Abstract
Viral infections are a major global health problem, representing a significant cause of mortality with an unfavorable continuously amplified socio-economic impact. The increased drug resistance and constant viral replication have been the trigger for important studies regarding the use of nanotechnology in antiviral therapies. Nanomaterials offer unique physico-chemical properties that have linked benefits for drug delivery as ideal tools for viral treatment. Currently, different types of nanomaterials namely nanoparticles, liposomes, nanospheres, nanogels, nanosuspensions and nanoemulsions were studied either in vitro or in vivo for drug delivery of antiviral agents with prospects to be translated in clinical practice. This review highlights the drug delivery nanosystems incorporating the major antiviral classes and their transport across specific barriers at cellular and intracellular level. Important reflections on nanomedicines currently approved or undergoing investigations for the treatment of viral infections are also discussed. Finally, the authors present an overview on the requirements for the design of antiviral nanotherapeutics.
1. Social Impact and Economic Burden of Viral Infectious Diseases
Today, we are living through a so-called fourth great transitional period, after the other three waves of epidemiological transitions, namely early agrarian-based settlements, early Eurasian civilizations and European expansionism (more details can be found in McMichael’s description [1]). The current configuration and variety of infectious diseases closely followed the combined evolutions in demography, environment, technology, social change and behaviours. Medicine itself has created new opportunities for microbes either through blood transfusions, organ transplants, the use of hypodermic syringes or the excessive use of antibiotics thus contributing to the induction of iatrogenic effects in some treatments for infections such as hepatitis C, HIV and others [1].
In recent decades, old concerns have been reactivated at both the official and the general public levels regarding infectious diseases as a threat to public health. McMichael [1] analyzed the reflection of this issue in social media and noticed the “emergence and resurgence” of infectious diseases (determined by environmental, sociological and economic changes) and a so-called “public anxiety” set on this topic.
Despite their widespread and increasing transmission, there is still a poor understanding of global economic impact of viral diseases, which makes difficult to evaluate the societal costs and the cost-effectiveness of preventive efforts. The issue of estimating a general impact of viral infections involves several aspects that hinder this approach, namely: the variety of viral infections, the incidence of associated co-morbidities, social and psychological issues having economic repercussions (hidden costs), the variety of treatments (only direct-acting antiviral (DAA) and vaccinations can be used in the evaluation) and the presence of negative externalities [2], meaning that the disease consequences are not limited to their patients infected or potentially but also to the related families who may experience social distress as well. Also, human mobility and long-distance trade have increased; ever-larger cities, often girded with slums, have become highways for microbial traffic; poverty perpetuates vulnerability to infectious disease; and sexual practices, drug injecting, intensified food production and much modern medical technology all create new “audience” for microbial opportunism, and new management issues for public health decision makers [1].
For all these reasons, a global assessment of socio-economic impact seems practically impossible or, if contrary, it could not meet all the criteria to be relevant. Rather, the impact can be split by relevant categories of viral infections, were the literature is more precise but even so, there remain some inconsistencies regarding the unification of the methodologies from the various studies that will ensure the comparability of the data. Figure 1 presents statistical facts related to the most “burden-generator” viral infection diseases [3,4,5,6,7].
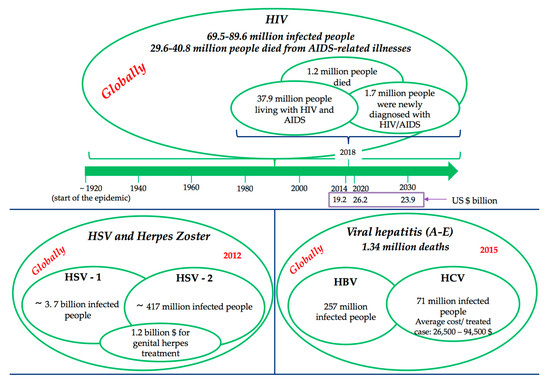
Figure 1.
Global impact of viral diseases.
HIV/AIDS, considered as one of the major burdens of disease globally, became a chronic disease after the introduction of multiple antiretroviral therapy (ART), and therefore it needs to provide long-term care and support for the ill person, demanding a higher level of treatment costs for the HIV-affected households. Consequently, HIV/AIDS causes depletion of savings and productive assets, and increases the indebtedness of the HIV-affected households [8,9]. Moreover, the higher health care expenditure of the households reduces investment for nutritional food for the family members, investment for farming or business, and the children education. Death during the working age of the patient is a major factor in the economic impact of HIV/AIDS. The household level impact of HIV/AIDS includes direct costs, including medical and non-medical costs, and productivity costs such as loss of labour time, as a result of the morbidity of HIV positive household members, as well as time spent by others caring for them [10]. This evidence suggests that HIV/AIDS places significant economic pressure on households trying to pay for health care costs, and trying to make up for lost income.
The difficulty in accurately quantifying and explaining the morbidity and mortality related to viral hepatitis stems from the fact that hepatitis deaths are caused by five distinct viruses (hepatitis A–E) with different routes of transmission, or from the fact that death occurs decades after infection, and that when people die with hepatitis-related liver cancer and cirrhosis, these deaths are not always linked to the underlying infection.
Although antiviral therapy appears to be expensive (for example, average cost for a treated HCV case ranges from $26,500 to $94,500 [11]), is considered to be also cost effective when compared with other well-accepted medical interventions [12] due to sustained viral response to therapy, the cost savings and quality-of-life improvement and prolongation of life expectancy from the prevention of HCV complications. In the era of new DAAs, the statement “provide treatment to HCV-patients” generates savings compared to not provide it. Low and middle-income countries may consider HCV-treatment as a cost-saving intervention for the health system, not only in a long-term horizon, but in 5–10 years [5]. Economic impact of no treatment is higher than treatment costs itself. But still, the enthusiasm for DAA therapies, however, has been tempered by two major concerns: the price, still very high, of these medications, and, the challenges patients and clinicians face with respect to drug access in many countries.
HSV and herpes zoster (varicella-zosterian virus—VZV reactivation) are some of the most common infections in humans, with no effective treatment available at this time. The impact on the health degree varies from a very small impairment to severe, disabling forms, in most cases limiting methods are applied to local infection and reducing side effects (pain, manifestations dermis etc.). Also, specific psychological issues are related with this disease, such as negative feelings correlated to the condition following diagnosis, in particular if they have acquired the genital form of the disease. Feelings can include depression, fear of rejection, feelings of isolation, fear of being found out, and self-destructive feelings [6].
As mentioned above, all the available reports present crude estimates rather than precise measures of the economic costs of the illness, because most of the costs are calculated directly on interventions or on medical budgetary chapters, without taking into account the societal losses. Moreover, different approaches are discrepant (can be explained, at least in part, with the influence of compliance to treatment and possible under sampling of subpopulations in the data set) [7]. Also, limitations must be placed on the ability to generalize the results beyond the sample. Moreover, not only the cost matters. The costs of an intervention have to be compared with the results of interventions because the effectiveness of treatments and the efficiency can produce societal gains that must be offset by losses [13].
The usual most accurate methodology to estimate a burden (associated with a disease) is the so-called “cost-of-Illness” [10], and measures all the costs of a particular disease, including the direct, indirect, and intangible dimensions. It is widely accepted that estimating the total social cost of a disease is useful in establishing policy decisions [14]. But there are also other methods as the cross-sectional surveys of samples of primary and secondary care physicians, analyzing health care resource utilization or approaches based on the analysis of a large administrative data sets, such as values of spending or drugs consuming lists [15]. Other approaches are used to estimate the number of patients seeking medical treatment, the average medical expenditures (as health inputs employed per unit multiplied by number of units) and estimated national costs. These comprehensive studies can often be advantageous in allocating total national expenditures among the major diagnostic categories [16]. However, regardless of the method, such analyzes are not possible otherwise than inside countries (where the impact determined by cultural and social aspects can vary substantially). But even in the absence of global data of this nature, we still can extract from the information presented the relevant issue for the topic of this paper: the current arrangements in the management of viral infections (treatments, prevention and limitations of spread) are costly and less effective, unaffordable in some cases and burdensome for medical systems. For example, according to the current analysis of Globe Newswire Reports and Data [17], the global antiviral drugs market was valued at $49.87 billion in 2018 and is expected to reach $71.48 billion by year 2026. Sales of antivirals increased by approximately 20% each two years. Moreover, thanks to better diagnostics, innovative drugs and new therapeutics, the market is likely to witness even further future growth. However, the list of viral diseases for which antiviral therapies are available is still relatively short [18].
There are several factors that hinder the development of antiviral drugs:
- Dependence of viruses replication on host cell biosynthetic machinery [19], that leads to a limited number of virus-specific metabolic functions can be targeted by antiviral drugs without any damage to the host;
- the viruses’ functions are specific to each virus, preventing the development of a broad-spectrum antivirals fighting against different viruses that cause similar symptoms. Antivirals developed for some viruses (as HSV and HIV) can treat the acute illness, but do not cure the latent infection. This leads to recurrent or chronic diseases that require treatment for longer periods of time [18].
All these limitations prompted the need for a paradigm shift. The great challenge of antiviral therapies is to move on to developing new drug formulas. This involves changing the physico-chemical and bio-pharmaceutical properties of antiviral molecules using new scientific strategies during the preparation or in dosage configuration.
2. Viruses: Types, Current Therapy and Observed Drawbacks
Viruses are sub-microscopic intracellular parasitic particles of genetic material contained in a protein coat, totally dependent by host for cell replication, showing both living and non-living characteristics [20].
