Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals
Abstract
1. Introduction
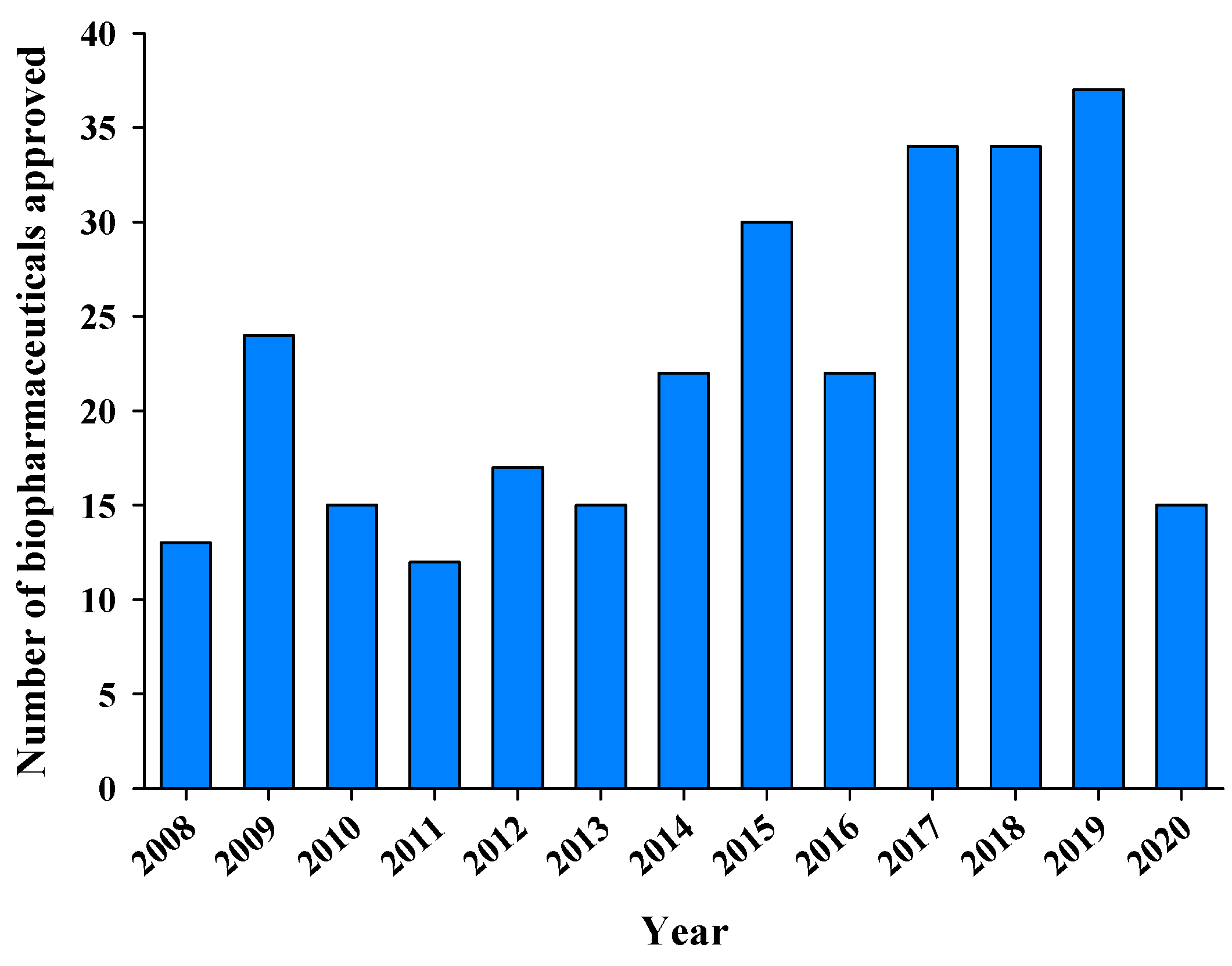
2. Overview of the Clinical and Commercial Success of Biopharmaceuticals
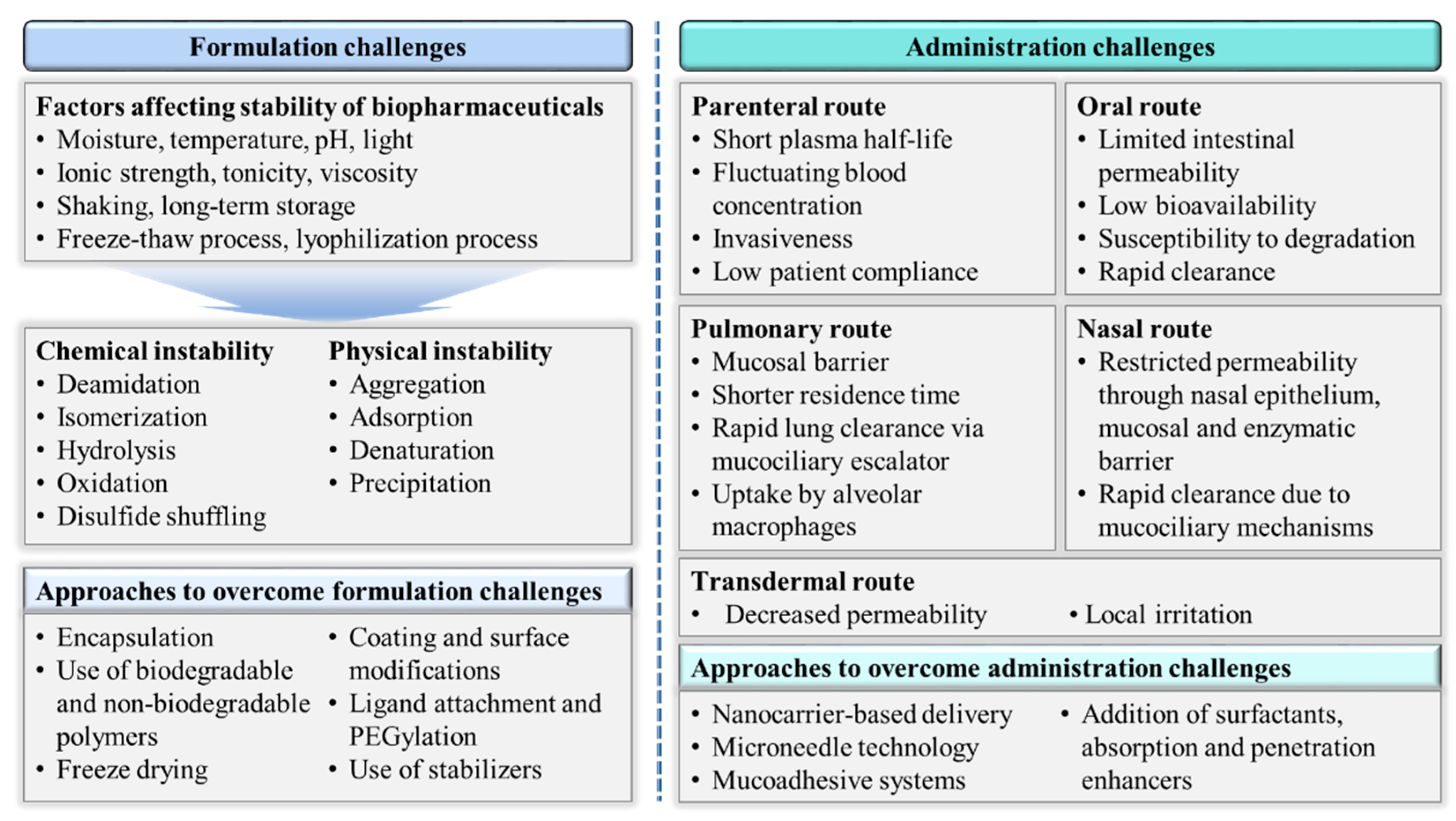
3. Challenges in the Successful Delivery of Biopharmaceuticals
3.1. Formulation Challenges
3.2. Administration Challenges
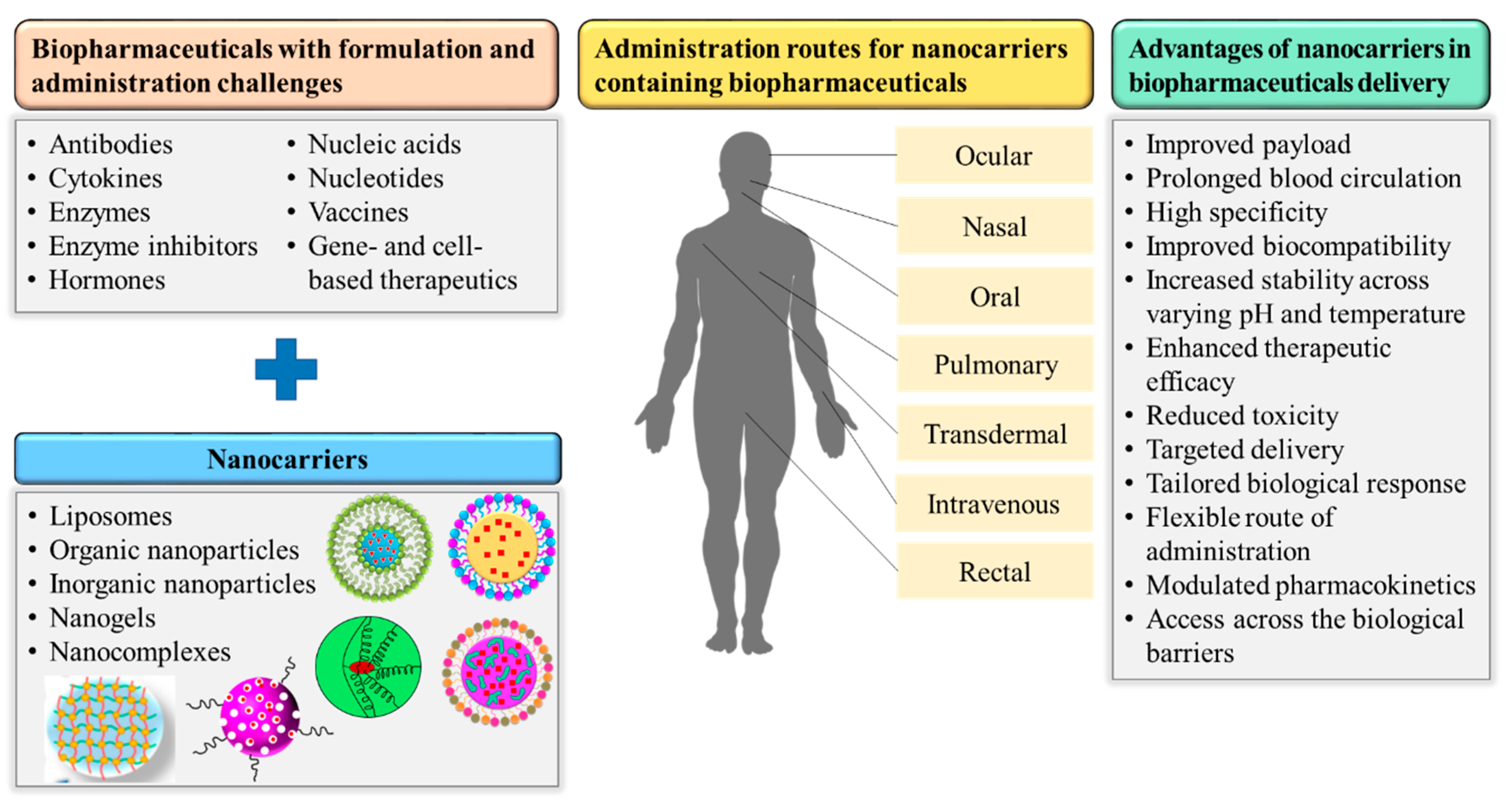
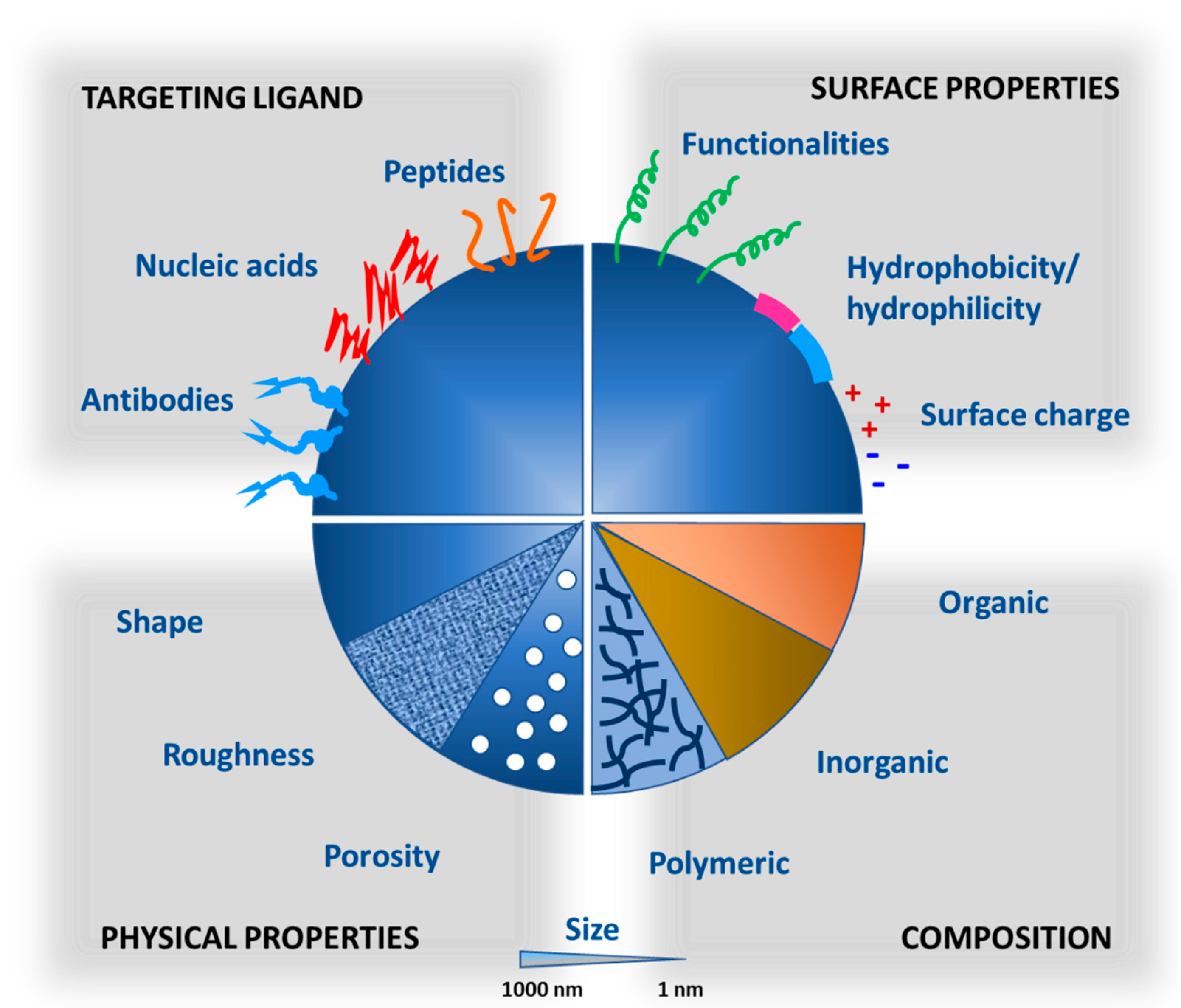
4. Applications of Nanocarriers in Successful Biopharmaceutical Delivery
4.1. Nanocarriers-Mediated Hormones Delivery
4.2. Nanocarriers-Mediated Cytokines Delivery
4.3. Nanocarriers-Mediated Nucleic Acid and Nucleotide Delivery
4.4. Nanocarriers-Mediated Vaccines Delivery
4.5. Nanocarriers-Mediated Antibodies Delivery
