Mechanochemical Formation of Racemic Praziquantel Hemihydrate with Improved Biopharmaceutical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Praziquantel Hemihydrate Preparation
2.2.2. Slurry Experiments
2.2.3. Differential Scanning Calorimetry and Thermogravimetric Analyses (DSC-TGA)
2.2.4. FT-Infrared Spectroscopy (FT–IR)
2.2.5. Polarimetry and Drug Recovery
2.2.6. Hot Stage Microscopy (HSM)
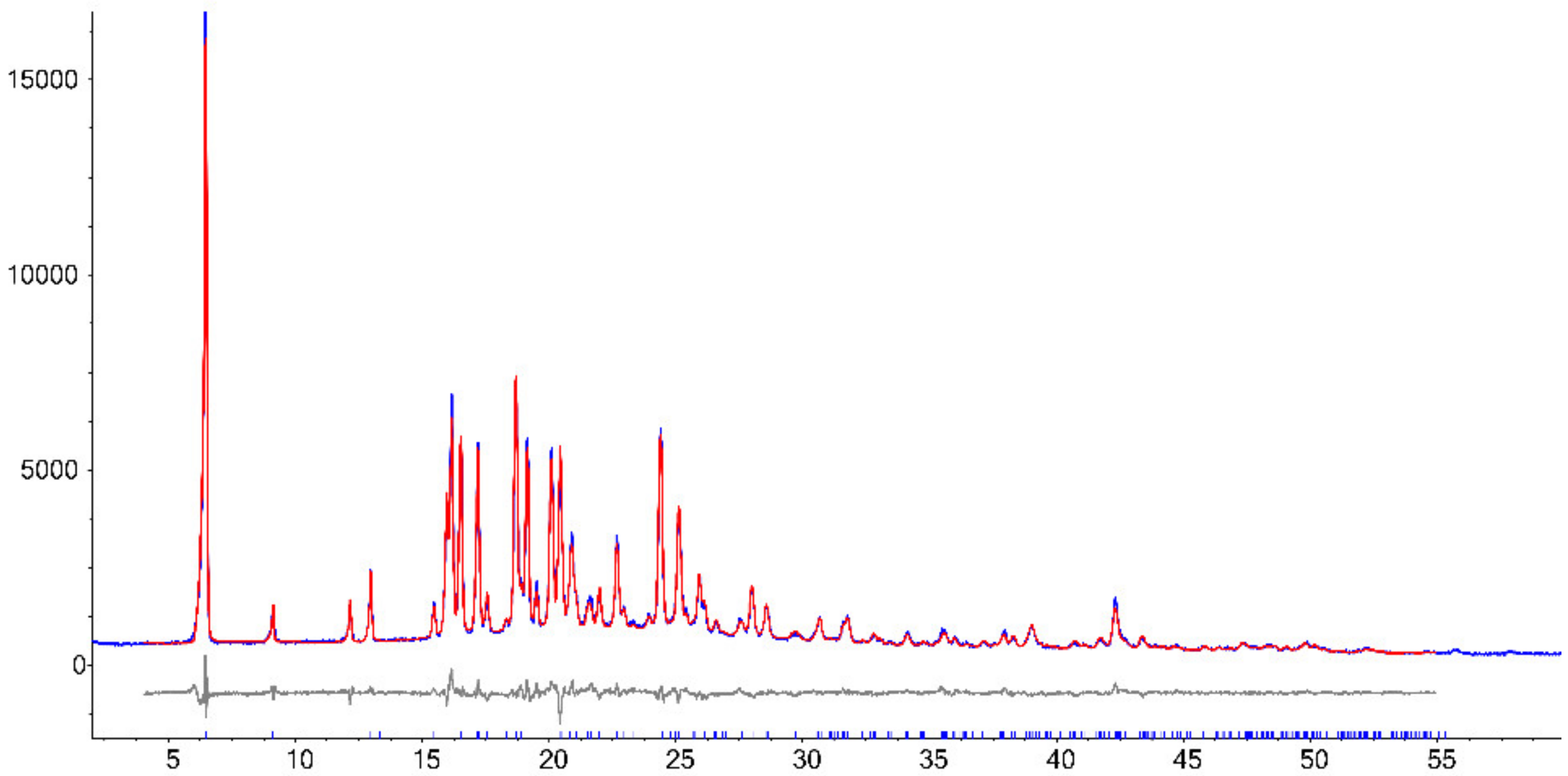
2.2.7. Powder X-ray Diffraction
2.2.8. Scanning Electron Microscopy
2.2.9. Solid-State NMR
2.2.10. Crystal Structure Determination from Powder X-ray Diffraction Data
2.2.11. Periodic DFT Calculations
2.2.12. Modeling of Solid-State NMR Spectra
2.2.13. Saturation Solubility and Intrinsic Dissolution Rate (IDR)
2.2.14. In Vitro Activity
2.2.15. Physical Stability under Several Conditions
3. Results
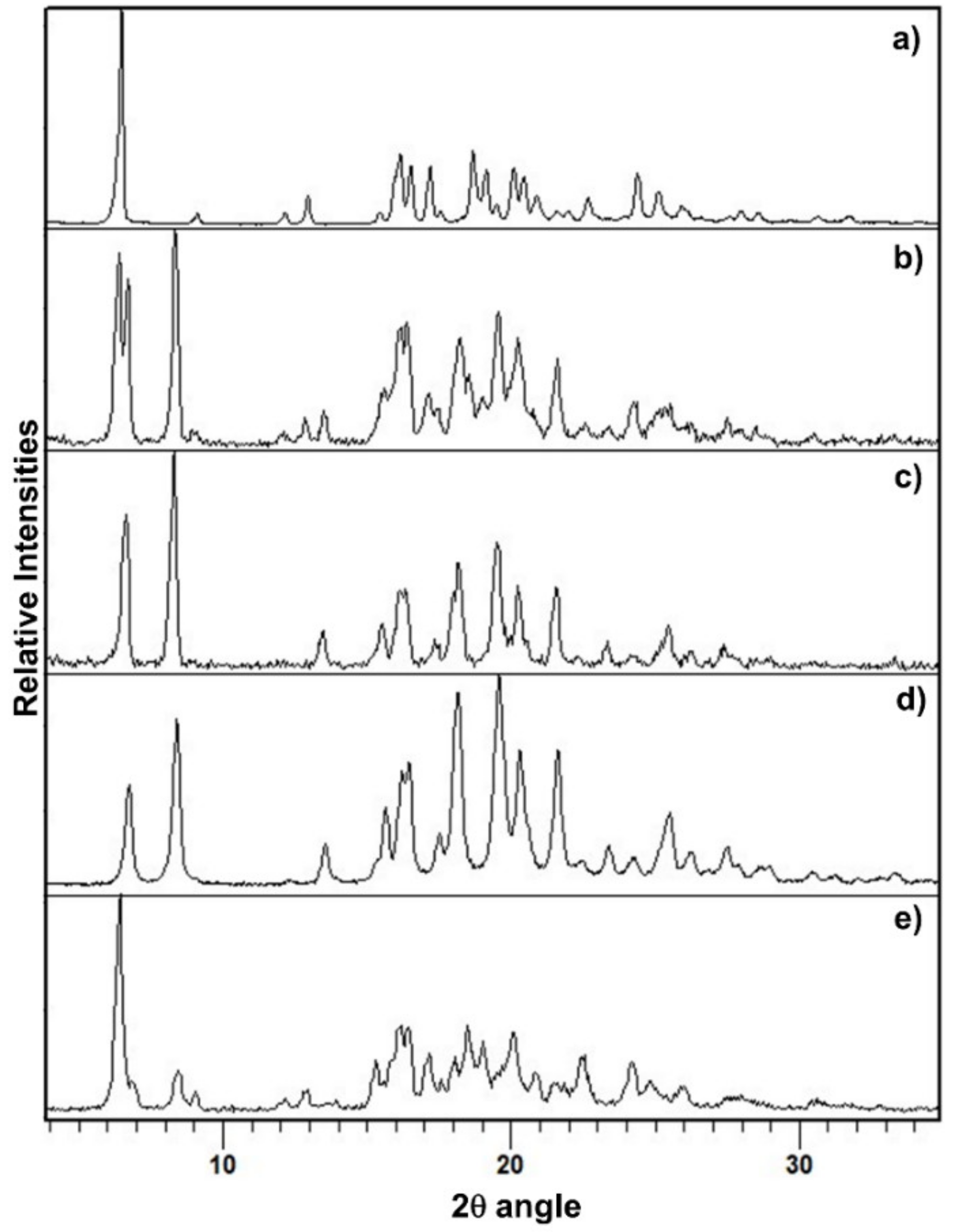
3.1. Mechanochemical Preparation of PZQ-HH Using PZQ Form A as Starting Material
3.2. Mechanochemical Preparation of PZQ-HH Using PZQ Form B as Starting Material
3.3. Mechanochemical Preparation of PZQ-HH Starting with PZQ Form A and Seeds of Preformed PZQ-HH
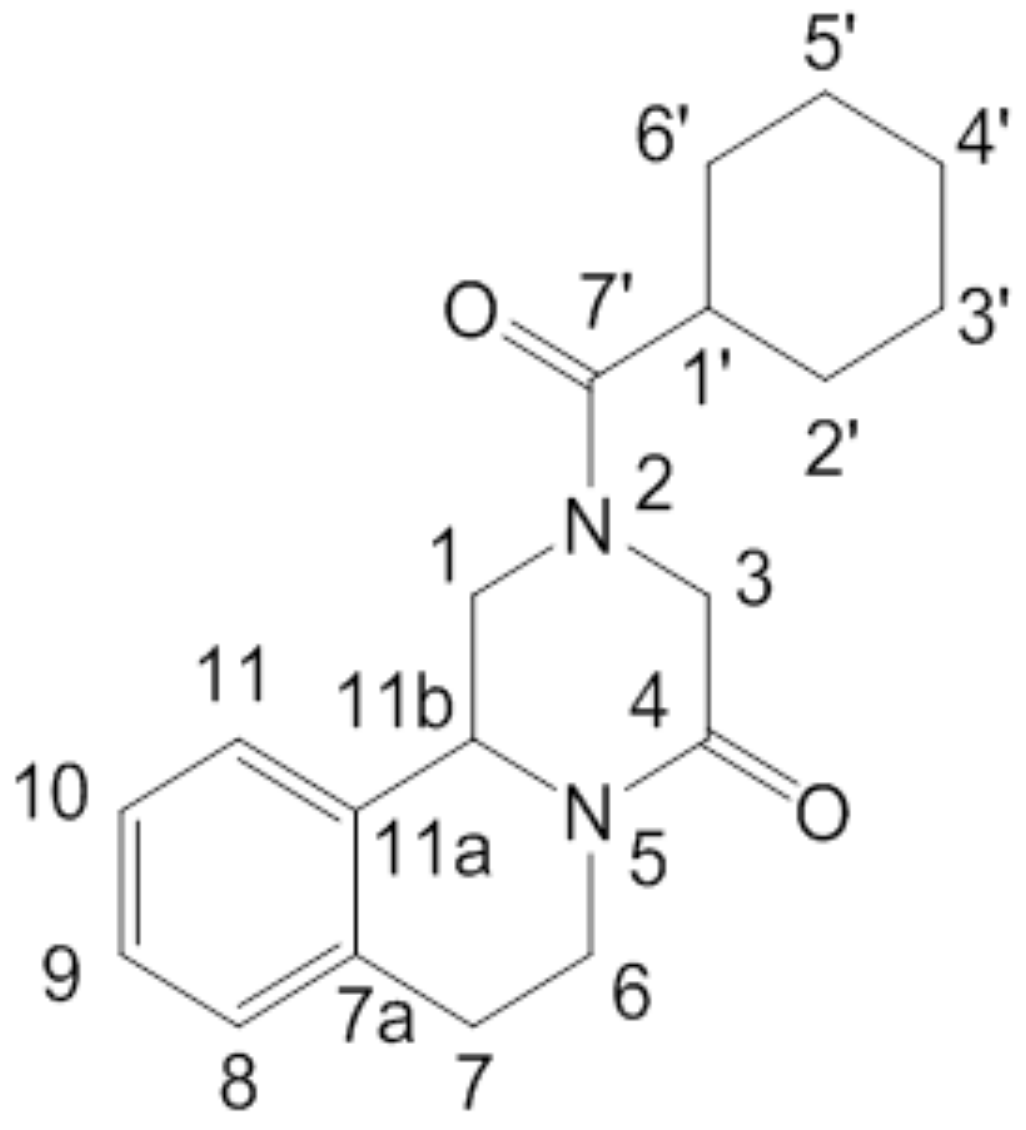
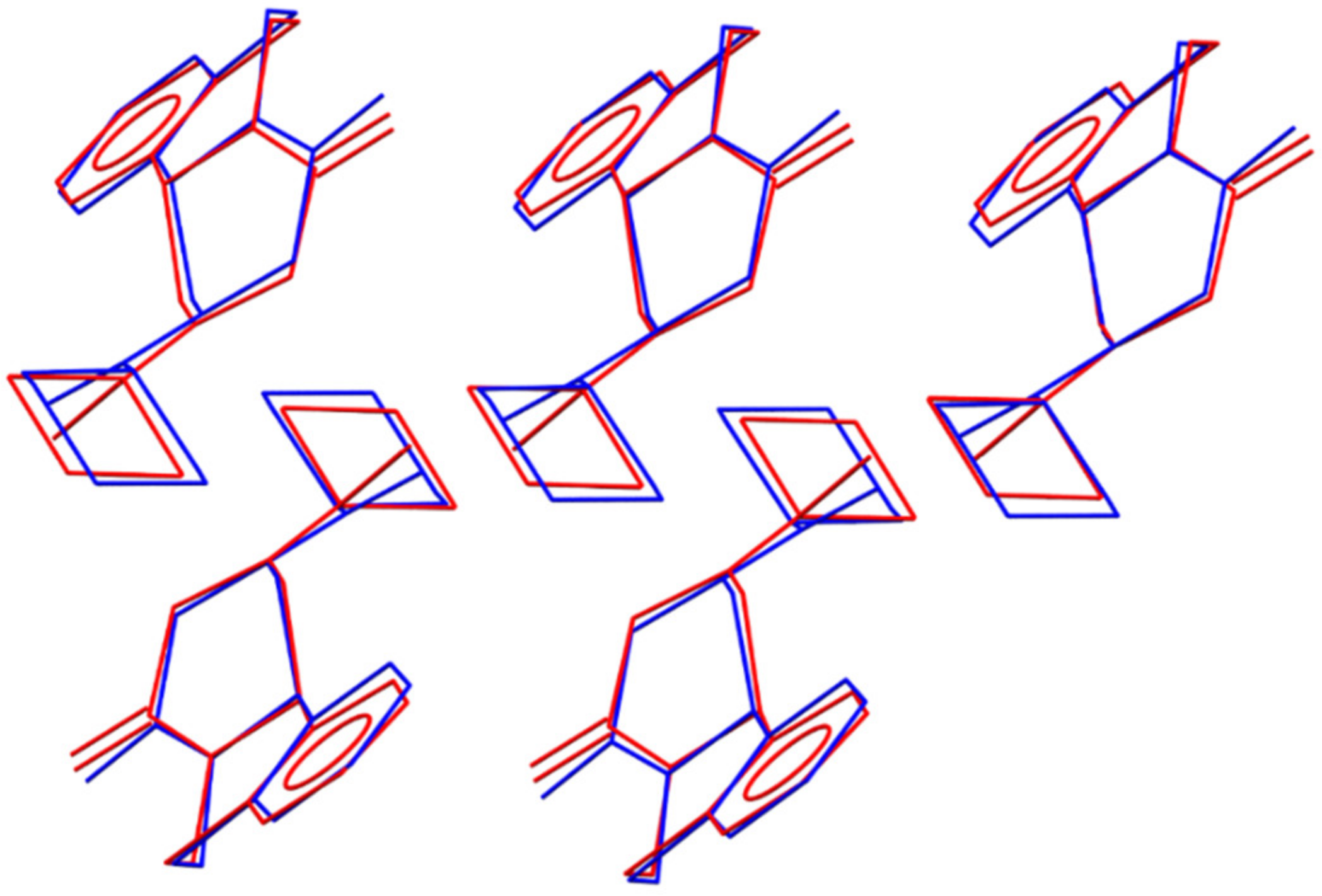
3.4. Characterizations of PZQ-HH and Crystal Structure Solution
3.4.1. Chemical Analyses
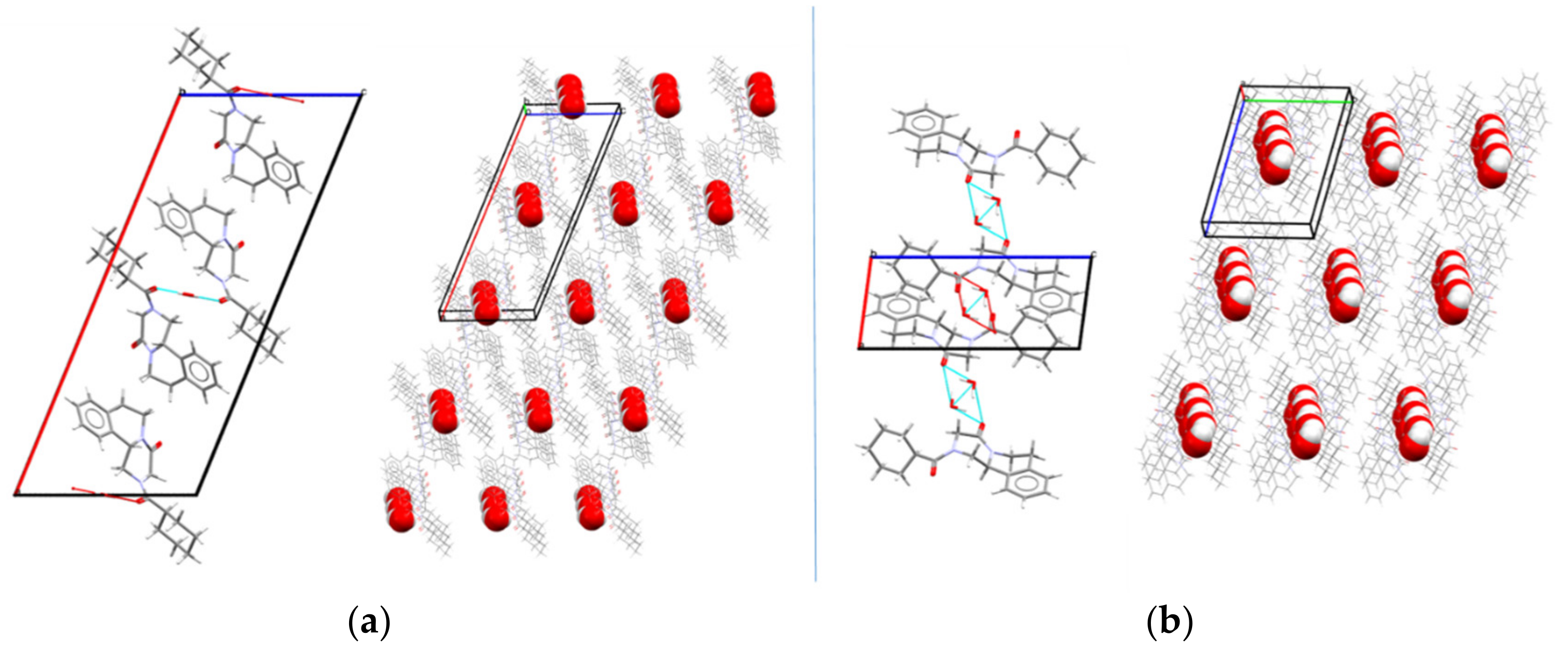
3.4.2. Crystal Structure Solution
3.4.3. Thermal Analyses
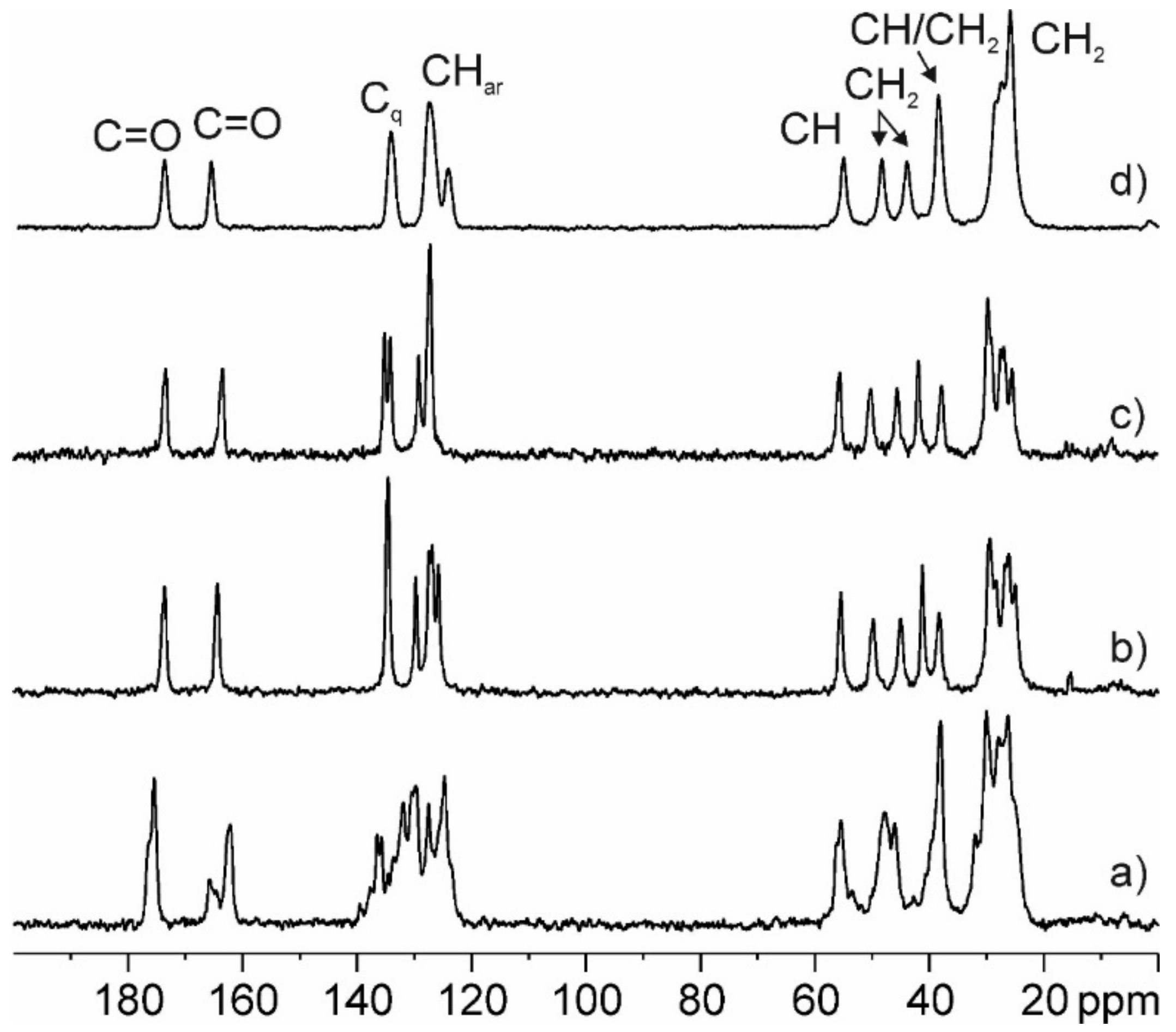
3.4.4. Experimental and GIPAW-DFT Calculated 13C CPMAS SS-NMR Spectra
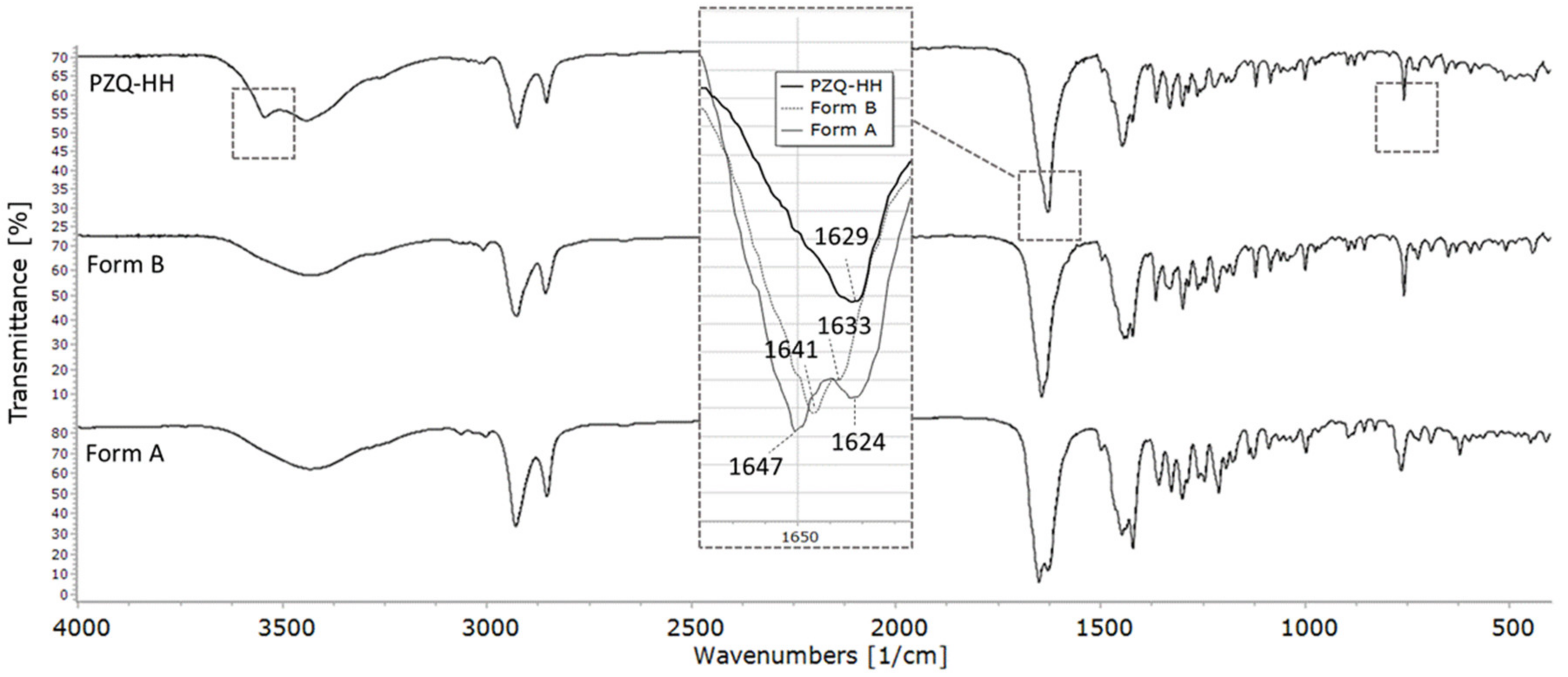
3.4.5. FT–IR Spectroscopy
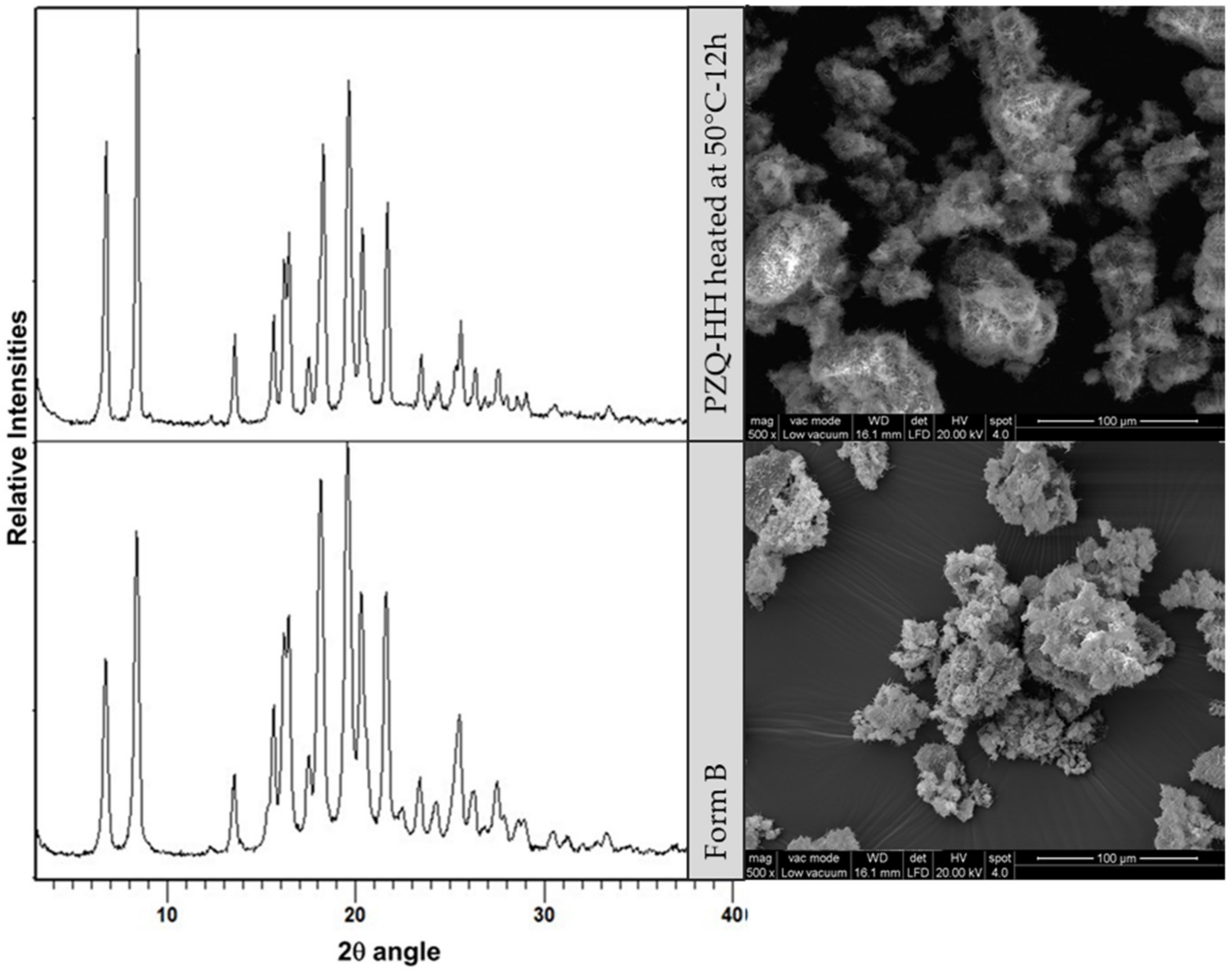
3.4.6. Morphological Analyses
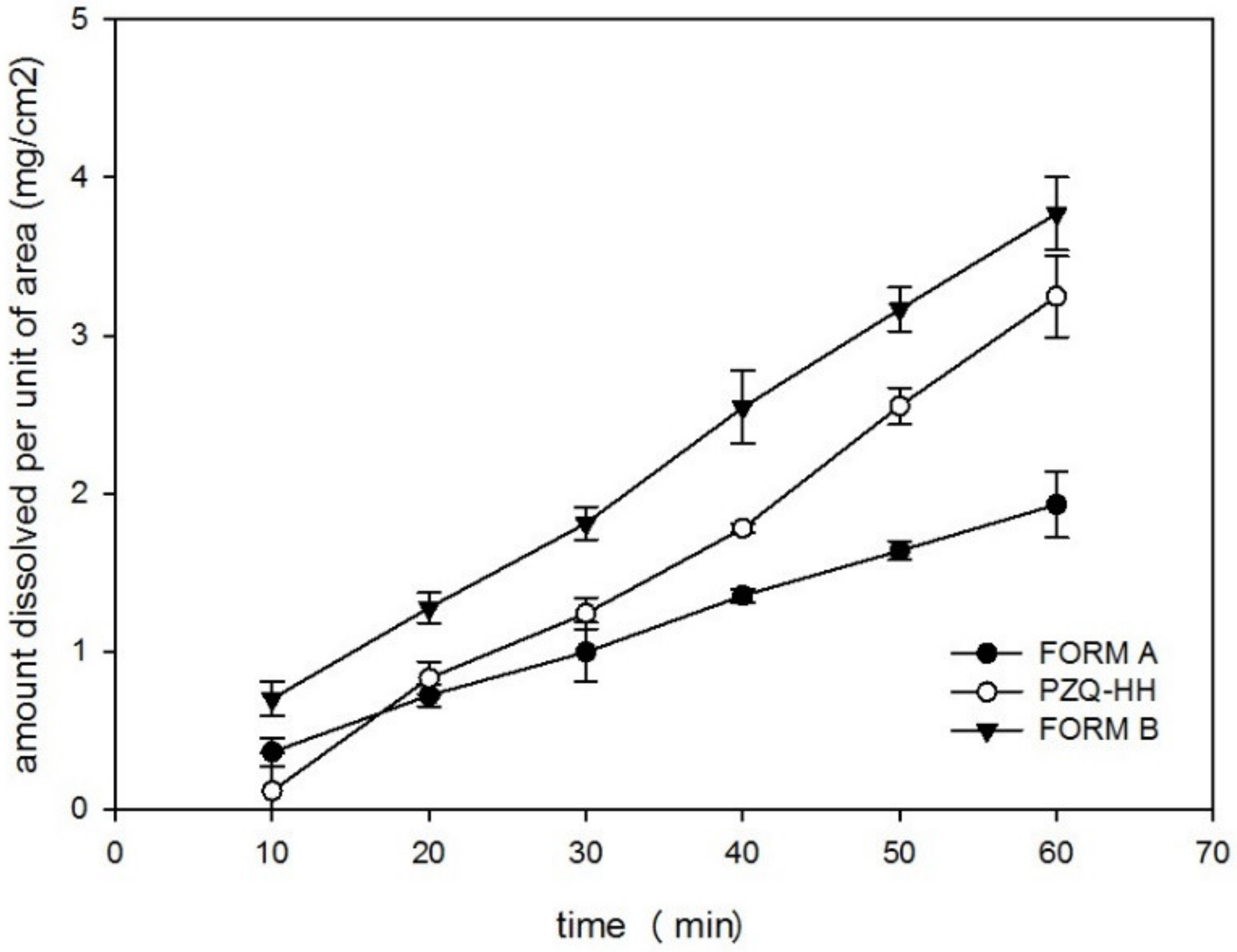
3.4.7. Saturation Solubility, IDR and Antischistosomal Activity
3.5. Physical Stability under Various Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/schistosomiasis/disease/en/ (assessed on 14 February 2020).