Living characteristics of the viruses are represented by the high rate of multiplication (only in living host cells) and by the ability to mutate. The non-living characteristics for viruses consist in acellularity (lack of cytoplasm and organelles), the replication only by using host cell’s metabolic machinery and the composition with DNA or RNA [20]. In humans, viral infections are responsible for different diseases as briefly presented in Table 1.

Table 1.
Common viral infections.
According to International Committee on Taxonomy of Viruses (ICTV) there are 1 phylum, 2 subphylia, six classes, 10 orders, seven suborders, 89 families, 36 subfamilies, 387 genera, 59 subgenera and 2202 species [43]. Currently, viruses are classified based on their type of nucleic acid (DNA, RNA, single-stranded, double-stranded) and their way of replication, known as Baltimore classification [44], divided as seven Baltimore classes:
- I—dsDNA viruses (e.g., adenoviruses, herpesviruses, poxviruses): enter to the host nucleus and are dependent by host cell polymerases to replicate viral genome. The virus may induce the cell to forcefully undergo cell division, which may lead to transformation of the cell and, ultimately, to cancer.
- II—ssDNA viruses (+ strand or “sense”) DNA (e.g., parvoviruses), consists of viruses that have a single-stranded DNA genome of the same polarity as the mRNA. Excepting Parvoviruses, most of them have circular genomes and are replicating within nucleus.
- III—dsRNA viruses (e.g., reoviruses): not dependent by host replication polymerases and their replication (monocistronic) is realized into capsid (in cytoplasm).
- IV—(+)ssRNA viruses (+ strand or sense) RNA (e.g., picornaviruses, Togaviruses): the RNA can be directly accessed by ribosomes of the host to form proteins, and use a simple reproduction pathway (viruses with polycistronic mRNA) or a more complex transcription pathway (for which subgenomic mRNAs, ribosomal frameshifting, and proteolytic processing of polyproteins may be used).
- V—(−)ssRNA viruses (− strand or antisense) RNA (e.g., orthomyxoviruses, rhabdoviruses), that first must be transcribed by viral polymerases (positive-sense) before can be directly accessed by host ribosomes to form proteins.
- VI—ssRNA-RT viruses (+ strand or sense) RNA with DNA intermediate in life-cycle (e.g., retroviruses), which use the reverse transcriptase to convert the positive-sense RNA into DNA. They are using DNA to create the templates and those are spliced into host genome by integrase.
- VII—dsDNA-RT viruses DNA with RNA intermediate in life-cycle (e.g., hepadnaviruses), dsDNA viruses that replicate through a single-stranded RNA intermediate, which use a pregenome RNA as a template and conversion to DNA is done by a viral reverse transcriptase.
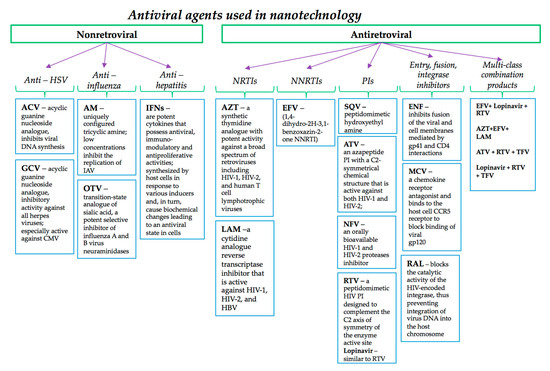
There are distinct stages of viral replication (cell entry, uncoating, transcription of viral genome, translation of viral proteins, post-translational modifications and assembly of virion components) and the classes of antiviral agents that can act at each stage, the correspondence between stage of replication and classes of selective inhibitors being described in detail in reference [45] and their pharmacological properties as it follows in the next section.
3. Biological Barriers Security System
The first line of defence [85] that any substance encounters is the biological barriers penetration into the organism. The “security” system includes physiological barriers, such as blood-brain barrier (BBB), epithelium, stratum corneum, air-blood lung barrier [86], reproductive system barrier, etc. all of which control the extracellular and intracellular access and trafficking of foreign substances such as bacteria, viruses, fungi, and chemicals [87] but also provide selective access to “suitable candidates” such as nutrition and/or therapy molecules.
It is well established that more than one mechanism may be involved in intracellular drug delivery. The mechanisms involved in nano-based intracellular drug delivery include passive diffusion of free drug, non-specific phagocytosis of the nanocarrier, nanocarrier uptake by pinocytosis, and receptor-mediated endocytosis [88]. In this section, we will discuss in particular how to overcome biological barriers such as mucus, skin, cell membrane and BBB in antiviral therapy.
3.1. Mucus
The gastrointestinal tract, respiratory system, the urogenital cavities, eyes and mouth, are all covered with mucosal membranes. The highly adhesive mucus acts as protective layer as well as for lubrication purposes. Although large molecules cannot pass, small ones along with viruses can easily penetrate. These are also the reasons that drug delivery is so challenging. Mucus contains 95% of water along with mucin fibres, lipids, salts, cholesterol and proteins. It is continuously produced but the thickness, pH and amount differ by its position.
Two strategies have to be followed for passing through mucus, mainly depending how fast the turnover is: fast mucosal penetration or highly adhesive particles (slow turnover) to increase the drug’s residence on the targeted mucosa.
Mucoadhesion can be the resultant of interactions like hydrophobic, hydrogen bonding, ionic bonding or van der Waals ones. Other possible attractive interactions can be covalent bond formation between catechols, maleimides, thiols, and acrylates with domains of mucin glycoproteins rich in cysteine. Chitosan, alginate, pectin or cellulose polymers are mostly used for achieving adhesion on mucosa. Furthermore, it is known that thiolation of the mentioned polymers develops high mucoadhesive properties.
Mucopenetration is the second strategy found to permeate the mucus layers by two potential mechanisms: active strategy characterized by the interaction with the mucus and chemically shifting the features of the mucus or their own structures and passive strategy that uses hydrophilicity enhancers to penetrate the mucus [89].
The classical HSV therapy includes daily dosing of orally administered ACV [90] that is effective in most cases, and of course problematic in other cases, for example, in long-term use of ACV patients report resistance against the drug followed by renal injury [91,92]. Another issue produced by standard HSV treatment in this case for the topical form of trifluridine (TFT) and ganciclovir (ACV analog) gels, is denoted by low retention time on vaginal and corneal mucus followed by multiple doses up to 10 times [93].
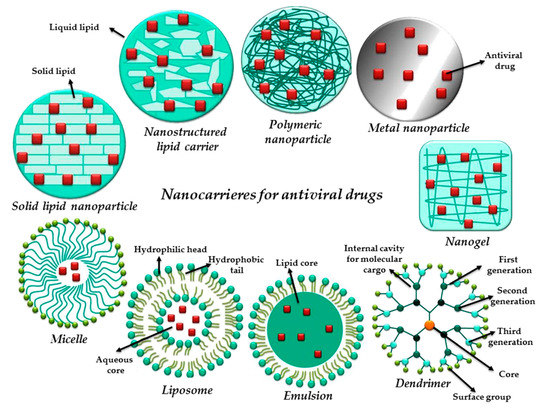
The nanotechnology field offers a great deal of drug delivery modalities in order to overpass the biological barriers, deliver efficiently the incorporated active principle in a controlled and targeted manner, reduce circulating drug levels and attenuate the renal damage. A recent review points out the nanogels based on the above-mentioned materials capabilities as an adequate example to pass through these types of biological barriers [94]. For example, the synthesized nano-drug delivery micelles based on chitosan-g-oligo (NiPAam) copolymers stabilised by ionotropic crosslinking by Raskin et al. [95], gave good results for delivering antiretroviral drugs (EFV) through mucosa. More studies on how ACV penetrates different barriers can be found in Table 2 and detailed in Section 5.
It is important to mention a “special barrier” namely the ocular mucus that changes completely in 5 to 8 min, making the drug absorption unfavourable. In addition, the eye is protected by blood-anterior chamber and blood-retinal barriers. In case of drug administration, a combination of penetration and adhesion of the substances is necessary. Polymers like phosphotyrosine could be the solution. It was demonstrated that if intestinal alkaline phosphatase is present, polymers can manifest a zeta potential change, thus causing their immobilization after penetration. Another kind of construct for combining adhesion and penetration is through thiolated systems with mucolytic enzymes, pH dependent. Therefore, at acid pH, the absence of disulphide bonds formation with cysteine-rich domains in mucins does not manifest mucosal adhesion unless they are near the epithelium [94].
3.2. Skin
Skin, as the largest organ of our body, protects us from microorganisms and chemicals, regulates our body temperature and maintains hydroelectrolytic balance. The two layers of the skin are the epidermis and the dermis. The first one is avascular and is composed of stratified, keratinized squamous epithelium, in four layers from bottom up: basale, spinosum, granulosum and corneum. Thick skin (palms and soles) has a fifth layer (under the most superficial corneum) called lucidum. The dermis consists of two layers (reticular and the more superficial papillary) of connective tissue of elastin and collagenous fibres and has in its component blood and lymphatic vessels network, nerves, touch receptors (Meissner corpuscles), adipocytes, phagocytes, hair follicles and sweat glands [96].
Topical, through skin drug delivery, has a local effect, requiring less drug for the targeted outcome. Transdermal therapy results in fewer side effects with no need of regular treatment but systemic distribution of the drug. Both methods of treatment have a common blockage, stratum corneum. To pass through this barrier, different approaches were developed based mostly on disrupting this structure chemically with substances like surfactants, alcohols, esters, amines, terpenes, alkanes phospholipids, or mechanically by using ultrasounds, micro needling, magnetophoresis, iontophoresis, electroporation or lasers. Excessive use, though, can damage the skin.
