Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. SLN Preparation and Characterization
2.3. Cell Lines
2.4. Co-Culture Models
2.5. Doxorubicin Accumulation
2.6. Cytotoxicity
2.7. Rhodamine 123 Efflux
2.8. Immunoblotting
2.9. Flow Cytometry
2.10. Quantitative Real Time-PCR (qRT-PCR)
2.11. Reactive Oxygen Species (ROS) Measurement
2.12. Nuclear Factor-kB (NF-kB) and Hypoxia Inducible Factor-1α (HIF-1α) Activity.
2.13. Chromatin Immunoprecipitation (ChIP)
2.14. In Vivo Tumor Growths and Hematochemical Parameters
2.15. Statistical Analysis
3. Results
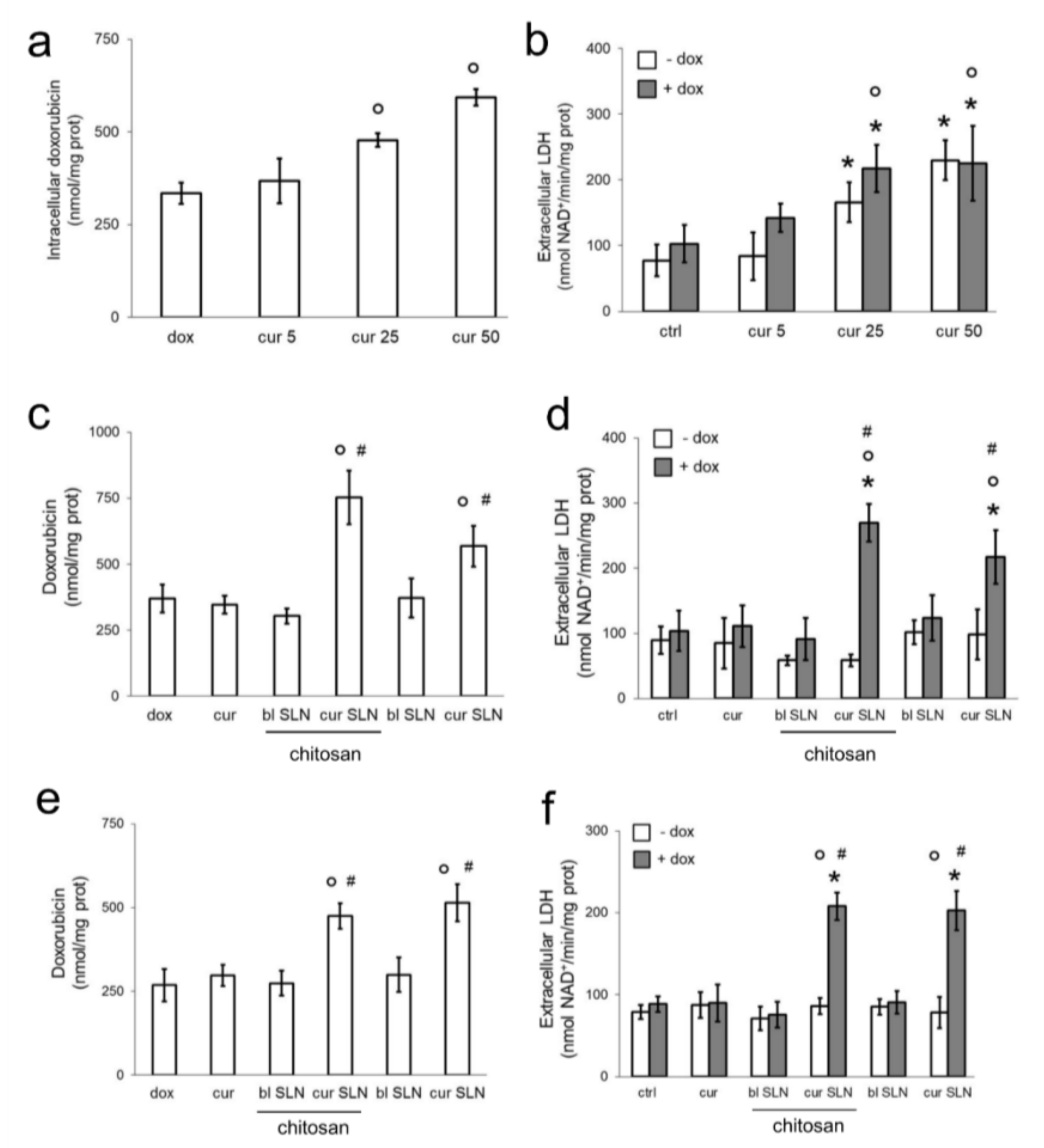
3.1. CURC-Loaded SLNs Are More Effective than Free CURC in Increasing Doxorubicin Efficacy in Resistant Triple Negative Breast Cancer Cells
3.2. CURC-Loaded SLNs Decrease Pgp Activity and Expression
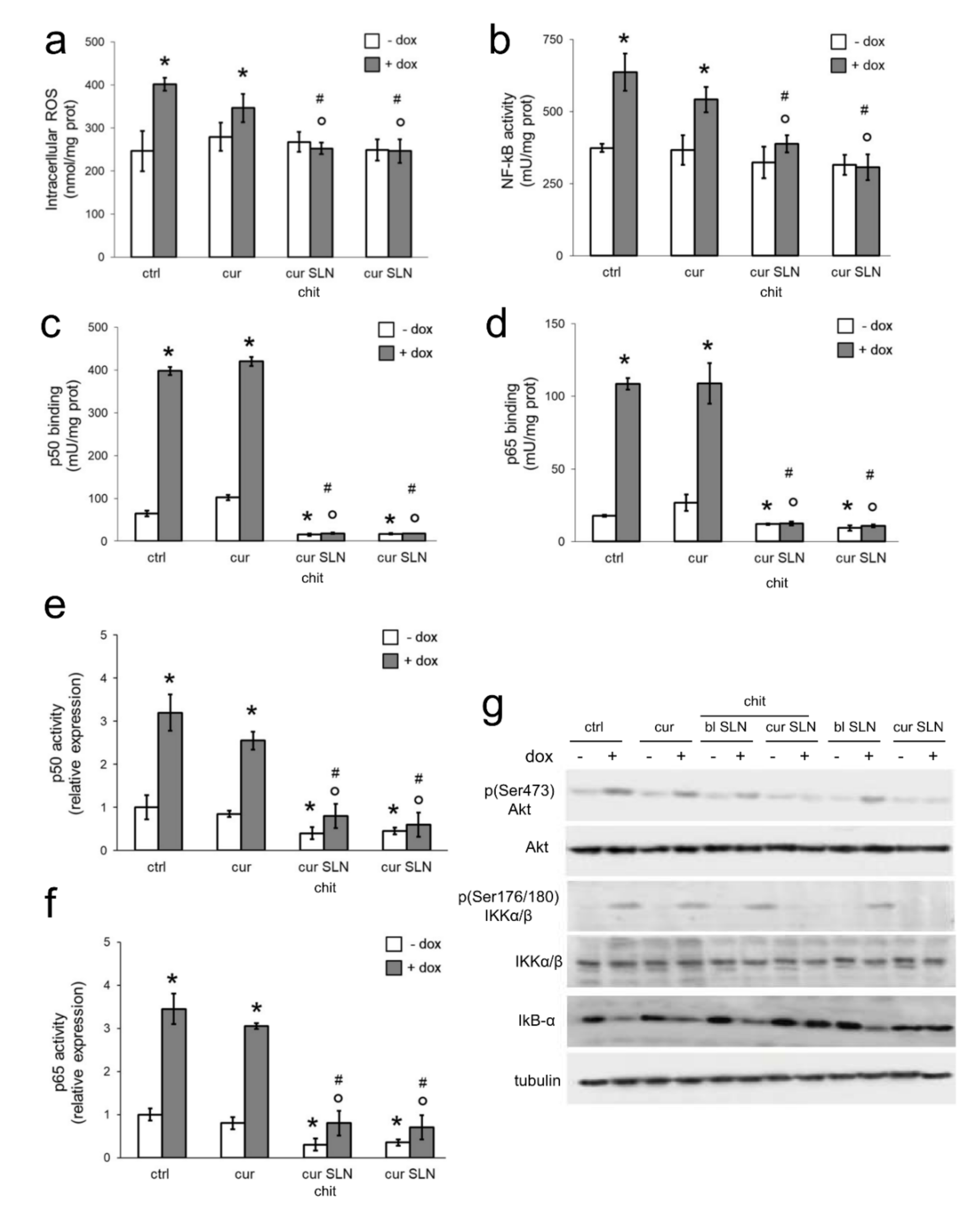
3.3. CURC-Loaded SLNs Decrease Pgp Transcription by Reducing Intracellular ROS and NF-kB Activity
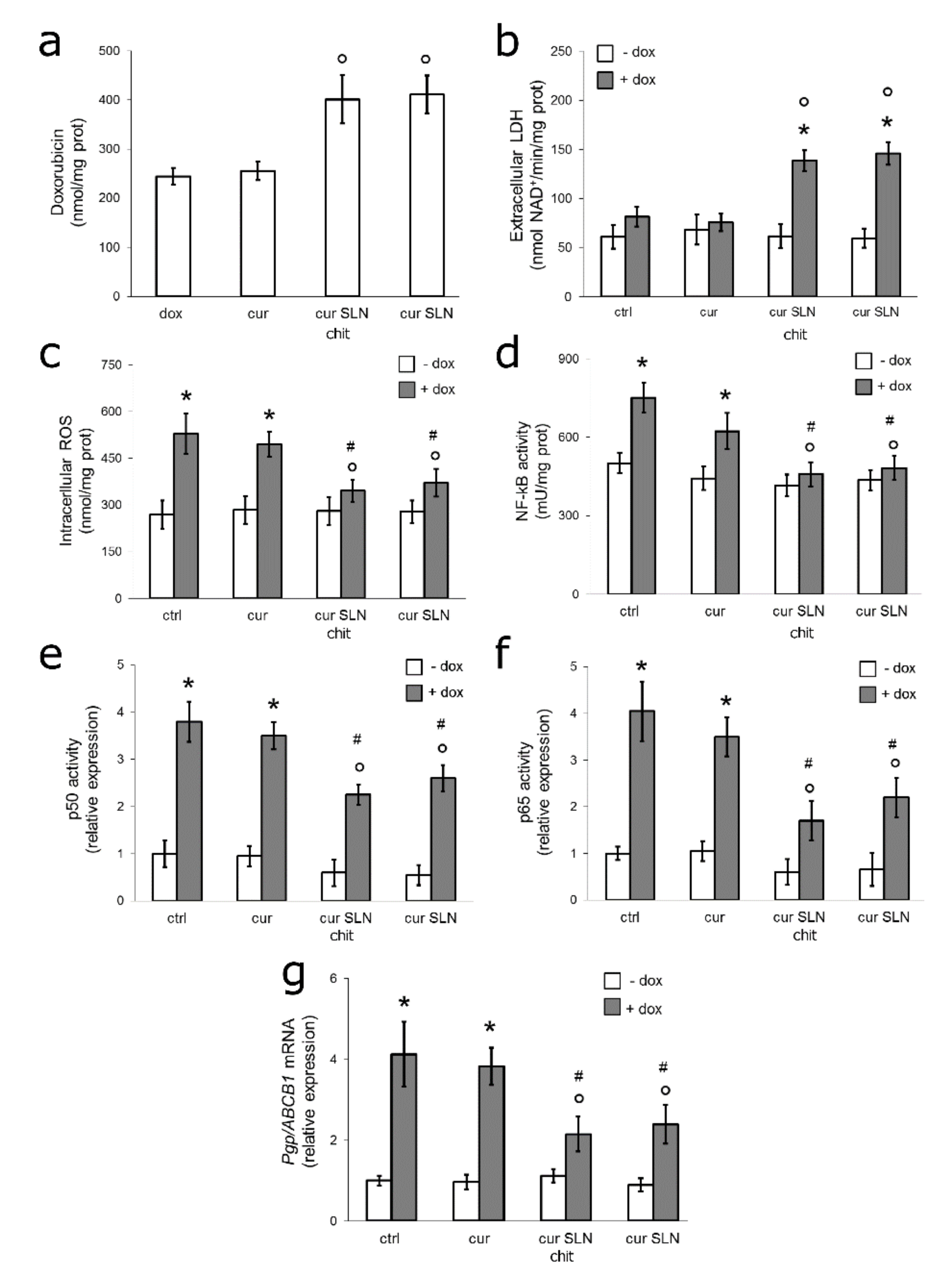
3.4. CURC-Loaded SLNs Restore Doxorubicin Sensitivity by Down-Regulating ROS/NF-kB/Pgp Axis in Resistant TNBC Cells Co-Cultured with Macrophages
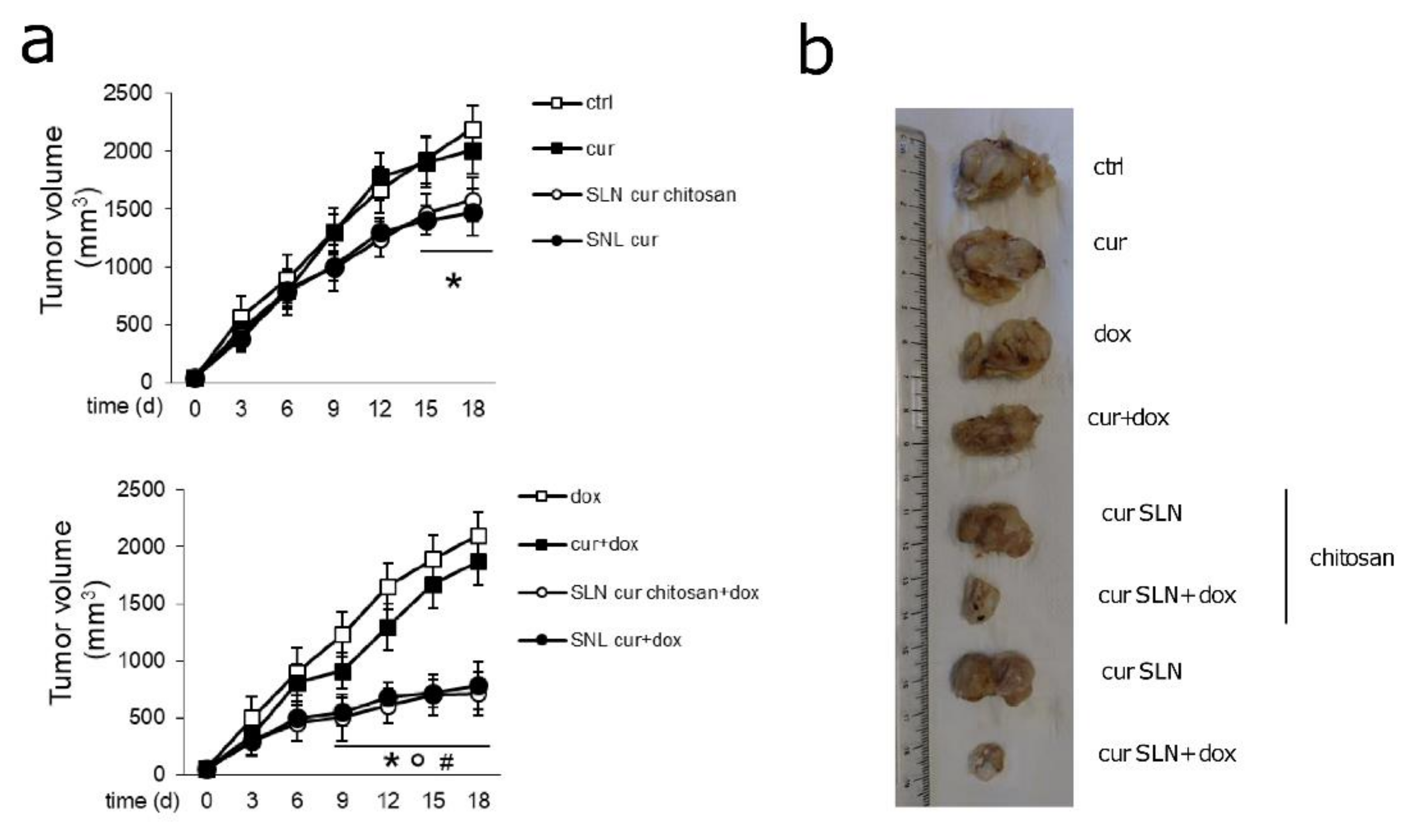
3.5. CURC-Loaded SLNs Are Effective and Safe in Preclinical Models of Pgp-Expressing Mammary JC Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Ween, M.P.; Armstrong, M.A.; Oehler, M.K.; Ricciardelli, C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit. Rev. Oncol. Hematol. 2015, 96, 220–256. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Shapira, A.; Livney, Y.D.; Broxterman, H.J.; Assaraf, Y.G. Nanomedicine for targeted cancer therapy: Towards the overcoming of drug resistance. Drug Resist. Update 2011, 14, 150–163. [Google Scholar] [CrossRef]