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Sousa-Figueiredo, J.C.; Betson, M.; Stothard, J.R. Treatment of schistosomiasis in African infants and preschool-aged children: Downward extension and biometric optimization of the current praziquantel dose pole. Int. Health 2012, 4, 95–102. [Google Scholar] [CrossRef] [PubMed]
- El-Arini, S.K.; Leuenberger, H. Dissolution properties of praziquantel-PVP systems. Pharm. Acta Helv. 1998, 73, 89–94. [Google Scholar] [CrossRef]
- Stelma, F.F.; Talla, I.; Sow, S.; Kongs, A.; Niang, M.; Polman, K.; Deelder, A.M.; Gryseels, B. Efficacy and side effects of praziquantel in an epidemic focus of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 1995, 53, 167–170. [Google Scholar] [CrossRef]
- Dayan, A.D. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003, 86, 141–159. [Google Scholar] [CrossRef]
- World Health Organization. Report of the WHO Informal Consultation on the use of praziquantel during pregnancy/lactation and albendazole/mebendazole in children under 24 months. 2002. Available online: https://apps.who.int/iris/handle/10665/68041 (accessed on 4 March 2020).
- Cedillo-Cruz, A.; Aguilar, M.I.; Flores-Alamo, M.; Palomares-Alonso, F.; Jung-Cook, H. A straightforward and efficient synthesis of praziquantel enantiomers and their 4′-hydroxy derivatives. Tetrahedron Asymmetry 2014, 25, 133–140. [Google Scholar] [CrossRef]
- Meister, I.; Ingram-Sieber, K.; Cowan, N.; Todd, M.; Robertson, M.N.; Meli, C.; Patra, M.; Gasser, G.; Keiser, J. Activity of praziquantel enantiomers and main metabolites against Schistosoma mansoni. Antimicrob. Agents Chemother. 2014, 58, 5466–5472. [Google Scholar] [CrossRef]
- Kovač, J.; Vargas, M.; Keiser, J. In vitro and in vivo activity of R- and S-praziquantel enantiomers and the main human metabolite trans-4-hydroxy-praziquantel against Schistosoma haematobium. Parasites Vectors 2017, 10, 365. [Google Scholar] [CrossRef]
