Abstract
Topical drug delivery is an attractive alternative to conventional methods because of advantages such as non-invasive delivery, by-pass of first pass metabolism, and improved patient compliance. However, several factors such as skin, physicochemical properties of the drug, and vehicle characteristics influence the permeation. Within a formulation, critical factors such as concentration of drug, physical state of drug in the formulation, and organoleptic properties affect the flux across the skin. The aim of the study was to develop and investigate topical semisolid preparations (creams and gels) with ibuprofen as the model drug and investigate the effect of various formulation parameters on the in-vitro performance across the Strat-M® membrane using flow-through cells. In addition, the physical stability of the developed formulations was investigated by studying viscosity, pH, and appearance. All the formulations developed in the study had appealing appearance with smooth texture and no signs of separation. Viscosity and pH of the formulations were acceptable. Cumulative amount of drug permeated at the end of 24 h was highest for clear gel (3% w/w ibuprofen; F6: 739.6 ± 36.1 µg/cm2) followed by cream with high concentration of ibuprofen in suspended form (5% w/w; F3: 320.8 ± 17.53 µg/cm2), emulgel (3% w/w ibuprofen; F5: 178.5 ± 34.5 µg/cm2), and cream with solubilized ibuprofen (3% w/w; F2A: 163.2 ± 9.36 µg/cm2). Results from this study showed that permeation of ibuprofen was significantly influenced by formulation parameters such as concentration of ibuprofen (3% vs. 5% w/w), physical state of ibuprofen (solubilized vs. suspended), formulation type (cream vs. gel), mucoadhesive agents, and viscosity (high vs. low). Thus, findings from this study indicate that pharmaceutical formulation scientists should explore these critical factors during the early development of any new topical drug product in order to meet pre-determined quality target product profile.
1. Introduction
Topical/transdermal drug delivery refers to the delivery of drugs via skin and is an attractive alternative to conventional methods such as oral and parenteral routes. Advantages associated with topical/transdermal delivery include non-invasive delivery, bypass of first pass metabolism, prolonged duration of action, reduced dosing frequency, constant levels of drug in the plasma, reduced drug toxicity/adverse events, improved patient compliance, and others [1,2,3]. However, skin acts as a major barrier for the entry of drugs and foreign compounds due to the presence of stratum corneum, a thin keratin-rich layer (15 µm) of dead cells embedded in an intricate lipid environment made of cholesterol, ceramides, and free fatty acids [4,5,6]. In addition, several other factors such as physicochemical properties of the drug (lipophilicity, solubility, molecular weight or size, and hydrogen bonding) and characteristics of a formulation/vehicle or a drug delivery system influence the permeation [7]. To overcome these challenges, several physical and chemical methods have been employed to enhance the transport of drugs through the skin. Physical methods include approaches such as microneedles, thermal ablation, radiofrequency, iontophoresis, ballistic liquid jets, laser, and others [4,8,9,10,11]. However, these methods are known to cause irritation to the skin due to mechanical, thermal, magnetic, and electrical energy [8]. Chemical methods include the use of penetration enhancers such as propylene glycol, ethanol, transcutol, and others to enhance the drug transport through the skin. They increase the diffusion of drugs through the skin by interacting and altering the complex structure of skin and thus enhancing the partition of drug into different layers [12,13]. Several penetration enhancers have been approved in the market, but their application in topical and transdermal formulations is limited as there is no clear understanding on how these agents enhance drug transport [14]. In addition to penetration enhancers, several other excipients/additives such as solvents, co-solvents, surfactants, humectants, thickening agents, and others are used in the development of topical/transdermal formulation. These agents act as inactive ingredients and control the extent of absorption (thermodynamic activity and partition coefficient), maintain the viscosity and pH, improve the stability as well as organoleptic properties, and increase the bulk of the formulation [15,16]. Similar to every other dosage form, topical formulation development program also involves pre-formulation development, formulation development, performance (in vitro and in vivo), and stability. A well-designed Quality Target Product Profile (QTPP) provides a structure to ensure that a formulation scientist embarks on a product development program that is efficient and yet defines a listing of all relevant medical, technical, and scientific information required to reach the desired commercial development outcome [17]. However, formulation scientists face several challenges while developing a drug product with desirable QTPP. In case of topical product development, achieving the target flux is a challenge as it is dependent of several factors.
Percutaneous drug absorption is a process which involves steps such as (i) dissolution and release of drug from the vehicle/formulation, (ii) partition of drug into the stratum corneum, (iii) diffusion of the solubilized drug across the stratum corneum, and (iv) penetration of drug into the layers of the skin [18]. The goal in the development of any topical/transdermal drug formulation is to achieve maximum flux across the skin without any drug build-up. Critical factors which influence the flux across the skin include concentration of drug in the vehicle/formulation, physical state of drug in the formulation, and other formulation properties. Concentration of drug in the formulation is important as a proportional increase in the flux can be achieved by increasing the concentration of the dissolved drug. According to Fick’s law of diffusion (Equation (1)), at a higher concentration above the solubility, the excess drug in the formulation acts as a reservoir and helps in maintaining constant flux for a prolonged period and thus increases the permeation [19]. Physical state of the drug in the formulation (solubilized drug vs. dispersed/suspended drug) can also significantly affect the permeation. It is known that greater flux is achieved when the drug is in solubilized form compared to suspended form. Enhanced permeation is attributed to increase in thermodynamic activity and partition with solubilized drug. Thus, the solubilized systems have advantages such as increased efficacy at lower concentrations, low drug irritation potential and cost efficient [16]. In addition to the above, formulation properties such as type of formulation (monophasic vs. multiphasic systems), viscosity, pH, and other organoleptic properties significantly affect the transport of drug across the skin. Therefore, pharmaceutical formulation scientists must consider all the above factors in the development of any new topical drug product. Since the inception of topical dosage forms, numerous excipients have been investigated for their application in conventional dosage forms, such as creams, gels, and ointments. Although several excipients have been approved by the United States Food and Drug Administration (USFDA) for topical use, formulation scientists face challenges in the development of topical drug product with desired permeation profile. In addition, efforts have also been made in development of novel formulations, such as microemulsions, nanoparticulate drug delivery systems, eutectic mixtures, patches, and others to enhance the permeation of drugs across the skin [20,21]. In general, there is a lack of scientific evaluation on how the formulation properties, such as concentration of drug, physical state of the drug, and formulation type, influence the bioavailability of conventional topical dosage forms, such as creams and gels. Although several studies have evaluated the effect of formulation properties on transdermal permeation of drugs, the scientific data show that the research was limited to only one factor, such as concentration of drug [22], concentration of excipients [7,23], and formulation type [24,25]. Therefore, the current study was designed to provide ready information to the formulators who design and develop topical semisolid formulations on the impact of formulation properties (concentration of drug, physical state of the drug, mucoadhesive agents, and formulation type) on transdermal permeation.