- Stuart, E.C.; Jarvis, R.M.; Rosengren, R.J. In Vitro mechanism of action for the cytotoxicity elicited by the combination of epigallocatechin gallate and raloxifene in MDA-MB-231 cells. Oncol. Rep. 2010, 24, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Willers, C.; Svitina, H.; Rossouw, M.J.; Swanepoel, R.A.; Hamman, J.H.; Gouws, C. Models used to screen for the treatment of multidrug resistant cancer facilitated by transporter-based efflux. J. Cancer Res. Clin. Oncol. 2019, 145, 1949–1976. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Zinzi, L.; Capparelli, E.; Cantore, M.; Contino, M.; Leopoldo, M.; Colabufo, N.A. Small and Innovative Molecules as New Strategy to Revert MDR. Front. Oncol. 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.M.J.; Zhang, L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharmacol. 2012, 83, 1104–1111. [Google Scholar] [CrossRef]
- Limtrakul, P.; Anuchapreeda, S.; Buddhasukh, D. Modulation of human multidrug-resistance MDR-1 gene by natural curcumoids. BMC Cancer 2004, 4, 13. [Google Scholar] [CrossRef]
- Lopes-Rodrigues, V.; Sousa, E.; Vasconcelos, M.H. Curcumin as a Modulator of P-Glycoprotein in Cancer: Challenges and Perspectives. Pharmaceuticals 2016, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanomedicine: A road to cancer therapeutics. Curr. Pharm. Des. 2013, 19, 1994–2010. [Google Scholar] [CrossRef]
- Guri, A.; Gülseren, I.; Corredig, M. Utilization of solid lipid nanoparticles for enhanced delivery of CURC in cocultures of HT29-MTX and Caco-2 cells. Food Funct. 2013, 4, 1410–1419. [Google Scholar] [CrossRef]
- Nakhlband, A.; Eskandani, M.; Saeedi, N.; Ghafari, S.; Omidi, Y.; Barar, J.; Garjani, A. Marrubiin-loaded solid lipid nanoparticles’ impact on TNF-α treated umbilical vein endothelial cells: A study for cardioprotective effect. Colloids Surf. B Biointerfaces 2018, 164, 299–307. [Google Scholar] [CrossRef]