- Bagchus, W.M.; Bezuidenhout, D.; Harrison-Moench, E.; Kourany-Lefoll, E.; Wolna, P.; Yalkinoglu, O. Relative bioavailability of orally dispersible tablet formulations of levo- and racemic praziquantel: Two phase I studies. Clin. Transl. Sci. 2019, 12, 66–76. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, H.; Ji, J.; Nie, L.; Sun, D. A quantification method for determination of racemate Praziquantel and R-enantiomer in rat plasma for comparison of their pharmacokinetics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1048, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Sekljic, H.; Fuchs, S.; Bothe, H.; Schollmeyer, D.; Miculka, C. Taste, a new incentive to switch to (R)-praziquantel in schistosomiasis treatment. PLoS Negl. Trop. Dis. 2009, 3, e357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Wang, J.K.; Ching, C.B. Investigation of the phase diagrams of chiral praziquantel. Chirality 1993, 18, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Wei, C.C.; Xu, Z.Y.; Yuan, H.C.; Lian, W.N.; Yang, Q.J.; Chen, M.; Jiang, Q.W.; Wang, C.Z.; Zhang, S.J.; et al. Comparison of the therapeutic efficacy and side effects of a single dose of levo-praziquantel with mixed isomer praziquantel in 278 cases of schistosomiasis japonica. Am. J. Trop. Med. Hyg. 1991, 45, 345–349. [Google Scholar] [CrossRef]
- Espinosa-Lara, J.C.; Guzman-Villanueva, D.; Arenas-García, J.I.; Herrera-Ruiz, D.; Rivera-Islas, J.; Román-Bravo, P.; Morales-Rojas, H.; Höpfl, H. Cocrystals of active pharmaceutical ingredients praziquantel in combination with oxalic, malonic, succinic, maleic, fumaric, glutaric, adipic, and pimelic acids. Cryst. Growth Des. 2013, 13, 169–185. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Zanolla, D.; Perissutti, B.; Passerini, N.; Chierotti, M.R.; Hasa, D.; Voinovich, D.; Gigli, L.; Demitri, N.; Geremia, S.; Keiser, J.; et al. A new soluble and bioactive polymorph of Praziquantel. Eur. J. Pharm. Biopharm. 2018, 127, 19–28. [Google Scholar] [CrossRef]
