Are Nutraceuticals Beneficial in Chronic Kidney Disease?
Abstract
1. Introduction
2. Nutraceuticals
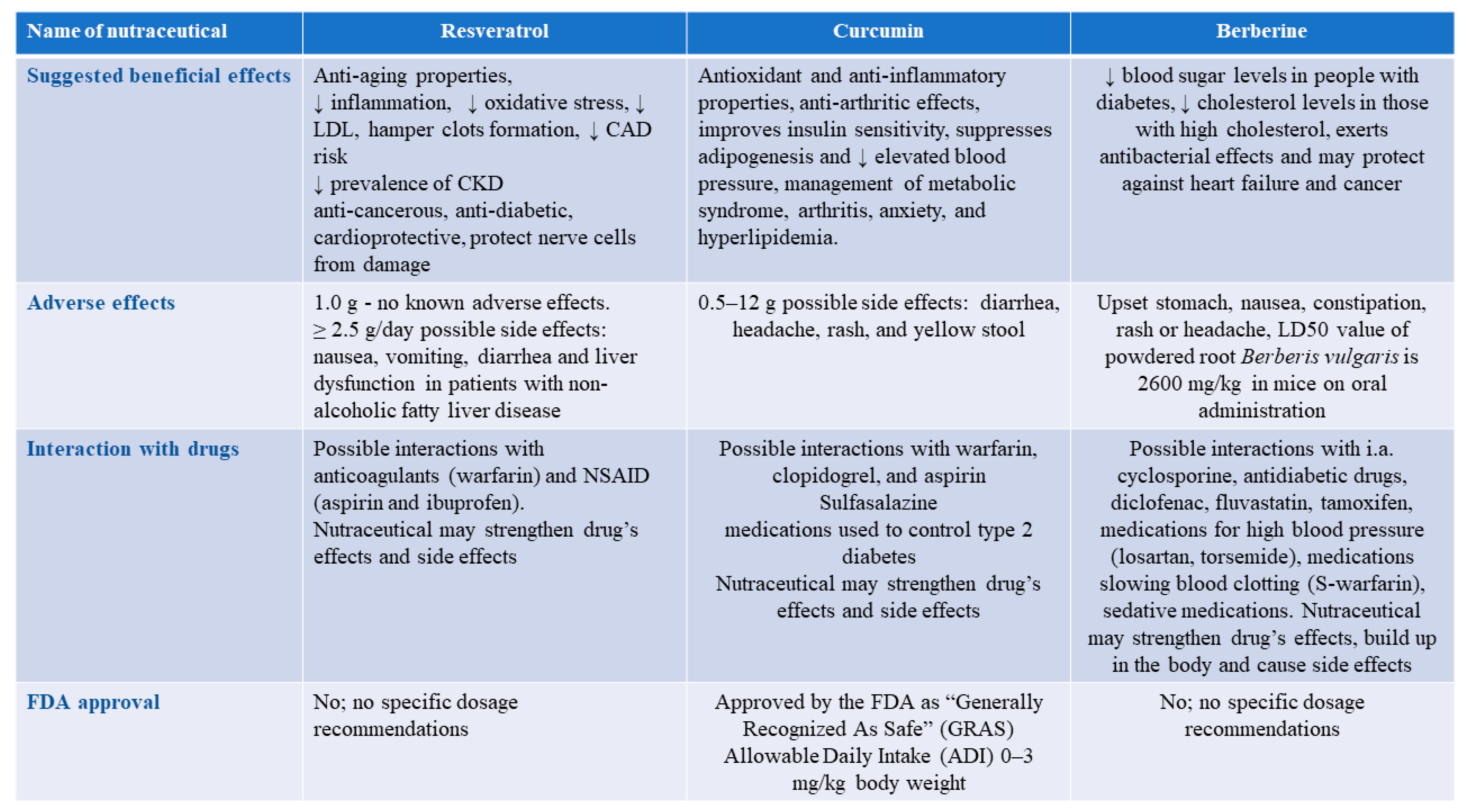
2.1. Polyphenols
2.1.1. Curcumin
2.1.2. Animal Studies
2.1.3. Human Studies
2.1.4. Resveratrol
2.1.5. In Vitro/Animal Studies
2.1.6. Human Studies
2.1.7. Green Tea and Coffee
2.1.8. In Vitro/Animal Studies
2.1.9. Human Studies
2.1.10. Caffeine
2.1.11. Human Studies
2.1.12. Animal Studies
2.2. Vitamin D Supplementation
Human Studies
2.3. Polyunsaturated Fatty Acids (PUFA)
2.3.1. Animal Studies
2.3.2. Human Studies
2.4. Conjugated Linolenic Acid (CLA)
Animal Studies
2.5. Red Yeast Rice and Berberine
Human Studies
2.6. Menaquinone-7 (MK-7)
2.6.1. In Vitro/Animal Studies
2.6.2. Human Studies
3. Combined Use of Nutraceuticals
4. Are Nutraceuticals Always Safe?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. Jama 2007, 298, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Keith, D.S.; Nichols, G.A.; Gullion, C.M.; Brown, J.B.; Smith, D.H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 2004, 164, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Gehr, T.W.; Ghosh, S. Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Sica, D.A.; Kalantar-Zadeh, K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am. J. Nephrol. 2013, 38, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Du, C. Could grape-based food supplements prevent the development of chronic kidney disease? Crit. Rev. Food Sci. Nutr. 2020, 60, 3054–3062. [Google Scholar] [CrossRef]
- Bergeron, R.; Previs, S.F.; Cline, G.W.; Perret, P.; Russell, R.R., 3rd; Young, L.H.; Shulman, G.I. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes 2001, 50, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif. 2001, 19, 53–61. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef]
- Choi, B.H.; Kang, K.S.; Kwak, M.K. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules 2014, 19, 12727–12759. [Google Scholar] [CrossRef]
- Cybulsky, A.V. Growth factor pathways in proliferative glomerulonephritis. Curr. Opin. Nephrol. Hypertens. 2000, 9, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kruse, N.T. Nutraceuticals as a potential adjunct therapy toward improving vascular health in CKD. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R719–R732. [Google Scholar] [CrossRef]
- Ghiadoni, L.; Cupisti, A.; Huang, Y.; Mattei, P.; Cardinal, H.; Favilla, S.; Rindi, P.; Barsotti, G.; Taddei, S.; Salvetti, A. Endothelial dysfunction and oxidative stress in chronic renal failure. J. Nephrol. 2004, 17, 512–519. [Google Scholar]
- Rossi, S.H.; McQuarrie, E.P.; Miller, W.H.; Mackenzie, R.M.; Dymott, J.A.; Moreno, M.U.; Taurino, C.; Miller, A.M.; Neisius, U.; Berg, G.A.; et al. Impaired renal function impacts negatively on vascular stiffness in patients with coronary artery disease. BMC Nephrol. 2013, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Cosola, C.; Sabatino, A.; di Bari, I.; Fiaccadori, E.; Gesualdo, L. Nutrients, nutraceuticals, and xenobiotics affecting renal health. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Ajay, A.K.; Vig, S.; Sabbisetti, V. Mechanism of action of functional lipids and metabolites for patients with chronic kidney disease. Funct. Foods Health Dis. 2019, 9, 412–429. [Google Scholar] [CrossRef]
- Sulaiman, M.K. Diabetic nephropathy: Recent advances in pathophysiology and challenges in dietary management. Diabetol. Metab. Syndr. 2019, 11, 7. [Google Scholar] [CrossRef]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Cosola, C.; Gesualdo, L.; Fiaccadori, E. Intestinal microbiota in type 2 diabetes and chronic kidney disease. Curr. Diab. Rep. 2017, 17, 16. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Uribarri, J.; Castillo, M. Usefulness of dietary components as sustainable nutraceuticals for chronic kidney disease. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Oxford, UK, 2019; pp. 323–331. [Google Scholar] [CrossRef]
- Singh, R.; Geetanjali. Nutraceuticals: Promising health product. Int. Res. J. Med. Sci. 2013, 1, 14–17. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, A.; Mantzioris, E.; Cross, G.; Deo, P.; Garg, S.; Hill, A.M. Nutraceuticals: Reviewing their role in chronic disease prevention and management. Pharmaceut. Med. 2019, 33, 291–309. [Google Scholar] [CrossRef]
- Salis, S. Role of nutraceuticals and probiotics in chronic kidney disease. J. Renal. Nutr. Metab. 2018, 4, 47. [Google Scholar] [CrossRef]
- Burnett, A.J.; Livingstone, K.M.; Woods, J.L.; McNaughton, S.A. Dietary supplement use among Australian adults: Findings from the 2011–2012 national nutrition and physical activity survey. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z. Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: A perspective on plant biotechnology application. Recent Pat. Biotechnol. 2007, 1, 75–97. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Concepts and strategy of functional food science: The European perspective. Am. J. Clin. Nutr. 2000, 71, 1660S–1664S. [Google Scholar] [CrossRef]
- German, J.B.; Walzem, R.L. The health benefits of wine. Ann. Rev. Nutr. 2000, 20, 561–593. [Google Scholar] [CrossRef]
- Găman, M.-A.; Epingeac, M.; Diaconu, C.; Găman, A. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J. Diabet. 2020, 11, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; LaRocca, T.J.; Martens, C.R.; Seals, D.R. Healthy lifestyle-based approaches for successful vascular aging. J. Appl. Physiol. 2018, 125, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.; Esmail, R.; Donya, S.M. Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods. Asian Pac. J. Trop. Biomed. 2014, 4, 618–627. [Google Scholar] [CrossRef]