Analysing the literature data, we can suggest that there are different processes and mechanisms that govern the penetration of small/large molecules through skin barrier. According to Schneider et al. [97] review there are two general pathways for skin absorption: through skin appendages or through the stratum corneum and the underlying layers. The lipophilic statum corneum medium determines the first mechanism of skin penetration, namely absorption of lipophilic compounds [98]. The three transport routes of substances across stratum corneum can be classified in transcellular, intercellular and trans-appendageal pathways as defined by Liang et al. [98]. More examples are available but the conclusion is the same: “the full understanding of the penetration or absorption processes is still under evaluation” due to the challenges associated with delivering complex burdens through the skin barrie [99].
As specified in Section 2, the current antiviral therapy for HSV infection includes topical formulations of ACV that is unable permeate stratum corneum and target the virus site at the basal epidermis due to its polarity and solubility, leading to poor clinical efficacy due to delayed antiviral activity and sub-inhibitory concentrations [100]. Nanotechnology strategies [97] seem to facilitate the “admission fees” due to the rationally design and innovative functionalities of the synthesized nano-platforms as presented in Figure 3.
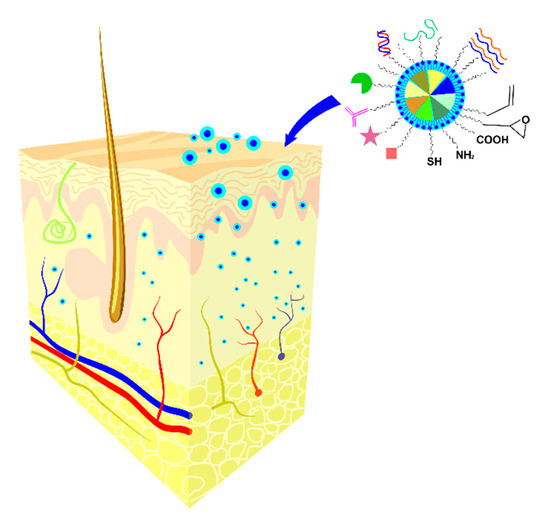
Figure 3.
Schematic illustration of nano-scale carrier systems and their interactions with the dermal barrier.
One more problem in dermal drug passing is related to inflammation skin pathology. The barrier is changed, and the drug resides much less on the targeted site because of fast penetration. The thermoresponsive drug delivery nanogels used in this purpose have encouraging results for overcoming the above-mentioned problems. pH sensitive nanogels can also be utilised for controlled medicinal release.
Thermoresponsive nanogels can be controlled through irradiation with infrared lamp. Another way of skin penetration is the hair follicle. The stratum corneum is less intact in the lower infundibulum, so the nanoparticle’s (NPs) passage is dependent on size and not on composition [94].
3.3. Cell Membrane
The cell membrane separates the content of a cell from the exterior surroundings. Besides the standard protection around the cell, the cell membrane controls what substances go in and out. The composition is based on a bilayer of phospholipids, internally hydrophobic (tails) and externally hydrophilic (heads) with different proteins and cholesterol between them. The membrane is permeable selectively, permitting only some materials to pass through its lipid layer by active (through protein pumps or vesicles) or passive (diffusion) processes of transportation. Water passes the membrane through a process called osmosis, which occurs when an imbalance of solutes appears outside and inside the cell [101].
In most of the cases, hydrophobic and small molecules can pass through diffusion. Nanoscale drug delivery systems depend upon an active mechanism (endocytosis). Over this process, the cell unit takes in ions, solid particles and molecules. There are studies that prove that positive charged nanogels can bind the membrane of the cell (negative charge) through electrostatic intercommunication. More than that, receptor-mediated endocytosis can provide a mechanism through which selectively attracted cell groups are targeted. In addition, hydrophobic nano-platforms can grow the adhesion to the membrane and the amount of drug entered in the cell [94].
3.4. Blood-Brain Barrier
The BBB is a highly selective semipermeable structure composed of five parts: the basement membrane, the astrocytes, the immune cells, the pericytes and an endothelial cell layer of capillaries. The area between basement membrane and the neurons is called Virchow-Robin space. In this region there is interstitial fluid in which reside microglia. All the above components are called neurovascular unit. The kinetics of this unit is crucial to the role of BBB and its states of illness. The capillaries of BBB compose a layer of squamous epithelial cells that fold to form a circular vessel. These cells are linked with strong connections for blocking entrance or exit of materials through central nervous system. Protein transportation facilitates the selective flow of molecules through vessel lumens, essential biomolecules being in higher concentrations, like glucose and at the same time eliminating toxins.
The BBB is a physical and metabolic “obstacle”, physiologically important and active, which survey blood-brain traffic and control it, restricting the paracellular diffusion between the endothelial cells (microvessels) and the efflux pumps activity that quickly expel back into the capillary lumen a wide variety of xenobiotics. BBB integrity and function is critically influenced by what is now referred to as “the extended neurovascular unit” that incorporates not only microvascular endothelial cells and adjacent pericytes, astrocytes and neurons, but also neighbouring smooth muscle cells and microglia in the brain, and blood cells in the capillary lumen such as polymorphonuclear cells, lymphocytes and monocytes [102].
Transport at the BBB level is assured by numerous transport mechanisms that provides to the brain the necessary nutrients and also protects from the toxic xenobiotics. The main transport mechanisms are represented by free diffusion of small lipophilic substances or by active transport (carrier mediated, receptor mediated and active efflux transport).
Active efflux transport is assured by two major types of transporters that extrude metabolic waste, xenobiotics and a large number of drugs from the brain back into the blood. The first superfamily of BBB efflux transporters is the solute carrier proteins (SLC) superfamily, being represented at the level of BBB by SLC22 and SLCO (SLC21) efflux transporters. The second is the ATP-binding cassette (ABC) efflux transporter family represented by permeability glycoprotein (P-gp), breast cancer resistance protein (BCRP) and the multidrug resistance associated proteins (MRPs) [103,104,105].
Based on their localisation, the ABC efflux transporters prevent lipophilic and amphiphilic environmental toxic compounds or drugs, including anti-inflammatory, immunosuppressive, anti-infectious, antineoplastic drugs, some antiepileptic, antidepressant and psychotropic agents, and drug conjugates by an energy-dependent, unidirectional direct transport mechanism, from entering specific substrates [106]. The BBB’s efflux machinery does an excellent job of recognizing xenobiotics, but a poor job on distinguishing between toxicants and therapeutic drugs, creating an important obstacle to treatment of brain cancer, epilepsy and neuro AIDS [107].
The penetration of the BBB for drug delivery, although challenging, captivates the interest of numerous researchers in antiviral therapy since the mechanisms by which for example HSV-1 penetrates the CNS remain unclear. The most likely routes include retrograde transport via the olfactory or trigeminal nerve fibres, occasionally leading to herpes simplex encephalitis (HSE) caused by HSV-1 [108].
Another studied virus that is involved in encephalitis and BBB disruption is HIV, known to cause severe neurological disorders and leading to HIV-related encephalitis [109] since BBB is impermeable to 98% of antiretroviral drugs [110]. The possible mechanism responsible for BBB disruption in HIV-1 encephalitis is considered a “Trojan horse” mechanism, where HIV infects specific T-lymphocytes and circulating monocytes, then entering to CNS through BBB gaps and followed by inflammatory reactions [111], but, in the last years nanotechnology has been intensely explored and several experimental attempts have been carried out in order to enhance the BBB permeability toward antiretroviral drugs, briefly described below based on the nano-based formulation composition, since it is well known that the size and surface functionalization influence transport properties within tissues:
- polymeric polybutylcyanoacrylate (PBCA) nanoparticles with two incorporeated antiretroviral drugs (AZT and lamivudine) showed a 8–20 and 10–18 fold increase in BBB permeation, by three possible mechanisms as presented by the authors: prolonged interaction interval between drug-loaded nanoparticles and brain-microvascular endothelial cells elevated the concentration gradient between blood and the brain, Polysorbate 80 covering on the periphery of nanoparticles was able to be absorbed and degraded nanoparticles improved drug absorption [112];
- spherical transferrin coated-PEGylated albumin nanoparticles encapsulating AZT prepared by ultra-emulsification method using chemical cross-linking by glutaraldehyde gained an access across the BBB through the transferrin receptor mediated endocytosis on the membrane [113];
- transferrin-conjugated quantum rod nanoparticles conjugated with saquinavir crossed an in vitro BBB model by exploiting a receptor-mediated transport [114];
- magnetic liposomal nanoformulations of azidothymidine 5′-triphosphate (the active form of azidothymidine) migrate across BBB in vitro, either directly or by a monocyte-mediated transport, under the influence of an external magnetic field [115];
- novel nanodrug consisting of an iron oxide nanoparticle coated with PMA amphiphilic polymer and functionalized with the antiretroviral peptide enfuvirtide crossed the BBB by a passive diffusion, probably mediated by the absorption of the amphiphilic coating on the cell membrane [116].
As briefly presented the preliminary results are more than encouraging. Certainly, future investigations on the mechanisms about BBB disruption are needed along with novel, innovative, safe and efficacious therapeutic approaches.
8. Conclusions
Treating or improving treatment success rate for viral diseases are fundamental responsibilities. The established potential and boosted progress of nanotechnology in antiviral therapy development generates great expectations for new therapeutic innovative strategies for attacking or eradicating viral disorders. At present, studies explored numerous and diverse nano-platforms including nanoparticles, liposomes, micelles, with different compositions, size, with single or combined entrapped drugs that may serve as potential antiviral drug delivery transporters. These nano-based systems have exhibited versatile features to improve the identified current therapy drawback. However, the clinical use of a nano-based antiviral formulation to date based on our knowledge has turned out just a few approved or under clinical trials nanoformulations, mainly vaccines, despite more than 22 years of constant efforts. It is expected in the upcoming years that part of this “success preclinical story” to be scaled-up, translated and applied for better outcome, convenience and access for patients.
