Development and Characterization of Biointeractive Gelatin Wound Dressing Based on Extract of Punica granatum Linn
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Procedure and Sample Preparation
2.3. HPLC Instrumentation and Chromatographic Condition
2.4. Antioxidant Activity
2.5. Preparation of Gelatin-Based Membrane Loaded with Aqueous Extract of P. granatum
2.6. Characterization
2.6.1. Mechanical Properties
2.6.2. Water Vapor Transmission Rates (WVTRs)
2.6.3. Swelling Index
2.6.4. Infrared Spectroscopy (FTIR)
2.6.5. Thermal Analysis
2.6.6. Color Measurement
2.6.7. Scanning Electron Microscopy (SEM)
2.7. Wound-Healing Assay
2.7.1. Animals
2.7.2. Surgical Procedures and Groups
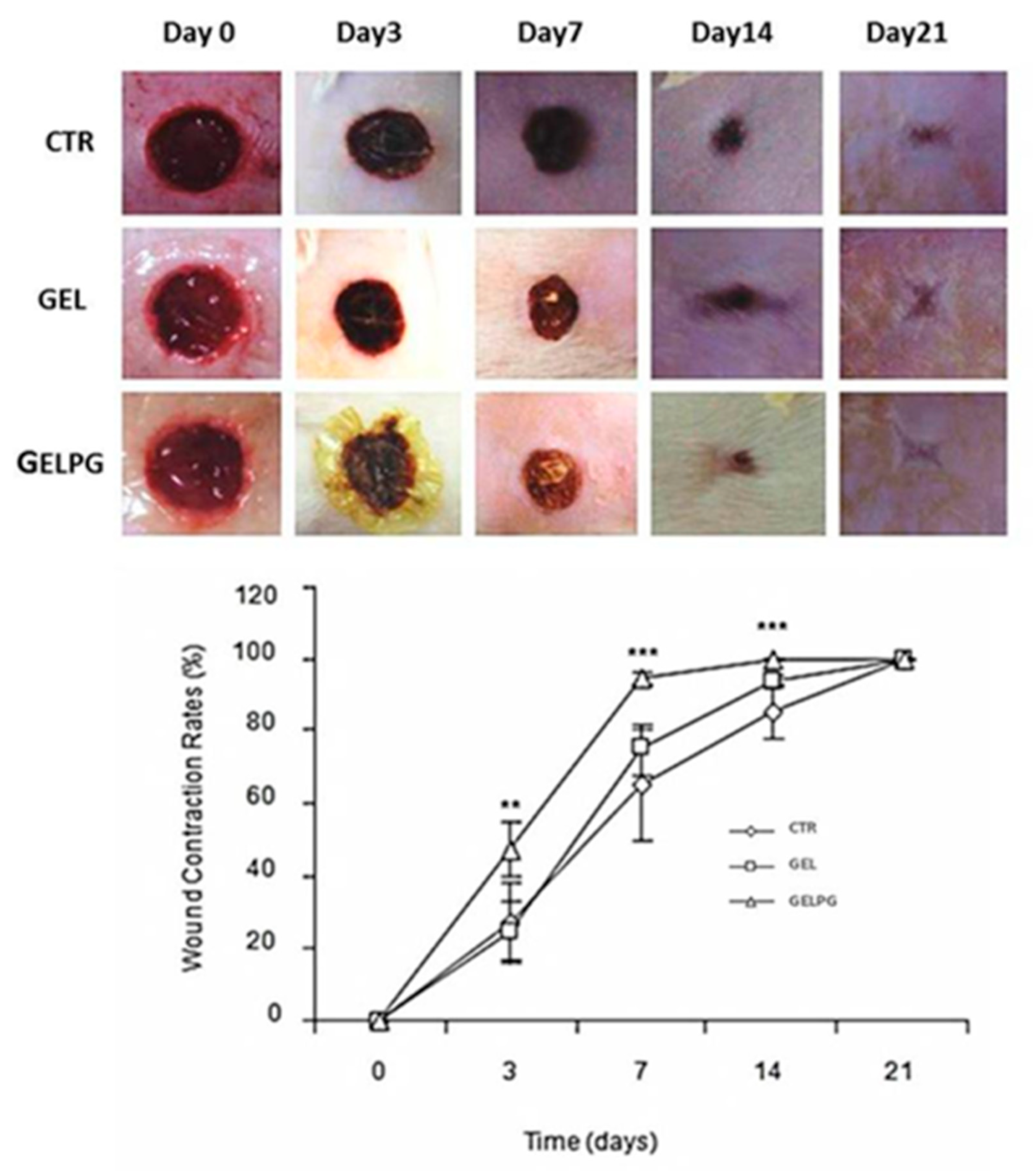
2.7.3. Assessment of the Wound Closure Rates (WCR)
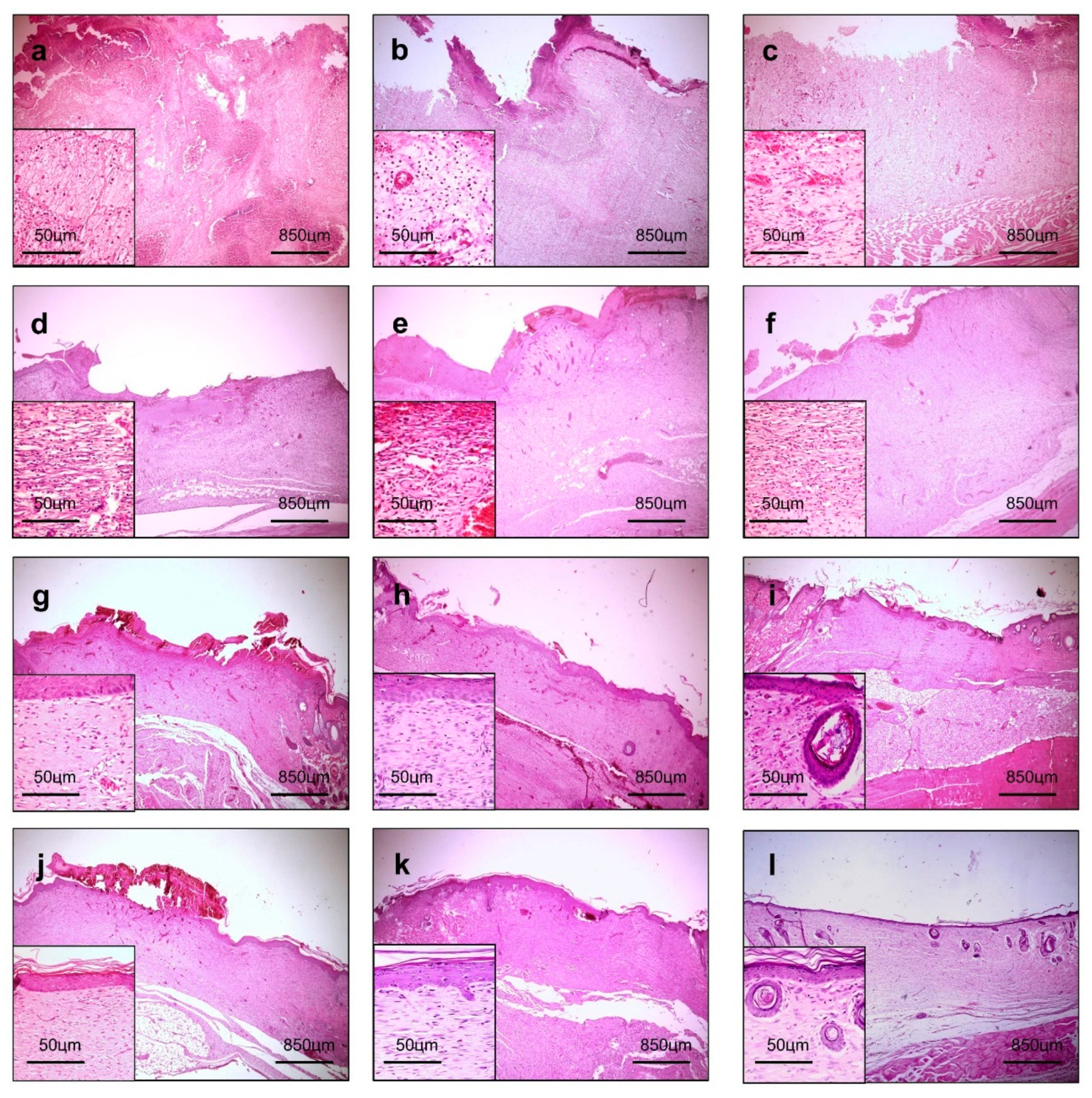
2.7.4. Histological Procedures and Morphological Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Pomegranate Peel Extracts
3.2. Characterization of the Membranes
3.3. Assessment of the Wound Closure Rates (WCR)
3.4. Morphological Analysis of the Wound-Healing Process
3.5. Morphological Analysis of the Collagenization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hayouni, E.A.; Miled, K.; Boubaker, S.; Bellasfar, Z.; Abedrabba, M.; Iwaski, H.; Oku, H.; Matsui, T.; Limam, F.; Hamdi, M. Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomed. Int. J. Phytother. Phytopharm. 2011, 18, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.; Grocott, P.; Probst, S.; Clarke, E. Current practice in the management of wound odour: An international survey. Int. J. Nurs. Stud. 2014, 51, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.R.; Maia, R.C.A.P.; Rannier, L.; Andrade, L.N.; Chaud, M.V.