- Rehman, M.U.; Khan, M.A.; Khan, W.S.; Shafique, M.; Khan, M. Fabrication of Niclosamide loaded solid lipid nanoparticles: In Vitro characterization and comparative in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1926–1934. [Google Scholar] [CrossRef]
- Baek, J.S.; Na, Y.G.; Cho, C.W. Sustained cytotoxicity of wogonin on breast cancer cells by encapsulation in solid lipid nanoparticles. Nanomaterials 2018, 8, 159. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 2018, 23, 1587. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Peira, E.; Sapino, S.; Dianzani, C.; Barge, A.; Muntoni, E.; Morel, S.; Gallarate, M. Stearoyl-chitosan coated nanoparticles obtained by microemulsion cold dilution technique. Int. J. Mol. Sci. 2018, 19, 3833. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.L.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: CURC Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials 2019, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Dong, Y.; Peng, L.; Yang, M.; Niu, L.; Liu, Z.; Xie, J. Tumor-associated macrophages affect the biological behavior of lung adenocarcinoma A549 cells through the PI3K/AKT signaling pathway. Oncol. Lett. 2019, 18, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Yin, M.; Min, W. 3D Co-culture System of Tumor-associated Macrophages and Ovarian Cancer Cells. Bio. Protoc. 2018, 8, E2815. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Miraglia, E.; Viarisio, D.; Costamagna, C.; Pescarmona, G.; Ghigo, D.; Bosia, A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [Google Scholar]
