Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective
Abstract
1. Introduction
2. Targeting the Blood Brain Barrier
2.1. Passive Targeting
2.2. Active Targeting
3. Stimuli-Responsive Targeting
4. Targeting by Administration Route
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mensch, J.; Oyarzabal, J.; Mackie, C.; Augustijns, P. In vivo, in vitro and in silico methods for small molecule transfer across the BBB. J. Pharm. Sci. 2009, 98, 4429–4468. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.R. Assessment of blood-brain barrier penetration: In silico, in vitro and in vivo. Curr. Drug Metab. 2002, 3, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Prediction of blood-brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov. Today Technol. 2004, 1, 407–416. [Google Scholar] [CrossRef]
- Ciura, K.; Dziomba, S. Application of separation methods for in vitro prediction of blood–brain barrier permeability—The state of the art. J. Pharm. Biomed. Anal. 2020, 177. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview—Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Moura, R.P.; Martins, C.; Pinto, S.; Sousa, F.; Sarmento, B. Blood-brain barrier receptors and transporters: An insight on their function and how to exploit them through nanotechnology. Expert Opin. Drug Deliv. 2019, 16, 271–285. [Google Scholar] [CrossRef]
- Kwok, S.S.; Bu, Y.S.; Lo, A.C.Y.; Chan, T.C.Y.; So, K.F.; Lai, J.S.M.; Shih, K.C. A Systematic Review of Potential Therapeutic Use of Lycium Barbarum Polysaccharides in Disease. Biomed. Res. Int. 2019, 2019, 4615745. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, M.; He, Z.Z. Low-Molecular-Weight Fucoidan Attenuates Mitochondrial Dysfunction and Improves Neurological Outcome After Traumatic Brain Injury in Aged Mice: Involvement of Sirt3. Cell. Mol. Neurobiol. 2016, 36, 1257–1268. [Google Scholar] [CrossRef]
- Yang, Y.M.; Liu, P.F.; Chen, L.X.; Liu, Z.J.; Zhang, H.X.; Wang, J.J.; Sun, X.S.; Zhong, W.Q.; Wang, N.; Tian, K.; et al. Therapeutic effect of Ginkgo biloba polysaccharide in rats with focal cerebral ischemia/reperfusion (I/R) injury. Carbohyd. Polym. 2013, 98, 1383–1388. [Google Scholar] [CrossRef]
- Huang, S.C.; Mao, J.X.; Ding, K.; Zhou, Y.; Zeng, X.L.; Yang, W.J.; Wang, P.P.; Zhao, C.; Yao, J.; Xia, P.; et al. Polysaccharides from Ganoderma lucidum Promote Cognitive Function and Neural Progenitor Proliferation in Mouse Model of Alzheimer’s Disease. Stem Cell Rep. 2017, 8, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hao, J.W.; Zheng, Y.; Su, R.J.; Liao, Y.J.; Gong, X.L.; Liu, L.M.; Wang, X.M. Fucoidan Protects Dopaminergic Neurons by Enhancing the Mitochondrial Function in a Rotenone-induced Rat Model of Parkinson’s Disease. Aging Dis. 2018, 9, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Shi, S.S.; Chen, Q.; Lin, S.Q.; Wang, R.; Wang, S.Z.; Chen, C.M. Antitumor and Immunomodulatory Activities of Ganoderma lucidum Polysaccharides in Glioma-Bearing Rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Lai, I.C.; Kuo, H.C.; Chuang, S.E.; Lee, H.L.; Whang-Peng, J.; Yao, C.J.; Lai, G.M. Epigenetic modification and differentiation induction of malignant glioma cells by oligo-fucoidan. Mar. Drugs 2019, 17, 525. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.A.; Letourneur, D.; Chauvierre, C. Polysaccharide Nanosystems for Future Progress in Cardiovascular Pathologies. Theranostics 2014, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Overcoming the blood-brain barrier: Functionalised chitosan nanocarriers. Pharmaceutics 2020, 12, 1013. [Google Scholar] [CrossRef]
- Cortés, H.; Alcalá-Alcalá, S.; Caballero-Florán, I.H.; Bernal-Chávez, S.A.; Ávalos-Fuentes, A.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Floran, B.; et al. A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier. Membranes 2020, 10, 212. [Google Scholar] [CrossRef]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef]
- Zhang, T.T.; Li, W.; Meng, G.M.; Wang, P.; Liao, W.Z. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef]
- Radwan, R.R.; Abdel Ghaffar, A.M.; Ali, H.E. Gamma radiation preparation of chitosan nanoparticles for controlled delivery of memantine. J. Biomater. Appl. 2020, 34, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.F.; Molnar, E.; Toman, P.; Tsibouklis, J.; Pilkington, G.J.; Gorecki, D.C.; Barbu, E. In Vitro Assessment of Alkylglyceryl-Functionalized Chitosan Nanoparticles as Permeating Vectors for the Blood-Brain Barrier. Biomacromolecules 2012, 13, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Ibegbu, D.M.; Boussahel, A.; Cragg, S.M.; Tsibouklis, J.; Barbu, E. Nanoparticles of alkylglyceryl dextran and poly(ethyl cyanoacrylate) for applications in drug delivery: Preparation and characterization. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 265–279. [Google Scholar] [CrossRef][Green Version]