Equation (1): Fick’s law of diffusion, where J is the steady-state flux of the drug molecule through the skin (µg/cm2·h), K is the permeability coefficient (cm/s), ΔC is the difference in concentration (µg/cm3), D is the diffusion coefficient (cm2/s), Kp is the apparent partition coefficient, and h is the thickness of the layer of skin (cm).
Non-steroidal anti-inflammatory drugs (NSAIDs) are used to treat local pain and inflammation associated with injuries, rheumatoid arthritis, osteoarthritis, and other musculoskeletal problems [7,26]. Although, NSAIDs are very effective, their oral absorption is associated with severe gastric irritation leading to gastric bleeding and ulcers. Therefore, topical/transdermal delivery of NSAIDs is preferred as it bypasses hepatic first pass metabolism and also results in targeted effect at the site of inflammation/pain [24]. Majority of the NSAIDs (salicylates, acetic acid derivatives, enol acid derivatives, and propionic acid derivatives) approved by USFDA have similar physicochemical properties (molecular mass, logP, and pKa) [27,28,29,30]. Hence, it can be assumed that there may be similarities in transdermal permeation for these compounds [29]. Among these agents, ibuprofen is the most commonly used NSAID. Ibuprofen (α-methyl-4-(2-methylpropyl) benzeneacetic acid) is a weak acid (pKa 4.5–4.6), thus the pH of the skin (~4.8) favors passive diffusion as majority of the molecules will be in unionized form. However, poor aqueous solubility (0.084 and 0.685 mg/L at pH 4.5 and 5.54, respectively) limits the skin permeation of ibuprofen. Ibuprofen is considered as an attractive candidate for topical/percutaneous delivery due to the physicochemical properties (low molecular weight (MW: 206.29 g·mol−1), suitable partition coefficient (logP: 3.68), and short elimination half-life (t1/2 2–4 h), [7]. Currently, topical formulations of ibuprofen are not approved in the United States. Therefore, taking into account all the above factors and availability of drug for research purposes, ibuprofen was chosen as the model drug for our study. The main goal of the study was to prepare semisolid formulations and investigate the effect of concentration of drug, formulation type and physical state of drug on transdermal permeation of ibuprofen. All the excipients (except Sepineo SE 68) used were approved by the USFDA for topical use and were within the limits listed in the inactive ingredient database. In the present study, we have developed ibuprofen topical creams at two concentrations (3% and 5% w/w)—emulgel (3% w/w) and clear non-aqueous gel (3% w/w). Further, in-vitro permeation studies were performed across the Strat-M® membrane to study the effect of various formulation parameters on the permeation of ibuprofen.
2. Materials
Ibuprofen, hydroxy propyl methyl cellulose (HPMC) (MW: 86,000, viscosity 4000 cps at 2% solution), and hydroxy propyl cellulose were purchased from Acros Organics (Fair Lawn, NJ, USA). Absolute ethanol, sorbitan monolaurate (Span 20), sodium chloride, glacial acetic acid, acetonitrile (HPLC grade) were procured from Fisher Chemicals (Fair Lawn, NJ, USA). Deionized water used in all the experiments was obtained from in-house Milli-Q® IQ 7000 Ultrapure Water System (EMD Millipore, Bedford, MA, USA). Mineral oil NF and white petrolatum were purchased from PCCA (Houston, TX, USA). Tefose® 63 (mixture of PEG-6 stearate NF/JPE and Ethylene glycol palmitostearate EP/NF/JPE) and Transcutol® (Diethylene glycol monoethyl ether EP/NF) were gift samples from Gattefossé (Paramus, NJ, USA). Kollicream® IPM (isopropyl myristate), Kollicream® OA (oleyl alcohol), Kollisolv® MCT 70 (medium-chain triglycerides), Kollisolv® PEG 400 (polyethylene glycol 400), Kollisolv® PG (propylene glycol), Kolliphor® CS 20 (macrogol cetostearyl ether 20/polyoxyl 20 cetostearyl ether), Kolliphor® PS 80 (polysorbate 80), Kolliphor® CS A (cetostearyl alcohol (type A)), Kolliwax® CA (cetyl alcohol), and Kolliwax® SA (stearyl alcohol) were generous samples from BASF (Tarrytown, NY, USA). Glycerol monostearate was obtained from Alfa Aesar (Ward Hill, MA, USA). Strat-M® membrane and glycerol were procured from Sigma-Aldrich (St. Louis, MO, USA). Carbopol 974P (Carbomer Homopolymer Type B) was a sample from Lubrizol Life Sciences (Cleveland, OH, USA). Sepineo™ P600 (acrylamide/sodium acryloyldimethyl taurate copolymer/isohexadecane and Polysorbate 80) and Sepineo™ SE 68 (cetearyl alcohol, cetearyl glucoside) were gift samples from Seppic Inc (Fairfield, NJ, USA).
3. Methods
3.1. Solubility of Ibuprofen in Solvents
The solubility of ibuprofen in liquid excipients was determined using visual solubility protocol. In this method, the excipients were accurately weighed (2.5 g) in individually-labelled 20 mL scintillation vials. To these vials, accurately weighed aliquots of ibuprofen (~5 mg for glycerol due to poor solubility and ~25 mg for other excipients) was added and tightly closed. Further, the vials were placed in a shaking water bath (Fisher Scientific, Waltham, MA, USA) maintained at 25 °C for at least 15 min to allow proper mixing. After 15 min, the vials were visually inspected, and additional aliquots of ibuprofen were added periodically (every 15 min) until saturation was achieved. Following this, the vials were placed in the shaking water bath for 24 h and visually inspected the following day. The final weight of the vials was measured to determine the approximate solubility of ibuprofen in each excipient and reported as mg/g and percentage (%).
3.2. Formulation of Ibuprofen Creams and Gels
3.2.1. Optimization of Formulations
Optimization of all the formulations was performed by evaluating the effect of different concentrations of excipients on the stability, excipient instability, viscosity, and any visual changes in the formulations (Appendix A). After optimization, stable formulations which provided acceptable appearance and viscosity were chosen for further evaluation. Compositions of all the optimized creams, emulgel and clear gel are provided in Table 1, Table 2, and Table 3, respectively. All the formulations had differences in composition since the main aim of this study was to evaluate on how formulation parameters such as concentration and physical state of the drug, formulation type and mucoadhesive agents influence the permeation of ibuprofen.

Table 1.
Composition of optimized creams in the study.

Table 2.
Composition of optimized Emulgel in the study.

Table 3.