4.6. Nanocarriers-Mediated Delivery of Enzymes and Enzyme Inhibitors
4.7. Nanocarriers-Mediated Delivery of Gene- and Cell-Based Therapies
5. Hurdles in the Clinical Translation and Commercialization of Nanocarriers
5.1. Biological Hurdles
5.2. Technological Hurdles
5.3. Nanotoxicological Hurdles
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rader, R.A. (Re) defining biopharmaceutical. Nat. Biotechnol. 2008, 26, 743–751. [Google Scholar] [CrossRef]
- Silva, A.C.; Lopes, C.M.; Lobo, J.M.; Amaral, M.H. Delivery systems for biopharmaceuticals. Part I: Nanoparticles and microparticles. Curr. Pharm. Biotechnol. 2015, 16, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.; Storm, G.; Verrijk, R.; de Leede, L.; Jiskoot, W.; Hennink, W.E. Shifting paradigms: Biopharmaceuticals versus low molecular weight drugs. Int. J. Pharm. 2003, 266, 3–16. [Google Scholar] [CrossRef]
- Walsh, G. Biopharmaceutical benchmarks 2010. Nat. Biotechnol. 2010, 28, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Engen, J.R.; Mazzeo, J.R.; Jones, G.B. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat. Rev. Drug Discov. 2012, 11, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat. Rev. Drug Discov. 2002, 1, 457–462. [Google Scholar] [CrossRef]
- Ezan, E. Pharmacokinetic studies of protein drugs: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 1065–1073. [Google Scholar] [CrossRef]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Byeon, J.C.; Ahn, J.B.; Jang, W.S.; Lee, S.-E.; Choi, J.-S.; Park, J.-S. Recent formulation approaches to oral delivery of herbal medicines. J. Pharm. Investig. 