- Toman, P.; Lien, C.F.; Ahmad, Z.; Dietrich, S.; Smith, J.R.; An, Q.; Molnar, E.; Pilkington, G.J.; Gorecki, D.C.; Tsibouklis, J.; et al. Nanoparticles of alkylglyceryl-dextran-graft-poly(lactic acid) for drug delivery to the brain: Preparation and in vitro investigation. Acta Biomater. 2015, 23, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Boussahel, A.; Ibegbu, D.M.; Lamtahri, R.; Maucotel, J.; Chuquet, J.; Lefranc, B.; Leprince, J.; Roldo, M.; Le Mevel, J.C.; Gorecki, D.; et al. Investigations of octylglyceryl dextran-graft-poly(lactic acid) nanoparticles for peptide delivery to the brain. Nanomedicine 2017, 12, 879–892. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Lalatsa, A.; Gorecki, D.C.; Barbu, E. Engineering butylglyceryl-modified polysaccharides towards nanomedicines for brain drug delivery. Carbohyd. Polym. 2020, 236, 116060. [Google Scholar] [CrossRef]
- Blanco, S.; Peralta, S.; Morales, M.E.; Martinez-Lara, E.; Pedrajas, J.R.; Castan, H.; Peinado, M.A.; Ruiz, M.A. Hyaluronate Nanoparticles as a Delivery System to Carry Neuroglobin to the Brain after Stroke. Pharmaceutics 2020, 12, 40. [Google Scholar] [CrossRef]
- Lalatsa, A.; Schatzlein, A.G.; Garrett, N.L.; Moger, J.; Briggs, M.; Godfrey, L.; Iannitelli, A.; Freeman, J.; Uchegbu, I.F. Chitosan amphiphile coating of peptide nanofibres reduces liver uptake and delivers the peptide to the brain on intravenous administration. J. Control. Release 2015, 197, 87–96. [Google Scholar] [CrossRef]
- Tunesi, M.; Raimondi, I.; Russo, T.; Colombo, L.; Micotti, E.; Brandi, E.; Cappelletti, P.; Cigada, A.; Negro, A.; Ambrosio, L.; et al. Hydrogel-based delivery of Tat-fused protein Hsp70 protects dopaminergic cells in vitro and in a mouse model of Parkinson’s disease. NPG Asia Mater. 2019, 11, 28. [Google Scholar] [CrossRef]
- Bezem, M.T.; Johannessen, F.G.; Jung, K.C.K.; Gundersen, E.T.; Jorge-Finnigan, A.; Ying, M.; Betbeder, D.; Herfindal, L.; Martinez, A. Stabilization of Human Tyrosine Hydroxylase in Maltodextrin Nanoparticles for Delivery to Neuronal Cells and Tissue. Bioconjugate Chem. 2018, 29, 493–502. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Caires, A.J.; Carvalho, S.M.; Capanema, N.S.V.; Carvalho, I.C.; Mansur, H.S. Dual-functional supramolecular nanohybrids of quantum dot/biopolymer/chemotherapeutic drug for bioimaging and killing brain cancer cells in vitro. Colloid Surf. B 2019, 184, 110507. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.C.; Mansur, H.S.; Mansur, A.A.P.; Carvalho, S.M.; de Oliveira, L.C.A.; Leite, M.D. Luminescent switch of polysaccharide-peptide-quantum dot nanostructures for targeted-intracellular imaging of glioblastoma cells. J. Mol. Liq. 2020, 304, 112759. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Hu, Y.L.; Gao, J.Q. Brain Localization and Neurotoxicity Evaluation of Polysorbate 80-Modified Chitosan Nanoparticles in Rats. PLoS ONE 2015, 10, e0134722. [Google Scholar] [CrossRef] [PubMed]
- Girotra, P.; Thakur, A.; Kumar, A.; Singh, S.K. Identification of multi-targeted anti-migraine potential of nystatin and development of its brain targeted chitosan nanoformulation. Int. J. Biol. Macromol. 2017, 96, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Sinha, P.; Laha, B.; Maiti, S.; Bhattacharyya, U.K.; Nayak, A.K. Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting. J. Drug Deliv. Sci. Technol. 2018, 48, 21–29. [Google Scholar] [CrossRef]
- Mansur, A.A.P.; Carvalho, S.M.; Lobato, Z.I.P.; Leite, M.D.; Cunha, A.D.; Mansur, H.S. Design and Development of Polysaccharide-Doxorubicin-Peptide Bioconjugates for Dual Synergistic Effects of Integrin-Targeted and Cell-Penetrating Peptides for Cancer Chemotherapy. Bioconjugate Chem. 2018, 29, 1973–2000. [Google Scholar] [CrossRef]
- Yang, L.; Gao, S.Y.; Asghar, S.; Liu, G.H.; Song, J.; Wang, X.; Ping, Q.N.; Zhang, C.; Xiao, Y.Y. Hyaluronic acid/chitosan nanoparticles for delivery of curcuminoid and its in vitro evaluation in glioma cells. Int. J. Biol. Macromol. 2015, 72, 1391–1401. [Google Scholar] [CrossRef]
- Xu, Y.R.; Asghar, S.; Yang, L.; Chen, Z.P.; Li, H.Y.; Shi, W.W.; Li, Y.B.; Shi, Q.Q.; Ping, Q.N.; Xiao, Y.Y. Nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG containing curcumin: In vitro evaluation and pharmacokinetics in rats. Int. J. Biol. Macromol. 2017, 102, 1083–1091. [Google Scholar] [CrossRef]
- Yang, L.Q.; Song, X.; Gong, T.; Jiang, K.J.; Hou, Y.Y.; Chen, T.J.; Sun, X.; Zhang, Z.R.; Gong, T. Development a hyaluronic acid ion-pairing liposomal nanoparticle for enhancing anti-glioma efficacy by modulating glioma microenvironment. Drug Deliv. 2018, 25, 388–397. [Google Scholar] [CrossRef]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef]
- Fan, R.R.; Chuan, D.; Hou, H.; Chen, H.F.; Han, B.; Zhang, X.N.; Zhou, L.X.; Tong, A.P.; Xu, J.G.; Guo, G. Development of a hybrid nanocarrier-recognizing tumor vasculature and penetrating the BBB for glioblastoma multi -targeting therapy. Nanoscale 2019, 11, 11285–11304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Yang, Y.T.; Zhang, Y.H.; Huang, M.; Zhou, Z.J.; Luo, W.X.; Tang, J.; Wang, J.G.; Xiao, Q.; Chen, H.J.; et al. Dual-Targeting Heparin-Based Nanoparticles that Re-Assemble in Blood for Glioma Therapy through Both Anti-Proliferation and Anti-Angiogenesis. Adv. Funct. Mater. 2016, 26, 7873–7885. [Google Scholar] [CrossRef]