Author Contributions
All authors contributed equally to this manuscript. G.D. and F.-D.C. were responsible for the review concept and design. G.D., C.-M.U., F.-D.C., C.-T.M., I.G., D.B., B.-I.T., C.R., L.T. and E.R. were responsible for draft preparation. G.D. and F.-D.C. were responsible for the review and editing of the manuscript. G.D., C.R., L.T., B.-I.T. and E.R. were responsible for visualization and funding acquisition. G.D. and F.-D.C. were responsible for the revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research and Innovation, CNCS-UEFISCDI, project number PN-III-P1-1.1-PD-2016-1642, within PNCDI III.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AIDS: acquired immunodeficiency syndrome; ACV: acyclovir; Ag: silver; AM: amantadine; ART: antiretroviral therapy; ATV: atazanavir; AZT: zidovudine; BMVECs: brain microvascular endothelial cells; Au: gold; CCK-8: Cell Counting Kit-8; CH: cholesterol; CMV: cytomegalovirus; Cs: chitosan; DAA: direct-acting antiviral; DSC: differential scanning calorimetry; DLS: dynamic light-scattering; DNA: Deoxyribonucleic acid; DsDNA: double stranded DNA; DRV: darunavir; DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine; EFV: efavirenz; EVG: elvitegravir; FDA: Food and Drug Administration; FTIR: Fourier-transform infrared spectroscopy; GCV: gangiclovir; GMP: good manufacturing practice; AuNPs: gold nanoparticles; HBV: hepatitis B virus; HCV: hepatitis C virus; HSV: herpes Simplex; HIV: human immunodeficiency virus; HPAC: highly porous activated carbon; HPMC: hydroxypropyl methylcellulose; IAV: influenza A virus; IFNs: interferons; LAM: lamivudine; LNPs: lipid nanoparticles; MNPs: magnetic nanoparticles; MPEG: N-(carbonylmethoxypolyethyleneglycol-2000); DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt; MTT: [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide]; MTS: [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]; NaDC: sodium deoxycholate; NEs: nanoemuslions; NiPAam: N-isopropylacrylamide; NLCs: nanostructured lipid carriers; NPs: nanoparticles; NRTIs: nucleoside and nucleotide reverse transcriptase inhibitors; NNRTIs: non-nucleoside reverse transcriptase inhibitors; NS: nanospheres; OTV: oseltamivir; PBS: phosphate buffer solution; PC: phosphatidylcholine; PCL: poly(ε-caprolactone); PEG: poly(ethylene glycol); PEO-PCL: poly(ethylene oxide)-modified poly(ε-caprolactone); PG: polyglycerol; PLGA: poly lactic-co-glycolic acid; PIs: protease inhibitors; pMBA: p-mercaptobenzoic acid; PVA: polyvinyl alcohol; PVP: poly(vinylpyrrolidone); QRs: quantum rods; RAL: raltegravir; REF: reference; rHDL: recombinant high density lipoproteins; RNA: Ribonucleic acid; RTIs: Reverse transcriptase inhibitors; RTV: ritonavir; SCM: sodium carboxymethylcellulose; SLNs: solid lipid nanoparticles; SLS: sodium lauryl sulphate; SAHA: suberoylanilide hydroxamic acid; SEM: scanning electron microscopy; SeNPs: selenium nanoparticles; Se@AM: selenium nanoparticles with amantadine; siRNA: small interfering RNA; ssDNA: single stranded DNA; ssRNA: single stranded RNA; TAF: tenofovir alafenamide; TEM: transmission electron microscopy; Tf: transferrin; TFV: tenofovir; UNAIDS: The Joint United Nations Programme on HIV/AIDS; VZV: varicella-zosterian virus; ζ-potential: zeta potential.
References
- McMichael, A.J. Environmental and social influences on emerging infectious diseases: Past, present and future. Philos. Trans. R. Soc. Lond. B 2004, 359, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Szucs, P.A.; Richman, P.B.; Mandell, M. Triage Nurse Application of the Ottawa Knee Rule. Acad. Emerg. Med. 2001, 8, 112–116. [Google Scholar] [CrossRef] [PubMed]
- The Joint United Nations Programme on HIV/AIDS. Available online: https://www.unaids.org/en (accessed on 27 November 2019).
- UNAIDS. Global Statistics Report 2019. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 27 November 2019).
- WHO. Global Hepatits Report. 2017. Available online: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en (accessed on 25 November 2019).
- WHO. Herpes Simplex Virus Key Facts. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 20 November 2019).
- Szucs, T.D.; Berger, K.; Fisman, D.N.; Harbarth, S. The estimated economic burden of genital herpes in the United States. An analysis using two costing approaches. BMC Infect. Dis. 2001, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, T.; García Goñi, M.; Muñoz-Fernández, M.A. Socio-economic impact of antiretroviral treatment in HIV patients. An economic review of cost savings after introduction of HAART. AIDS Rev. 2009, 11, 79–90. [Google Scholar]
- Tseng, A.; Seet, J.; Phillips, E.J. The evolution of three decades of antiretroviral therapy: Challenges, triumphs and the promise of the future: Three decades of antiretroviral therapy. Br. J. Clin. Pharmacol. 2015, 79, 182–194. [Google Scholar] [CrossRef]
- Fleßa, S.; Marschall, P. Socio-Economic Impact of Antiviral Intervention. In Antiviral Strategies; Kräusslich, H.-G., Bartenschlager, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 189, pp. 347–374. ISBN 978-3-540-79085-3. [Google Scholar] [CrossRef]
- Woolston, S.L.; Kim, N. Cost and Access to Direct-Acting Antiviral Agents. Hepatoma Res. 2018, 4, 3. [Google Scholar]
- Iyengar, S.; Tay-Teo, K.; Vogler, S.; Beyer, P.; Wiktor, S.; de Joncheere, K.; Hill, S. Prices, Costs, and Affordability of New Medicines for Hepatitis C in 30 Countries: An Economic Analysis. PLoS Med. 2016, 13, e1002032. [Google Scholar] [CrossRef]
- Noyes, K.; Holloway, R.G. Evidence from cost-effectiveness research. Neurotherapeutics 2004, 1, 348–355. [Google Scholar] [CrossRef]
- Byford, S. Economic Note: Cost of illness studies. BMJ 2000, 320, 1335. [Google Scholar] [CrossRef]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Jo, C. Cost-of-illness studies: Concepts, scopes, and methods. Clin. Mol. Hepatol. 2014, 20, 327. [Google Scholar] [CrossRef]
- Reports and Data. Available online: www.globenewswire.com (accessed on 10 November 2019).
- Lembo, D.; Cavalli, R. Nanoparticulate Delivery Systems for Antiviral Drugs. Antivir. Chem. Chemother. 2010, 21, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. How Viral and Intracellular Bacterial Pathogens Reprogram the Metabolism of Host Cells to Allow Their Intracellular Replication. Front. Cell. Infect. Microbiol. 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, M.H.V. Virus Species. In Genetics and Evolution of Infectious Dissease; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–19. [Google Scholar]
- Heikkinen, T.; Järvinen, A. The common cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef]
- Faith, S.C.; Durrani, A.F.; Jhanji, V. Cytomegalovirus keratitis. Curr. Opin. Ophthalmol. 2018, 29, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Kalezic, T.; Mazen, M.; Kuklinski, E.; Asbell, P. Herpetic eye disease study: Lessons learned. Curr. Opin. Ophthalmol. 2018, 29, 340–346. [Google Scholar] [CrossRef]
- Jhanji, V.; Chan, T.C.Y.; Li, E.Y.M.; Agarwal, K.; Vajpayee, R.B. Adenoviral keratoconjunctivitis. Surv. Ophthalmol. 2015, 60, 435–443. [Google Scholar] [CrossRef]
- Venkatesan, A.; Murphy, O.C. Viral Encephalitis. Neurol. Clin. 2018, 36, 705–724. [Google Scholar] [CrossRef]
- Ruuskanen, O.; Lahti, E.; Jennings, L.C.; Murdoch, D.R. Viral pneumonia. Lancet 2011, 377, 1264–1275. [Google Scholar] [CrossRef]
- Hékimian, G.; Combes, A. Myocardites. La Revue de Médecine Interne 2017, 38, 531–538. [Google Scholar] [CrossRef]
- Rose, N.R. Viral myocarditis. Curr. Opin. Rheumatol. 2016, 28, 383–389. [Google Scholar] [CrossRef]
- Op de Beeck, A.; Eizirik, D.L. Viral infections in type 1 diabetes mellitus—Why the β cells? Nat. Rev. Endocrinol. 2016, 12, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Thuener, J. Hepatitis A and B Infections. Prim. Care 2017, 44, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Hepatitis, C. BMJ 358, j2861. Available online: https://bestpractice.bmj.com/topics/en-us/128 (accessed on 31 October 2019).