- Zanolla, D.; Perissutti, B.; Cerreia Vioglio, P.; Chierotti, M.R.; Gigli, L.; Demitri, N.; Passerini, N.; Albertini, B.; Franceschinis, E.; Keiser, J.; et al. Exploring mechanochemical parameters using a DoE approach: Crystal structure solution from synchrotron XRPD and characterization of a new praziquantel polymorph. Eur. J. Pharm. Sci. 2019, 140, 105084. [Google Scholar] [CrossRef]
- Salazar-Rojas, D.; Maggio, R.M.; Kaufman, T.S. Preparation and characterization of a new solid form of praziquantel, an essential anthelmintic drug. Praziquantel racemic monohydrate. Eur. J. Pharm. Sci. 2020, 105267. [Google Scholar] [CrossRef]
- Perissutti, B.; Passerini, N.; Trastullo, R.; Keiser, J.; Zanolla, D.; Zingone, G.; Voinovich, D.; Albertini, B. An explorative analysis of process and formulation variables affecting comilling in a vibrational mill: The case of Praziquantel. J. Pharm. 2017, 553, 402–412. [Google Scholar]
- Zanolla, D.; Perissutti, B.; Passerini, N.; Invernizzi, S.; Voinovich, D.; Bertoni, S.; Melegari, C.; Milotti, G.; Albertini, B. Milling and comilling Praziquantel at cryogenic and room temperatures: Assessment of the process-induced effects on drug properties. J. Pharm. Biomed. Anal. 2018, 153, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Šagud, I.; Zanolla, D.; Perissutti, B.; Passerini, N.; Škorić, I. Identification of degradation products of praziquantel during the mechanochemical activation. J. Pharm. Biomed. Anal. 2018, 159, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Khankari, R.K.; Grant, D.J.W. Pharmaceutical hydrates. Thermochim. Acta 1995, 248, 61–79. [Google Scholar] [CrossRef]
- Franklin, S.J.; Younis, U.S.; Myrdal, P.B. Estimating the aqueous solubility of pharmaceutical hydrates. J. Pharm. Sci. 2016, 105, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.R. Structural aspects of hydrates and solvates. In Polymorphism in Pharmaceutical Solids; Brittain, H.G., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 125–181. [Google Scholar]