- Fernandes, J.; Ghate, M.V.; Mallik, S.B.; Lewis, S.A. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharmaceut. 2018, 547, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Waddad, A.Y.; Ramharack, P.; Soliman, M.E.S.; Govender, T. Grafted hyaluronic acid N-acetyl-L-methionine for targeting of LAT1 receptor: In-silico, synthesis and microscale thermophoresis studies. Int. J. Biol. Macromol. 2019, 125, 767–777. [Google Scholar] [CrossRef]
- Jaruszewski, K.M.; Curran, G.L.; Swaminathan, S.K.; Rosenberg, J.T.; Grant, S.C.; Ramakrishnan, S.; Lowe, V.J.; Poduslo, J.F.; Kandimalla, K.K. Multimodal Nanoprobes to target cerebrovascular amyloid in Alzheimer’s disease brain. Biomaterials 2014, 35, 1967–1976. [Google Scholar] [CrossRef]
- Agyare, E.K.; Jaruszewski, K.M.; Curran, G.L.; Rosenberg, J.T.; Grant, S.C.; Lowe, V.J.; Ramakrishnan, S.; Paravastu, A.K.; Poduslo, J.F.; Kandimalla, K.K. Engineering theranostic nanovehicles capable of targeting cerebrovascular amyloid deposits. J. Control. Release 2014, 185, 121–129. [Google Scholar] [CrossRef]
- Gu, J.J.; Al-Bayati, K.; Ho, E.A. Development of antibody-modified chitosan nanoparticles for the targeted delivery of si A across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res. 2017, 7, 497–506. [Google Scholar] [CrossRef]
- Ashrafi, H.; Azadi, A. Chitosan-based hydrogel nanoparticle amazing behaviors during transmission electron microscopy. Int. J. Biol. Macromol. 2016, 84, 31–34. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef]
- Gao, H.L. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Ramge, P.; Unger, R.E.; Oltrogge, J.B.; Zenker, D.; Begley, D.; Kreuter, J.; von Briesen, H. Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate (PBCA)-nanoparticles by human and bovine primary brain capillary endothelial cells. Eur. J. Neurosci. 2000, 12, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.V.; Roney, C.A.; Antich, P.P.; Bonte, F.J.; Raghu, A.V.; Aminabhavi, T.M. Quinoline-n-butylcyanoacrylate-based nanoparticles for brain targeting for the diagnosis of Alzheimer’s disease. Wires Nanomed. Nanobiotechnol. 2010, 2, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.Y.; Zhang, Z.H.; Zhang, Y.L.; Lv, H.X.; Zhou, J.P.; Li, C.C.; Hou, L.L.; Zhang, Q. Dual-functional liposomes based on pH-responsive cell-penetrating peptide and hyaluronic acid for tumor-targeted anticancer drug delivery. Biomaterials 2012, 33, 9246–9258. [Google Scholar] [CrossRef]
- Peura, L.; Malmioja, K.; Huttunen, K.; Leppanen, J.; Hamalainen, M.; Forsberg, M.M.; Rautio, J.; Laine, K. Design, Synthesis and Brain Uptake of LAT1-Targeted Amino Acid Prodrugs of Dopamine. Pharm. Res. Dordr. 2013, 30, 2523–2537. [Google Scholar] [CrossRef]
- Morales-Cruz, M.; Delgado, Y.; Castillo, B.; Figueroa, C.M.; Molina, A.M.; Torres, A.; Milian, M.; Griebenow, K. Smart Targeting To Improve Cancer Therapeutics. Drug Des. Dev. Ther. 2019, 13, 3753–3772. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Concheiro, A.; Iemma, F.; Alvarez-Lorenzo, C. pH/redox dual-sensitive dextran nanogels for enhanced intracellular drug delivery. Eur. J. Pharm. Biopharm. 2017, 117, 324–332. [Google Scholar] [CrossRef]
- Makharza, S.A.; Cirillo, G.; Vittorio, O.; Valli, E.; Voli, F.; Farfalla, A.; Curcio, M.; Iemma, F.; Nicoletta, F.P.; El-Gendy, A.A.; et al. Magnetic graphene oxide nanocarrier for targeted delivery of cisplatin: A perspective for glioblastoma treatment. Pharmaceuticals 2019, 12, 76. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Curcio, M.; Cirillo, G.; Spataro, T.; Vittorio, O.; Picci, N.; Hampel, S.; Iemma, F.; Nicoletta, F.P. Recent Advances in the Synthesis and Biomedical Applications of Nanocomposite Hydrogels. Pharmaceutics 2015, 7, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Fishman, P.S.; Frenkel, V. Focused Ultrasound: An Emerging Therapeutic Modality for Neurologic Disease. Neurotherapeutics 2017, 14, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Appelboom, G.; Detappe, A.; LoPresti, M.; Kunjachan, S.; Mitrasinovic, S.; Goldman, S.; Chang, S.D.; Tillement, O. Stereotactic modulation of blood-brain barrier permeability to enhance drug delivery. Neuro-Oncology 2016, 18, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.T.; Fu, C.P.; Li, M.; Li, X.P.; Wang, M.Y.; He, L.; Zhang, L.M.; Peng, Y. A pH-sensitive hyaluronic acid prodrug modified with lactoferrin for glioma dual-targeted treatment. Mater. Sci. Eng. C 2016, 67, 159–169. [Google Scholar] [CrossRef]
- Tian, C.H.; Asghar, S.; Xu, Y.R.; Chen, Z.P.; Zhang, M.; Huang, L.; Ye, J.X.; Ping, Q.N.; Xiao, Y.Y. The effect of the molecular weight of hyaluronic acid on the physicochemical characterization of hyaluronic acid-curcumin conjugates and in vitro evaluation in glioma cells. Colloid Surf. B 2018, 165, 45–55. [Google Scholar] [CrossRef]