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; da Costa, L.P.; Souto, E.B.; et al. Silver nanoparticles-composing alginate/gelatin hydrogel improves wound healing in vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hissae Yassue-Cordeiro, P.; Zandonai, C.H.; Pereira Genesi, B.; Santos Lopes, P.; Sanchez-Lopez, E.; Garcia, M.L.; Camargo Fernandes-Machado, N.R.; Severino, P.; Ferreira da Silva, C. Development of Chitosan/Silver Sulfadiazine/Zeolite Composite Films for Wound Dressing. Pharmaceutics 2019, 11, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siritienthong, T.; Ratanavaraporn, J.; Aramwit, P. Development of ethyl alcohol-precipitated silk sericin/polyvinyl alcohol scaffolds for accelerated healing of full-thickness wounds. Int. J. Pharm. 2012, 439, 175–186. [Google Scholar] [CrossRef]
- Nunes, P.S.; Albuquerque-Júnior, R.L.C.; Cavalcante, D.R.R.; Dantas, M.D.M.; Cardoso, J.C.; Bezerra, M.S.; Souza, J.C.C.; Serafini, M.R.; Quitans, L.J., Jr.; Bonjardim, L.R.; et al. Collagen-Based Films Containing Liposome-Loaded Usnic Acid as Dressing for Dermal Burn Healing. J. Biomed. Biotechnol. 2011, 2011, 761593. [Google Scholar] [CrossRef]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef]
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.C.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef]
- De Almeida, E.B.; Cordeiro Cardoso, J.; Karla de Lima, A.; de Oliveira, N.L.; de Pontes-Filho, N.T.; Oliveira Lima, S.; Leal Souza, I.C.; de Albuquerque-Júnior, R.L.C. The incorporation of Brazilian propolis into collagen-based dressing films improves dermal burn healing. J. Ethnopharmacol. 2013, 147, 419–425. [Google Scholar] [CrossRef]
