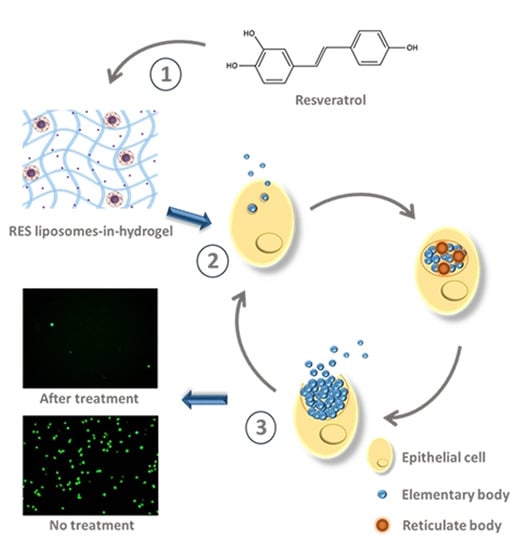
Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RES Liposomes
2.3. Characterization of RES Liposomes
2.4. Preparation of RES Liposomes-In-Hydrogel Formulation
2.5. In Vitro RES Release
2.6. McCoy Cell Culture and Propagation of Chlamydia trachomatis
2.7. Inhibition of NO Production
2.8. Statistical Analyses
3. Results
3.1. Characteristics of Liposomes-in-Hydrogel Formulation
3.2. C. trachomatis RES Challenge In Vitro
3.3. Anti-Inflammatory Effect of RES Liposomes-In-Hydrogel
4. Discussion
4.1. RES Liposomes-In-Hydrogel Formulation
4.2. Anti-Chlamydial Activity
4.3. Anti-Inflammatory Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Puolakkainen, M. Laboratory diagnosis of persistent human chlamydial infection. Front. Cell. Infect. Microbiol. 2013, 3, e99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Fransis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.; Schwebke, J.R.; Hoornenborg, E. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, 235–279. [Google Scholar] [CrossRef]
- Sandoz, K.M.; Rockey, D.D. Antibiotic resistance in Chlamydiae. Future Microbiol. 2010, 5, 1427–1442. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Lanjouw, E.; Ouburg, S.; de Vries, H.; Stary, A.; Radcliffe, K.; Unemo, M. 2015 European guideline on the management of Chlamydia trachomatis infections. Int. J. STD AIDS 2016, 27, 333–348. [Google Scholar] [CrossRef]
- van Dooremalen, W.T.M.; Verweij, S.P.; den Hartog, J.E.; Kebbi-Beghdadi, C.; Ouburg, S.; Greub, G.; Morré, S.A.; Ammerdorffer, A. Screening of Chlamydia trachomatis and Waddlia chondrophila Antibodies in Women with Tubal Factor Infertility. Microorganisms 2020, 8, 918. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Tostrup, M.J.; Moueffaq, S.; Škalko-Basnet, N. Localized therapy of vaginal infections and inflammation: Liposomes-in-hydrogel delivery system for polyphenols. Pharmaceutics 2019, 11, e53. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Potroz, M.G.; Cho, N.J. Natural products for the treatment of trachoma and Chlamydia trachomatis. Molecules 2015, 20, 4180–4203. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Catinella, G.; Dallavalle, S.; Pinto, A. Stilbenoids: A Natural Arsenal against Bacterial Pathogens. Antibiotics 2020, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Potroz, M.G.; Teh, S.W.; Cho, N.J. Natural products for the treatment of chlamydiaceae infections. Microorganisms 2016, 4, e39. [Google Scholar] [CrossRef] [PubMed]
- Petyaev, I.M.; Zigangirova, N.A.; Morgunova, E.Y.; Kyle, N.H.; Fedina, E.D.; Bashmakov, Y.K. Resveratrol inhibits propagation of Chlamydia trachomatis in McCoy cells. Biomed. Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Ur-Rehman, T.; Slepenkin, A.; Chu, H.; Blomgren, A.; Dahlgren, M.K.; Zetterström, C.E.; Peterson, E.M.; Elofsson, M.; Gylfe, Å. Pre-clinical pharmacokinetics and anti-chlamydial activity of salicylidene acylhydrazide inhibitors of bacterial type III secretion. J. Antibiot. 2012, 65, 397–404. [Google Scholar] [CrossRef][Green Version]
- Zetterström, C.E.; Hasselgren, J.; Salin, O.; Davis, R.A.; Quinn, R.J.; Sundin, C.; Elofsson, M. The resveratrol tetramer (-)-hopeaphenol inhibits type III secretion in the Gram-negative pathogens Yersinia pseudotuberculosis and Pseudomonas aeruginosa. PLoS ONE 2013, 8, e81969. [Google Scholar] [CrossRef] [PubMed]
- Ende, R.; Derré, I. A Coinfection Model to Evaluate Chlamydia Inc Protein Interactions. In Chlamydia Trachomatis: Methods and Protocols, 1st ed.; Brown, A.C., Ed.; Humana: New York, NY, USA, 2019; Volume 2042, pp. 205–218. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Mahony, J.B. Enumeration of Viable Chlamydia from Infected Animals Using Immunofluorescent Microscopy. In Chlamydia Trachomatis: Methods and Protocols, 1st ed.; Brown, A.C., Ed.; Humana: New York, NY, USA, 2019; Volume 2042, pp. 237–244. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Acharya, G.; Škalko-Basnet, N. PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. 2017, 113, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Vanić, Ž.; Tho, I.; Škalko-Basnet, N. Chitosan-coated liposomes for topical vaginal therapy: Assuring localized drug effect. Int. J. Pharm. 2014, 472, 94–101. [Google Scholar] [CrossRef]