- Riganti, C.; Costamagna, C.; Doublier, S.; Miraglia, E.; Polimeni, M.; Bosia, A.; Ghigo, D. The NADPH oxidase inhibitor apocynin induces nitric oxide synthesis via oxidative stress. Toxicol. Appl. Pharmacol. 2008, 228, 277–285. [Google Scholar] [CrossRef]
- Riganti, C.; Voena, C.; Kopecka, J.; Corsetto, P.A.; Montorfano, G.; Enrico, E.; Costamagna, C.; Rizzo, A.M.; Ghigo, D.; Bosia, A. Liposome-encapsulated doxorubicin reverses drug resistance by inhibiting P-glycoprotein in human cancer cells. Mol. Pharm. 2011, 8, 683–700. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Gulino, G.R.; Volante, M.; Ghigo, D.; Kopecka, J. Two repeated low doses of doxorubicin are more effective than a single high dose against tumors overexpressing P-glycoprotein. Cancer Lett. 2015, 360, 219–226. [Google Scholar] [CrossRef]
- Gazzano, E.; Rolando, B.; Chegaev, K.; Salaroglio, I.C.; Kopecka, J.; Pedrini, I.; Saponara, S.; Sorge, M.; Buondonno, I.; Stella, B.; et al. Folate-targeted liposomal nitrooxy-doxorubicin: An effective tool against P-glycoprotein-positive and folate receptor-positive tumors. J. Control. Release 2018, 270, 37–52. [Google Scholar] [CrossRef]
- Kopecka, J.; Salzano, G.; Campia, I.; Lusa, S.; Ghigo, E.; De Rosa, G.; Riganti, C. Insights in the chemical components of liposomes responsible for P-glycoprotein inhibition. Nanomedicine 2014, 10, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Campia, I.; Jacobs, A.; Frei, A.P.; Ghigo, D.; Wollscheid, B.; Riganti, C. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget 2015, 6, 6776–6793. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.J.; Kim, B.J.; Rah, S.Y.; Chung, S.M.; Im, M.J.; Kim, U.H. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp. Mol. Med. 2006, 38, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Mohajeri, M.; Sahebkar, A. Protective effects of curcumin against doxorubicin-induced toxicity and resistance: A review. Crit. Rev. Oncol. Hematol. 2018, 122, 30–51. [Google Scholar] [CrossRef]