Composition of optimized clear non-aqueous gel in the study.
3.2.2. Formulation of Creams
Compositions of the creams developed is provided in Table 1. Ibuprofen creams were developed at two different strengths (3% w/w (F1A, F1B, F2A, F2B) and 5% w/w (F3, F4)) using a water-in-oil (w/o) emulsion method. Briefly, ibuprofen was accurately weighed and transferred into a 250 mL beaker containing all the required oil phase components for each formulation. In another 100 mL beaker, accurately weighed water soluble components were dissolved in water. Both the beakers were placed in a water bath and heated to 65 ± 2 °C. Once both the phases reached approximately similar temperature, the aqueous phase was added to the oil phase and homogenized at 5000 rpm using a high shear homogenizer (Fisherbrand™ 850, Waltham, MA, USA) for 10 min to form an emulsion. After homogenization, the emulsion was allowed to cool down to room temperature by mixing it using an overhead stirrer (IKA RW20 Digital, Wilmington, NC, USA) at 300 rpm for 2 h until a smooth cream was obtained. Sepineo P600 was added to the mixture during the process of homogenization for formulations F3 and F5.
3.2.3. Formulation of Emulgel
Composition of ibuprofen emulgel (F5) (3% w/w) is provided in Table 2. Ibuprofen (3 g) was dissolved in ethanol (30 mL) and water was added to ibuprofen solution. To this mixture, accurately weighed Sepineo P600 was added immediately and vigorously mixed using a glass rod until a smooth emulgel was formed.
3.2.4. Formulation of Clear Non-Aqueous Gel
Composition of ibuprofen clear non aqueous gel (F6) (3% w/w) is provided in Table 3. Ibuprofen was weighed and added to a mixture of propylene glycol, ethanol, transcutol, and glycerin to obtain a clear solution. To this clear solution, PEG 400 was added and mixed on a magnetic stirrer. Accurately weighed HPC (4 g) was dispersed in the mixture and allowed to thicken at room temperature using an overhead mixer (IKA RW20 Digital, Wilmington, NC, USA) at 500 rpm for 2 h.
3.3. Polarized Light Microscopy
Polarized light microscopy was used to study the microscopic features of the optimized creams and gels. All the formulations were applied on a microscopic glass slide and evenly spread with a coverslip. The cover slipped slides were observed under Amscope® PZ300 series polarized light microscope (Amscope, Irvine, CA, USA) in the transmission mode at 180× magnification and photomicrographs were captured on a laboratory PC.
3.4. HPLC Analysis of Ibuprofen
The amount of ibuprofen in the samples was quantified using Waters Alliance e2695 HPLC equipped with 2998 photodiode array detector and Empower 3.0 software. The analysis was carried out on a reverse phase Phenomenex® C18 column (250 × 4.6 mm; 5 µm particle size) at 25 °C. The mobile phase was a mixture (60:40) of acetonitrile and water (adjusted to pH 3.8 with acetic acid) at a constant flow rate of 1.5 mL/min. Samples (60 µL) were injected into the column using autosampler and monitored at 220 nm. Retention time of ibuprofen was 6.5 min. All the samples injected were filtered through 0.45 µm membrane filter.
3.5. Measurement of Viscosity and pH
Rheological experiments were conducted to measure the viscosity of the optimized formulations. Measurements were performed at room temperature using a Viscolead-one digital viscometer (Fungilab Inc. New York, NY, USA) equipped with a spindle rotor (R6) set at 20 rpm. The method was validated by using 2% HPMC gel (4000 cps) as a control. The pH of the formulations was evaluated on day 0 and day 60 using a calibrated Mettler Toledo InLab® pH meter equipped with LE422 micro pH electrode (Mettler Toledo, Columbus, OH, USA).
3.6. In-Vitro Permeation Studies
In-vitro permeation studies were conducted using a PermeGear® ILC-07 automated system (PermeGear, Riegelsville, PA, USA) equipped with seven in-line flow-through diffusion cells, made of Kel-F. Each diffusion cell had a donor and receptor chamber clamped with threaded rods and adjustable locking nuts. Receptor chambers (volume: 254 µL receptor) had inlet and outlet ports connected to the Tygon tubings having 1/4-28 HPLC fittings. Temperature of the cells was maintained at 32 °C using Julabo BC4 circulating water bath (Seelbach, Germany). Diameter of the diffusional area was 1 cm (total diffusional area: 0.785 cm2) and the cells were connected to a 7-channel peristaltic pump® IPC (Ismatec, Zurich, Switzerland) which draws receptor solution from a reservoir (Figure 1). Strat-M® was used as a diffusion membrane, which was mounted on the cells and sandwiched between the donor and receptor chambers using the adjustable locking nuts. Formulations (~10 mg ibuprofen) were placed on the diffusion membrane and the receptor fluid (10% v/v ethanol) was allowed to flow at a rate of 4 mL/h for 24 h. At pre-determined time intervals, the receptor fluid was collected in 20 mL scintillation vials and analyzed using HPLC to determine the amount of ibuprofen permeated through the Strat-M® membrane.
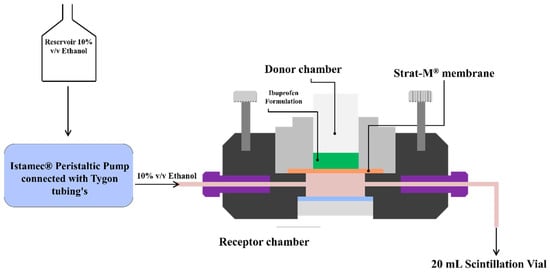
Figure 1.
Permegear ILC-07® automated flow-through cells.
3.7. Permeation Data Analysis
The permeation profile from the formulations was plotted as cumulative amount of ibuprofen permeated vs. time. The flux (µg/cm2/h) and lag-time (h) estimates were generated using Skin and Membrane Permeation Data Analysis (SAMPA) software, version 1.04, a free software tool used for skin and membrane permeation data analysis [31].
3.8. Physical Stability
Physical stability studies were conducted for all the formulations at 25 ± 2 °C and at 40 ± 2 °C. All the samples were transferred to glass scintillation vials, closed tightly and stored at 25 ± 2 °C and 40 ± 2 °C. Samples were evaluated for stability, changes in color, and any other physical instability for 90 days.
3.9. Statistical Analysis
All the data was statistically analyzed using GraphPad Prism software (Version 5.0, San Diego, CA, USA). Permeation data analysis was performed using SAMPA software, version 1.04. A p-value of <0.05 was considered as statistically significant.