- Bajpai, A.; Scott, H.S.; Pham, T.; Chen, K.-J.; Space, B.; Lusi, M.; Perry, M.L.; Zaworotko, M.J. Towards an understanding of the propensity for crystalline hydrate formation by molecular compounds. IUCrJ 2016, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Gal, S. Phenomenological study of water vapor sorption isotherms of solid sorbents. Chimia 1968, 22, 409–425. [Google Scholar]
- Griesser, U.J. The importance of solvates. In Polymorphism: In the Pharmaceutical Industry; Hilfiker, R., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 211–233. [Google Scholar]
- Braun, D.E.; Griesser, U.J. Supramolecular organization of nonstoichiometric drug hydrates: Dapsone. Front. Chem. 2018, 6, 31. [Google Scholar] [CrossRef]
- Roszkowski, P.; Maurin, J.K.; Czarnocki, Z. Enantioselective synthesis of (R)-(−)-praziquantel (PZQ). Tetrahedron Asymmetry 2006, 17, 1415–1419. [Google Scholar] [CrossRef]
- Altomare, A.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Rizzi, R.; Werner, P.-E. New Techniques for Indexing: N-TREOR in EXPO. J. Appl. Crystallogr. 2000, 33, 1180–1186. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS-Academic; Version 4.1; Coehlo Software: Brisbane, Australia, 2007. [Google Scholar]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Björkman, T. CIF2Cell: Generating geometries for electronic structure programs. Comput. Phys. Commun. 2011, 182, 1183–1186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.P. Special points for Brillouinzone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Van De Streek, J.; Neumann, M.A. Validation of molecular crystal structures from powder diffraction data with dispersion-corrected density functional theory (DFT-D). Acta Crystallogr. 2014, B70, 1020–1032. [Google Scholar] [CrossRef]
- Pickard, C.J.; Mauri, F. All-electron magnetic response with pseudopotentials: NMR chemical shifts. Phys. Rev. B 2001, 21, 245101. [Google Scholar] [CrossRef]
- Yates, J.R.; Pickard, C.J.; Mauri, F. Calculation of NMR chemical shifts for extended systems using ultrasoft pseudopotentials. Phys. Rev. B 2007, 76, 0201. [Google Scholar] [CrossRef]