- Rizzetto, M. Hepatitis D Virus: Introduction and Epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021576. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Hepatitis E: Discovery, global impact, control and cure. WJG 2016, 22, 7030. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.; Gershon, A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef]
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Microbiol. 2014, 12, 197–210. [Google Scholar] [CrossRef]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Human Herpesviruses 6A, 6B, and 7. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Ramdass, P.; Mullick, S.; Farber, H.F. Viral Skin Diseases. Prim. Care Clin. Off. Pract. 2015, 42, 517–567. [Google Scholar] [CrossRef]
- Schaffer, J.V.; Berger, E.M. Molluscum Contagiosum. JAMA Dermatol. 2016, 152, 1072. [Google Scholar] [CrossRef]
- Krenzer, M.E. Viral Gastroenteritis in the Adult Population. Crit. Care Nurs. Clin. N. Am. 2012, 24, 541–553. [Google Scholar] [CrossRef]
- Eckardt, A.J.; Baumgart, D.C. Viral Gastroenteritis in Adults. PRI 2011, 6, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Thongprachum, A.; Khamrin, P.; Maneekarn, N.; Hayakawa, S.; Ushijima, H. Epidemiology of gastroenteritis viruses in Japan: Prevalence, seasonality, and outbreak: Epidemiology of Gastroenteritis Viruses in Japan. J. Med. Virol. 2016, 88, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Cone, M.M.; Whitlow, C.B. Sexually Transmitted and Anorectal Infectious Diseases. Gastroenterol. Clin. N. Am. 2013, 42, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Virus Taxonomy: 2018b Release. Available online: https://talk.ictvonline.org/taxonomy (accessed on 30 November 2019).
- Baltimore, D. Expression of animal virus genomes. Bacteriol. Rev. 1971, 35, 235–241. [Google Scholar] [CrossRef]
- Brunton, L.L.; Hilal-Dandan, R.; Knollmann, B. The Pharmacological Basis of Therapeutics, 13th ed.; McGraw-Hill Education-Europe: New York, NY, USA, 2017; ISBN 978-1-259-58473-2. [Google Scholar]
- Sacks, S.L.; Wilson, B. Famciclovir/Penciclovir. In Antiviral Chemotherapy 5; Mills, J., Volberding, P.A., Corey, L., Eds.; Springer: Boston, MA, USA, 1999; Volume 458, pp. 135–147. ISBN 978-1-4613-7150-2. [Google Scholar]
- Hitchcock, M.J.M.; Jaffe, H.S.; Martin, J.C.; Stagg, R.J. Cidofovir, a New Agent with Potent Anti-Herpesvirus Activity. Antivir. Chem. Chemother. 1996, 7, 115–127. [Google Scholar] [CrossRef]
- Perry, C.M.; Barman Balfour, J.A. Fomivirsen. Drugs 1999, 57, 375–380. [Google Scholar] [CrossRef]
- Mathiesen, S.; Dam, E.; Roge, B.; Joergensen, L.B.; Laursen, A.L.; Gerstoft, J.; Clavel, F. Long-term foscarnet therapy remodels thymidine analogue mutations and alters resistance to zidovudine and lamivudine in HIV-1. Antivir. Ther. 2007, 12, 335–343. [Google Scholar]
- Leung, D.T.; Sacks, S.L. Docosanol: A topical antiviral for herpes labialis. Exp. Opin. Pharmacother. 2004, 5, 2567–2571. [Google Scholar] [CrossRef]
- Miller, W.H.; Miller, R.L. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem. Pharmacol. 1982, 31, 3879–3884. [Google Scholar] [CrossRef]
- King, D.H. History, pharmacokinetics, and pharmacology of acyclovir. J. Am. Acad. Dermatol. 1988, 18, 176–179. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Baggett, H.C.; Brooks, W.A.; Feikin, D.R.; Hammitt, L.L.; Higdon, M.M.; Howie, S.R.C.; Deloria Knoll, M.; Kotloff, K.L.; Levine, O.S.; et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet 2019, 394, 757–779. [Google Scholar] [CrossRef]
- Strasfeld, L.; Chou, S. Antiviral Drug Resistance: Mechanisms and Clinical Implications. Infect. Dis. Clin. N. Am. 2010, 24, 413–437. [Google Scholar] [CrossRef]
- McGavin, J.K.; Goa, K.L. Ganciclovir: An Update of its Use in the Prevention of Cytomegalovirus Infection and Disease in Transplant Recipients. Drugs 2001, 61, 1153–1183. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Härter, G.; Schubert, A.; Bunjes, D.; Mertens, T.; Michel, D. Antiviral treatment of cytomegalovirus infection and resistant strains. Exp. Opin. Pharmacother. 2009, 10, 191–209. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antiviral agents active against influenza a viruses. Nat. Rev. Drug Discov. 2006, 5, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Cheng, J.X.; Liu, M.-T.; King, K.; Peng, J.-Y.; Zhang, X.-Q.; Wang, C.-H.; Shresta, S.; Schooley, R.T.; Liu, Y.-T. Inhibitory and combinatorial effect of diphyllin, a v-ATPase blocker, on influenza viruses. Antivir. Res. 2013, 99, 371–382. [Google Scholar] [CrossRef]
- Huss, M.; Wieczorek, H. Inhibitors of V-ATPases: Old and new players. J. Exp. Biol. 2009, 212, 341–346. [Google Scholar] [CrossRef]
- Yeganeh, B.; Ghavami, S.; Kroeker, A.L.; Mahood, T.H.; Stelmack, G.L.; Klonisch, T.; Coombs, K.M.; Halayko, A.J. Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L270–L286. [Google Scholar] [CrossRef]
- Ison, M.G. Antiviral Treatments. Clin. Chest Med. 2017, 38, 139–153. [Google Scholar] [CrossRef]
- Jefferson, T.; Jones, M.A.; Doshi, P.; Del Mar, C.B.; Hama, R.; Thompson, M.J.; Spencer, E.A.; Onakpoya, I.J.; Mahtani, K.R.; Nunan, D.; et al. Neuraminidase inhibitors for preventing and treating influenza in adults and children. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- De Jong, M.D.; Thanh, T.T.; Khanh, T.H.; Hien, V.M.; Smith, G.J.D.; Chau, N.V.; Cam, B.V.; Qui, P.T.; Ha, D.Q.; Guan, Y.; et al. Oseltamivir Resistance during Treatment of Influenza A (H5N1) Infection. N. Engl. J. Med. 2005, 353, 2667–2672. [Google Scholar] [CrossRef] [PubMed]
- Te, H.S.; Randall, G.; Jensen, D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol. Hepatol. 2007, 3, 218–225. [Google Scholar]
- Krečmerová, M. Amino Acid Ester Prodrugs of Nucleoside and Nucleotide Antivirals. MRMC 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Clinical Potential of the Acyclic Nucleoside Phosphonates Cidofovir, Adefovir, and Tenofovir in Treatment of DNA Virus and Retrovirus Infections. Clin. Microbiol. Rev. 2003, 16, 569–596. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, J.Y.; Ahn, S.H. An evaluation of entecavir for the treatment of chronic hepatitis B infection in adults. Exp. Rev. Gastroenterol. Hepatol. 2016, 10, 177–186. [Google Scholar] [CrossRef]
- Matthews, S. Telbivudine for the management of chronic hepatitis B virus infection. Clin. Ther. 2007, 29, 2635–2653. [Google Scholar] [CrossRef]
- Amarapurkar, D.N. Telbivudine: A new treatment for chronic hepatitis B. WJG 2007, 13, 6150. [Google Scholar] [CrossRef]
- Antiretroviral Drugs Used in the Treatment of HIV Infection. Available online: https://www.fda.gov/patients/hiv-treatment/antiretroviral-drugs-used-treatment-hiv-infection (accessed on 26 October 2019).
- FDA-Approved HIV Medicines. Available online: https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/58/fda-approved-hiv-medicines (accessed on 26 October 2019).