- Thu, H.E.; Zulfakar, M.H.; Ng, S.F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double membrane based on lidocaine-coated polymyxin-alginate nanoparticles for wound healing: In vitro characterization and in vivo tissue repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef] [PubMed]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.C.; Thomazzi, S.M. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a cosmeceutical source: Pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, E.; Hosseinimehr, S.J.; Akbari, J.; Azadbakht, M.; Azizi, S. The Effects of Punica granatum Flower Extract on Skin Injuries Induced by Burn in Rats. Adv. Pharmacol. Sci. 2017, 2017, 3059745. [Google Scholar] [CrossRef] [Green Version]
- Murthy, K.N.; Reddy, V.K.; Veigas, J.M.; Murthy, U.D. Study on wound healing activity of Punica granatum peel. J. Med. Food 2004, 7, 256–259. [Google Scholar] [CrossRef]
- Viudamartos, M.; Fernández-López, J.; Pérez-Álvarez, J. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. J. Clin. Ther. 2008, 13, 128–144. [Google Scholar]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Zaid, M.A.; Afaq, F.; Syed, D.N.; Dreher, M.; Mukhtar, H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem. Photobiol. 2007, 83, 882–888. [Google Scholar] [CrossRef]
- Park, H.M.; Moon, E.; Kim, A.J.; Kim, M.H.; Lee, S.; Lee, J.B.; Park, Y.K.; Jung, H.S.; Kim, Y.B.; Kim, S.Y. Extract of Punica granatum inhibits skin photoaging induced by UVB irradiation. Int. J. Dermatol. 2010, 49, 276–282. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, P. Assessment of the histological state of the healing wound. Plast. Aesthetic Res. 2015, 2, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Sultana, J.; Molla, M.; Kamal, M.; Shahidullah, M.; Begum, F.; Bashar, M. Histological differences in wound healing in Maxillofacial region in patients with or without risk factors. Bangladesh J. Pathol. 2009, 24, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Bombardelli, E.; Bombardelli, V. Twenty years’ experience in the botanical health food market. Fitoterapia 2005, 76, 495–507. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Vadhanam, M.V.; Kausar, H.; Jeyabalan, J.; Schultz, D.J.; Gupta, R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012, 49, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Shi, L.; Fan, L.; Ding, Y.; Zhao, S.; Liu, Y.; Ma, C. Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L.) leaves. Ind. Crop. Prod. 2013, 42, 587–594. [Google Scholar] [CrossRef]
- Peh, K.; Khan, T.; Ch’ng, H. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2000, 3, 303–311. [Google Scholar]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Fadini, A.L.; Rocha, F.S.; Alvim, I.D.; Sadahira, M.S.; Queiroz, M.B.; Alves, R.M.V.; Silva, L.B. Mechanical properties and water vapour permeability of hydrolysed collagen–cocoa butter edible films plasticised with sucrose. Food Hydrocoll. 2013, 30, 625–631. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Q.; Du, Y. Alginate/gelatin blend films and their properties for drug controlled release. J. Membr. Sci. 2006, 280, 37–44. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A Review of the Potential Benefits of Plants Producing Berries in Skin Disorders. Antioxidants 2020, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Suzuki, N.; Masamune, A.; Kikuta, K.; Watanabe, T.; Satoh, K.; Shimosegawa, T. Ellagic Acid Inhibits Pancreatic Fibrosis in Male Wistar Bonn/Kobori Rats. Dig. Dis. Sci. 2009, 54, 802–810. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef] [Green Version]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (-)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate Inflammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Skin Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Naldaiz-Gastesi, N.; Bahri, O.A.; De Munain, A.L.; Mccullagh, K.J.A.; Izeta, A. Thepanniculus carnosusmuscle: An evolutionary enigma at the intersection of distinct research fields. J. Anat. 2018, 233, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, D.E.; Jones, W.D.; Ruge, F.; Harding, K.G.; Moore, K. The role of lymphocytes in human dermal wound healing. Br. J. Dermatol. 2000, 143, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Masi, E.C.D.J.D.; Campos, A.C.L.; Masi, F.D.J.D.; Ratti, M.A.S.; Shin Ike, I.; Masi, R.D.J.D. The influence of growth factors on skin wound healing in rats. J. Braz. J. Otorhinolaryngol. 2016, 82, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Hwang, S.M.; Yoon, J.J.; Lee, S.M.; Kyung, E.H.; Kim, J.S.; Kang, D.G.; Lee, H.S. Inhibitory effect of Thuja orientalis on TNF-α-induced vascular inflammation. Phytother. Res. 2010, 24, 1489–1495. [Google Scholar] [CrossRef]