- Hurler, J.; Škalko-Basnet, N. Potentials of chitosan-based delivery systems in wound therapy: Bioadhesion study. J. Funct. Biomater. 2012, 3, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, S.; Hokynar, K.; Mannonen, L.; Paavonen, J.; Hiltunen-Back, E.; Puolakkainen, M. Transcriptional expression of the ompA, cpaf, tarp, and tox genes of Chlamydia trachomatis clinical isolates at different stages of the developmental cycle. Microorganisms 2019, 7, e153. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Hussain, H.; Tho, I.; Škalko-Basnet, N. Liposomal delivery system enhances anti-inflammatory properties of curcumin. J. Pharm. Sci. 2012, 101, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kristl, J.; Teskač, K.; Caddeo, C.; Abramović, Z.; Šentjurc, M. Improvements of cellular stress response on resveratrol in liposomes. Eur. J. Pharm. Biopharm. 2009, 73, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Nøhr-Meldgaard, K.; Ovsepian, A.; Ingmer, H.; Vestergaard, M. Resveratrol enhances the efficacy of aminoglycosides against Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Fiod Riccio, B.V.; Fonseca-Santos, B.; Colerato Ferrari, P.; Chorilli, M. Characteristics, biological properties and analytical methods of trans-resveratrol: A review. Crit. Rev. Anal. Chem. 2019, 50, 339–358. [Google Scholar] [CrossRef]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Mohammadinejad, R.; Ashrafizadeh, M. Drug delivery systems for resveratrol, a non-flavonoid polyphenol: Emerging evidence in last decades. J. Drug. Deliv. Sci. Technol. 2019, 51, 591–604. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B: Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to improve resveratrol systemic and topical bioavailability: An update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41. [Google Scholar] [CrossRef]
- Vanić, Ž.; Škalko-Basnet, N. Hydrogels as intrinsic antimicrobials. In Hydrogels Based on Natural Polymers, 1st ed.; Chen, Y., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 309–328. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Carlson, E.E. Tiny things with enormous impact: Nanotechnology in the fight against infectious disease. ACS Infect. Dis. 2018, 4, 1432–1435. [Google Scholar] [CrossRef]
- Caddeo, C.; Teskač, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2018, 363, 183–191. [Google Scholar] [CrossRef]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.; Storm, G. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Chabot, G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Xu, C.; Hosseinidoust, Z. Preserving the efficacy of glycopeptide antibiotics during nanoencapsulation in liposomes. ACS Infect. Dis. 2019, 5, 1794–1801. [Google Scholar] [CrossRef]
- Pavelić, Ž.; Škalko-Basnet, N.; Filipović-Grčić, J.; Martinac, A.; Jalšenjak, I. Development and in vitro evaluation of a liposomal vaginal delivery system for acyclovir. J. Control. Release 2005, 1–2, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Z.; Rukavina, Z.; Manner, S.; Fallarero, A.; Uzelac, L.; Kralj, M.; Klarić, D.A.; Bogdanov, A.; Raffai, T.; Virok, D.P.; et al. Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections. Int. J. Nanomed. 2019, 14, 5957–5976. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Martini, S.; Ciani, L.; Lamponi, S.; Rebmann, H.; Rossi, C.; Ristori, S. Using liposomes as carriers for polyphenolic compounds: The case of trans-resveratrol. PLoS ONE 2012, 7, e41438. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Hurler, J.; Ferderber, K.; Golja Gašparović, P.; Škalko-Basnet, N.; Filipović-Grčić, J. Novel vaginal drug delivery system: Deformable propylene glycol liposomes-in-hydrogel. J. Liposome Res. 2014, 24, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The antimicrobial properties of chitosan can be tailored by formulation. Mar. Drugs 2020, 18, e96. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Žakelj, S.; Mravljak, J.; Pajk, S.; Kristl, A.; Schubert, R.; Škalko-Basnet, N. The effect of lipid composition and liposome size on the release properties of liposomes-in-hydrogel. Int. J. Pharm. 2013, 456, 49–57. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; Del Prado-Audelo, M.L.; Ortega-Peña, S.; Mendoza-Muñoz, N.; Urbán-Morlán, Z.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H. Modifications in Vaginal Microbiota and Their Influence on Drug Release: Challenges and Opportunities. Pharmaceutics 2019, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.M.; Carvalho, S.G.; Araujo, V.H.S.; Carvalho, G.C.; Gremião, M.P.D.; Chorilli, M. Recent advances in hydrogels as strategy for drug delivery intended to vaginal infections. Int. J. Pharm. 2020, 590, 119867. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 2015, 5, 10029. [Google Scholar] [CrossRef]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 26, 1533–1538. [Google Scholar] [CrossRef]
- Sundin, C.; Zetterström, C.E.; Vo, D.D.; Brkljača, R.; Urban, S.; Elofsson, M. Exploring resveratrol dimers as virulence blocking agents–Attenuation of type III secretion in Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Basnet, P.; Mishchenko, E.; Luppi, B.; Škalko-Basnet, N. Utilizing liposomal quercetin and gallic acid in localized treatment of vaginal candida infections. Pharmaceutics 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Gupta, A.; Rotello, V.M. Nanomaterials for the treatment of bacterial biofilms. ACS Infect. Dis. 2016, 2, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, K.K.; Borden, E.; Omtri, R.S.; Boyapati, S.P.; Smith, M.; Lebby, K.; Mulpuru, M.; Gadde, M. Ability of chitosan gels to disrupt bacterial biofilms and their applications in the treatment of bacterial vaginosis. J. Pharm. Sci. 2013, 102, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Agents that inhibit bacterial biofilm formation. Future Med. Chem. 2015, 7, 647–671. [Google Scholar] [CrossRef]
- O’Connell, C.M.; Ferone, M.E. Chlamydia trachomatis genital infections. Microb. Cell 2016, 3, 390–403. [Google Scholar] [CrossRef]



| Formulation | Vesicle Size (nm) | PDI * | Zeta Potential (mV) | Entrapment Efficiency (%) |
|---|---|---|---|---|
| Plain liposomes | 156 ± 21 | 0.073 | −0.56 ± 0.86 | - |
| RES liposomes | 158 ± 22 | 0.077 | −6.72 ± 2.47 | 85 ± 2 |
| RES Formulation | 50 ng/mL | 500 ng/mL | 2500 ng/mL |
|---|---|---|---|
| Control * | 3 ± 3 | 11 ± 2 | 24 ± 1 |
| RES liposomes-in-hydrogel | 15 ± 2 | 37 ± 5 a | 58 ± 4 b |
| Control * in hydrogel | 10 ± 3 | 18 ± 3 a | 51 ± 1 b |
| RES liposomes | 9 ± 2 | 12 ± 2 | 16 ± 6 |
| Plain liposomes-in-hydrogel ** | 17 ± 5 | 21 ± 1 | 46 ± 3 |
| Plain hydrogel ** | 0 ± 1 | 7 ± 4 | 54 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jøraholmen, M.W.; Johannessen, M.; Gravningen, K.; Puolakkainen, M.; Acharya, G.; Basnet, P.; Škalko-Basnet, N. Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection. Pharmaceutics 2020, 12, 1203. https://doi.org/10.3390/pharmaceutics12121203
Jøraholmen MW, Johannessen M, Gravningen K, Puolakkainen M, Acharya G, Basnet P, Škalko-Basnet N. Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection. Pharmaceutics. 2020; 12(12):1203. https://doi.org/10.3390/pharmaceutics12121203
Chicago/Turabian StyleJøraholmen, May Wenche, Mona Johannessen, Kirsten Gravningen, Mirja Puolakkainen, Ganesh Acharya, Purusotam Basnet, and Nataša Škalko-Basnet. 2020. "Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection" Pharmaceutics 12, no. 12: 1203. https://doi.org/10.3390/pharmaceutics12121203
APA StyleJøraholmen, M. W., Johannessen, M., Gravningen, K., Puolakkainen, M., Acharya, G., Basnet, P., & Škalko-Basnet, N. (2020). Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection. Pharmaceutics, 12(12), 1203. https://doi.org/10.3390/pharmaceutics12121203