- Hasa, D.; Pastore, M.; Arhangelskis, M.; Gabriele, B.; Cruz-Cabeza, A.J.; Rauber, G.S.; Bond, A.D.; Jones, W. On the kinetics of solvate formation through mechanochemistry. CrystEngComm 2019, 21, 2097–2104. [Google Scholar] [CrossRef]
- Tian, F.; Qu, H.; Zimmermann, A.; Munk, T.; Jørgensen, A.C.; Rantanen, J. Factors affecting crystallization of hydrates. J. Pharm. Pharmacol. 2010, 62, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.D.; Kudla, R.A.; Day, G.M.; Mueller, L.J.; Beran, G.J.O. Benchmark fragment-based 1H, 13C, 15N and 17O chemical shift predictions in molecular crystals. Phys. Chem. Chem. Phys. 2016, 18, 21686–21709. [Google Scholar] [CrossRef] [PubMed]
- Mayoka, G.; Keiser, J.; Haeberli, G.; Chibale, K. Structure-activity relationship and in vitro ADMET studies of N-aryl 3-Trifluoromethyl Pyrido [1,2-A] benzimidazoles that are efficacious in a mouse model of schistosomiasis. ACS Infect. Dis. 2018, 5, 406–417. [Google Scholar]
- Linberg, K.; Zafar, N.A.; Etter, M.; Michalchuk, A.A.L.; Rademann, K.; Emmerling, F. A Comparative Study of the Ionic Cocrystals NaX (α-d-Glucose)2 (X = Cl, Br, I). Cryst. Growth Des. 2019, 19, 4293–4299. [Google Scholar] [CrossRef]
- Kulla, H.; Haferkamp, S.; Akhmetova, I.; Röllig, M.; Maierhofer, C.; Rademann, K.; Emmerling, F. In situ investigations of mechanochemical one-pot syntheses. Angew. Chem. Int. Ed. 2018, 57, 5930–5933. [Google Scholar] [CrossRef]
- Uzarevic, K.; Halasz, I.; Friščić, T. Real-time and in situ monitoring of mechanochemical reactions: A new playground for all chemists. J. Phys. Chem. Lett. 2015, 6, 4129–4140. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W.; Motherwell, W.D.S. Screening for pharmaceutical cocrystal hydrates via neat and liquid-assisted grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef]
- Hasa, D.; Jones, W. Screening for new pharmaceutical solid forms using mechanochemistry: A practical guide. Adv. Drug Deliv. Rev. 2016, 117, 147–161. [Google Scholar] [CrossRef]
- Ostwald, W. Studien über die Bildung und Umwandlung fester Körper. Z. Phys. 1897, 22, 289–330. [Google Scholar] [CrossRef]