- Costa, R.M.; Magalhães, A.S.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M.; Silva, B.M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: A comparative study with green tea (Camellia sinensis). Food Chem. Toxicol. 2009, 47, 860–865. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2012, 70, 234–240. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Almomen, S.M.; Guan, Q.; Liang, P.; Yang, K.; Sidiqi, A.M.; Levin, A.; Du, C. Daily intake of grape powder prevents the progression of kidney disease in obese type 2 diabetic ZSF1 rats. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Luciano, R.L. Acute kidney injury from cherry concentrate in a patient with CKD. Am. J. Kidney Dis. 2014, 63, 503–505. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef]
- Akazawa, N.; Choi, Y.; Miyaki, A.; Tanabe, Y.; Sugawara, J.; Ajisaka, R.; Maeda, S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr. Res. 2012, 32, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Draijer, R.; de Graaf, Y.; Slettenaar, M.; de Groot, E.; Wright, C.I. Consumption of a polyphenol-rich grape-wine extract lowers ambulatory blood pressure in mildly hypertensive subjects. Nutrients 2015, 7, 3138–3153. [Google Scholar] [CrossRef]
- Nardini, M.; Natella, F.; Scaccini, C. Role of dietary polyphenols in platelet aggregation. A review of the supplementation studies. Platelets 2007, 18, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Reuland, D.J.; McCord, J.M.; Hamilton, K.L. The role of Nrf2 in the attenuation of cardiovascular disease. Exerc. Sport Sci. Rev. 2013, 41, 162–168. [Google Scholar] [CrossRef]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Caliceti, C.; Fogacci, F.; Giovannini, M.; Calabria, D.; Colletti, A.; Veronesi, M.; Roda, A.; Borghi, C. Effect of apple polyphenols on vascular oxidative stress and endothelium function: A translational study. Mol. Nutr. Food Res. 2017, 61, 1700373. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary antioxidant supplements and uric acid in chronic kidney disease: A review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef]
- Gliozzi, M.; Malara, N.; Muscoli, S.; Mollace, V. The treatment of hyperuricemia. Int. J. Cardiol. 2016, 213, 23–27. [Google Scholar] [CrossRef]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Liang, L.; Liu, Q.; Duan, W.; Jiang, Y.; Zhang, L. Autophagy is a major mechanism for the dual effects of curcumin on renal cell carcinoma cells. Eur. J. Pharmacol. 2018, 826, 24–30. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. Biofactors 2013, 39, 14–20. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Hernández-Reséndiz, S.; Correa, F.; García-Niño, W.R.; Buelna-Chontal, M.; Roldán, F.J.; Ramírez-Camacho, I.; Delgado-Toral, C.; Carbó, R.; Pedraza-Chaverrí, J.; Tapia, E.; et al. Cardioprotection by curcumin post-treatment in rats with established chronic kidney disease. Cardiovasc. Drugs Ther. 2015, 29, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverri, J.; et al. The effect of dietary supplementation with curcumin on redox status and nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: A pilot study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.; Lapierre, C.; Cruz-Correa, M.; Muruve, N.; Rosario, R.; Fromkin, B.; Braun, M.; Copley, J. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: A randomized placebo controlled trial. Transplantation 2005, 80, 1556–1559. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, H.; Peng, H.; Huang, F.; Zhong, J.; Zhou, J. Molecular mechanisms of curcumin renoprotection in experimental acute renal injury. Front. Pharmacol. 2017, 8, 912. [Google Scholar] [CrossRef]
- Sun, P.P.; Perianayagam, M.C.; Jaber, B.L. Endotoxin-binding affinity of sevelamer: A potential novel anti-inflammatory mechanism. Kidney Int. Suppl. 2009, S20–S25. [Google Scholar] [CrossRef]
- Bentala, H.; Verweij, W.R.; Huizinga-Van der Vlag, A.; van Loenen-Weemaes, A.M.; Meijer, D.K.; Poelstra, K. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock 2002, 18, 561–566. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Salloum, F.N.; Abbate, A.; Krieg, R.; Sica, D.A.; Gehr, T.W.; Kukreja, R.C. Curcumin prevents cardiac remodeling secondary to chronic renal failure through deactivation of hypertrophic signaling in rats. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H975–H984. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Krieg, R.; Massey, H.D.; Sica, D.A.; Fakhry, I.; Ghosh, S.; Gehr, T.W. Curcumin and enalapril ameliorate renal failure by antagonizing inflammation in 5/6 nephrectomized rats: Role of phospholipase and cyclooxygenase. Am. J. Physiol. Renal. Physiol. 2012, 302, F439–F454. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijón, E.; Medina-Campos, O.N.; Macías-Ruvalcaba, N.A.; León-Contreras, J.C.; Hernández-Pando, R.; García-Arroyo, F.E.; Cristóbal, M.; Sánchez-Lozada, L.G.; et al. Curcumin prevents mitochondrial dynamics disturbances in early 5/6 nephrectomy: Relation to oxidative stress and mitochondrial bioenergetics. Biofactors 2017, 43, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Chaves, L.; Eadon, M.T.; Chang, A.; Quigg, R.J.; Alexander, J.J. Curcumin alleviates immune-complex-mediated glomerulonephritis in factor-H-deficient mice. Immunology 2013, 139, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, J.J.; Bowden, R.G.; Deike, E.; Griggs, J.; Wilson, R.; Shelmadine, B.; Cooke, M.; Beaujean, A. The use of an anti-inflammatory supplement in patients with chronic kidney disease. J. Complement. Integr. Med. 2013, 10. [Google Scholar] [CrossRef]