- Tian, C.H.; Asghar, S.; Xu, Y.R.; Chen, Z.P.; Zhang, J.W.; Ping, Q.N.; Xiao, Y.Y. Tween 80-modified hyaluronic acid-ss-curcumin micelles for targeting glioma: Synthesis, characterization and their in vitro evaluation. Int. J. Biol. Macromol. 2018, 120, 2579–2588. [Google Scholar] [CrossRef]
- Lv, W.; Xu, J.P.; Wang, X.Q.; Li, X.R.; Xu, Q.W.; Xin, H.L. Bioengineered Boronic Ester Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment. ACS Nano 2018, 12, 5417–5426. [Google Scholar] [CrossRef]
- Aguilera, G.; Berry, C.C.; West, R.M.; Gonzalez-Monterrubio, E.; Angulo-Molina, A.; Arias-Carrion, O.; Mendez-Rojas, M.A. Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood-brain barrier. Nanoscale Adv. 2019, 1, 671–685. [Google Scholar] [CrossRef]
- Shevtsov, M.; Nikolaev, B.; Marchenko, Y.; Yakovleva, L.; Skvortsov, N.; Mazur, A.; Tolstoy, P.; Ryzhov, V.; Multhoff, G. Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs). Int. J. Nanomed. 2018, 13, 1471–1482. [Google Scholar] [CrossRef]
- Das, M.; Wang, C.Y.; Bedi, R.; Mohapatra, S.S.; Mohapatra, S. Magnetic micelles for DNA delivery to rat brains after mild traumatic brain injury. Nanomedicine 2014, 10, 1539–1548. [Google Scholar] [CrossRef]
- Wang, X.Q.; Chang, Y.Y.; Zhang, D.X.; Tian, B.M.; Yang, Y.; Wei, F. Transferrin-conjugated drug/dye-co-encapsulated magnetic nanocarriers for active-targeting fluorescent/magnetic resonance imaging and anti-tumor effects in human brain tumor cells. RSC Adv. 2016, 6, 105661–105675. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Kievit, F.M.; Veiseh, O.; Chiarelli, P.A.; Fang, C.; Wang, K.; Hatzinger, S.J.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M.Q. Redox-Responsive Magnetic Nanoparticle for Targeted Convection-Enhanced Delivery of O-6-Benzylguanine to Brain Tumors. ACS Nano 2014, 8, 10383–10395. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, K.; Patel, S.; Singh, R.; Patel, K.; Sawant, K. Hyaluronic acid tethered pH-responsive alloy-drug nanoconjugates for multimodal therapy of glioblastoma: An intranasal route approach. Mater. Sci. Eng. C 2019, 98, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. Redox-responsive nano-carriers as tumor-targeted drug delivery systems. Eur. J. Med. Chem. 2018, 157, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.J.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef]
- Meng, F.H.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef]
- Panagiotou, S.; Saha, S. Therapeutic benefits of nanoparticles in stroke. Front. Neurosci. 2015, 9, 182. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.N.; Yu, P.; Niu, J.Q.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.D.; Niu, X.B. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef]
- Lerra, L.; Farfalla, A.; Sanz, B.; Cirillo, G.; Vittorio, O.; Voli, F.; Grand, M.L.; Curcio, M.; Nicoletta, F.P.; Dubrovska, A.; et al. Graphene oxide functional nanohybrids with magnetic nanoparticles for improved vectorization of doxorubicin to neuroblastoma cells. Pharmaceutics 2019, 11, 3. [Google Scholar] [CrossRef]
- Vittorio, O.; Voliani, V.; Faraci, P.; Karmakar, B.; Iemma, F.; Hampel, S.; Kavallaris, M.; Cirillo, G. Magnetic catechin-dextran conjugate as targeted therapeutic for pancreatic tumour cells. J. Drug Target. 2014, 22, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Graham-Gurysh, E.G.; Moore, K.M.; Schorzman, A.N.; Lee, T.; Zamboni, W.C.; Hingtgen, S.D.; Bachelder, E.M.; Ainslie, K.M. Tumor Responsive and Tunable Polymeric Platform for Optimized Delivery of Paclitaxel to Treat Glioblastoma. ACS Appl. Mater. Inter. 2020, 12, 19345–19356. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Zarrintaj, P.; Kamrava, S.K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutiérrez, T.J.; Saeb, M.R. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr. Polym. 2019, 224. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.M.; Li, X.G.; Wang, C.; Hao, P.; Song, W.; Li, M.L.; Zhao, W.; Gao, Y.D.; Yang, Z.Y. Functional hyaluronate collagen scaffolds induce NSCs differentiation into functional neurons in repairing the traumatic brain injury. Acta Biomater. 2016, 45, 182–195. [Google Scholar] [CrossRef]
- Cassano, R.; Trapani, A.; Di Gioia, M.L.; Mandracchia, D.; Pellitteri, R.; Tripodo, G.; Trombino, S.; Di Gioia, S.; Conese, M. Synthesis and characterization of novel chitosan-dopamine or chitosan-tyrosine conjugates for potential nose-to-brain delivery. Int. J. Pharmaceut. 2020, 589. [Google Scholar] [CrossRef]
- Liu, S.S.; Yang, S.L.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2018, 13, 72–81. [Google Scholar] [CrossRef]
- Bhavna, S.; Ali, M.; Ali, R.; Bhatnagar, A.; Baboota, S.; Ali, J. Donepezil nanosuspension intended for nose to brain targeting: In vitro and in vivo safety evaluation. Int. J. Biol. Macromol. 2014, 67, 418–425. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Shak, A.T.; Xi, L.W.; Safian, N.H.; Choudhury, H.; Lim, W.M.; Shahzad, N.; Alhakamy, N.A.; Anwer, M.K.; Radhakrishnan, A.K.; et al. Nose to brain delivery of rotigotine loaded chitosan nanoparticles in human SH-SY5Y neuroblastoma cells and animal model of Parkinson’s disease. Int. J. Pharmaceut. 2020, 579, 119148. [Google Scholar] [CrossRef]