- Belenguer, A.M.; Lampronti, G.I.; Cruz-Cabeza, A.J.; Hunter, C.A.; Sanders, J.K. Solvation and surface effects on polymorph stabilities at the nanoscale. Chem. Sci. 2016, 7, 6617–6627. [Google Scholar] [CrossRef]
- Trask, A.V.; Shan, N.; Motherwell, W.D.S.; Jones, W.; Feng, S.; Tan, R.B.H.; Carpenter, K.J. Selective polymorph transformation via solvent-drop grinding. Chem. Commun. 2005, 7, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.V.; Rahn, P.D.; Sarapu, A.C.; Vanderwielens, A.J. Physical characterization of erythromycin: Anhydrate, monohydrate, and dihydrate crystalline solids. J. Pharm. Sci. 1978, 67, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Niwa, T.; Takeuchi, H.; Hino, T.; Itoh, Y.; Furuyama, S. Characterization of polymorphs of tranilast anhydrate and tranilast monohydrate when crystallized by two solvent change spherical crystallization techniques. J. Pharm. Sci. 1991, 80, 472–478. [Google Scholar] [CrossRef] [PubMed]




| Initial Polymorph | Method/Technique | Conditions/Duration | Outcome |
|---|---|---|---|
| A | LAG with water 1 | Two-step 2 | HH |
| A | LAG with water | One-step | A |
| B | LAG with water | One-step | HH |
| A | Slurry | 7 days | A |
| B | Slurry | 3 days 3 | HH |
| A | LAG with water | One-step | HH |
| with seeds of PZQ-HH | |||
| HH | RT | 4 months | B |
| HH | 50 °C/under vacuum | Overnight | B |
| HH | Milling (25 Hz) | 60 min | B |
| HH | 25 °C–60 °C | Dynamic heating | B |
| Atom | Group | Form A | Form B | Form C | PZQ-HH | |
|---|---|---|---|---|---|---|
| Exp | Exp | Exp | Exp | Calc 1 | ||
| 7′ | C=O | 175.4, 176.2 sh | 173.6 | 173.3 | 173.6 | 173.8 |
| 4 | C=O | 165.8, 164.6, 162.1 | 164.3 | 163.4 | 165.4 | 165.4 |
| 7a | Cq | 137.7, 136.5 | 134.6 | 135.1 | 134.1 | 136.3 |
| 11a | Cq | 135.8, 134.6 | 134.6 | 134.0 | 134.1 | 136.2 |
| 8 | CH | 129.7, 127.5, 124.8 | 129.8 | 129.1 | 127.3 | 129.4 |
| 11 | CH | 133.7, 132.0, 130.5 | 127.5 | 127.5 | 127.3 | 129.9 |