- Ye, J. Regulation of PPARgamma function by TNF-alpha. Biochem. Biophys. Res. Commun. 2008, 374, 405–408. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Massey, H.D.; Krieg, R.; Fazelbhoy, Z.A.; Ghosh, S.; Sica, D.A.; Fakhry, I.; Gehr, T.W. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: Role of inflammation. Am. J. Physiol. Renal. Physiol. 2009, 296, F1146–F1157. [Google Scholar] [CrossRef]
- Shin, S.K.; Ha, T.Y.; McGregor, R.A.; Choi, M.S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011, 55, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, C.; Sun, H.; Luo, T.; Tan, Y.; Tian, D.; Guo, Z. Curcumin inhibits monocyte chemoattractant protein-1 expression and enhances cholesterol efflux by suppressing the c-Jun N-terminal kinase pathway in macrophage. Inflamm. Res. 2014, 63, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.T.; Zingg, J.M.; Kwan, P.; Noble, T.; Smith, D.; Meydani, M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis 2014, 232, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice--role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaei, Z.M.; Mohammad, T.U.; Ali, L.K. Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak. J. Biol. Sci. 2014, 17, 1237–1241. [Google Scholar] [CrossRef]
- Kon, V.; Linton, M.F.; Fazio, S. Atherosclerosis in chronic kidney disease: The role of macrophages. Nat. Rev. Nephrol. 2011, 7, 45–54. [Google Scholar] [CrossRef]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Maccarrone, M.; Taccone-Gallucci, M.; Finazzi-Agrò, A. 5-Lipoxygenase-mediated mitochondrial damage and apoptosis of mononuclear cells in ESRD patients. Kidney Int. Suppl. 2003, S33–S36. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant supplementation in renal replacement therapy patients: Is there evidence? Oxid. Med. Cell Longev. 2019, 2019, 9109473. [Google Scholar] [CrossRef]
- Jhun, J.; Min, H.-K.; Na, H.S.; Kwon, J.y.; Ryu, J.; Cho, K.-H.; Choi, J.; Jung, K.; Lee, S.-Y.; Kim, S.J.; et al. Combinatmarion treatment with Lactobacillus acidophilus LA-1, vitamin B, and curcumin ameliorates the progression of osteoarthritis by inhibiting the pro-inflammatory mediators. Immunol. Lett. 2020, 228, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 2010, 12, R127. [Google Scholar] [CrossRef]
- Todhunter, P.G.; Kincaid, S.A.; Todhunter, R.J.; Kammermann, J.R.; Johnstone, B.; Baird, A.N.; Hanson, R.R.; Wright, J.M.; Lin, H.C.; Purohit, R.C. Immunohistochemical analysis of an equine model of synovitis-induced arthritis. Am. J. Vet. Res. 1996, 57, 1080–1093. [Google Scholar] [PubMed]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Shakibaei, M.; Schulze-Tanzil, G.; John, T.; Mobasheri, A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: An immunomorphological study. Ann. Anat. 2005, 187, 487–497. [Google Scholar] [CrossRef]
- Gaedeke, J.; Noble, N.A.; Border, W.A. Curcumin blocks multiple sites of the TGF-beta signaling cascade in renal cells. Kidney Int. 2004, 66, 112–120. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, H.; Du, Y.; Zhu, Y.; Wang, X. Curcumin inhibits transforming growth factor-beta activity via inhibition of Smad signaling in HK-2 cells. Am. J. Nephrol. 2010, 31, 332–341. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, S.; Mao, X.; Wang, W.; Tai, H. Inhibition effect of curcumin on TNF-α and MMP-13 expression induced by advanced glycation end products in chondrocytes. Pharmacology 2013, 91, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Cao, Y.; Huang, T.; Song, D.X.; Tao, H.R. Therapeutic potential of hyaluronic acid/chitosan nanoparticles for the delivery of curcuminoid in knee osteoarthritis and an in vitro evaluation in chondrocytes. Int. J. Mol. Med. 2018, 42, 2604–2614. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Y.; Li, C.; Yan, Y.; Ao, L.; Yu, B.; Fang, W.; Liu, J.; Li, Y. Levo-corydalmine alleviates vincristine-induced neuropathic pain in mice by inhibiting an NF-kappa B-dependent CXCL1/CXCR2 signaling pathway. Neuropharmacology 2018, 135, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: Role of NF-κB inhibition. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1694–H1699. [Google Scholar] [CrossRef]
- Li, J.Y.; Huang, W.Q.; Tu, R.H.; Zhong, G.Q.; Luo, B.B.; He, Y. Retraction Note: Resveratrol rescues hyperglycemia-induced endothelial dysfunction via activation of Akt. Acta Pharmacol. Sin. 2020, 41, 1262. [Google Scholar] [CrossRef]
- Yousuf, S.; Atif, F.; Ahmad, M.; Hoda, N.; Ishrat, T.; Khan, B.; Islam, F. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009, 1250, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Ray, P.S.; Maulik, G.; Maulik, N.; Engelman, R.M.; Bertelli, A.A.; Bertelli, A.; Das, D.K. Myocardial protection with red wine extract. J. Cardiovasc. Pharmacol. 2000, 35, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Förstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Förstermann, U.; Li, H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric. Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, D.R.; Terzibasi, E.; Genade, T.; Cattaneo, A.; Domenici, L.; Cellerino, A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006, 16, 296–300. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Habermeier, A.; Closs, E.I.; Thum, T.; Spanier, G.; Lu, Q.; Oelze, M.; Torzewski, M.; Lackner, K.J.; et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther. 2010, 335, 149–154. [Google Scholar] [CrossRef]
- Ferrero, M.E.; Bertelli, A.E.; Fulgenzi, A.; Pellegatta, F.; Corsi, M.M.; Bonfrate, M.; Ferrara, F.; de Caterina, R.; Giovannini, L.; Bertelli, A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am. J. Clin. Nutr. 1998, 68, 1208–1214. [Google Scholar] [CrossRef]
- Leikert, J.F.; Räthel, T.R.; Wohlfart, P.; Cheynier, V.; Vollmar, A.M.; Dirsch, V.M. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 2002, 106, 1614–1617. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef]