4. Results and Discussion
4.1. Solubility in Solvents
The solubility of ibuprofen in different solvents is provided in Table 4. Results show that transcutol and propylene glycol provided the greater solubility (300 mg/g), whereas glycerol provided lowest solubility of ibuprofen (4 mg/g). The order of ibuprofen solubility in various solvents was transcutol = propylene glycol > isopropyl myristate > polyethylene glycol 400 > oleyl alcohol = polysorbate 80 > medium chain triglycerides > mineral oil > glycerol. Results from the solubility studies are in agreement with the literature where solvents/co-solvents such as transcutol, propylene glycol, oleyl alcohol, and isopropyl myristate enhance the solubility of poorly-soluble compounds [1].

Table 4.
Visual solubility of ibuprofen in solvents.
4.2. pH and Viscosity
All the optimized formulations developed in the study had appealing appearance with smooth texture and no signs of phase separation. Physical evaluation was done by pressing a small quantity of formulation between the thumb and index finder. It was observed that all the formulations were homogeneous and consistent without any coarse particles. The color of all the creams and emulgel was observed to be white to translucent white (Table 5). Viscosity is an important factor for semisolid formulations as it may influence the release of drug by altering the diffusion rate from the vehicles. Results for the viscosity and pH of all the formulations are provided in Table 5. Viscosity of the formulations ranged from 1872 to 32,655 cps. There was no significant change in the pH of the formulations over 60 days. The range of pH of the formulations was 4.2 to 5.95, which is close to the pH of human skin, hence there is minimal risk of skin irritation expected. In addition all excipients used in the formulations were approved by the USFDA (except Sepineo SE 68) for dermatological applications, and the concentrations used were within the limits listed in the inactive ingredients database for approved drug products [32]. Thus, there is minimal potential risk expected of skin drying, sensory reactions, and alterations in skin hydration with the formulations.

Table 5.
Viscosity and pH of ibuprofen creams and gels.
4.3. Polarized Light Microscopy
Polarized light microscopy was used to study the presence of ibuprofen particulates in the formulations. Polarized light microphotographs of all the formulations and control (ibuprofen in mineral oil) are provided in Figure 2. Table 6 summarizes the observations from polarized light microphotographs. It can be observed from the images that ibuprofen is in solubilized form (no crystals) in formulations F2A, F2B, and F6, whereas in all the other formulations presence of crystals in the images indicate that the drug was suspended in the formulation. Moreover, presence of a higher number of ibuprofen crystals in formulations F3 and F4 is due to the higher concentration of ibuprofen in F3 and F4 (5% w/w) compared to other formulations (3% w/w).
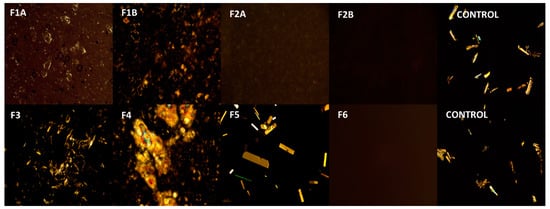
Figure 2.
Polarized light microphotographs of formulations. Images were captured using Amscope polarized light microscope at 180× magnification. F1A, ibuprofen suspended cream (3% w/w) with Tefose 63, F1B, ibuprofen suspended cream (3% w/w) without Tefose 63, F2A, ibuprofen solubilized cream (3% w/w) with HPMC, F2A, ibuprofen solubilized cream (3% w/w) without HPMC, F3, ibuprofen suspended cream (5% w/w), F4 ibuprofen suspended cream (5% w/w), F5, ibuprofen emulgel (3% w/w), F6, ibuprofen clear non aqueous gel (3% w/w).

Table 6.
Observations from polarized light microscopy.
4.4. In-Vitro Permeation Studies
Cumulative amount and flux of drug permeated through the Strat-M® membrane at the end of 24 h for all the formulations is provided in Figure 3 and Table 7, respectively. Recently, there has been a significant rise in using synthetic artificial membranes (cellulose acetate, Strat-M®, Parallel Artificial Membrane Permeability Assay (PAMPA)) and 3-D cultured human skin models as an alternative to human and animal skin in the development of topical and transdermal formulations [33]. In 2018, European Medicines Agency’s draft guideline on quality and equivalence of topical products has recommended the use of synthetic membranes to better understand and characterize performance of a finished topical dosage form [34]. Moreover, synthetic membranes are inexpensive and easily resourced with superior data reproducibility [35,36]. Therefore, for our studies, Strat-M® was used as a diffusion membrane. Strat-M® is a multilayered synthetic membrane (300 µm thickness) similar to skin and made up of several tightly-packed layers of polyester sulfone. Several studies have been reported in the literature comparing the ability of Strat-M® membrane to predict the permeation of hydrophilic and lipophilic compounds such as diclofenac, hydrocortisone, caffeine, amphotericin B, and capsaicin. Results have shown that the Strat-M® membrane had better correlation to human skin with minimal lot-to-lot variability, safety, and storage limitations [37,38,39]. Uchida et al. evaluated the skin permeabilities of 13 chemical compounds using Strat-M® membrane and compared them to human and animal skins. Results confirmed that permeability coefficients, diffusion, and partition parameters were well correlated between the Strat-M® membrane and human and animal skin [33]. Recently, Haq et al. compared the Strat-M® membrane with human skin on permeation of nicotine. Results showed that there was again a high correlation between human skin and Strat-M® with R2 value of 0.99 [40]. Hence, we used Strat-M® as a diffusion membrane for our studies.
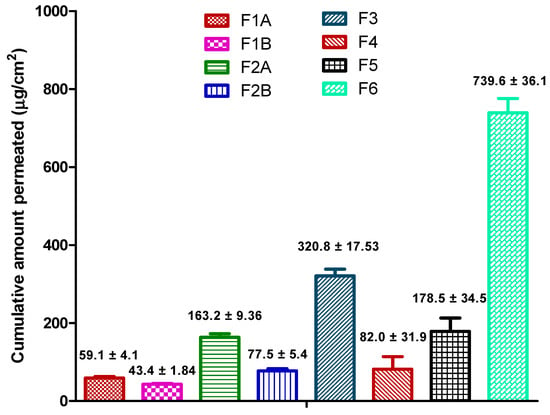
Figure 3.
Cumulative amount of drug permeated from the formulations at the end of 24 h.

Table 7.
Cumulative amount, flux, and lag-time of drug permeated from the formulations at the end of 24 h (mean ± SD).