- Gallant, J.E.; Gerondelis, P.Z.; Wainberg, M.A.; Shulman, N.S.; Haubrich, R.H.; St Clair, M.; Lanier, E.R.; Hellmann, N.S.; Richman, D.D. Nucleoside and nucleotide analogue reverse transcriptase inhibitors: A clinical review of antiretroviral resistance. Antivir. Ther. 2003, 8, 489–506. [Google Scholar]
- Perry, C.M.; Faulds, D. Lamivudine: A Review of its Antiviral Activity, Pharmacokinetic Properties and Therapeutic Efficacy in the Management of HIV Infection. Drugs 1997, 53, 657–680. [Google Scholar] [CrossRef]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A Review of the Toxicity of HIV Medications. J. Med. Toxicol. 2014, 10, 26–39. [Google Scholar] [CrossRef]
- McConville, C.; Boyd, P.; Major, I. Efficacy of Tenofovir 1% Vaginal Gel in Reducing the Risk of HIV-1 and HSV-2 Infection. Clin. Med. Insights Women’s Health 2014, 7. [Google Scholar] [CrossRef]
- Sluis-Cremer, N.; Temiz, N.; Bahar, I. Conformational Changes in HIV-1 Reverse Transcriptase Induced by Nonnucleoside Reverse Transcriptase Inhibitor Binding. CHR 2004, 2, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, Z.; Chu, Y. HIV protease inhibitors: A review of molecular selectivity and toxicity. HIV 2015, 95. [Google Scholar] [CrossRef] [PubMed]
- Latinovic, O.; Kuruppu, J.; Davis, C.; Le, N.; Heredia, A. Pharmacotherapy of HIV-1 Infection: Focus on CCR5 Antagonist Maraviroc. Clin. Med. Ther. 2009, 1, CMT.S2365. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Morris-Natschke, S.L.; Lee, K.-H. HIV entry inhibitors and their potential in HIV therapy. Med. Res. Rev. 2009, 29, 369–393. [Google Scholar] [CrossRef]
- Lai, W.; Huang, L.; Chen, C. HIV entry inhibitors: Progress in development and application. Yao Xue Xue Bao 2010, 45, 131–140. [Google Scholar]
- Hajimahdi, Z.; Zarghi, A. Progress in HIV-1 Integrase Inhibitors: A Review of their Chemical Structure Diversity. Iran. J. Pharm. Res. 2016, 15, 595–628. [Google Scholar]
- Steigbigel, R.T.; Cooper, D.A.; Kumar, P.N.; Eron, J.E.; Schechter, M.; Markowitz, M.; Loutfy, M.R.; Lennox, J.L.; Gatell, J.M.; Rockstroh, J.K.; et al. Raltegravir with Optimized Background Therapy for Resistant HIV-1 Infection. N. Engl. J. Med. 2008, 359, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Cocohoba, J.; Dong, B.J. Raltegravir: The first HIV integrase inhibitor. Clin. Ther. 2008, 30, 1747–1765. [Google Scholar] [CrossRef]
- Hicks, C.; Gulick, R.M. Raltegravir: The First HIV Type 1 Integrase Inhibitor. Clin. Infect. Dis. 2009, 48, 931–939. [Google Scholar] [CrossRef]
- Avila-Olias, M.; Pegoraro, C.; Battaglia, G.; Canton, I. Inspired by nature: Fundamentals in nanotechnology design to overcome biological barriers. Ther. Deliv. 2013, 4, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Hirn, S.; Möller, W.; Schleh, C.; Wenk, A.; Celik, G.; Lipka, J.; Schäffler, M.; Haberl, N.; Johnston, B.D.; et al. Air–Blood Barrier Translocation of Tracheally Instilled Gold Nanoparticles Inversely Depends on Particle Size. ACS Nano 2014, 8, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Leong, W.; Leong, K.W.; Chen, C.; Zhao, Y. Walking the line: The fate of nanomaterials at biological barriers. Biomaterials 2018, 174, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Aalinkeel, R.; Law, W.-C.; Reynolds, J.; Nair, B.B.; Sykes, D.E.; Yong, K.-T.; Roy, I.; Prasad, P.; Schwartz, S. Anti-HIV-1 nanotherapeutics: Promises and challenges for the future. IJN 2012, 5301. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Piñón-Segundo, E.; Mendoza-Muñoz, N.; Zambrano-Zaragoza, M.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Approaches in Polymeric Nanoparticles for Vaginal Drug Delivery: A Review of the State of the Art. IJMS 2018, 19, 1549. [Google Scholar] [CrossRef] [PubMed]
- Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease: Effect on prevention of epithelial keratitis and stromal keratitis. Arch. Ophthalmol. 2000, 118, 1030–1036. [Google Scholar]
- Durai, R. Drug delivery approaches of an antiviral drug: A comprehensive review. Asian J. Pharm. 2015, 9. [Google Scholar] [CrossRef]
- Wood, A.J.J.; Whitley, R.J.; Gnann, J.W. Acyclovir: A Decade Later. N. Engl. J. Med. 1992, 327, 782–789. [Google Scholar] [CrossRef]
- Yaldiz, M.; Solak, B.; Kara, R.O.; Cosansu, N.; Erdem, M.T. Comparison of Famciclovir, Valaciclovir, and Brivudine Treatments in Adult Immunocompetent Patients with Herpes Zoster. Am. J. Ther. 2018, 25, e626–e634. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Blanco, E.R.O.; Gugliotta, L.M.; Alvarez Igarzabal, C.I.; Calderón, M. Crossing biological barriers with nanogels to improve drug delivery performance. J. Control. Release 2019, 307, 221–246. [Google Scholar] [CrossRef]
- Raskin, M.M.; Schlachet, I.; Sosnik, A. Mucoadhesive nanogels by ionotropic crosslinking of chitosan-g-oligo(NiPAam) polymeric micelles as novel drug nanocarriers. Nanomedicine 2016, 11, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Heyden, R.J. Layers of the Skin. In Anatomy and Physiology. Available online: https://opentextbc.ca/anatomyandphysiology (accessed on 11 November 2019).
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermat. Endocrinol. 2009, 1, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xu, Z.; Grice, J.; Zvyagin, A.; Roberts, M.; Liu, X. Penetration of Nanoparticles into Human Skin. CPD 2013, 19, 6353–6366. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Brenza, T.M.; Binnebose, A.M.; Phanse, Y.; Kanthasamy, A.G.; Gendelman, H.E.; Salem, A.K.; Bartholomay, L.C.; Bellaire, B.H.; Narasimhan, B. Nano-enabled delivery of diverse payloads across complex biological barriers. J. Control. Release 2015, 219, 548–559. [Google Scholar] [CrossRef]
- Prabhu, P.; Patravale, V.; Joshi, M. Nanocarriers for Effective Topical Delivery of Anti-Infectives. CNANO 2012, 8, 491–503. [Google Scholar] [CrossRef]
- Heyden, R.J. The Cell Membrane. In Anatomy and Physiology. Available online: https://opentextbc.ca/anatomyandphysiology/chapter/the-cell-membrane (accessed on 11 November 2019).
- McCaffrey, G.; Davis, T.P. Physiology and Pathophysiology of the Blood-Brain Barrier: P-Glycoprotein and Occludin Trafficking as Therapeutic Targets to Optimize Central Nervous System Drug Delivery. J. Investig. Med. 2012, 60, 1131–1140. [Google Scholar] [CrossRef]
- Ek, C.J.; Wong, A.; Liddelow, S.A.; Johansson, P.A.; Dziegielewska, K.M.; Saunders, N.R. Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol. Lett. 2010, 197, 51–59. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Leandro, K.; Bicker, J.; Alves, G.; Falcão, A.; Fortuna, A. ABC transporters in drug-resistant epilepsy: Mechanisms of upregulation and therapeutic approaches. Pharmacol. Res. 2019, 144, 357–376. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1043661819300726 (accessed on 18 February 2020). [CrossRef]
- Mahringer, A.; Fricker, G. ABC transporters at the blood–brain barrier. Exp. Opin. Drug Metab. Toxicol. 2016, 12, 499–508. [Google Scholar] [CrossRef]
- Miller, D. Regulation of ABC transporters at the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 395–403. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, K.; He, Q.; Lei, Q.; Lu, W. Mechanisms of Blood-Brain Barrier Disruption in Herpes Simplex Encephalitis. J. Neuroimmune Pharmacol. 2019, 14, 157–172. [Google Scholar] [CrossRef]
- Al-Obaidi, M.M.J.; Bahadoran, A.; Wang, S.M.; Manikam, R.; Raju, C.S.; Sekaran, S.D. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018, 62, 16–27. [Google Scholar] [CrossRef]
- Sagar, V.; Pilakka-Kanthikeel, S.; Pottathil, R.; Saxena, S.K.; Nair, M. Towards nanomedicines for neuroAIDS: Nanomedicines for neuroAIDS. Rev. Med. Virol. 2014, 24, 103–124. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, X.P.; McArthur, J.C.; Scott, J.; Gartner, S. Analysis of human immunodeficiency virus type 1 gp160 sequences from a patient with HIV dementia: Evidence for monocyte trafficking into brain. J. Neurovirol. 2000, 6 (Suppl. 1), S70–S81. [Google Scholar]
- Kuo, Y.-C.; Chen, H.-H. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate–sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood–brain barrier. Int. J. Pharm. 2006, 327, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Mahor, S.; Rawat, A.; Gupta, P.N.; Dubey, P.; Khatri, K.; Vyas, S.P. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J. Drug Target. 2006, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Shusta, E.V. Blood–Brain Barrier Transport of Therapeutics via Receptor-Mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Saiyed, Z.; Gandhi, N.; Nair, M. Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood & ndash; brain barrier. IJN 2010, 157. [Google Scholar] [CrossRef]
- Fiandra, L.; Colombo, M.; Mazzucchelli, S.; Truffi, M.; Santini, B.; Allevi, R.; Nebuloni, M.; Capetti, A.; Rizzardini, G.; Prosperi, D.; et al. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1387–1397. [Google Scholar] [CrossRef]