- Gracia-Sancho, J.; Villarreal, G., Jr.; Zhang, Y.; García-Cardeña, G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 2010, 85, 514–519. [Google Scholar] [CrossRef]
- Hammad, A.S.A.; Ahmed, A.F.; Heeba, G.H.; Taye, A. Heme oxygenase-1 contributes to the protective effect of resveratrol against endothelial dysfunction in STZ-induced diabetes in rats. Life Sci. 2019, 239, 117065. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [PubMed]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.X.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology 2017, 153, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Busch, F.; Shayan, P.; Shakibaei, M. Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells. J. Biol. Chem. 2014, 289, 22048–22062. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2alpha. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Dvir-Ginzberg, M.; Mobasheri, A.; Kumar, A. The role of sirtuins in cartilage homeostasis and osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 43. [Google Scholar] [CrossRef]
- Buhrmann, C.; Popper, B.; Aggarwal, B.B.; Shakibaei, M. Resveratrol downregulates inflammatory pathway activated by lymphotoxin α (TNF-β) in articular chondrocytes: Comparison with TNF-α. PLoS ONE 2017, 12, e0186993. [Google Scholar] [CrossRef]
- Limagne, E.; Lançon, A.; Delmas, D.; Cherkaoui-Malki, M.; Latruffe, N. Resveratrol interferes with IL1-β-induced pro-inflammatory paracrine interaction between primary chondrocytes and macrophages. Nutrients 2016, 8. [Google Scholar] [CrossRef]
- Jespersen, T.; Kruse, N.; Mehta, T.; Kuwabara, M.; Noureddine, L.; Jalal, D. Light wine consumption is associated with a lower odd for cardiovascular disease in chronic kidney disease. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Janecka, A.; Gorlach, S. Overview of metabolism and bioavailability enhancement of polyphenols. J. Agric. Food Chem. 2013, 61, 12183–12199. [Google Scholar] [CrossRef] [PubMed]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef]
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013, 32, 894–903. [Google Scholar] [CrossRef]
- Riegsecker, S.; Wiczynski, D.; Kaplan, M.J.; Ahmed, S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013, 93, 307–312. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef]
- Chen, W.C.; Hayakawa, S.; Shimizu, K.; Chien, C.T.; Lai, M.K. Catechins prevents substance P-induced hyperactive bladder in rats via the downregulation of ICAM and ROS. Neurosci. Lett. 2004, 367, 213–217. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-gallate attenuates oxidative stress and inflammation in obstructive nephropathy via NF-κB and Nrf2/HO-1 signalling pathway regulation. Basic Clin. Pharmacol. Toxicol. 2015, 117, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Wongmekiat, O.; Peerapanyasut, W.; Kobroob, A. Catechin supplementation prevents kidney damage in rats repeatedly exposed to cadmium through mitochondrial protection. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 385–394. [Google Scholar] [CrossRef]
- Hsu, S.P.; Wu, M.S.; Yang, C.C.; Huang, K.C.; Liou, S.Y.; Hsu, S.M.; Chien, C.T. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am. J. Clin. Nutr. 2007, 86, 1539–1547. [Google Scholar] [CrossRef]
- Park, C.S.; Kim, W.; Woo, J.S.; Ha, S.J.; Kang, W.Y.; Hwang, S.H.; Park, Y.W.; Kim, Y.S.; Ahn, Y.K.; Jeong, M.H.; et al. Green tea consumption improves endothelial function but not circulating endothelial progenitor cells in patients with chronic renal failure. Int. J. Cardiol. 2010, 145, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, D.; Su, B.; Wei, J.; Găman, M.-A.; Sedanur Macit, M.; Borges do Nascimento, I.J.; Guimaraes, N.S. The effect of green tea supplementation on obesity: A systematic review and dose–response meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Jhee, J.H.; Nam, K.H.; An, S.Y.; Cha, M.U.; Lee, M.; Park, S.; Kim, H.; Yun, H.R.; Kee, Y.K.; Park, J.T.; et al. Effects of coffee intake on incident chronic kidney disease: A community-based prospective cohort study. Am. J. Med. 2018, 131, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Herber-Gast, G.C.; van Essen, H.; Verschuren, W.M.; Stehouwer, C.D.; Gansevoort, R.T.; Bakker, S.J.; Spijkerman, A.M. Coffee and tea consumption in relation to estimated glomerular filtration rate: Results from the population-based longitudinal doetinchem cohort study. Am. J. Clin. Nutr. 2016, 103, 1370–1377. [Google Scholar] [CrossRef]
- Tofovic, S.P.; Jackson, E.K. Effects of long-term caffeine consumption on renal function in spontaneously hypertensive heart failure prone rats. J. Cardiovasc. Pharmacol. 1999, 33, 360–366. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Eng. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D., and VDR activation. Kidney Int. Suppl. 2011, 1, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontán, F.; Gonzales-Candia, B.; Luna, E.; Caravaca, F. Relative importance of the determinants of serum levels of 25-hydroxy vitamin D in patients with chronic kidney disease. Nefrología 2016, 36, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.P.; Pereira, L.A.L. Native vitamin D in pre-dialysis chronic kidney disease. Nefrología 2019, 39, 18–28. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Schold, J.D.; Arrigain, S.; Jolly, S.E.; Jain, A.; Schreiber, M.J.; Simon, J.F.; Srinivas, T.R.; Nally, J.V. Low 25-hydroxyvitamin d levels and mortality in non–dialysis-dependent CKD. Am. J. Kidney Dis. 2011, 58, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Ravani, P.; Malberti, F.; Tripepi, G.; Pecchini, P.; Cutrupi, S.; Pizzini, P.; Mallamaci, F.; Zoccali, C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009, 75, 88–95. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD update work group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [CrossRef]