4.4.1. Effect of Mucoadhesive Agents (F1A vs. F1B and F2A vs. F2B)
As per the formulation composition (Table 1), F1A and F1B, F2A and F2B had the same surfactant system and concentration of ibuprofen but the only difference was the addition of mucoadhesive agents such as Tefose 63 in F1A and HPMC in F2A. Permeation data show that the addition of mucoadhesive agents in the formulations (F1A and F2A) resulted in higher permeation of ibuprofen (F1A: 59.1 ± 4.1 µg/cm2 vs. F1B: 43.4 ± 1.84 µg/cm2 and F2A: 163.2 ± 9.36 µg/cm2 vs. 77.5 ± 5.4 µg/cm2). Addition of mucoadhesive agents in the formulation could have resulted in increased retention, prolonged contact, and reduced leakage of formulation and thus increasing the permeation. Additionally, these agents increase level of hydration in the membrane interface which in turn decreases the diffusional path length and thus favoring the transport of ibuprofen [24,41]. In addition, mucoadhesive agents could have increased the concentration gradient due to prolonged contact of the formulation at the membrane interface and resulting in enhanced permeation [42,43]. Addition of mucoadhesive agents could be advantageous in the development of topical formulations intended for vaginal applications where bio-adhesion of mucoadhesive polymers, such as tefose 63 and HPMC, increase the retention and absorption of drugs [44,45,46].
4.4.2. Effect of Physical State of the Drug (F1A vs. F2A)
Physical state of drug in the formulation (solubilized vs. suspended) could significantly affect the release of drug from the formulation. Polarized light microscopy (Figure 2) revealed that even though ibuprofen concentration was the same (3% w/w) in F1A and F2A, drug was in solubilized form in F2A and suspended form in F1A. Permeation results show that cumulative amount of drug permeated at the end of 24 h was significantly higher in F2A compared to F1A (163.2 ± 9.36 µg/cm2 vs. 59.1 ± 4.1 µg/cm2). Enhanced permeation in F2A could be attributed to increased thermodynamic activity with the solubilized formulation. Additionally, ibuprofen could have been dissolved in the oil phase resulting in no dispersed drug particles in the formulation. Thus, solubilized drug increased the partition into the membrane and thus resulted in enhanced permeation [16]. These types of systems are suitable for enhanced permeation of lipophilic drugs, like ibuprofen, which tend to dissolve in the oil phase and partition across the skin.
4.4.3. Effect of Drug Concentration in the Formulation (F1A vs. F3 and F2A vs. F4)
F1A vs. F3
As per the formulation composition table provided in the earlier section (Table 1), F1A and F3 had the same surfactant system to stabilize the formulation, but F3 had higher concentration of drug (5% w/w) compared to F1A (3% w/w). Polarized light microscopy results revealed that ibuprofen existed in a suspended form in both the formulations (Figure 2). As expected, permeation data showed that cumulative amount of drug ibuprofen permeated at the end of 24 h was significantly higher with F3 compared to F1A (320.8 ± 17.53 µg/cm2 vs. 59.1 ± 4.1 µg/cm2). Enhanced permeation of ibuprofen could be attributed to the higher concentration of ibuprofen in F3, which acted as a reservoir and helped in maintaining concentration gradient for a prolonged period [19]. These types of systems should be considered for drugs that require high doses, such as NSAIDs (ibuprofen and diclofenac) and lidocaine, where the goal is to achieve maximum flux across the skin for improving efficacy.
F2A vs. F4
As per the formulation composition table (Table 1) provided in the earlier section, F2A and F4 had the same surfactant system to stabilize the cream formulation but F4 had higher concentration of drug (5% w/w) compared to F2A (3% w/w). Polarized light microscopy results revealed that ibuprofen existed in a suspended form in F4 and solubilized form in F2A (Figure 2). Interestingly, permeation results showed that although F4 had higher ibuprofen concentration compared to F2A (5% w/w vs. 3% w/w), cumulative amount of drug permeated at the end of 24 h was less compared to F2A (82.0 ± 31.9 µg/cm2 vs. 163.2 ± 9.36 µg/cm2). Enhanced permeation with F2A formulation could be attributed to increased thermodynamic activity and partition of the solubilized drug in the formulation [47]. Solubilized systems are preferred for highly-potent drugs such as fentanyl, progesterone, and testosterone, requiring very less concentration of drug for activity. Formulations with highly-potent drugs have a high risk if there is residual drug leftover after the intended use period, which may impact the product’s quality, efficacy, and safety. Recently USFDA has released a guidance on minimizing the residual drug in the formulations. One of the recommendations was to design formulations with optimal drug delivery and minimal residual drug [48]. Thus, the approach of using systems with solubilized drugs could minimize the residual drug and maintain the desired permeation rate of drugs throughout the usage period.
4.4.4. Effect of Formulation Type
Solubilized Cream vs. Non-Aqueous Gel (F6 vs. F2A)
Although the concentration of ibuprofen was similar and the drug was in solubilized form in formulations F6 and F2A, cumulative amount of drug permeated was significantly higher with gel compared to solubilized cream (F6: 739.6 ± 36.1 µg/cm2 vs. F2A: 163.2 ± 9.36 µg/cm2). Enhanced permeation could be due to low viscosity of the gel which resulted in high drug release from the formulation. Moreover, higher concentration of permeation enhancers in the formulation could have increased the solubility of ibuprofen and enhanced the diffusivity across the membrane.
Emulgel vs. Cream (F5 vs. F1A and F2A)
Permeation data show that cumulative amount of drug permeated at 24 h was higher with emulgel even though ibuprofen was available as suspended form in the formulation (F5: 178.5 ± 34.5 µg/cm2 vs. F2A: 163.2 ± 9.36 µg/cm2 vs. F1A: 59.1 ± 4.1 µg/cm2). Emulgels, also known as emulsified gels, are biphasic systems, containing aqueous gel dispersed with lipid phase, and are closely related to cream. It is reported in the literature that emulgels provide better solubility for poorly water-soluble drugs like ibuprofen and enhance the skin permeability. Higher permeation of ibuprofen with emulgels compared to creams could be attributed to increased thermodynamic activity of ibuprofen, due to a change in the vehicle where ibuprofen was more soluble, or due to a different interaction of the formulation with the membrane [24,49,50].
Clear Gel vs. Emulgel (F6 vs. F5)
At the end of 24 h, significantly higher amount of ibuprofen was permeated across the membrane with clear non aqueous gel (739.6 ± 36.1 µg/cm2) compared to emulgel (178.5 ± 34.5 µg/cm2). Although, concentration of ibuprofen in the formulation was similar in both the formulations, low viscosity and presence of ibuprofen in solubilized form in the gel formulation resulted in enhanced permeation. As discussed earlier, low viscosity of the clear gel resulted in greater permeation due to immediate and higher release of ibuprofen from formulation. Additionally, high thermodynamic activity and partition of ibuprofen could have resulted in enhanced permeation.
4.5. Permeation Data Analysis
The cumulative amount of ibuprofen permeated was plotted as a function of time and the permeation profiles are shown in Figure 4. All the formulations showed significant lag in ibuprofen permeation except F2A and F2B. This could be due to the presence of drug in solubilized form in F2A and F2B (Table 7).