- McNeil, S.E. (Ed.) Unique Benefits of Nanotechnology to Drug Delivery and Diagnostics. In Characterization of Nanoparticles Intended for Drug Delivery; Humana Press: Totowa, NJ, USA, 2011; Volume 697, pp. 3–8. ISBN 978-1-60327-197-4. [Google Scholar]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef]
- Gagliardi, M. Biomimetic and bioinspired nanoparticles for targeted drug delivery. Ther. Deliv. 2017, 8, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Singh, S. Targeted nanomedicines: Effective treatment modalities for cancer, AIDS and brain disorders. Nanomedicine 2009, 4, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C. Role of nanotechnology in HIV/AIDS vaccine development. Adv. Drug Deliv. Rev. 2016, 103, 76–89. [Google Scholar] [CrossRef]
- Milovanovic, M.; Arsenijevic, A.; Milovanovic, J.; Kanjevac, T.; Arsenijevic, N. Nanoparticles in Antiviral Therapy. In Antimicrobial Nanoarchitectonics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 383–410. ISBN 978-0-323-52733-0. [Google Scholar]
- Cao, S.; Woodrow, K.A. Nanotechnology approaches to eradicating HIV reservoirs. Eur. J. Pharm. Biopharm. 2019, 138, 48–63. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, J. Preparation and ocular pharmacokinetics of ganciclovir liposomes. AAPS J. 2007, 9, E371. [Google Scholar] [CrossRef]
- Feng, M.; Cai, Q.; Shi, X.; Huang, H.; Zhou, P.; Guo, X. Recombinant high-density lipoprotein complex as a targeting system of nosiheptide to liver cells. J. Drug Target. 2008, 16, 502–508. [Google Scholar] [CrossRef]
- Feng, M. Liver targeting and anti-HBV activity of reconstituted HDL–acyclovir palmitate complex. Eur. J. Pharm. Biopharm. 2008, 68, 688–693. [Google Scholar] [CrossRef]
- Kim, S.I.; Shin, D.; Lee, H.; Ahn, B.-Y.; Yoon, Y.; Kim, M. Targeted delivery of siRNA against hepatitis C virus by apolipoprotein A-I-bound cationic liposomes. J. Hepatol. 2009, 50, 479–488. [Google Scholar] [CrossRef]
- Kim, S.-S.; Peer, D.; Kumar, P.; Subramanya, S.; Wu, H.; Asthana, D.; Habiro, K.; Yang, Y.-G.; Manjunath, N.; Shimaoka, M.; et al. RNAi-mediated CCR5 Silencing by LFA-1-targeted Nanoparticles Prevents HIV Infection in BLT Mice. Mol. Ther. 2010, 18, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Pollock, S.; Dwek, R.A.; Burton, D.R.; Zitzmann, N. N-Butyldeoxynojirimycin is a broadly effective anti-HIV therapy significantly enhanced by targeted liposome delivery. AIDS 2008, 22, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Gagné, J.-F.; Désormeaux, A.; Perron, S.; Tremblay, M.J.; Bergeron, M.G. Targeted delivery of indinavir to HIV-1 primary reservoirs with immunoliposomes. BBA Biomembr. 2002, 1558, 198–210. [Google Scholar] [CrossRef]
- Clayton, R.; Ohagen, A.; Nicol, F.; Del Vecchio, A.M.; Jonckers, T.H.M.; Goethals, O.; Van Loock, M.; Michiels, L.; Grigsby, J.; Xu, Z.; et al. Sustained and specific in vitro inhibition of HIV-1 replication by a protease inhibitor encapsulated in gp120-targeted liposomes. Antivir. Res. 2009, 84, 142–149. [Google Scholar] [CrossRef]
- Yadavalli, T.; Ames, J.; Agelidis, A.; Suryawanshi, R.; Jaishankar, D.; Hopkins, J.; Thakkar, N.; Koujah, L.; Shukla, D. Drug-encapsulated carbon (DECON): A novel platform for enhanced drug delivery. Sci. Adv. 2019, 5, eaax0780. [Google Scholar] [CrossRef]
- Yang, X.; Shah, S.J.; Wang, Z.; Agrahari, V.; Pal, D.; Mitra, A.K. Nanoparticle-based topical ophthalmic formulation for sustained release of stereoisomeric dipeptide prodrugs of ganciclovir. Drug Deliv. 2015, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Zhao, M.; Xia, Y.; Wang, C.; Xu, T.; Zhu, B. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. IJN 2018, 13, 2005–2016. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Xia, Y.; Zhao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. IJN 2017, 12, 5733–5743. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Zhao, M.; Guo, M.; Xu, T.; Wang, C.; Xia, H.; Zhu, B. Reversal of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with amantadine. RSC Adv. 2016, 6, 89679–89686. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Chen, Y.-T.; Fang, Z.-S.; Chang, W.-S.; Chen, H.-W. Antiviral efficacy of nanoparticulate vacuolar ATPase inhibitors against influenza virus infection. IJN 2018, 13, 8579–8593. [Google Scholar] [CrossRef]
- Chiellini, E.E.; Chiellini, F.; Solaro, R. Bioerodible Polymeric Nanoparticles for Targeted Delivery of Proteic Drugs. J. Nanosci. Nanotechnol. 2006, 6, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lakshmi, Y.S.; Bhaskar, C.; Golla, K.; Kondapi, A.K. Improved Safety, Bioavailability and Pharmacokinetics of Zidovudine through Lactoferrin Nanoparticles during Oral Administration in Rats. PLoS ONE 2015, 10, e0140399. [Google Scholar] [CrossRef] [PubMed]
- Kirimlioğlu, G.Y.; Öztürk, A.A. Preparation and in vitro characterization of lamivudine loaded nanoparticles prepared by acid and/or ester terminated PLGA for effective oral anti-retroviral therapy. JRP 2019, 23, 897–913. [Google Scholar] [CrossRef]
- Sankar, V.; Madhura Keerthi, L.; Nilaykumar, P. Formulation and In-vitro Evaluation of Zidovudine-Lamivudine Nanoparticles. Indian J. Pharm. Educ. Res. 2012, 46, 192–196. [Google Scholar]
- Shah, L.K.; Amiji, M.M. Intracellular Delivery of Saquinavir in Biodegradable Polymeric Nanoparticles for HIV/AIDS. Pharm. Res. 2006, 23, 2638–2645. [Google Scholar] [CrossRef]
- Tang, X.; Liang, Y.; Liu, X.; Zhou, S.; Liu, L.; Zhang, F.; Xie, C.; Cai, S.; Wei, J.; Zhu, Y.; et al. PLGA-PEG Nanoparticles Coated with Anti-CD45RO and Loaded with HDAC Plus Protease Inhibitors Activate Latent HIV and Inhibit Viral Spread. Nanoscale Res. Lett. 2015, 10, 413. [Google Scholar] [CrossRef]
- Venkatesh, D.N.; Baskaran, M.; Karri, V.V.S.R.; Mannemala, S.S.; Radhakrishna, K.; Goti, S. Fabrication and in vivo evaluation of Nelfinavir loaded PLGA nanoparticles for enhancing oral bioavailability and therapeutic effect. Saudi Pharm. J. 2015, 23, 667–674. [Google Scholar] [CrossRef]
- Kumar, P.; Lakshmi, Y.; Kondapi, A. An oral formulation of efavirenz-loaded lactoferrin nanoparticles with improved biodistribution and pharmacokinetic profile. HIV Med. 2017, 18, 452–462. [Google Scholar] [CrossRef]
- Zhang, G. The mixed lineage kinase-3 inhibitor URMC-099 improves therapeutic outcomes for long-acting antiretroviral therapy. Nanomedicine 2016, 12, 109–122. [Google Scholar] [CrossRef]
- Savage, A.C.; Tatham, L.M.; Siccardi, M.; Scott, T.; Vourvahis, M.; Clark, A.; Rannard, S.P.; Owen, A. Improving maraviroc oral bioavailability by formation of solid drug nanoparticles. Eur. J. Pharm. Biopharm. 2019, 138, 30–36. [Google Scholar] [CrossRef]
- Shibata, A.; McMullen, E.; Pham, A.; Belshan, M.; Sanford, B.; Zhou, Y.; Goede, M.; Date, A.A.; Destache, C.J. Polymeric Nanoparticles Containing Combination Antiretroviral Drugs for HIV Type 1 Treatment. AIDS Res. Hum. Retrovir. 2013, 29, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Prathipati, P.K.; Mandal, S.; Pon, G.; Vivekanandan, R.; Destache, C.J. Pharmacokinetic and Tissue Distribution Profile of Long Acting Tenofovir Alafenamide and Elvitegravir Loaded Nanoparticles in Humanized Mice Model. Pharm. Res. 2017, 34, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lakshmi, Y.S.; Kondapi, A.K. Triple Drug Combination of Zidovudine, Efavirenz and Lamivudine Loaded Lactoferrin Nanoparticles: An Effective Nano First-Line Regimen for HIV Therapy. Pharm. Res. 2017, 34, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Lauster, D.; Glanz, M.; Bardua, M.; Ludwig, K.; Hellmund, M.; Hoffmann, U.; Hamann, A.; Böttcher, C.; Haag, R.; Hackenberger, C.P.R.; et al. Multivalent Peptide-Nanoparticle Conjugates for Influenza-Virus Inhibition. Angew. Chem. Int. Ed. 2017, 56, 5931–5936. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Balachandran, Y.L.; Hao, Y.; Hao, X.; Li, R.; Nan, Z.; Zhang, H.; Shao, Y.; Liu, Y. Amantadine Surface-Modified Silver Nanorods Improves Immunotherapy of HIV Vaccine Against HIV-Infected Cells. ACS Appl. Mater. Interfaces 2018, 10, 28494–28501. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Roy, I.; Xu, G.; Yong, K.-T.; Ding, H.; Aalinkeel, R.; Reynolds, J.L.; Sykes, D.E.; Nair, B.B.; Lin, E.Y.; et al. Enhancing the Delivery of Anti Retroviral Drug “Saquinavir”; Across the Blood Brain Barrier Using Nanoparticles. CHR 2010, 8, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef]
- Patel, G.V.; Patel, V.B.; Pathak, A.; Rajput, S.J. Nanosuspension of efavirenz for improved oral bioavailability: Formulation optimization, in vitro, in situ and in vivo evaluation. Drug Dev. Ind. Pharm. 2014, 40, 80–91. [Google Scholar] [CrossRef]
- Akhter, S.; Talegaonkar, S.; Khan, Z.I.; Jain, G.K.; Khar, R.K.; Ahmad, F.J. Assessment of Ocular Pharmacokinetics and Safety of Ganciclovir Loaded Nanoformulations. J. Biomed. Nanotechnol. 2011, 7, 144–145. [Google Scholar] [CrossRef]