- Sprague, S.M.; Silva, A.L.; Al-Saghir, F.; Damle, R.; Tabash, S.P.; Petkovich, M.; Messner, E.J.; White, J.A.; Melnick, J.Z.; Bishop, C.W. Modified-release calcifediol effectively controls secondary hyperparathyroidism associated with vitamin D insufficiency in chronic kidney disease. Am. J. Nephrol. 2014, 40, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Sprague, S.M.; Crawford, P.W.; Melnick, J.Z.; Strugnell, S.A.; Ali, S.; Mangoo-Karim, R.; Lee, S.; Petkovich, P.M.; Bishop, C.W. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am. J. Nephrol. 2016, 44, 316–325. [Google Scholar] [CrossRef]
- Pilz, S.; Iodice, S.; Zittermann, A.; Grant, W.B.; Gandini, S. Vitamin D status and mortality risk in CKD: A meta-analysis of prospective studies. Am. J. Kidney Dis. 2011, 58, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Căpuşa, C.; Stefan, G.; Stancu, S.; Ilyes, A.; Dorobanţu, N.; Mircescu, G. Subclinical cardiovascular disease markers and vitamin D deficiency in non-dialysis chronic kidney disease patients. Arch. Med. Sci. 2016, 12, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Rodriguez-Ortiz, M.E.; Duny, Y.; Rodriguez, M.; Daurès, J.P.; Argilés, A. Vitamin D treatment and mortality in chronic kidney disease: A systematic review and meta-analysis. Am. J. Nephrol. 2013, 37, 239–248. [Google Scholar] [CrossRef]
- Selamet, U.; Katz, R.; Ginsberg, C.; Rifkin, D.E.; Fried, L.F.; Kritchevsky, S.B.; Hoofnagle, A.N.; Bibbins-Domingo, K.; Drew, D.; Harris, T.; et al. Serum calcitriol concentrations and kidney function decline, heart failure, and mortality in elderly community-living adults: The health, aging, and body composition study. Am. J. Kidney Dis. 2018, 72, 419–428. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, A.K.; Lal, A.; Kumar, V.; Singhal, M.; Billot, L.; Gupta, K.L.; Banerjee, D.; Jha, V. A Randomized trial of vitamin d supplementation on vascular function in CKD. J. Am. Soc. Nephrol. 2017, 28, 3100–3108. [Google Scholar] [CrossRef]
- Aytaç, M.B.; Deveci, M.; Bek, K.; Kayabey, Ö.; Ekinci, Z. Effect of cholecalciferol on local arterial stiffness and endothelial dysfunction in children with chronic kidney disease. Pediatr. Nephrol. 2016, 31, 267–277. [Google Scholar] [CrossRef]
- Thadhani, R.; Appelbaum, E.; Pritchett, Y.; Chang, Y.; Wenger, J.; Tamez, H.; Bhan, I.; Agarwal, R.; Zoccali, C.; Wanner, C.; et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The PRIMO randomized controlled trial. Jama 2012, 307, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Fang, F.; Chan, J.; Wen, Y.Y.; Qing, S.; Chan, I.H.; Lo, G.; Lai, K.N.; Lo, W.K.; Lam, C.W.; et al. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J. Am. Soc. Nephrol. 2014, 25, 175–186. [Google Scholar] [CrossRef]
- Levin, G.P.; Robinson-Cohen, C.; de Boer, I.H.; Houston, D.K.; Lohman, K.; Liu, Y.; Kritchevsky, S.B.; Cauley, J.A.; Tanaka, T.; Ferrucci, L.; et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. Jama 2012, 308, 1898–1905. [Google Scholar] [CrossRef]
- Jean, G.; Souberbielle, J.C.; Chazot, C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Virani, S.S. Omega-3 Fatty Acids (Fish Oil) supplementation and albuminuria: Not a slam dunk. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, T.; Kondo, H.; Nii, H.; Kimura, K.; Egami, Y.; Oka, Y.; Yoshida, M.; Kida, E.; Ye, Y.; Akahoshi, S.; et al. Furan fatty acid as an anti-inflammatory component from the green-lipped mussel Perna canaliculus. Proc. Natl. Acad. Sci. USA 2011, 108, 17533–17537. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Aguila, M.B.; Pinheiro, A.R.; Aquino, J.C.; Gomes, A.P.; Mandarim-de-Lacerda, C.A. Different edible oil beneficial effects (canola oil, fish oil, palm oil, olive oil, and soybean oil) on spontaneously hypertensive rat glomerular enlargement and glomeruli number. Prostaglandins Other Lipid Mediat. 2005, 76, 74–85. [Google Scholar] [CrossRef]
- Yokoyama, M.; Tanigawa, K.; Murata, T.; Kobayashi, Y.; Tada, E.; Suzuki, I.; Nakabou, Y.; Kuwahata, M.; Kido, Y. Dietary polyunsaturated fatty acids slow the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Nutr. Res. 2010, 30, 217–225. [Google Scholar] [CrossRef]
- Zanetti, M.; Gortan Cappellari, G.; Barbetta, D.; Semolic, A.; Barazzoni, R. Omega 3 polyunsaturated fatty acids improve endothelial dysfunction in chronic renal failure: Role of eNOS activation and of oxidative stress. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Saglimbene, V.M.; Wong, G.; van Zwieten, A.; Palmer, S.C.; Ruospo, M.; Natale, P.; Campbell, K.; Teixeira-Pinto, A.; Craig, J.C.; Strippoli, G.F.M. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Baggio, B.; Musacchio, E.; Priante, G. Polyunsaturated fatty acids and renal fibrosis: Pathophysiologic link and potential clinical implications. J. Nephrol. 2005, 18, 362–367. [Google Scholar] [PubMed]
- Lee, C.C.; Sharp, S.J.; Wexler, D.J.; Adler, A.I. Dietary intake of eicosapentaenoic and docosahexaenoic acid and diabetic nephropathy: Cohort analysis of the diabetes control and complications trial. Diabetes Care 2010, 33, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Bowden, R.G.; Jitomir, J.; Wilson, R.L.; Gentile, M. Effects of omega-3 fatty acid supplementation on lipid levels in end-stage renal disease patients. J. Ren. Nutr. 2009, 19, 259–266. [Google Scholar] [CrossRef]
- Svensson, M.; Schmidt, E.B.; Jørgensen, K.A.; Christensen, J.H. The effect of n-3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: A randomized placebo-controlled intervention study. Nephrol. Dial Transplant. 2008, 23, 2918–2924. [Google Scholar] [CrossRef]