- Comerford, K.M.; Wallace, T.J.; Karhausen, J.; Louis, N.A.; Montalto, M.C.; Colgan, S.P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002, 62, 3387–3394. [Google Scholar]
- Bentires-Alj, M.; Barbu, V.; Fillet, M.; Chariot, A.; Relic, B.; Jacobs, N.; Gielen, J.; Merville, M.P.; Bours, V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 2003, 22, 90–97. [Google Scholar] [CrossRef]
- Karabay, A.Z.; Koc, A.; Ozkan, T.; Hekmatshoar, Y.; Altinok Gunes, B.; Sunguroglu, A.; Buyukbingol, Z.; Atalay, A.; Aktan, F. Expression analysis of Akirin-2, NFκB-p65 and β-catenin proteins in imatinib resistance of chronic myeloid leukemia. Hematology 2018, 23, 765–770. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Baldwin, A.S. NF-kappaB as a therapeutic target in cancer. Trends Mol. Med. 2002, 8, 385–389. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.; Carmody, R. The Regulation of NF-κB Subunits by Phosphorylation. Cells. 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Arslan, S.Ç.; Scheidereit, C. It takes two to tango: IκBs, the multifunctional partners of NF-κB. Immunol. Rev. 2012, 246, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Liu, C.; He, X.; Liu, X.; Yu, J.; Zhang, M.; Yu, F.; Wang, Y. RPS15A promotes gastric cancer progression via activation of the Akt/IKK-β/NF-κB signalling pathway. J. Cell Mol. Med. 2019, 23, 2207–2218. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, S.; Hu, Y.; Feng, Y.; Li, M.; Bian, Z.; Zhang, J.; Qin, Y.; Qi, X.; Zhou, L.; et al. The Immune-microenvironment Confers Chemoresistance of Colorectal Cancer through Macrophage-Derived IL6. Clin. Cancer Res. 2017, 23, 7375–7387. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, E577. [Google Scholar] [CrossRef]