- Corbett, S.; Daniel, J.; Drayton, R.; Field, M.; Steinhardt, R.; Garrett, N. Evaluation of the Anti-inflammatory Effects of Ellagic Acid. J. PeriAnesthesia Nurs. 2010, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Fang, Y. Anti-inflammatory gallic Acid and wedelolactone are G protein-coupled receptor-35 agonists. Pharmacology 2012, 89, 211–219. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsu, Y.F.; Lin, T.C. Effects of punicalagin and punicalin on carrageenan-induced inflammation in rats. Am. J. Chin. Med. 1999, 27, 371–376. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Gryglewski, R.J.; Szczeklik, A.; Wandzilak, M. The effect of six prostaglandins, prostacyclin and iloprost on generation of superoxide anions by human polymorphonuclear leukocytes stimulated by zymosan or formyl-methionyl-leucyl-phenylalanine. Biochem. Pharmacol. 1987, 36, 4209–4213. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chou, C.W.; Senthil Kumar, K.J.; Fu, K.T.; Wang, H.M.; Hsu, L.S.; Kuo, Y.H.; Wu, C.R.; Chen, S.C.; Yang, H.L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- Kannan, M.M.; Quine, S.D. Ellagic acid ameliorates isoproterenol induced oxidative stress: Evidence from electrocardiological, biochemical and histological study. Eur. J. Pharmacol. 2011, 659, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pirbalouti, A.G.; Koohpayeh, A.; Karimi, I. The wound healing activity of flower extracts of Punica granatum and Achillea kellalensis in Wistar rats. Acta Pol. Pharm. 2010, 67, 107–110. [Google Scholar] [PubMed]