- Vernaglione, L.; Cristofano, C.; Chimienti, S. Omega-3 polyunsaturated fatty acids and proxies of cardiovascular disease in hemodialysis: A prospective cohort study. J. Nephrol. 2008, 21, 99–105. [Google Scholar]
- Ogawa, J.; Kishino, S.; Ando, A.; Sugimoto, S.; Mihara, K.; Shimizu, S. Production of conjugated fatty acids by lactic acid bacteria. J. Biosci. Bioeng. 2005, 100, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Sinclair, A.J.; Xu, C.; Li, D. Incorporation and metabolism of punicic acid in healthy young humans. Mol. Nutr. Food Res. 2009, 53, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Ogborn, M.R.; Nitschmann, E.; Bankovic-Calic, N.; Weiler, H.A.; Fitzpatrick-Wong, S.; Aukema, H.M. Dietary conjugated linoleic acid reduces PGE2 release and interstitial injury in rat polycystic kidney disease. Kid. Int. 2003, 64, 1214–1221. [Google Scholar] [CrossRef]
- Cicero, A. Effect of a lipid-lowering nutraceutical on pulse-wave-velocity in hypercholesterolemic patients with or without chronic kidney disease. Open Hypertens. J. 2013, 5, 18–22. [Google Scholar] [CrossRef]
- Cicero, A.F.; Tartagni, E.; Borghi, C. Nutraceuticals with lipid-lowering activity: Do they have any effect beyond cholesterol reduction? Clin. Lipidol. 2012, 7, 549–559. [Google Scholar] [CrossRef]
- Affuso, F.; Ruvolo, A.; Micillo, F.; Saccà, L.; Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 656–661. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ghanbari, N.; shekari, M.; Reiner, Ž.; Amirani, E.; Hallajzadeh, J.; Mirsafaei, L.; Asemi, Z. The effect of berberine supplementation on obesity parameters, inflammation and liver function enzymes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Crepaldi, G.; Maggi, S.; Galli, F.; D’Angelo, A.; Calò, L.; Giannini, S.; Miozzo, D.; Gallieni, M. Vitamin K, bone fractures, and vascular calcifications in chronic kidney disease: An important but poorly studied relationship. J. Endocrinol. Investig. 2010, 34, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Yasufumi, N.; Yusuke, H.; Fumiaki, I.; Toshio, K. Effect of simvastatin (MK-733) on the regulation of cholesterol synthesis in Hep G2 cells. Biochem. Pharmacol. 1990, 40, 843–850. [Google Scholar] [CrossRef]
- Lupo, M.G.; Biancorosso, N.; Brilli, E.; Tarantino, G.; Adorni, M.P.; Vivian, G.; Salvalaio, M.; Dall’Acqua, S.; Sut, S.; Neutel, C.; et al. Cholesterol-lowering action of a novel nutraceutical combination in uremic rats: Insights into the molecular mechanism in a hepatoma cell line. Nutrients 2020, 12, 436. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.; van der Meer, I.M.; Hofman, A.; Witteman, J.C. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef]
- Keyzer, C.A.; Vermeer, C.; Joosten, M.M.; Knapen, M.H.; Drummen, N.E.; Navis, G.; Bakker, S.J.; de Borst, M.H. Vitamin K status and mortality after kidney transplantation: A cohort study. Am. J. Kidney Dis. 2015, 65, 474–483. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3–5. Pol. Arch. Med. Wewn. 2015, 125, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Galassi, A.; Ciceri, P.; Messa, P.; Nigwekar, S. Vitamin K in chronic kidney disease. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Ferroni, A.; Ertek, S. Tolerability and safety of commonly used dietary supplements and nutraceuticals with lipid-lowering effects. Expert Opin. Drug Saf. 2012, 11, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Leena, M.M.; Silvia, M.G.; Vinitha, K.; Moses, J.A.; Anandharamakrishnan, C. Synergistic potential of nutraceuticals: Mechanisms and prospects for futuristic medicine. Food Funct. 2020, 11, 9317–9337. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug. Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Rather, M.A.; Bhat, B.A.; Qurishi, M.A. Multicomponent phytotherapeutic approach gaining momentum: Is the “one drug to fit all” model breaking down? Phytomedicine 2013, 21, 1–14. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Mazzoli, S.; Bechi, A.; Addonisio, P.; Mondaini, N.; Pagliai, R.C.; Bartoletti, R. Serenoa repens associated with Urtica dioica (ProstaMEV) and curcumin and quercitin (FlogMEV) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: Results from a prospective randomised study. Int. J. Antimicrob. Agents 2009, 33, 549–553. [Google Scholar] [CrossRef]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ novel formulations: The good, the bad, the unknown and patents involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef] [PubMed]
- Nounou, M.I.; Ko, Y.; Helal, N.A.; Boltz, J.F. Adulteration and counterfeiting of online nutraceutical formulations in the United States: Time for intervention? J. Diet Suppl. 2018, 15, 789–804. [Google Scholar] [CrossRef]
- Asghar, A.; Randhawa, M.A.; Masood, M.M.; Abdullah, M.; Irshad, M.A. Chapter 10-nutraceutical formulation strategies to enhance the bioavailability and efficiency: An overview. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Waltham, MA, USA, 2018; pp. 329–352. [Google Scholar] [CrossRef]
- Schmitt, J.; Ferro, A. Nutraceuticals: Is there good science behind the hype? Br. J. Clin. Pharmacol. 2013, 75, 585–587. [Google Scholar] [CrossRef]
- Gil, F.; Hernández, A.F.; Martín-Domingo, M.C. Chapter 58-toxic contamination of nutraceuticals and food ingredients. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 825–837. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- NKF. Use of Herbal Supplements in Chronic Kidney Disease. Available online: https://kidneyhi.org/use-of-herbal-supplements-in-chronic-kidney-disease (accessed on 26 January 2021).