- Llano, S.; Gómez, S.; Londoño, J.; Restrepo, A. Antioxidant activity of curcumoids. Phys. Chem. Chem. Phys. 2019, 21, 3752–3760. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumi. Adv. Exp. Med. Biol. 2014, 595, 105–125. [Google Scholar] [CrossRef]
- Moghadamtousi, Z.S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- López-Lázaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.; Inostroza-Riquelme, M.; Contreras-Orellana, P.; Diaz-Garcia, V.; Lara, P.; Vivanco-Palma, A.; Cárdenas, A.; Miranda, V.; Robert, P.; Leyton, L.; et al. Curcumin-loaded nanoemulsion: A new safe and effective formulation to prevent tumor reincidence and metastasis. Nanoscale 2018, 10, 22612–22622. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B Biointerfaces 2013, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, F.; Xu, T.; Sun, J. Tetrandrine prevents multidrug resistance in the osteosarcoma cell line, U-2OS, by preventing Pgp overexpression through the inhibition of NF-κB signaling. Int. J. Mol. Med. 2017, 39, 993–1000. [Google Scholar] [CrossRef]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J. Cell Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Hossain, M.M.; Banik, N.L.; Ray, S.K. Synergistic anti-cancer mechanisms of curcumin and paclitaxel for growth inhibition of human brain tumor stem cells and LN18 and U138MG cells. Neurochem. Int. 2012, 61, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Takeya, M. CAFs and TAMs: Maestros of the tumour microenvironment. J. Pathol. 2017, 241, 313–315. [Google Scholar] [CrossRef]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Székely, B.; Silber, A.L.M.; Pusztai, L. New Therapeutic Strategies for Triple-Negative Breast Cancer. Oncology 2017, 31, 130–137. [Google Scholar]