- Feliciani, C.; Ruocco, E.; Zampetti, A.; Toto, P.; Amerio, P.; Tulli, A.; Amerio, P.; Ruocco, V. Tannic acid induces in vitro acantholysis of keratinocytes via IL-1alpha and TNF-alpha. Int. J. Immunopathol. Pharmacol. 2007, 20, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, J.E.; Green, C.J.; Motterlini, R. Involvement of the Heme Oxygenase–Carbon Monoxide Pathway in Keratinocyte Proliferation. Biochem. Biophys. Res. Commun. 1997, 241, 215–220. [Google Scholar] [CrossRef] [PubMed]
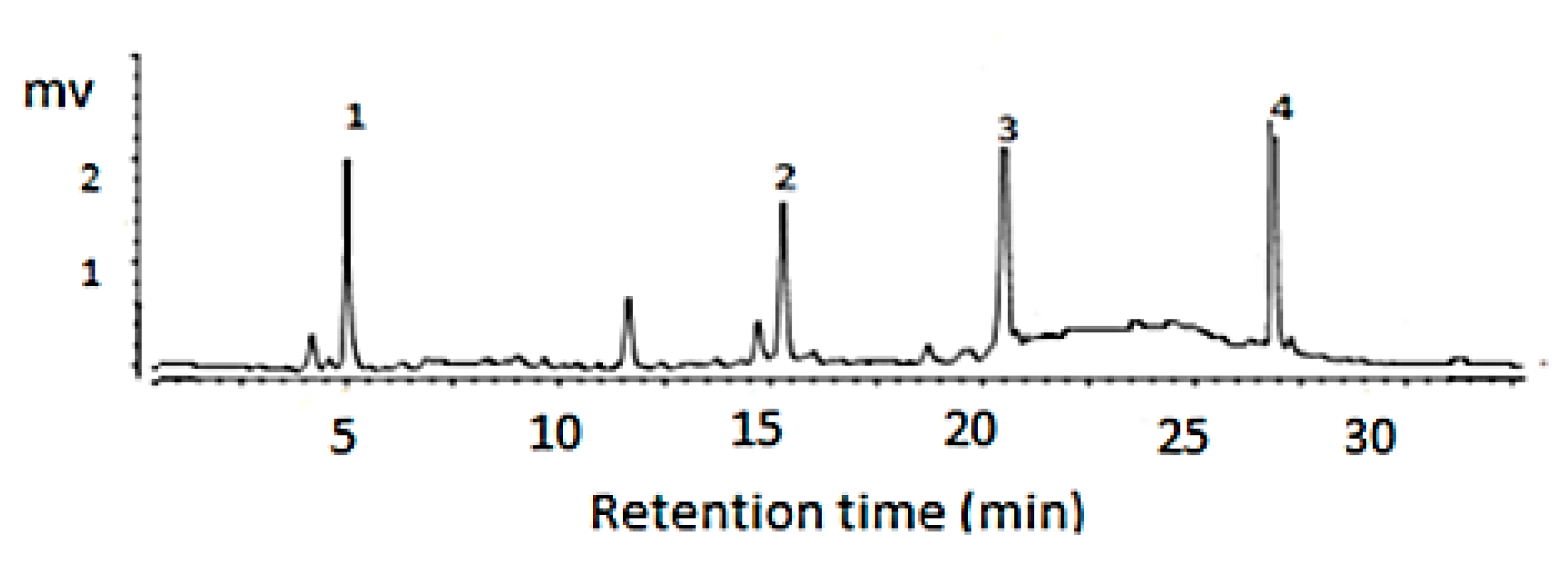
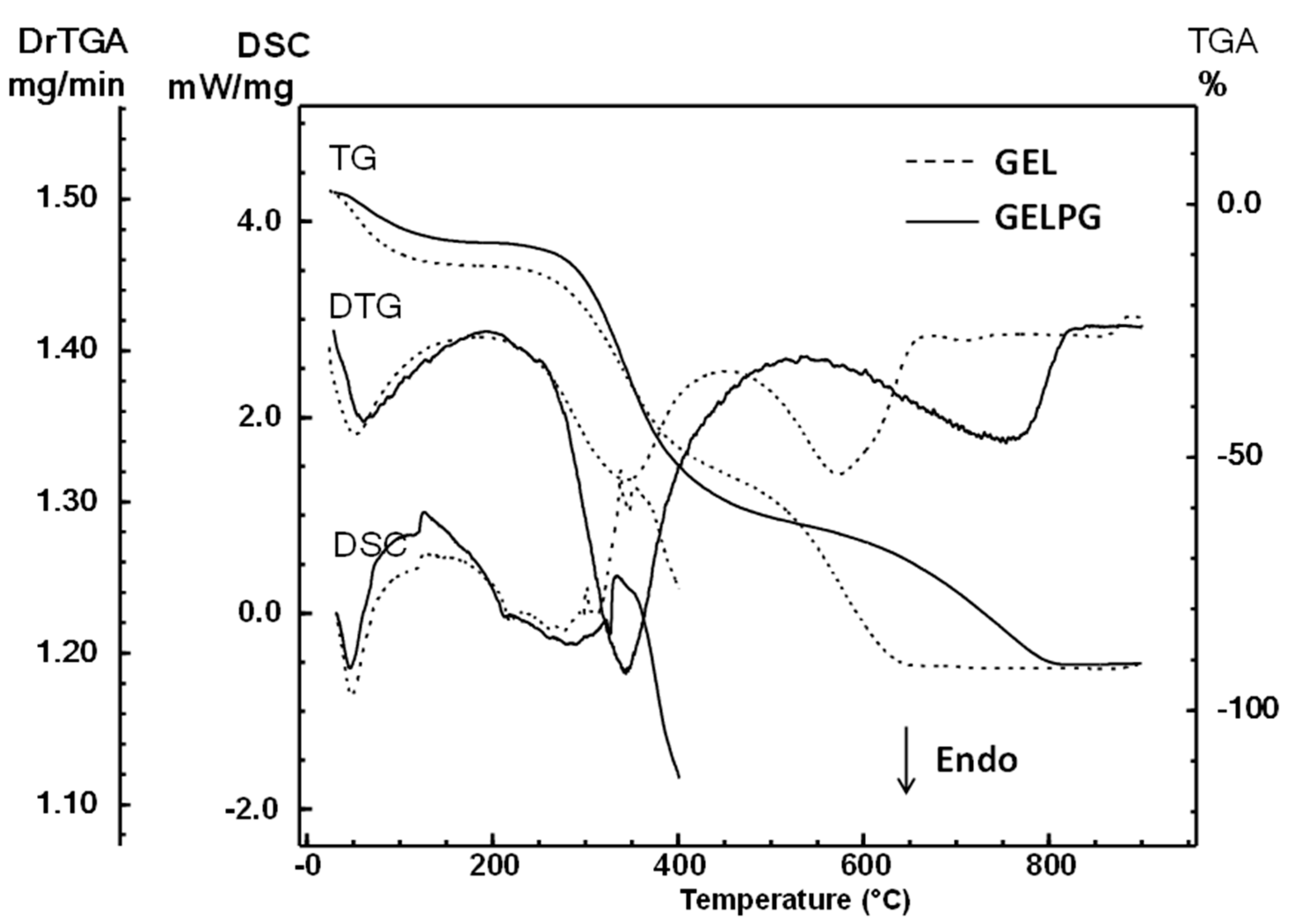
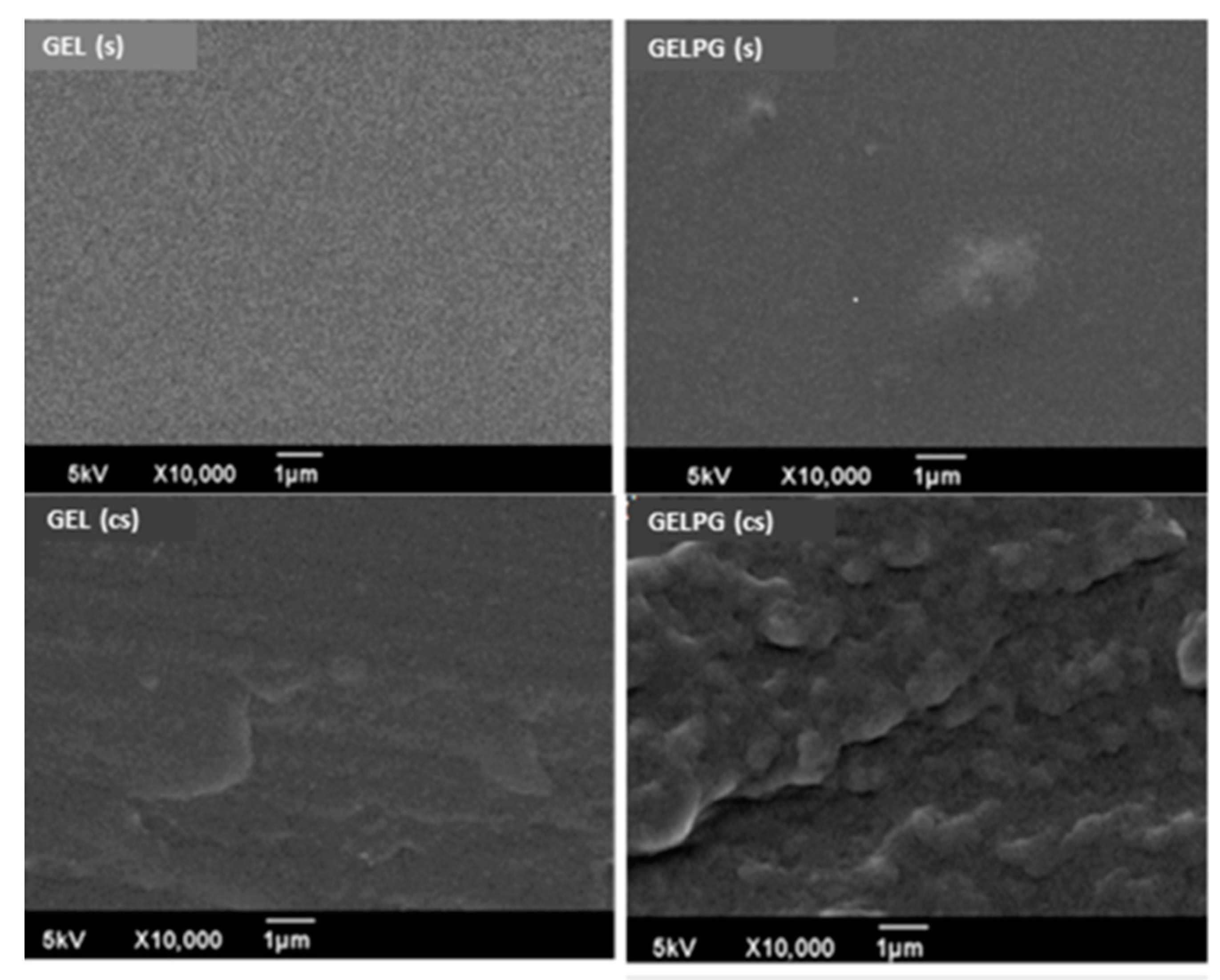
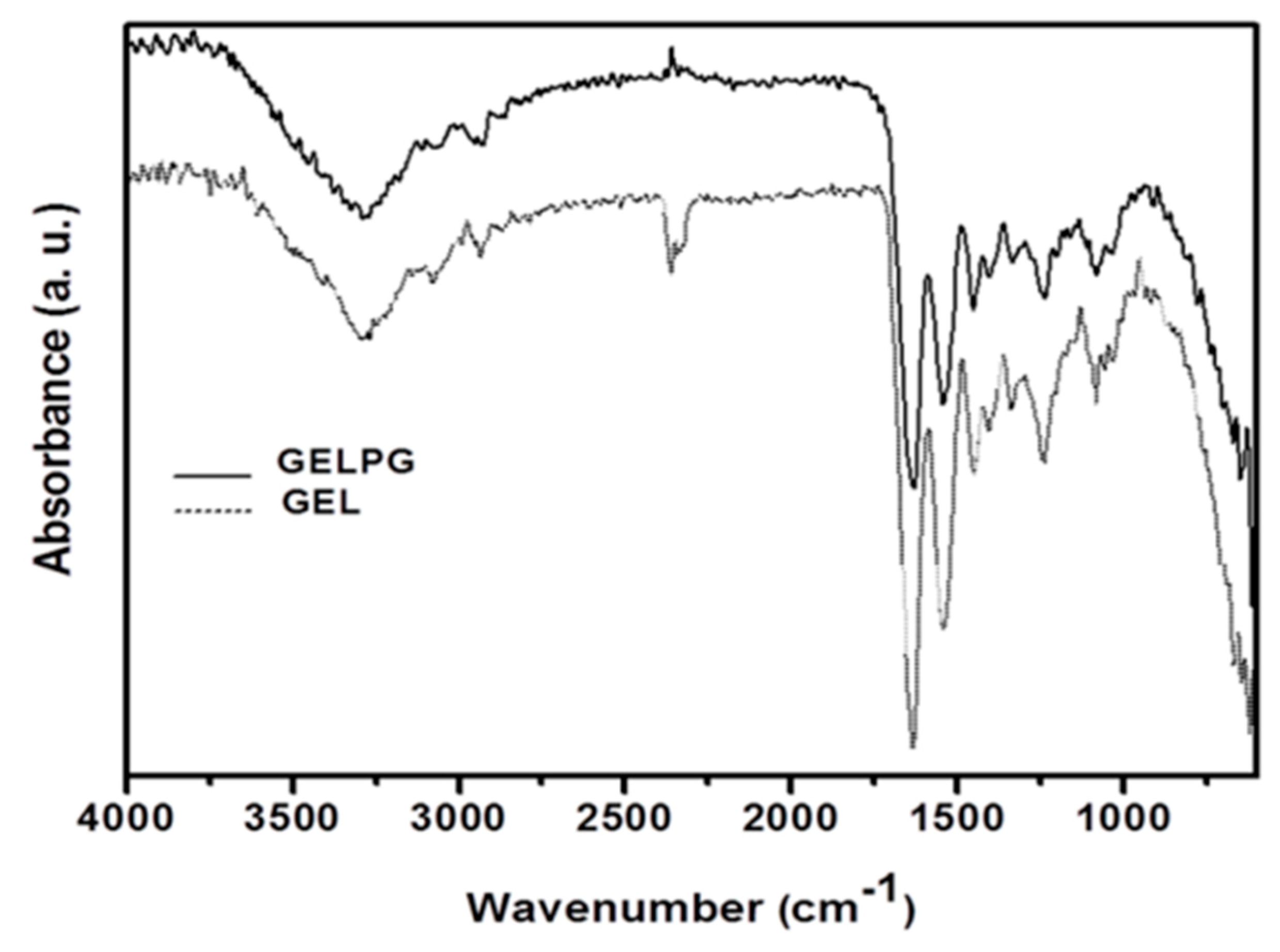

| Compound | Regression Equation | R2 | mg/g Extract |
|---|---|---|---|
| Gallic acid | y = 1.234x + 24.578 | 0.9998 | 32.24 |
| Ellagic acid | y = 2.109x + 13.875 | 0.9998 | 41.67 |
| Analysis | Parameters | GEL | GELPG | p-Values * |
|---|---|---|---|---|
| Mechanical properties | Young modulus (MPa) | 633.3 ± 128.5 | 639.3 ± 82.0 | 0.917 |
| Elongation (%) | 7.1 ± 2.5 | 8.7 ± 2.4 | 0.178 | |
| Maximal tension (MPa) | 43.3 ± 9.1 | 54.6 ± 7.3 | 0.021 | |
| Colorimetry | ΔE | 18.6 ± 0.6 | 15.0 ± 2.8 | 0.162 |
| Δa | −3.9 ± 0.2 | −3.7 ± 0.4 | 0.557 | |
| Δb | −12.3 ± 0.9 | 10.6 ± 4.1 | 0.011 | |
| ΔL | 13.3 ± 1.0 | 9.7 ± 0.6 | 0.006 | |
| Swelling behavior | Swelling index (%) after 3 h/pH 7.2 | 253.2 ± 23.9 | 412.5 ± 54.2 | 0.033 |
| Barrier properties | Permeability (g∙mm/d∙m2∙KPa) | 14.0 ± 1.9 | 12.4 ± 1.3 | 0.003 |
| Groups | Experimental Time (days) | |||
|---|---|---|---|---|
| Inflammation a | Wound-Healing Histological Grading b | |||
| Day 3 | Day 7 | Day 14 | 21 | |