- Yu, J.; Zhou, Z.; Tay-Sontheimer, J.; Levy, R.H.; Ragueneau-Majlessi, I. Intestinal drug interactions mediated by OATPs: A systematic review of preclinical and clinical findings. J. Pharm. Sci. 2017, 106, 2312–2325. [Google Scholar] [CrossRef]
- Diamond, B.J.; Bailey, M.R. Ginkgo biloba: Indications, mechanisms, and safety. Psychiatr. Clin. N. Am. 2013, 36, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Rider, C.V.; Nyska, A.; Cora, M.C.; Kissling, G.E.; Smith, C.; Travlos, G.S.; Hejtmancik, M.R.; Fomby, L.M.; Colleton, C.A.; Ryan, M.J.; et al. Toxicity and carcinogenicity studies of Ginkgo biloba extract in rat and mouse: Liver, thyroid, and nose are targets. Toxicol. Pathol. 2014, 42, 830–843. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Kochi, T.; Ideta, T.; Miyazaki, T.; Moriwaki, H. Chemopreventive potential of green tea catechins in hepatocellular carcinoma. Int. J. Mol. Sci. 2015, 16, 6124–6139. [Google Scholar] [CrossRef]
- Gupta, R.C.; Srivastava, A.; Lall, R. Toxicity potential of nutraceuticals. Methods Mol. Biol. 2018, 1800, 367–394. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.; Attias, S.; Ben-Arye, E.; Goldstein, L.; Schiff, E. Adverse events associated with interactions with dietary and herbal supplements among inpatients. Br. J. Clin. Pharmacol. 2017, 83, 836–845. [Google Scholar] [CrossRef]
- Mouly, S.; Lloret-Linares, C.; Sellier, P.O.; Sene, D.; Bergmann, J.F. Is the clinical relevance of drug-food and drug-herb interactions limited to grapefruit juice and Saint-John’s Wort? Pharmacol. Res. 2017, 118, 82–92. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed]
| Type of Study/Study Group | Nutraceutical/Dose | Effects of Nutraceutical Administration | Ref |
|---|---|---|---|
| Animal Studies | |||
| Curcumin | |||
| Nephrectomized rats (5/6Nx) | Curcumin (120 mg/kg/day) dissolved in 0.05% CMC via oral gavages during 30 days |
| [59] |
| 5/6 nephrectomized (Nx) rats | Curcumin (75 mg/kg) and enalapril (10 mg/kg), 10 weeks |
| [66] |
| Sprague-Dawley rats with 5/6 nephrectomy (Nx) induced CRF/in vitro (mesangial cells) | Untreated (Nx), curcumin-treated (curcumin), and enalapril-treated (enalapril) groups |
| [71] |
| 5/6 nephrectomized (Nx) Sprague-Dawley rats | (1) control (sham), (2) Nx, (3) Nx + curcumin (150 mg/kg bid), and (4) Nx + enalapril (15 mg/kg bid) as positive control, 7 weeks |
| [65] |
| Apoferritin-induced CSS model in Cfh-deficient (Cfh(-/-)) mice | Curcumin treatment (30 mg/kg) given every day |
| [68] |
| Resveratrol | |||
| Streptozotocin-diabetic rats | RSV (10 mg/kg) in presence or absence of an HO-1 blocker, Zinc protoporphyrin (ZnPP). |
| [108] |
| Female C57BL/6J mice and ApoE−/− mice with a C57BL/6 genetic background | Resveratrol |
| [109] |
| Green Tea and Coffee | |||
| Anesthetized rat bladder | Green tea extract (catechins), 2 weeks |
| [122] |
| Unilateral ureteral obstruction (UUO) mice model | Epigallocatechin-3-gallate (EGCG) (50 mg/kg/day), 2 weeks |
| [123] |
| Male Wistar rats | Cadmium (CdCl2 2 mg/kg, i.p.) and cadmium plus catechin (25, 50, and 100 mg/kg, orally), 4 weeks |
| [124] |
| Polyunsaturated fatty acids (PUFA) | |||
| 5/6 nephrectomized male Wistar rats (CKD) and sham operated animals (SHAM) | n-3 PUFA enriched diet (CKD + PUFA, n = 10) vs. standard diet, 6 weeks. |
| [155] |
| Conjugated Linolenic Acid (CLA) | |||
| Han:SPRD-cy rat model of polycystic kidney disease (PKD), heterozygotes | Diets containing corn oil with a CLA enriched oil (1% of diet by weight as CLA) vs. corn oil (control), 8 weeks |
| [165] |
| Human Trials | |||
| Curcumin | |||
| Randomized double-blind placebo-controlled clinical trial of 101 Mexican patients with nondiabetic or diabetic proteinuric CKD | Placebo or 320 mg/day curcumin for 8 weeks |
| [60] |
| 43 dialysis dependent cadaveric kidney recipients | 480 mg of curcumin and 20 mg of quercetin. Control (placebo), low dose (one capsule, one placebo), and high dose (two capsules). |
| [61] |
| 16 patients with CKD | Herbal supplement composed of Curcuma longa and Boswellia serrata, or placebo. |
| [69] |
| A randomized, double-blind, and placebo-controlled study of 40 patients with overt type 2 diabetic nephropathy | Study group: 3 capsules (daily) with 500 mg turmeric (22.1 mg of active curcumin) for 2 months.Control group: placebo |
| [78] |
| Resveratrol | |||
| Cross-sectional logistic regression of National Health and Nutrition Examination Survey (NHANES) | Wine. No consumption (0 glass per day), light (<1 glass per day), moderate (≥1 glasses per day). |
| [116] |
| Green Tea and Coffee | |||
| 6 healthy subjects and 54 hemodialysis patients | Three different doses (0, 455, and 910 mg) of oral catechins vs. oral vitamin C (500 mg) |