- Mittal, D.; Md, S.; Hasan, Q.; Fazil, M.; Ali, A.; Baboota, S.; Ali, J. Brain targeted nanoparticulate drug delivery system of rasagiline via intranasal route. Drug Deliv. 2016, 23, 130–139. [Google Scholar] [CrossRef]
- Mandlik, S.K.; Ranpise, N.S.; Mohanty, B.S.; Chaudhari, P.R. A coupled bimodal SPECT-CT imaging and brain kinetics studies of zolmitriptan-encapsulated nanostructured polymeric carriers. Drug Deliv. Transl. Res. 2018, 8, 797–805. [Google Scholar] [CrossRef]
- Jahromi, L.P.; Mohammadi-Samani, S.; Heidari, R.; Azadi, A. In vitro- and in vivo Evaluation of Methotrexate-Loaded Hydrogel Nanoparticles Intended to Treat Primary CNS Lymphoma via Intranasal Administration. J. Pharm. Pharm. Sci. 2018, 21, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Ashafaq, M.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia. Int. J. Biol. Macromol. 2016, 91, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Ho, P.C. Intranasal administration of brain-targeted HP-beta-CD/chitosan nanoparticles for delivery of scutellarin, a compound with protective effect in cerebral ischaemia. J. Pharm. Pharmacol. 2017, 69, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Van Woensel, M.; Wauthoz, N.; Rosiere, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.; Mathivet, T.; et al. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Application of Box-Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route. Int. J. Biol. Macromol. 2016, 89, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: Formulation, physicochemical and pharmacokinetic consideration. Eur. J. Pharm. Sci. 2016, 91, 196–207. [Google Scholar] [CrossRef]
- Sood, S.; Jain, K.; Gowthamarajan, K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloid Surf. B 2014, 113, 330–337. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Formulation and In-vivo Pharmacokinetic Consideration of Intranasal Microemulsion and Mucoadhesive Microemulsion of Rivastigmine for Brain Targeting. Pharm. Res. Dordr. 2018, 35, 8. [Google Scholar] [CrossRef]
- Abdou, E.M.; Kandil, S.M.; El Miniawy, H.M.F. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharmaceut. 2017, 529, 667–677. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. N,N,N-trimethyl chitosan modified flaxseed oil based mucoadhesive neuronanoemulsions for direct nose to brain drug delivery. Int. J. Biol. Macromol. 2018, 120, 2560–2571. [Google Scholar] [CrossRef]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.F.; Qin, N.; Sun, L.W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2017, 25, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Bshara, H.; Osman, R.; Mansour, S.; El-Shamy, A.A. Chitosan and cyclodextrin in intranasal microemulsion for improved brain buspirone hydrochloride pharmacokinetics in rats. Carbohyd. Polym. 2014, 99, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N. Rasagiline-encapsulated chitosan-coated PLGA nanoparticles targeted to the brain in the treatment of parkinson’s disease. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 677–690. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alrasheed, R.A.; Almatar, H.M.A.; Al-Ramadan, A.S.; Amir, M.; Sarafroz, M. Quantification and Evaluations of Catechin Hydrate Polymeric Nanoparticles Used in Brain Targeting for the Treatment of Epilepsy. Pharmaceutics 2020, 12, 203. [Google Scholar] [CrossRef]
- Ahmad, N.; Al-Subaiec, A.M.; Ahmad, R.; Sharma, S.; Alam, M.A.; Ashafaq, M.; Rub, R.A.; Ahmad, F.J. Brain-targeted glycyrrhizic-acid-loaded surface decorated nanoparticles for treatment of cerebral ischaemia and its toxicity assessment. Artif. Cells Nanomed. Biotechnol. 2019, 47, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Des Reis, L.G.; Scalia, S.; Dalpiaz, A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. 2018, 127, 250–259. [Google Scholar] [CrossRef]
- Hernando, S.; Herran, E.; Figueiro-Silva, J.; Pedraz, J.L.; Igartua, M.; Carro, E.; Hernandez, R.M. Intranasal Administration of TAT-Conjugated Lipid Nanocarriers Loading GDNF for Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 145–155. [Google Scholar] [CrossRef]
- Salade, L.; Wauthoz, N.; Deleu, M.; Vermeersch, M.; De Vriese, C.; Amighi, K.; Goole, J. Development of coated liposomes loaded with ghrelin for nose-to-brain delivery for the treatment of cachexia. Int. J. Nanomed. 2017, 12, 8531–8543. [Google Scholar] [CrossRef]
- Wang, Q.W.; Wong, C.H.; Chan, H.Y.E.; Lee, W.Y.; Zuo, Z. Statistical Design of Experiment (DoE) based development and optimization of DB213 in situ thermosensitive gel for intranasal delivery. Int. J. Pharmaceut. 2018, 539, 50–57. [Google Scholar] [CrossRef]
- Yang, M.B.; Zhang, Q.; Wang, Q.W.; Sorensen, K.K.; Boesen, J.T.; Ma, S.Y.; Jensen, K.J.; Kwan, K.M.; Ngo, J.C.K.; Chan, H.Y.E.; et al. Brain-Targeting Delivery of Two Peptidylic Inhibitors for Their Combination Therapy in Transgenic Polyglutamine Disease Mice via Intranasal Administration. Mol. Pharmaceut. 2018, 15, 5781–5792. [Google Scholar] [CrossRef] [PubMed]