- Salaroglio, I.C.; Gazzano, E.; Abdullrahman, A.; Mungo, E.; Castella, B.; Abd-Elrahman, G.E.F.A.; Massaia, M.; Donadelli, M.; Rubinstein, M.; Riganti, C.; et al. Increasing intratumor C/EBP-β LIP and nitric oxide levels overcome resistance to doxorubicin in triple negative breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 286. [Google Scholar] [CrossRef]

| Components | CURC-SLN (w/w %) | CURC-CS-SLN (w/w %) |
|---|---|---|
| TL | 4.22 | - |
| Cholesterol | - | 3.22 |
| CS | - | 0.43 |
| Epikuron®200 | 10.57 | 16.12 |
| s-EA | 14.09 | - |
| s-BL | - | 9.54 |
| CURC | 0.63 | 0.64 |
| Cremophor®RH60 | 3.52 | 9.54 |
| NaTC | 3.53 | 9.03 |
| 1,2 Propanediol | 14.09 | - |
| BenzOH | - | 6.71 |
| s-W | 49.35 | 44.77 |
| −Doxorubicin | Ctrl | CURC | CURC-CS-SLN | CURC-SLN |
| LDH (U/L) | 7091 ± 639 | 7189 ± 409 | 6781 ± 1021 | 7112 ± 678 |
| AST (U/L) | 89 ± 54 | 101 ± 43 | 121 ± 48 | 99 ± 29 |
| ALT (U/L) | 38 ± 8 | 41 ± 8 | 37 ± 10 | 34 ± 8 |
| AP (U/L) | 134 ± 49 | 139 ± 41 | 129 ± 29 | 139 ± 18 |
| Creatinine (mg/L) | 0.056 ± 0.005 | 0.062 ± 0.010 | 0.062 ± 0.009 | 0.054 ± 0.008 |
| CPK (U/L) | 314 ± 99 | 312 ± 83 | 382 ± 56 | 334 ± 39 |
| CPK-MB (ng/mL) | 0.109 ± 0.058 | 0.118 ± 0.032 | 0.129 ± 0.045 | 0.118 ± 0.041 |
| cTnI (pg/mL) | 1.011 ± 0.082 | 1.019 ± 0.032 | 1.008 ± 0.062 | 1.024 ± 0.029 |
| cTnT (pg/mL) | 2.182 ± 0.213 | 2.178 ± 0.101 | 2.189 ± 0.123 | 1.897 ± 0.162 |
| +doxorubicin | Ctrl | CURC | CURC-CS-SLN | CURC-SLN |
| LDH (U/L) | 7561 ± 761 | 7192 ± 506 | 7821 ± 821 | 6523 ± 801 |