| CTR | 3.0 ± 0.0 | 9.4 ± 1.2 | 12.8 ± 1.7 | 16.3 ± 1.4 |
| GEL | 2.6 ± 0.5 * | 10.2 ± 1.6 | 13.6 ± 1.9 | 17.1 ± 1.3 |
| GELPG | 2.4 ± 0.5 * | 11.7 ± 1.3 * | 15.1 ± 1.5 ** | 18.2 ± 1.2 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, M.F.; Cardoso, J.C.; Santos, T.S.; Tavares, L.A.; Pashirova, T.N.; Severino, P.; Souto, E.B.; Albuquerque-Junior, R.L.C.d. Development and Characterization of Biointeractive Gelatin Wound Dressing Based on Extract of Punica granatum Linn. Pharmaceutics 2020, 12, 1204. https://doi.org/10.3390/pharmaceutics12121204
do Nascimento MF, Cardoso JC, Santos TS, Tavares LA, Pashirova TN, Severino P, Souto EB, Albuquerque-Junior RLCd. Development and Characterization of Biointeractive Gelatin Wound Dressing Based on Extract of Punica granatum Linn. Pharmaceutics. 2020; 12(12):1204. https://doi.org/10.3390/pharmaceutics12121204
Chicago/Turabian Styledo Nascimento, Marismar F., Juliana C. Cardoso, Tarsizio S. Santos, Lívia A. Tavares, Tatiana N. Pashirova, Patricia Severino, Eliana B. Souto, and Ricardo L. C. de Albuquerque-Junior. 2020. "Development and Characterization of Biointeractive Gelatin Wound Dressing Based on Extract of Punica granatum Linn" Pharmaceutics 12, no. 12: 1204. https://doi.org/10.3390/pharmaceutics12121204
APA Styledo Nascimento, M. F., Cardoso, J. C., Santos, T. S., Tavares, L. A., Pashirova, T. N., Severino, P., Souto, E. B., & Albuquerque-Junior, R. L. C. d. (2020). Development and Characterization of Biointeractive Gelatin Wound Dressing Based on Extract of Punica granatum Linn. Pharmaceutics, 12(12), 1204. https://doi.org/10.3390/pharmaceutics12121204