| 10 | CH | 126.9 | 127.1 | 127.3 | 128.4 | |
| 9 | CH | 125.8 | 127.1 | 124.0 | 124.7 | |
| 11b | CH | 56.3, 55.5 | 55.5 | 55.5 | 54.9 | 55.1 |
| 3 | CH2 | 46.1 | 49.8 | 50.1 | 48.2 | 46.7 |
| 1 | CH2 | 47.9 | 45.0 | 45.5 | 43.9 | 41.4 |
| 1′ | CH | 39.7 | 41.2 | 41.8 | 38.4 | 36.8 |
| 6 | CH2 | 38.1 | 38.3 | 37.7 | 38.4 | 35.8 |
| 6’ | CH2 | 32.0, 30.1, 27.9, 26.3, 25.3 | 29.4 | 27.4 | 25.8 | 24.5 |
| 2’ | CH2 | 29.4 | 29.6 | 25.8 | 24.0 | |
| 7 | CH2 | 28.4 | 29.1 | 28.2 | 26.2 | |
| 4’ | CH2 | 26.7 | 25.4 | 25.8 | 23.5 | |
| 3’ | CH2 | 26.1 | 26.8 | 25.8 | 24.0 | |
| 5’ | CH2 | 25.0 | 25.4 | 27.3 | 25.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanolla, D.; Hasa, D.; Arhangelskis, M.; Schneider-Rauber, G.; Chierotti, M.R.; Keiser, J.; Voinovich, D.; Jones, W.; Perissutti, B. Mechanochemical Formation of Racemic Praziquantel Hemihydrate with Improved Biopharmaceutical Properties. Pharmaceutics 2020, 12, 289. https://doi.org/10.3390/pharmaceutics12030289
Zanolla D, Hasa D, Arhangelskis M, Schneider-Rauber G, Chierotti MR, Keiser J, Voinovich D, Jones W, Perissutti B. Mechanochemical Formation of Racemic Praziquantel Hemihydrate with Improved Biopharmaceutical Properties. Pharmaceutics. 2020; 12(3):289. https://doi.org/10.3390/pharmaceutics12030289
Chicago/Turabian StyleZanolla, Debora, Dritan Hasa, Mihails Arhangelskis, Gabriela Schneider-Rauber, Michele R. Chierotti, Jennifer Keiser, Dario Voinovich, William Jones, and Beatrice Perissutti. 2020. "Mechanochemical Formation of Racemic Praziquantel Hemihydrate with Improved Biopharmaceutical Properties" Pharmaceutics 12, no. 3: 289. https://doi.org/10.3390/pharmaceutics12030289
APA StyleZanolla, D., Hasa, D., Arhangelskis, M., Schneider-Rauber, G., Chierotti, M. R., Keiser, J., Voinovich, D., Jones, W., & Perissutti, B. (2020). Mechanochemical Formation of Racemic Praziquantel Hemihydrate with Improved Biopharmaceutical Properties. Pharmaceutics, 12(3), 289. https://doi.org/10.3390/pharmaceutics12030289