| AST (U/L) | 103 ± 44 | 132 ± 45 | 105 ± 36 | 137 ± 89 |
| ALT (U/L) | 36 ± 15 | 41 ± 18 | 39 ± 13 | 56 ± 19 |
| AP (U/L) | 138 ± 25 | 167 ± 56 | 139 ± 44 | 168 ± 41 |
| Creatinine (mg/L) | 0.083 ± 0.009 | 0.082 ± 0.009 | 0.093 ± 0.011 | 0.093 ± 0.010 |
| CPK (U/L) | 556 ± 89 * | 571 ± 89 * | 562 ± 81 * | 504 ± 81 * |
| CPK-MB (ng/mL) | 0.302 ± 0.71 * | 0.287 ± 0.045 * | 0.297 ± 0.062 * | 0.322 ± 0.016 * |
| cTnI (pg/mL) | 1.021 ± 0.039 | 1.033 ± 0.046 | 1.031 ± 0.067 | 1.019 ± 0.052 |
| cTnT (pg/mL) | 3.197 ± 0.209 * | 2.882 ± 0.172 * | 2.904 ± 0.209 * | 2.821 ± 0.178 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Ragab Hamed, A.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2020, 12, 96. https://doi.org/10.3390/pharmaceutics12020096
Fathy Abd-Ellatef G-E, Gazzano E, Chirio D, Ragab Hamed A, Belisario DC, Zuddas C, Peira E, Rolando B, Kopecka J, Assem Said Marie M, et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics. 2020; 12(2):96. https://doi.org/10.3390/pharmaceutics12020096
Chicago/Turabian StyleFathy Abd-Ellatef, Gamal-Eldein, Elena Gazzano, Daniela Chirio, Ahmed Ragab Hamed, Dimas Carolina Belisario, Carlo Zuddas, Elena Peira, Barbara Rolando, Joanna Kopecka, Mohamed Assem Said Marie, and et al. 2020. "Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells" Pharmaceutics 12, no. 2: 96. https://doi.org/10.3390/pharmaceutics12020096
APA StyleFathy Abd-Ellatef, G.-E., Gazzano, E., Chirio, D., Ragab Hamed, A., Belisario, D. C., Zuddas, C., Peira, E., Rolando, B., Kopecka, J., Assem Said Marie, M., Sapino, S., Ramadan Fahmy, S., Gallarate, M., Zaki Abdel-Hamid, A.-H., & Riganti, C. (2020). Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics, 12(2), 96. https://doi.org/10.3390/pharmaceutics12020096










