newton r = 0.997 | Newtonian | 3.50 ± 0.01 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moles-Aranda, C.; Calpena-Campmany, A.C.; Halbaut-Bellowa, L.; Díaz-Tomé, V.; Otero-Espinar, F.J.; Morales-Molina, J.A.; Clares-Naveros, B. Novel Polymeric Formulation for Removal of Gastrointestinal Polyps by Digestive Endoscopy. Pharmaceutics 2020, 12, 322. https://doi.org/10.3390/pharmaceutics12040322
Moles-Aranda C, Calpena-Campmany AC, Halbaut-Bellowa L, Díaz-Tomé V, Otero-Espinar FJ, Morales-Molina JA, Clares-Naveros B. Novel Polymeric Formulation for Removal of Gastrointestinal Polyps by Digestive Endoscopy. Pharmaceutics. 2020; 12(4):322. https://doi.org/10.3390/pharmaceutics12040322
Chicago/Turabian StyleMoles-Aranda, Cristina, Ana C. Calpena-Campmany, Lyda Halbaut-Bellowa, Victoria Díaz-Tomé, Francisco J. Otero-Espinar, José A. Morales-Molina, and Beatriz Clares-Naveros. 2020. "Novel Polymeric Formulation for Removal of Gastrointestinal Polyps by Digestive Endoscopy" Pharmaceutics 12, no. 4: 322. https://doi.org/10.3390/pharmaceutics12040322
APA StyleMoles-Aranda, C., Calpena-Campmany, A. C., Halbaut-Bellowa, L., Díaz-Tomé, V., Otero-Espinar, F. J., Morales-Molina, J. A., & Clares-Naveros, B. (2020). Novel Polymeric Formulation for Removal of Gastrointestinal Polyps by Digestive Endoscopy. Pharmaceutics, 12(4), 322. https://doi.org/10.3390/pharmaceutics12040322