- Akilo, O.D.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pradeep, P.; Modi, G.; Pillay, V. In situ thermo-co-electroresponsive mucogel for controlled release of bioactive agent. Int. J. Pharmaceut. 2019, 559, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Walgrave, H.; Salta, E.; Alvarez, D.M.; Castro-Lopez, V.; Loza, M.; Alonso, M.J. Nose-to-brain delivery of enveloped RNA cell permeating peptide nanocomplexes for the treatment of neurodegenerative diseases. Biomaterials 2020, 230, 119657. [Google Scholar] [CrossRef] [PubMed]
- Borodina, T.; Marchenko, I.; Trushina, D.; Volkova, Y.; Shirinian, V.; Zavarzin, I.; Kondrakhin, E.; Kovalev, G.; Kovalchuk, M.; Bukreeva, T. A novel formulation of zolpidem for direct nose-to-brain delivery: Synthesis, encapsulation and intranasal administration to mice. J. Pharm. Pharmacol. 2018, 70, 1164–1173. [Google Scholar] [CrossRef]
- Nasr, M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv. 2016, 23, 1444–1452. [Google Scholar] [CrossRef]
- Rajput, A.; Bariya, A.; Allam, A.; Othman, S.; Butani, S.B. In situ nanostructured hydrogel of resveratrol for brain targeting: In vitro-in vivo characterization. Drug Deliv. Transl. Res. 2018, 8, 1460–1470. [Google Scholar] [CrossRef]
- Galgatte, U.C.; Kumbhar, A.B.; Chaudhari, P.D. Development of in situ gel for nasal delivery: Design, optimization, in vitro and in vivo evaluation. Drug Deliv. 2014, 21, 62–73. [Google Scholar] [CrossRef]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V.S.R. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Ferraro, L.; Perrone, D.; Leo, E.; Iannuccelli, V.; Pavan, B.; Paganetto, G.; Beggiato, S.; Scalia, S. Brain Uptake of a Zidovudine Prodrug after Nasal Administration of Solid Lipid Microparticles. Mol. Pharmaceut. 2014, 11, 1550–1561. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Karavasili, C.; Fatouros, D.G. Smart materials: In situ gel-forming systems for nasal delivery. Drug Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.F.; Zhao, J.; Zhang, S.P.; Tong, T.T.; Zhuang, Q.N.; Jin, K.; Chen, W.; Tang, H. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery. Colloid Surf. B 2016, 147, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar] [PubMed]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharmaceut. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.X.; Wang, J.Q.; Zeng, Y.; Liu, G.; Chen, X.Y. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K. Scalable Nanomanufacturing—A Review. Micromachines 2017, 8, 20. [Google Scholar] [CrossRef]
- Dong, Y.C.; Ng, W.K.; Shen, S.C.; Kim, S.; Tan, R.B.H. Scalable ionic gelation synthesis of chitosan nanoparticles for drug delivery in static mixers. Carbohyd. Polym. 2013, 94, 940–945. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Kloet, S.K.; Kezic, S.; Kuper, F.; Park, M.V.D.Z.; Bellmann, S.; van der Zande, M.; Le Gac, S.; Krystek, P.; Peters, R.J.B.; et al. Progress and future of in vitro models to study translocation of nanoparticles. Arch. Toxicol. 2015, 89, 1469–1495. [Google Scholar] [CrossRef]



| PS Ref | System (Preparation) | Targeting | AR | Disease Drug | Performance | ||
|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | Outcome | |||||
| CH [21] | NP (γ-ray) | Enhanced permeation | Oral | Alzheimer MEM | --- | Albino rats | Neurological amelioration * # |
| AGCH [22] | NP (ion gelation) | Enhanced permeation (AG moieties) | systemic | --- --- | ECs bEnd3 | --- | Enhanced uptake § |
| AGDEX [23] | NP (e-polym) | Enhanced permeation (AG moieties) | systemic | --- DOX CUR | bEnd3 | --- | Sustained release § |
| AGDEX [24] | NP (el-spray) | Enhanced permeation (AG moieties) | systemic | --- DOX | bEnd3 | --- | Enhanced uptake § |
| AGDEX [25] | NPs (e-polym) | Enhanced permeation (AG moieties) | systemic | --- Ang II AnoxPep | --- | Trout/ mice | Neurological amelioration * |
| AGPS [26] | NP (chem-cross) | Enhanced permeation (AG moieties) | systemic | -- DOX; Ang II | bEnd3 | --- | Enhanced uptake § |
| HA/CH [27] | CSNP (e-polym) | Enhanced permeation (CH) | systemic | Ischemia NGB | --- | Wistar rat | Neurological amelioration # |
| PGCH [28] | CNF (sonication) | Enhanced permeation (PG moieties) | systemic | --- ENK-pp | --- | CD-1 mice | Improved pharmacokinetics # |
| HA [29] | COLL-HA (complex) | Prolonged delivery | topical | Parkinson Tat-Hsp70 | SH-SY5Y | CD-1; C57Bl/6N mice | Neurological amelioration * # |
| MDX [30] | NP (chem-cross) | Enhanced permeation | systemic | Parkinson TH | SH-SY5Y | Wild-type Bl-6 mice | Neurological amelioration # |
| CMC [31] | NPQD (magnetic stir) | Enhanced permeation | systemic | GBM DOX | U87 | --- | Enhanced uptake § |
| CMC [32] | NPQD (magnetic stir) | Enhanced permeation | systemic | GBM --- | U87 | --- | Enhanced uptake § |
| CH [33] | NP (ion gelation) | Ligand-receptor (Tween 80) | systemic | --- --- | --- | SD rats | Neurotoxicity # |
| CH [34] | NP (ion gelation) | Ligand-receptor (Tween 80) | systemic | Migraine NYS | --- | Swiss albino mice | Neurological amelioration * |