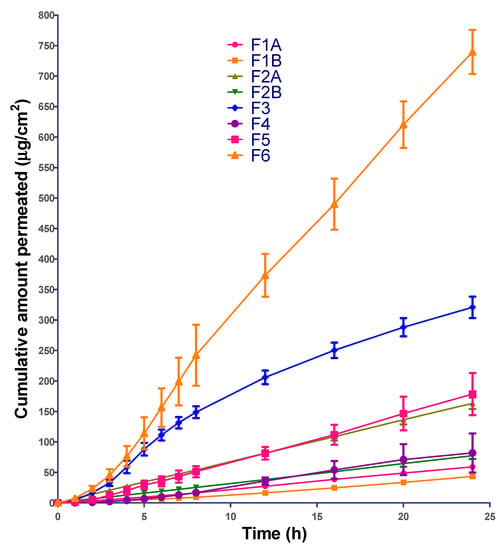
Figure 4.
In-vitro permeation profile of all the formulations.
4.6. Physical Stability
All the formulations were stored at 25 ± 2 °C and 40 ± 2 °C to observe any changes in the physical stability. As shown in the Figure 5, there were no observable changes in color and stability (phase separation, creaming, crystallization, and phase inversion) of all creams and gels. This suggests that all the formulations were physically stable throughout the storage period at both temperatures.
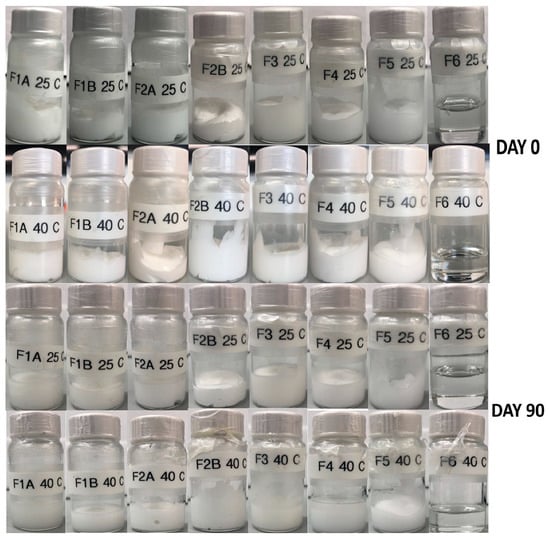
Figure 5.
Physical stability of creams and gels at 25 and 40 °C.
5. Conclusions
Formulation of a topical drug product for lipophilic drugs with desirable QTPP is challenging. Several factors such as physicochemical properties of the drug, formulation parameters, excipients in the formulation, and other parameters can affect the permeation profile. For successful topical product development, a thorough understanding of the impact of these factors on product performance is required. This study gives an example of a screening study approach that evaluates several excipients and formulation variables on product performance. It is already known that active drug accounts only for a minor fraction in the formulation and; therefore, it is important to understand how the excipients within the formulation interact with the active drug and influence the permeation. The aim of the present study was to investigate the effect of formulation parameters, such as concentration of drug, physical state of the drug, addition of mucoadhesive agents, and formulation type, on permeation of ibuprofen from semisolid formulations. For this, we successfully developed eight different semisolid formulations (creams, emulgel and gel) of ibuprofen and evaluated the effect of formulation parameters on the in-vitro permeation. All the formulations were of acceptable quality and remained physically stable over a period of 90 days at room temperature and 40 °C. Results from the present investigation noted considerable differences in permeation of ibuprofen across the Strat-M® membrane due to significant influence of formulation parameters, including concentration of ibuprofen (3% vs. 5% w/w), physical state of ibuprofen (solubilized vs. suspended), formulation type (cream vs. gel), mucoadhesive agents, and viscosity (high vs. low). It is clear according to the permeation profile that F6 (3% w/w ibuprofen clear non-aqueous gel) had the highest permeation rate among all formulations evaluated. Thus, findings from this study indicate that pharmaceutical formulation scientists should explore these critical factors during the early development of any new topical drug product in order to meet pre-determined QTPP. Additionally, as the majority of the NSAIDs have similar physicochemical properties, this article could serve as a ready reference to formulation scientists for selection of the formulation type to achieve desirable permeation profile.
Author Contributions
Conceptualization, P.K.B., A.J. and J.R.; methodology, P.K.B., B.A.C., H.S.C., and A.J.; software, P.K.B. and H.S.C.; formal analysis, P.K.B. and H.S.C.; investigation, J.R.; writing—original draft preparation, P.K.B.; writing—review and editing, B.A.C., A.J. and J.R.; supervision, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors would like to thank Gattefossé (Paramus, NJ, USA); BASF (Tarrytown, NY, USA), Seppic Inc. (Fairfield, NJ, USA) and Lubrizol Life Sciences (Cleveland, OH, USA) for providing samples to conduct this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Optimization of Formulations
Results for the optimization of ibuprofen formulations are provided in Table A1, Table A2 and Table A3. The goal of the optimization experiments was to formulate creams and gels with acceptable appearance, viscosity, and stability. During optimization, it was observed that several formulations had issues with stability, excipient incompatibility, and viscosity. Thus, the formulations with these issues were dropped and reformulated by changing the excipient concentration to achieve optimized formulations (Table A1, Table A2 and Table A3) with desired qualities for further studies.

Table A1.
Optimization of cream formulations.
Table A1.
Optimization of cream formulations.