| [125] |
| 40 patients with CKD requiring chronic dialysis | Catechin group: green tea (5 g/day for 1 month). Control group: water |
| [126] |
| 8717 subjects with normal renal function | 0 coffee per week, <1 cup per week, 1–6 cups per week, 1 cup per day, and ≥2 cups per day |
| [128] |
| 4722 participants from the Doetinchem Cohort Study | Coffee and tea consumption (in cups/day) |
| [129] |
| Vitamin D Supplementation | |||
| Randomized, double-blind, placebo-controlled trial of 78 CKD subjects with iPTH >70 pg/mL and serum total 25-hydroxyvitamin D <30 ng/mL. | Daily treatment for six weeks with oral MR calcifediol (30, 60, or 90 µg) or a placebo |
| [138] |
| A systematic review and meta-analysis, 14 observational studies (194,932 patients) | Vitamin D compounds (25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and synthetic derivatives) vs. placebo |
| [139] |
| Randomized, double-blind, placebo-controlled trial, 120 patients with nondiabetic CKD stage 3–4 and vitamin D deficiency | Cholecalciferol (300,000 IU) or matching placebo |
| [144] |
| 41 children with CKD and 24 healthy subjects with low 25-hydroxyvitamin D3 (25OHD) levels | Stoss vitamin D supplementation |
| [145] |
| Multinational, double-blind, randomized placebo-controlled trial, 227 patients with CKD, mild-to-moderate LVH, and preserved LVEF | Oral paricalcitol, 2 μg/d (n = 115), or matching placebo (n = 112). |
| [143] |
| Polyunsaturated Fatty Acids (PUFA) | |||
| Systematic review and meta-analysis of randomized controlled trials. Sixty trials (4129 participants). | n-3 PUFA supplementation |
| [156] |
| Analysis of longitudinal data from 1436 participants in the Diabetes Control and Complications Trial | Dietary n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs). Average intake of eicosapentaenoic and docosahexaenoic acid taken from diet histories. |
| [158] |
| Double-blind, permuted-block, randomized, placebo-controlled of ESRD patients | Fish-oil concentrate (study group) vs. corn-oil capsules (control group). Six 1-g soft-gel capsules each day for 6 months |
| [160] |
| Double-blind randomized placebo-controlled design, 206 HD patients with documented CVD | n-3 PUFA or a control treatment (olive oil) |
| [161] |
| 12-month, prospective, single-blind, sequential intervention, cohort study of 24 HD patients | Three consecutive 4-month study periods taking the following supplements: A (olive oil: 2 g/day), B (omega-3 PUFA: 2 g/day), C (olive oil: 2 g/day). |
| [162] |
| Red Yeast Rice and Berberine | |||
| Forty moderately hypercholesterolemic outpatients with mild-to-moderate CKD and 40 cross-matched hypercholesterolemic subjects without CKD | Combined nutraceutical containing red yeast rice (3 mg monacolin K) and berberine (500 mg). |
| [166] |
| Single centre, randomized, double-blind, placebo-controlled study, 50 hypercholesterolemic patients | Daily oral dose of NC (25 patients) or placebo, 6 weeks |
| [168] |
| Menaquinone-7 (MK-7) | |||
| 42 non-dialyzed patients with CKD | Vitamin K2 at a dose of 90 μg (menaquinone, MK-7) + 10 μg of cholecalciferol (K + D group) or 10 μg of cholecalciferol (group D): |
| [175] |
| Interventional randomized non-placebo-controlled trial with 3 parallel groups, 53 long-term hemodialysis patients in stable conditions | Menaquinone-7 (vitamin K(2)) treatment at 45, 135, or 360 μg/d for 6 weeks |
| [174] |
| Uremic rats | Control (high-phosphate diet), uremic (high-phosphate diet containing 0.5% adenine), and supplemented uremic diet (0.5% adenine, MK-7, magnesium carbonate, and Sucrosomial® Iron), 6 weeks |
| [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rysz, J.; Franczyk, B.; Kujawski, K.; Sacewicz-Hofman, I.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. Are Nutraceuticals Beneficial in Chronic Kidney Disease? Pharmaceutics 2021, 13, 231. https://doi.org/10.3390/pharmaceutics13020231
Rysz J, Franczyk B, Kujawski K, Sacewicz-Hofman I, Ciałkowska-Rysz A, Gluba-Brzózka A. Are Nutraceuticals Beneficial in Chronic Kidney Disease? Pharmaceutics. 2021; 13(2):231. https://doi.org/10.3390/pharmaceutics13020231
Chicago/Turabian StyleRysz, Jacek, Beata Franczyk, Krzysztof Kujawski, Izabela Sacewicz-Hofman, Aleksanda Ciałkowska-Rysz, and Anna Gluba-Brzózka. 2021. "Are Nutraceuticals Beneficial in Chronic Kidney Disease?" Pharmaceutics 13, no. 2: 231. https://doi.org/10.3390/pharmaceutics13020231
APA StyleRysz, J., Franczyk, B., Kujawski, K., Sacewicz-Hofman, I., Ciałkowska-Rysz, A., & Gluba-Brzózka, A. (2021). Are Nutraceuticals Beneficial in Chronic Kidney Disease? Pharmaceutics, 13(2), 231. https://doi.org/10.3390/pharmaceutics13020231