- Ren, J.; Zou, M.; Gao, P.; Wang, Y.; Cheng, G. Tissue distribution of borneol-modified ganciclovir-loaded solid lipid nanoparticles in mice after intravenous administration. Eur. J. Pharm. Biopharm. 2013, 83, 141–148. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Zastre, J.; Wong, H.-L.; Wu, X.Y.; Bendayan, R. Solid Lipid Nanoparticles Enhance the Delivery of the HIV Protease Inhibitor, Atazanavir, by a Human Brain Endothelial Cell Line. Pharm. Res. 2008, 25, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Kovochich, M.; Marsden, M.D.; Zack, J.A. Activation of Latent HIV Using Drug-Loaded Nanoparticles. PLoS ONE 2011, 6, e18270. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Freeling, J.P.; Koehn, J.; Shu, C.; Ho, R.J.Y. Evaluation of Atazanavir and Darunavir Interactions with Lipids for Developing pH-Responsive Anti-HIV Drug Combination Nanoparticles. J. Pharm. Sci. 2014, 103, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Freeling, J.P.; Koehn, J.; Shu, C.; Sun, J.; Ho, R.J.Y. Anti-HIV Drug-Combination Nanoparticles Enhance Plasma Drug Exposure Duration as Well as Triple-Drug Combination Levels in Cells Within Lymph Nodes and Blood in Primates. AIDS Res. Hum. Retrovir. 2015, 31, 107–114. [Google Scholar] [CrossRef]
- Arca-Lafuente, S.; Martínez-Román, P.; Mate-Cano, I.; Madrid, R.; Briz, V. Nanotechnology: A reality for diagnosis of HCV infectious disease. J. Infect. 2019. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Samandari, L.; Sheibani, S. HIV biosensors for early diagnosis of infection: The intertwine of nanotechnology with sensing strategies. Talanta 2020, 206, 120201. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Dhanasezhian, A.; Srivani, S.; Govindaraju, K.; Preetam, P.; Sasikala, S.; Ramesh Kumar, M.R. Anti-Herpes Simplex Virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J. Geo Mar. Sci. 2019, 48, 1252–1257. [Google Scholar]
- Singaravelu, G.; Arockiamary, J.S.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B 2007, 57, 97–101. [Google Scholar] [CrossRef]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Jacob Silva, P.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018, 17, 195–203. [Google Scholar] [CrossRef]
- Klimyte, E.M.; Smith, S.E.; Oreste, P.; Lembo, D.; Dutch, R.E. Inhibition of Human Metapneumovirus Binding to Heparan Sulfate Blocks Infection in Human Lung Cells and Airway Tissues. J. Virol. 2016, 90, 9237–9250. [Google Scholar] [CrossRef]
- Bhatia, S.; Camacho, L.C.; Haag, R. Pathogen Inhibition by Multivalent Ligand Architectures. J. Am. Chem. Soc. 2016, 138, 8654–8666. [Google Scholar] [CrossRef]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.-S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef]
- Argenta, D.F.; Silva, I.T.; Bassani, V.L.; Koester, L.S.; Teixeira, H.F.; Simões, C.M.O. Antiherpes evaluation of soybean isoflavonoids. Arch. Virol. 2015, 160, 2335–2342. [Google Scholar] [CrossRef]
- Argenta, D.F.; Bidone, J.; Koester, L.S.; Bassani, V.L.; Simões, C.M.O.; Teixeira, H.F. Topical Delivery of Coumestrol from Lipid Nanoemulsions Thickened with Hydroxyethylcellulose for Antiherpes Treatment. AAPS PharmSciTech 2018, 19, 192–200. [Google Scholar] [CrossRef]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef]
- Grande, F.; Ioele, G.; Occhiuzzi, M.A.; De Luca, M.; Mazzotta, E.; Ragno, G.; Garofalo, A.; Muzzalupo, R. Reverse Transcriptase Inhibitors Nanosystems Designed for Drug Stability and Controlled Delivery. Pharmaceutics 2019, 11, 197. [Google Scholar] [CrossRef]
- Joshy, K.S.; George, A.; Jose, J.; Kalarikkal, N.; Pothen, L.A.; Thomas, S. Novel dendritic structure of alginate hybrid nanoparticles for effective anti-viral drug delivery. Int. J. Biol. Macromol. 2017, 103, 1265–1275. [Google Scholar] [CrossRef]
- Sneha, R.; Vedha Hari, B.N.; Ramya Devi, D. Design of antiretroviral drug-polymeric nanoparticles laden buccal films for chronic HIV therapy in paediatrics. Colloid Interface Sci. Commun. 2018, 27, 49–59. [Google Scholar] [CrossRef]
- Urbaniak, T.; Musiał, W. Influence of Solvent Evaporation Technique Parameters on Diameter of Submicron Lamivudine-Poly-ε-Caprolactone Conjugate Particles. Nanomaterials 2019, 9, 1240. [Google Scholar] [CrossRef]
- Tshweu, L.; Katata, L.; Kalombo, L.; Swai, H. Nanoencapsulation of water-soluble drug, lamivudine, using a double emulsion spray-drying technique for improving HIV treatment. J. Nanopart. Res. 2013, 15, 2040. [Google Scholar] [CrossRef]
- Reddy, P.P.; Reddy, B.M.; Prakash, J.; Latha, K.; Prashanthi, D. Formulation and characterisation of chitosan based lamivudine nanoparticles. EJPMR 2014, 4, 377–383. [Google Scholar]
- Sanchez, C.G.; Molinski, S.V.; Gongora, R.; Sosulski, M.; Fuselier, T.; MacKinnon, S.S.; Mondal, D.; Lasky, J.A. The Antiretroviral Agent Nelfinavir Mesylate: A Potential Therapy for Systemic Sclerosis. Arthritis Rheumatol. 2018, 70, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bowman, M.-C.; Ballard, T.E.; Ackerson, C.J.; Feldheim, D.L.; Margolis, D.M.; Melander, C. Inhibition of HIV Fusion with Multivalent Gold Nanoparticles. J. Am. Chem. Soc. 2008, 130, 6896–6897. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Ganesan, S. Gold Nanoparticles as an HIV Entry Inhibitor. CHR 2012, 10, 643–646. [Google Scholar] [CrossRef]
- Garrido, C.; Simpson, C.A.; Dahl, N.P.; Bresee, J.; Whitehead, D.C.; Lindsey, E.A.; Harris, T.L.; Smith, C.A.; Carter, C.J.; Feldheim, D.L.; et al. Gold nanoparticles to improve HIV drug delivery. Future Med. Chem. 2015, 7, 1097–1107. [Google Scholar] [CrossRef]
- Singh, L.; Kruger, H.G.; Maguire, G.E.M.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. 2017, 4, 105–131. [Google Scholar] [CrossRef]
- Bovier, P.A. Epaxal®: A virosomal vaccine to prevent hepatitis A infection. Exp. Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef]
- He, H.; Yuan, D.; Wu, Y.; Cao, Y. Pharmacokinetics and Pharmacodynamics Modeling and Simulation Systems to Support the Development and Regulation of Liposomal Drugs. Pharmaceutics 2019, 11, 110. [Google Scholar] [CrossRef]
- Herzog, C.; Hartmann, K.; Künzi, V.; Kürsteiner, O.; Mischler, R.; Lazar, H.; Glück, R. Eleven years of Inflexal® V—A virosomal adjuvanted influenza vaccine. Vaccine 2009, 27, 4381–4387. [Google Scholar] [CrossRef]
- Package Insert PEG-IntronTM (Peginterferon alfa-2b) Powder for Injection, Schering Corporation. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/pegsche080701LB.htm (accessed on 16 January 2020).
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Package Insert PEGASYS® (Peginterferon Alfa-2a) Hoffmann-La Roche Inc. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/pegihof101602LB.htm (accessed on 16 January 2020).
- Influvac (Influenza Vaccine). Available online: https://bodyandhealth.canada.com/drug/getdrug/influvac (accessed on 16 January 2020).
- VivaGel® BV launched in Europe. Available online: https://starpharma.com/assets/asxannouncements/190627%20VivaGel%20BV%20launched%20in%20Europe%20-%20final%20.pdf (accessed on 16 January 2020).
- Safety. Tolerability and Immune Response to LC002, an Experimental Therapeutic Vaccine, in Adults Receiving HAART-ClinicalTrials.gov Identifier: NCT00270205. Available online: https://clinicaltrials.gov/ct2/show/NCT00270205?cond=NCT00270205&draw=2&rank=1 (accessed on 18 January 2020).
- Bioavailability of MK-1439 Experimental Nano Formulations in Healthy Adults (MK-1439-046)-ClinicalTrials.gov Identifier: NCT02549040. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02549040?term=NCT02549040&draw=2&rank=1 (accessed on 18 January 2020).
- Study of ARB-001467 in Subjects with Chronic HBV Infection Receiving Nucleos(t)Ide Analogue Therapy-ClinicalTrials.gov Identifier: NCT02631096. Available online: https://clinicaltrials.gov/ct2/show/NCT02631096?term=NCT02631096&draw=2&rank=1 (accessed on 18 January 2020).
- Odiba, A.; Ottah, V.; Ottah, C.; Anunobi, O.; Ukegbu, C.; Edeke, A.; Uroko, R.; Omeje, K. Therapeutic nanomedicine surmounts the limitations of pharmacotherapy. Open Med. 2017, 12. [Google Scholar] [CrossRef]
- Parboosing, R.; Maguire, G.E.M.; Govender, P.; Kruger, H.G. Nanotechnology and the Treatment of HIV Infection. Viruses 2012, 4, 488–520. [Google Scholar] [CrossRef]
- Li, M.; Al-Jamal, K.T.; Kostarelos, K.; Reineke, J. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. ACS Nano 2010, 4, 6303–6317. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles—Properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Ochekpe, N.A.; Olorunfemi, P.O.; Ngwuluka, N.C. Nanotechnology and drug delivery part 2: Nanostructures for drug delivery. Trop. J. Pharm. Res. 2009, 8. [Google Scholar] [CrossRef]
- Dey, P.; Bergmann, T.; Cuellar-Camacho, J.L.; Ehrmann, S.; Chowdhury, M.S.; Zhang, M.; Dahmani, I.; Haag, R.; Azab, W. Multivalent Flexible Nanogels Exhibit Broad-Spectrum Antiviral Activity by Blocking Virus Entry. ACS Nano 2018, 12, 6429–6442. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).