| Components | Formulation Trial (%w/w) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 (F1A) | 3 (F1B) | 4 | 5 (F2A) | 6 (F2B) | 7 | 8 (F3) | 9 | 10 (F4) | |
| Ibuprofen | 3 | 3 | 3 | 3 | 3 | 3 | 5 | 5 | 5 | 5 |
| Propylene glycol | 15 | 15 | 15 | - | - | - | - | - | - | - |
| Oleyl alcohol | - | - | - | 5 | 5 | 5 | - | - | - | - |
| Isopropyl Myristate | - | - | - | - | - | - | 10 | 10 | - | - |
| Transcutol | - | - | - | - | - | - | - | - | 15 | 15 |
| Mineral oil | 10 | 10 | 10 | - | - | - | 7 | 7 | - | - |
| White petrolatum | - | - | - | 10 | 10 | 10 | - | - | - | - |
| Polyethylene glycol 400 | - | - | - | - | - | - | 10 | 10 | - | - |
| Medium chain triglycerides | - | - | - | - | - | - | - | - | 10 | 10 |
| Glycerol | - | - | - | - | - | - | - | - | 3 | 5 |
| Span 20 | 3.8 | 3.8 | 3.8 | - | - | - | 4.6 | 4.7 | - | - |
| Kolliphor Polysorbate 80 | 3.3 | 3.3 | 3.3 | - | - | - | 2.1 | 1.3 | - | - |
| Kolliphor CS 20 | - | - | - | 4.1 | 4.1 | 4.1 | - | - | 5 | 5 |
| Glycerol monostearate | - | - | - | 2.5 | 2.5 | 2.5 | - | - | 1.1 | 1.1 |
| Cetyl alcohol | 4 | 7 | 7 | - | - | - | - | - | 4 | 7 |
| Stearyl alcohol | - | - | - | 5 | 10 | 10 | - | - | - | - |
| Cetostearyl alcohol | - | - | - | - | - | - | - | 5 | - | - |
| Sepineo SE68 | 3 | 5 | 5 | - | - | - | - | - | - | - |
| Sepineo P600 | - | - | - | - | - | - | 4 | 4 | - | - |
| Tefose 63 | 3 | 5 | - | - | - | - | 7 | 8 | - | - |
| HPMC | - | - | - | - | 1 | - | - | - | - | - |
| Carbopol 974 | - | - | - | - | - | - | - | - | 0.5 | 0.5 |
| Water | 54.9 | 47.9 | 52.9 | 70.4 | 64.4 | 65.4 | 50.3 | 45 | 56.4 | 51.4 |
| Observation | Emulsion was not stable | Thick white cream with no signs of separation | Less viscous white cream with no signs of separation | Less viscous cream with physical instability | Stable white cream with no signs of separation | Smooth white cream with no signs of separation | Physical instability was observed | Stable emulsion with no signs of instability | Physical instability was observed | Stable cream with no signs of separation |

Table A2.
Optimization of emulgel formulation.
Table A2.
Optimization of emulgel formulation.
| Components | Formulation Trial (%w/w) | ||
|---|---|---|---|
| 1 | 2 | 3 (F5) | |
| Ibuprofen | 3 | 3 | 3 |
| Ethanol | 10 | 20 | 30 |
| Sepineo P600 | 4 | 4 | 4 |
| Water | 83 | 73 | 63 |
| Observation | Physical instability. Precipitation of ibuprofen in the formulation | Slight precipitation of ibuprofen in the formulation | Translucent gel with acceptable viscosity |

Table A3.
Optimization of clear gel formulation.
Table A3.
Optimization of clear gel formulation.
| Components | Formulation Trial (%w/w) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 (F6) | |
| Ibuprofen | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Propylene glycol | 15 | 5 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Ethanol | - | - | - | - | 10 | 10 | 10 | 10 | 10 | |
| Transcutol | 5 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| HPMC | 1.5 | - | 1.5 | - | 1.5 | - | 1.5 | - | - | - |
| HPC | - | - | - | - | - | - | - | 1.5 | 3 | 4 |
| Carbopol 974 | - | 1.5 | - | 1.5 | - | 1.5 | - | - | - | - |
| Polyethylene glycol 400 | - | - | - | - | - | - | 75.5 | 55.5 | 54 | 53 |
| Water | 75.5 | 75.5 | 65.5 | 65.5 | 55.5 | 55.5 | - | - | - | - |
| Observation | Precipitation of drug was observed. Gel was not clear gel | Precipitation of drug was observed. Gel was not clear gel | Precipitation of drug was observed. Gel was not clear gel | Precipitation of drug was observed. Gel was not clear gel | Precipitation of drug was observed. Gel was not clear gel | Precipitation of drug was observed. Gel was not clear gel | HPMC was not dissolved in PEG 400 | Gel was clear but not viscous | Gel was clear but not viscous | Clear gel with acceptable viscosity |
References
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel chemical permeation enhancers for transdermal drug delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.; Díaz-Torres, R.; Rodríguez Cruz, I.; Domínguez-Delgado, C.; Angeles, S.R.E.; Melgoza, L. Nanocarriers for transdermal drug delivery. Res. Rep. Transdermal Drug Deliv. 2012, 2012, 3. [Google Scholar] [CrossRef]
- Bolla, P.K.; Meraz, C.A.; Rodriguez, V.A.; Deaguero, I.; Singh, M.; Yellepeddi, V.K.; Renukuntla, J. Clotrimazole loaded ufosomes for topical delivery: Formulation development and in-vitro studies. Molecules 2019, 24, 3139. [Google Scholar] [CrossRef]
- Pham, Q.D.; Björklund, S.; Engblom, J.; Topgaard, D.; Sparr, E. Chemical penetration enhancers in stratum corneum—Relation between molecular effects and barrier function. J. Control. Release 2016, 232, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Saino, V.; Monti, D.; Burgalassi, S.; Tampucci, S.; Palma, S.; Allemandi, D.; Chetoni, P. Optimization of skin permeation and distribution of ibuprofen by using nanostructures (coagels) based on alkyl vitamin C derivatives. Eur. J. Pharm. Biopharm. 2010, 76, 443–449. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Advanced topical formulations (ATF). Int. J. Pharm. 2016, 514, 52–57. [Google Scholar] [CrossRef]
- Djekic, L.; Martinovic, M.; Stepanović-Petrović, R.; Micov, A.; Tomić, M.; Primorac, M. Formulation of hydrogel-thickened nonionic microemulsions with enhanced percutaneous delivery of ibuprofen assessed in vivo in rats. Eur. J. Pharm. Sci. 2016, 92, 255–265. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Juluri, A.; Narasimha Murthy, S. Transdermal iontophoretic delivery of a liquid lipophilic drug by complexation with an anionic cyclodextrin. J. Control. Release 2014, 189, 11–18. [Google Scholar] [CrossRef]
- Juluri, A.; Modepalli, N.; Jo, S.; Repka, M.A.; Shivakumar, H.N.; Murthy, S.N. Minimally invasive transdermal delivery of iron-dextran. J. Pharm. Sci. 2013, 102, 987–993. [Google Scholar] [CrossRef]
- Juluri, A.; Peddikotla, P.; Repka, M.A.; Murthy, S.N. Transdermal iontophoretic delivery of propofol: A general anaesthetic in the form of its phosphate salt. J. Pharm. Sci. 2013, 102, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Hirata, K.; Hadgraft, J.; Lane, M.E. Influence of skin penetration enhancers on skin barrier function and skin protease activity. Eur. J. Pharm. Sci. 2014, 51, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Kang, Y.P.; Yoon, I.-S.; Kim, J.S.; Kwon, S.W.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Effect of permeation enhancers on transdermal delivery of fluoxetine: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 456, 362–369. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Garg, T.; Rath, G.; Goyal, A.K. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. 2015, 22, 969–987. [Google Scholar] [CrossRef]
- Brown, M.B.; Turner, R.; Lim, S.T. Topical product formulation development. Top. Transdermal Drug Deliv. 2011, 255–286. [Google Scholar] [CrossRef]
- ICH. Guidance for Industry: Q8 (R2) Pharmaceutical Development; U.S. Department of Health and Human Services: Washington, DC, USA, 2009; pp. 1–29. Available online: https://www.fda.gov/media/71535/download (accessed on 29 January 2020).