| CH [35] | NP (em-cross) | Ligand-receptor (Tween 80) | systemic | Parkinson ROP | --- | Wistar rats | Improved pharmacokinetics # |
| CMC [36] | CSNP (self-assembly) | Ligand-receptor/ transporter (RGD/L-Arg) | --- | GBM DOX | U-87 | CAM | Enhanced uptake § Synergism # |
| CH/HA [37] | NP (mixing) | HA | systemic | Glioma CUR | C6 | --- | Enhanced uptake § |
| CH/HA [38] | NP (mixing) | HA | systemic | Glioma CUR | C6 | SD rats | Enhanced uptake § Improved pharmacokinetic # |
| HA [39] | LPsNP (film hydration) | HA | systemic | Glioma DOX | C6 | BALB/c mice-SD rats | Enhanced uptake Synergism # |
| HA [40] | LNP (film hydration) | HA | --- | GBM DOX | A172; U87MG | --- | Enhanced uptake § |
| HA [41] | NPs (solvent evap) | Pep | systemic | GBM DTX | C6; U87; U251; BMVEC; NIH/3T3 | SD rats; BALB/c mice | Enhanced uptake § Synergism # |
| HP [42] | NPs (self-assembly) | cRGD; SWLAYPGAVSYR Peptides | systemic | Glioma --- | U251; U87; HUVEC; HEB | U251-xenograft mice | Enhanced uptake § Improved pharmacokinetics # |
| CH [43] | NP (ion gelation) | L-Val | systemic | Alzheimer SGT | --- | Wistar rats | Improved pharmacokinetics # |
| HA [44] | Prodrug (grafting) | NAA | --- | Brain tumor | --- | --- | Molecular docking § |
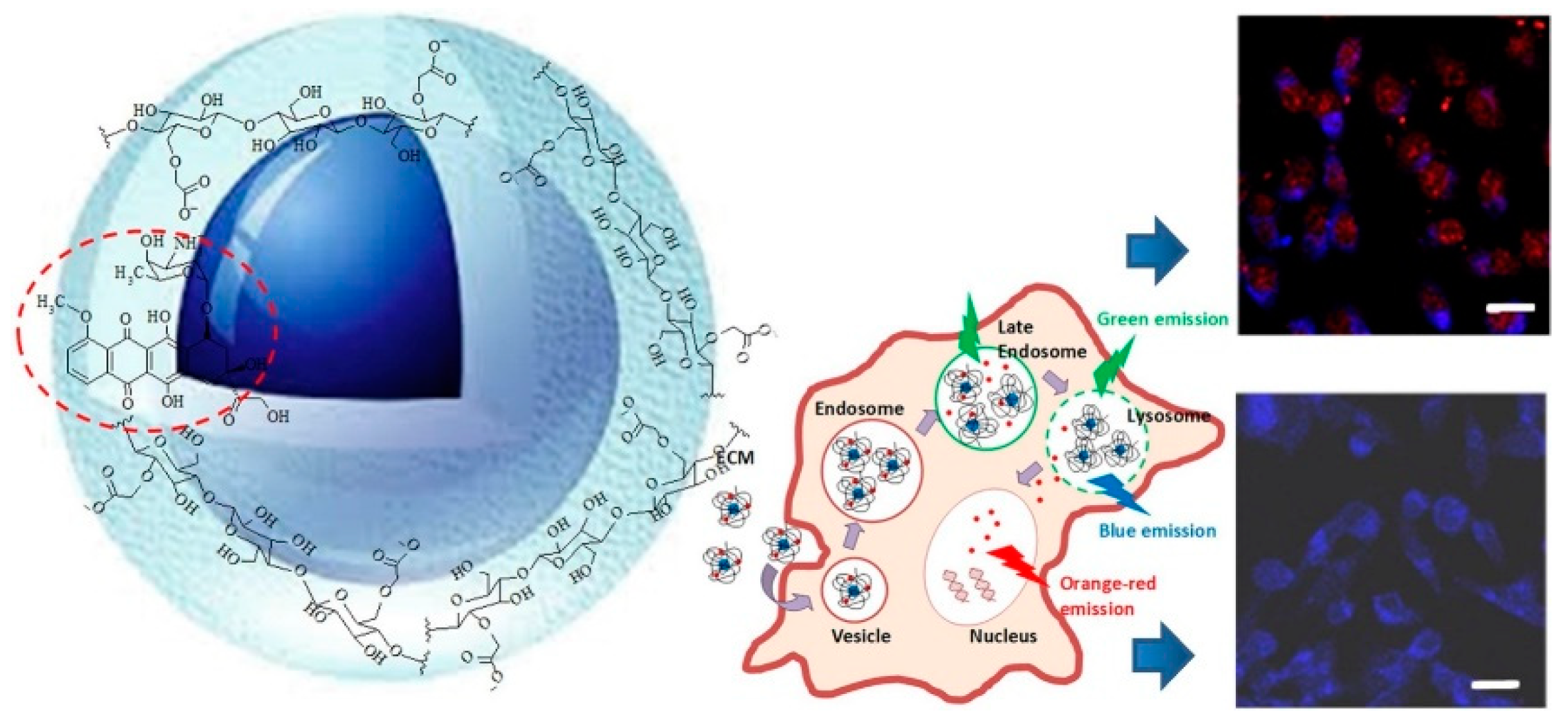
| CH [45] | NP (ion gelation) | Magnevist®/ IgG4.1 | systemic | Alzheimer CUR/DMT | hCMEC;D3 | B6/SJL mice; Tg2576 transgenic mice | Improved pharmacokinetics # |
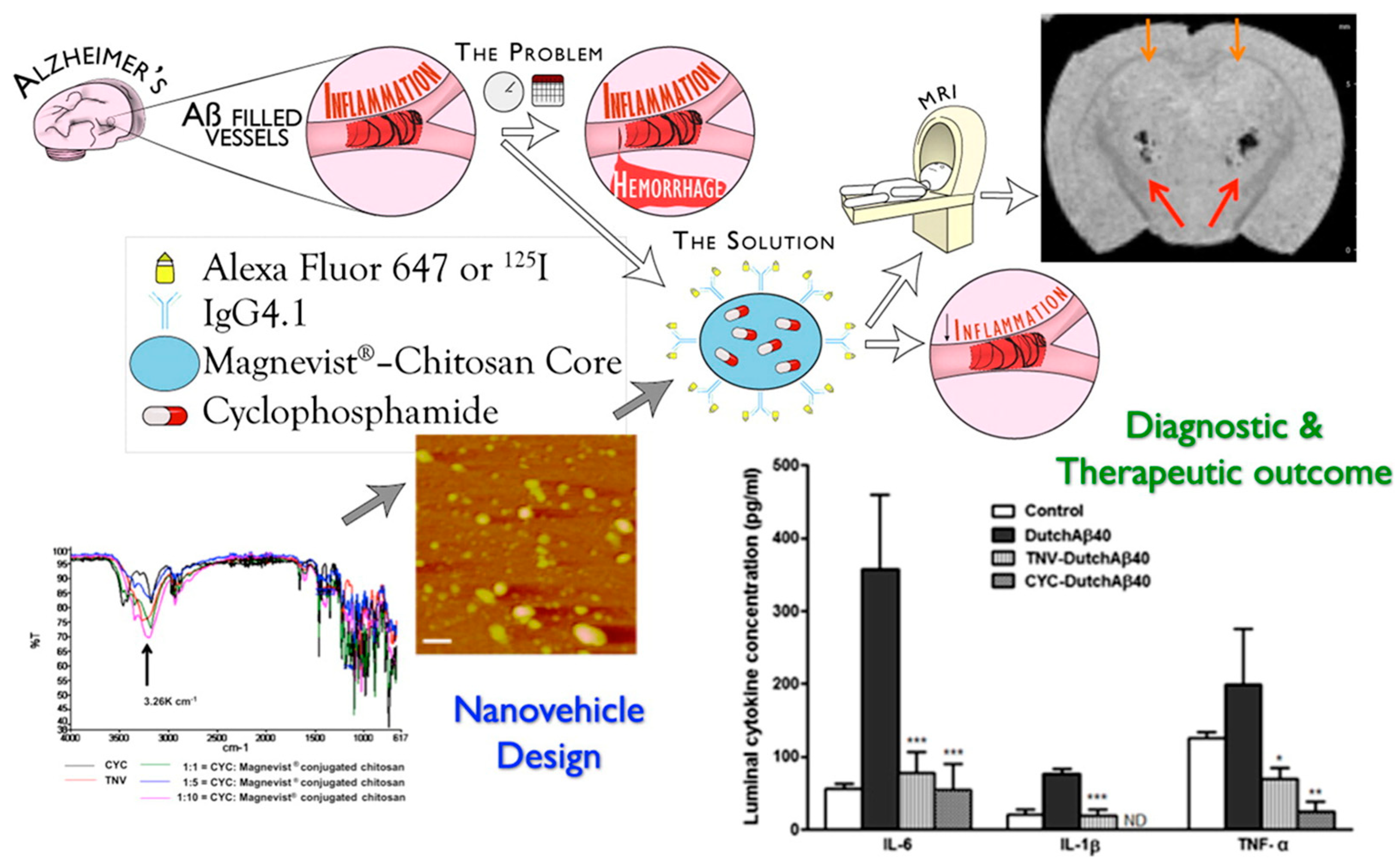
| CH [46] | NP (ion gelation) | Magnevist®/ F(ab′)2 | systemic | CAA CYP | hCMEC;D3 | B6SJLF1/J mice | Improved pharmacokinetics # |
| CH [47] | NP (ion gelation) | TfR/B2R | systemic | HIV SiRNA | U138-MG; hCMEC;D3 | --- | Enhanced uptake § |
| PS Ref | System (Preparation) | Stimuli | Active Targeting | AR | Disease Drug | Performance | ||
|---|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo | Outcome | ||||||
| HA [64] | Prodrug (condensation) | pH | Lf | systemic | Glioma DOX | U87 C6 | C6 orthotopic mice | Enhanced uptake § Improved pharmacokinetics # Synergism # |
| HA [65] | Micelles (self-assembly) | Redox | --- | systemic | Glioma CUR | G422 | --- | Enhanced uptake § |
| HA [66] | Micelles (self-assembly) | Redox | Tween80 | systemic | Glioma CUR | BEnd3 G422 | --- | Enhanced uptake § |
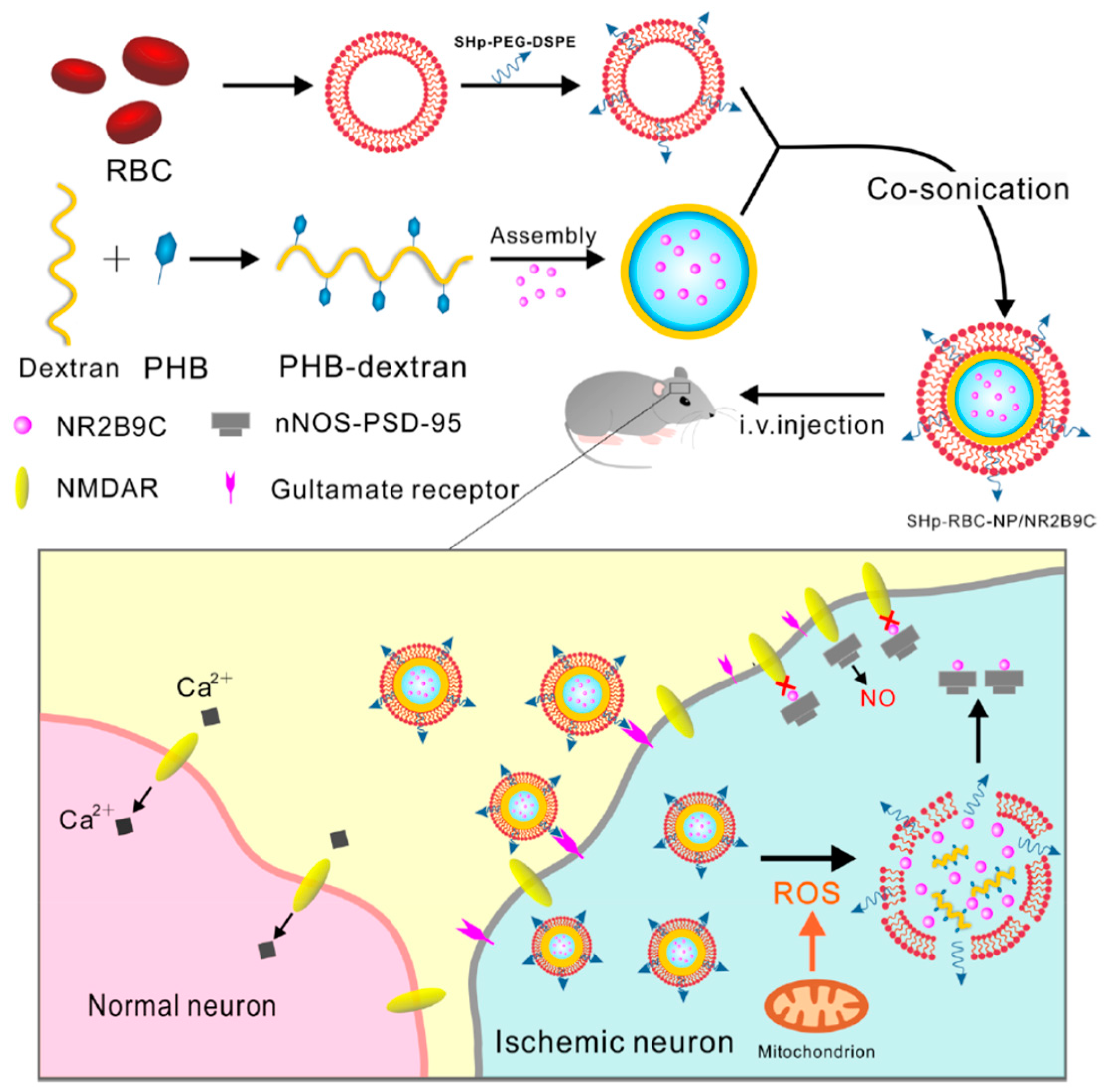
| PHB-DEX [67] | NP (sonication) | ROS | --- | systemic | Stroke --- | PC-12; BCECs | SD rat | Improved pharmacokinetics # Neurological amelioration # |
| CMC [68] | NP (precipitation) | Magnetic | --- | --- | --- DPM | HLMVE | --- | Enhanced uptake § |
| CH/ DEX [69] | NP (ion gelation) | Magnetic | --- | systemic | GBM --- | C6; U87 | C6 xenograft | Enhanced uptake § Synergism # |
| CH [70] | micelles (solvent evap) | Magnetic | --- | NB | TBI DNA | HT22 | SD rats | Enhanced uptake § Improved pharmacokinetics # |
| CH [71] | NP (precipitation) | Magnetic | Tf | systemic | GBM DOX | U251 MG | --- | Enhanced uptake § |
| CH [72] | NP (precipitation) | Magnetic/ Redox | CTX | CED | GBM BG | SF767 | GBM6 mice xenograft | Enhanced uptake § Improved pharmacokinetics # Synergism # |
| HA [73] | NP (chem-coat) | Magnetic/ pH | cAA; Lf; HA | NB | GBM LND | U87MG | Wistar rats | Enhanced uptake § Improved pharmacokinetics # |
| PS Ref | System (Preparation) | AR | Disease Drug | Performance | ||
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | Outcome | ||||
| Ace-DEX [82] | NF (extrusion) | Local | GBM PTX | U87 | Athymic nude mice | Controlled release § Improved pharmacokinetics # |
| CH/ALG/ AGAR [83] | Hydrogel (cryogelation) | Local | Parkinson DMT | OE-MSCs | --- | Neuronal differentiation § |
| HA [84] | Scaffold (mixing) | Local | TBI bFGF | NSCs | TBI rats | Stem cell differentiation § Neurological amelioration # |
| CCH [85] | Conjugate (chem-coup) | NB | Parkinson DA Tyr | OECs | --- | Enhanced uptake § |
| CMCH [86] | NP (desolvation) | NB | Epilepsy CBZ | --- | C57BL mice | Improved pharmacokinetics # |
| CH [87] | NSP (ion gelation) | NB | Alzheimer DNZ | --- | SD rats | Improved pharmacokinetics # |
| CH [88] | NP (ion gelation) | NB | Parkinson RGT | SH-SY5Y | SD rats | Enhanced uptake § Improved pharmacokinetics # Neurological amelioration *# |
| CG [89] | NP (ion gelation) | NB | Parkinson RGN | GNM | Swiss albino mice | Enhanced permeation § Improved pharmacokinetics # |
| CH [90] | NP (ion gelation) | NB | Migraine ZMT | --- | Swiss albino mice | Improved pharmacokinetics # |
| CH [91] | NP (ion gelation) | NB | CNS Lymphoma MTX | --- | SD rats | Improved pharmacokinetics # |
| CH [92] | NP (ion gelation) | NB | Ischemia RUT | GNM | Wistar rats | Improved pharmacokinetics # Neurological amelioration # |
| CH [93] | NP (ion gelation) | NB | Ischemia SCU | --- | C57BL mice | Improved pharmacokinetics # |
| CH [94] | NP (ion gelation) | NB | GBM anti-Gal-1 siRNA | GL261; HPC | GL261-WT; GL261-BFP orthotopic mice | Enhanced uptake § Neurological amelioration # |
| CH [95] | NP (ion gelation) | NB | Schizophrenia QF | GNM | SD rats | Enhanced permeation § Improved pharmacokinetics # |