- Ruela, A.L.M.; Perissinato, A.G.; De Lino, M.E.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S.; Al-Dhubiab, B.; Attimarad, M.; Harsha, S. Basic considerations in the dermatokinetics of topical formulations. Braz. J. Pharm. Sci. 2013, 49, 423–434. [Google Scholar] [CrossRef]
- Korting, H.C.; Schäfer-Korting, M. Carriers in the Topical Treatment of Skin Disease BT-Drug Delivery; Schäfer-Korting, M., Ed.; Springer: Berlin, Germany, 2010; pp. 435–468. [Google Scholar] [CrossRef]
- Patel, V.; Sharma, O.P.; Mehta, T. Nanocrystal: A novel approach to overcome skin barriers for improved topical drug delivery. Expert Opin. Drug Deliv. 2018, 15, 351–368. [Google Scholar] [CrossRef]
- Rhee, Y.-S.; Chang, S.-Y.; Park, C.-W.; Chi, S.-C.; Park, E.-S. Optimization of ibuprofen gel formulations using experimental design technique for enhanced transdermal penetration. Int. J. Pharm. 2008, 364, 14–20. [Google Scholar] [CrossRef]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Jameel, B.M.; Huynh, A.; Chadha, A.; Pandey, S.; Duncan, J.; Chandler, M.; Baki, G. Computer-based formulation design and optimization using Hansen solubility parameters to enhance the delivery of ibuprofen through the skin. Int. J. Pharm. 2019, 569, 118549. [Google Scholar] [CrossRef] [PubMed]
- Samaras, E.G.; Riviere, J.E.; Ghafourian, T. The effect of formulations and experimental conditions on in vitro human skin permeation—Data from updated EDETOX database. Int. J. Pharm. 2012, 434, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Celebi, D.; Guy, R.H.; Edler, K.J.; Scott, J.L. Ibuprofen delivery into and through the skin from novel oxidized cellulose-based gels and conventional topical formulations. Int. J. Pharm. 2016, 514, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Szabó-Révész, P. Modifying the physicochemical properties of NSAIDs for nasal and pulmonary administration. Drug Discov. Today Technol. 2018, 27, 87–93. [Google Scholar] [CrossRef]
- Shimizu, M.; Tatsuno, M.; Matsushita, R.; Totsuka, J.; Inoue, Y.; Ohta, K.; Kuniya, K.; Fujii, N.; Fukasawa, Y.; Watanabe, N.; et al. Correlation between the physicochemical property of some nonsteroidal anti-inflammatory drugs and changes in adenosine triphosphate, glutathione and hemoglobin in rat erythrocytes. Biol. Pharm. Bull. 2003, 26, 1155–1165. [Google Scholar] [CrossRef]
- Beetge, E.; Du Plessis, J.; Müller, D.G.; Goosen, C.; Van Rensburg, F.J. The influence of the physicochemical characteristics and pharmacokinetic properties of selected NSAID’s on their transdermal absorption. Int. J. Pharm. 2000, 193, 261–264. [Google Scholar] [CrossRef]
- Hadgraft, J.; Du Plessis, J.; Goosen, C. The selection of non-steroidal anti-inflammatory agents for dermal delivery. Int. J. Pharm. 2000, 207, 31–37. [Google Scholar] [CrossRef]
- Bezrouk, A.; Fiala, Z.; Kotingová, L.; Krulichová, I.S.; Kopečná, M.; Vávrová, K. SAMPA: A free software tool for skin and membrane permeation data analysis. Toxicol. In Vitro 2017, 44, 361–371. [Google Scholar] [CrossRef]
- USFDA. Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 28 July 2019).
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef]
- CHMP. Draft Guideline on Quality and Equivalence of Topical Products; European Medicines Agency: Amsterdam, The Netherlands, 2018; pp. 1–36. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 25 January 2020).
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Nallagundla, S.; Patnala, S.; Kanfer, I. Comparison of in vitro release rates of acyclovir from cream formulations using vertical diffusion cells. AAPS Pharm. Sci. Tech. 2014, 15, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane properties for permeability testing: Skin versus synthetic membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A mechanistic study to determine the structural similarities between artificial membrane strat-MTM and biological membranes and its application to carry out skin permeation study of amphotericin B nanoformulations. AAPS Pharm. Sci. Tech. 2018, 19, 1606–1624. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, J.A.; Kim, J.T.; Cha, D.S.; Cho, J.H.; Park, H.J.; Shin, G.H. Preparation of a capsaicin-loaded nanoemulsion for improving skin penetration. J. Agric. Food Chem. 2014, 62, 725–732. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M(R) synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Vaginal semisolid products: Technological performance considering physiologic parameters. Eur. J. Pharm. Sci. 2017, 109, 556–568. [Google Scholar] [CrossRef]
- Johal, H.S.; Garg, T.; Rath, G.; Goyal, A.K. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 2016, 23, 550–563. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; GremiÃ/poundso, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Peña, J.; Veiga, M.-D. Development of mucoadhesive vaginal films based on HPMC and zein as novel formulations to prevent sexual transmission of HIV. Int. J. Pharm. 2019, 570, 118643. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y.; Şenyiğit, Z.A.; Eraç, B.; Limoncu, M.H.; Baloğlu, E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: Formulation, preparation, and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2017, 22, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Palacin, C.; Guerrero, M.; Raga, M.; Romero, A.; Gulietta, A. Pharmaceutical Compositions of Sertaconazole for Vaginal Use. US Patent US20060165803A1, 17 December 2003. Available online: https://patents.google.com/patent/US20060165803A1/en (accessed on 12 February 2020).
- Otto, A.; Du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Residual Drug in Transdermal and Related Drug Delivery Systems. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/residual-drug-transdermal-and-related-drug-delivery-systems (accessed on 5 December 2019).
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Gohel, M.C.; Shelat, P.K. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: Optimization of formulation using D-optimal design. AAPS Pharm. Sci. Tech. 2012, 13, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Salim, N.; Jose García-Celma, M.; Escribano, E.; Nolla, J.; Llinàs, M.; Basri, M.; Solans, C.; Esquena, J.; Tadros, T.F. Formation of nanoemulsion containing ibuprofen by PIC method for topical delivery. Mater. Today Proc. 2018, 5, S172–S179. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).