| CH [96] | ME (water trit) | NB | Schizophrenia QF | GNM | SD rats | Enhanced permeation § Improved pharmacokinetics # |
| CH [97] | NE (emulsion) | NB | BBB overcoming CUR | SNM | --- | Enhanced permeation § |
| CH [98] | ME (water trit) | NB | Dementia RIV | SNM | SD rats | Enhanced permeation § Improved pharmacokinetics # |
| CH [99] | NE (water trit) | NB | Migraine ZMT | SNM | SD rats | Enhanced permeation § Improved pharmacokinetics # |
| TMCH [100] | NE (HP-homogen) | NB | Parkinson ROP | --- | Swiss albino mice | Improved pharmacokinetics # |
| CH [101] | CSLNP (HP-homogen) | NB | Glioma TMZ | --- | Winstar rats | Improved pharmacokinetics # Reduced toxicity # |
| CH [102] | CSNP (solv em evap) | NB | Depression DVX | porcine mucin | Wistar rats | Improved pharmacokinetics # Neurological amelioration * # |
| CH-Asp [103] | ME (water trit) | NB | Anxiety BUS | SNM | Wistar albino rats | Improved pharmacokinetics # |
| CH [104] | CSNP (solv em evap) | NB | Parkinson RGN | GNM | Wistar rat | Enhanced permeation § Improved pharmacokinetics # |
| CH [105] | CSNP (double em) | NB | Epilepsy CAT | GNM | Wistar rats | Enhanced permeation § Improved pharmacokinetics # |
| CH [106] | CSNP (solv em evap) | NB | Cerebral Ischemia GA | GNM | Albino Wistar Rats | Mucoadhesion § Enhanced permeation § Improved pharmacokinetics # |
| CH [107] | CSLM (melt em) | NB | --- REV | NCM460 | Wistar rats | Enhanced permeation § Improved pharmacokinetics # |
| CH [108] | CSNP (melt em) | NB | Parkinson GDNF | --- | C57BL/6J mice | Neurological amelioration # |
| CH [109] | CSL (film hydration) | NB | Cachexia GHRL | Calu3 | --- | Mucoadhesion § Enhanced permeation § |
| CH [110] | Gel (cold method) | NB | HIV DB213 | --- | SD rats; C57BL/6J mice | Improved pharmacokinetics # |
| CH [111] | Gel (cold method) | NB | Polyglutamine diseases QBP1/L1P3V8 | --- | SD rats; C57 WT mice; R6/2 HD transgenic mice | Improved pharmacokinetics # Neurological amelioration * # |
| CH/ HPMC [112] | Gel (cold method) | NB | --- CRM | --- | --- | Controlled release § |
| HA [113] | MP (ion interaction) | NB | Alzheimer --- | CHO | AppNL−G-F knock-in mouse | Enhanced uptake § Improved pharmacokinetics # |
| HA / DAEDEX [114] | CSNP (physical coating) | NB | Anxiolytics ZPD | --- | BALB/c mice | Neurological amelioration * |
| HA [115] | NE (spontaneous em) | NB | Neurodegenerative diseases REV; CUR | SNM | Albino rats | Enhanced permeation § Improved pharmacokinetics # |
| GG/XG [116] | Gel (melt em-probe sonication) | NB | Alzheimer REV | SNM | SD rats | Enhanced permeation § Improved pharmacokinetics # |
| GG [117] | Gel (cold gelation) | NB | Migraine SMP | SNM | SD rats | Enhanced permeation § Improved pharmacokinetics # Neurological amelioration * |
| Disease (Ref) | PS | Load | AR | Vectorization | Efficacy | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | L | Adm | Stimuli | Passive | Active | In Vitro | In Vivo | |||
| Alzheimer [21,43,45,46,87,98,113,115,116] | CH (70) HA (20) GG (10) | 60 | 40 | 40 | 40 | -- | 10 | 40 | 50 | 80 |
| Parkinson [29,30,85,88,89,100,104,108,115] | CH (67) HA (17) MDX (8) | 83 | 33 | 50 | 58 | -- | 25 | 17 | 58 | 83 |
| Brain tumors [31,32,36,39,40,41,42,44,64,65,66,69,71,72,73,82,91,94] | HA (47) CH (24) CMC (12) HP (6) DEX (6) | 74 | 58 | 21 | 16 | 37 | 16 | 58 | 74 | 42 |
| Ischemia [27,67,86,92,106] | CH (60) HA (20) DEX (20) | 80 | 40 | 60 | 60 | 20 | 20 | -- | 60 | 100 |
| TBI [70,84] | CH (50) HA (50) | 50 | 50 | 50 | 50 | 50 | 50 | -- | 50 | 100 |
| Migraine [34,90,99,117] | CH (75) GG (25) | 100 | 25 | 75 | 75 | -- | 25 | -- | 50 | 100 |
| HIV [47,110,119] | CH (100) | 100 | 33 | 67 | 67 | 0 | 0 | 33 | 67 | 67 |
| Psychiatric disorders [95,96,102,103,105,114] | CH (80) HA (20) | 100 | -- | 100 | 100 | -- | -- | -- | 80 | 100 |
| Epilepsy [86,105] | CH (100) | 100 | -- | 100 | -- | -- | 100 | -- | 50 | 100 |
| Other [109,111] | CH (100) | 100 | -- | 100 | 100 | -- | -- | -- | 50 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curcio, M.; Cirillo, G.; Rouaen, J.R.C.; Saletta, F.; Nicoletta, F.P.; Vittorio, O.; Iemma, F. Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective. Pharmaceutics 2020, 12, 1183. https://doi.org/10.3390/pharmaceutics12121183
Curcio M, Cirillo G, Rouaen JRC, Saletta F, Nicoletta FP, Vittorio O, Iemma F. Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective. Pharmaceutics. 2020; 12(12):1183. https://doi.org/10.3390/pharmaceutics12121183
Chicago/Turabian StyleCurcio, Manuela, Giuseppe Cirillo, Jourdin R. C. Rouaen, Federica Saletta, Fiore Pasquale Nicoletta, Orazio Vittorio, and Francesca Iemma. 2020. "Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective" Pharmaceutics 12, no. 12: 1183. https://doi.org/10.3390/pharmaceutics12121183
APA StyleCurcio, M., Cirillo, G., Rouaen, J. R. C., Saletta, F., Nicoletta, F. P., Vittorio, O., & Iemma, F. (2020). Natural Polysaccharide Carriers in Brain Delivery: Challenge and Perspective. Pharmaceutics, 12(12), 1183. https://doi.org/10.3390/pharmaceutics12121183