2019, 49, 17–26. [Google Scholar] [CrossRef]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.-S.; Lee, G.-Y.; Kim, J.-K. Potential of nanoparticulate carriers for improved drug delivery via skin. J. Pharm. Investig. 2019, 49, 485–517. [Google Scholar] [CrossRef]
- Luangtana-anan, M.; Nunthanid, J.; Limmatvapirat, S. Potential of different salt forming agents on the formation of chitosan nanoparticles as carriers for protein drug delivery systems. J. Pharm. Investig. 2019, 49, 37–44. [Google Scholar] [CrossRef]
- Din, F.u.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Qureshi, O.S.; Kim, H.S.; Cha, J.H.; Kim, H.S.; Kim, J.K. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomed. 2016, 11, 3813–3824. [Google Scholar]
- Khan, N.; Shah, F.A.; Rana, I.; Ansari, M.M.; Din, F.u.; Rizvi, S.Z.H.; Aman, W.; Lee, G.-Y.; Lee, E.-S.; Kim, J.-K.; et al. Nanostructured lipid carriers-mediated brain delivery of carbamazepine for improved in vivo anticonvulsant and anxiolytic activity. Int. J. Pharm. 2020, 577, 119033. [Google Scholar] [CrossRef]
- Rana, I.; Khan, N.; Ansari, M.M.; Shah, F.A.; Din, F.u.; Sarwar, S.; Imran, M.; Qureshi, O.S.; Choi, H.-I.; Lee, C.-H.; et al. Solid lipid nanoparticles-mediated enhanced antidepressant activity of duloxetine in lipopolysaccharide-induced depressive model. Colloids Surf. B Biointerfaces 2020, 194, 111209. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.; Asad, M.I.; Qindeel, M.; Afzal, I.; Dar, M.J.; Shah, K.U.; Zeb, A.; Khan, G.M.; Ahmed, N.; Din, F.-u. Polymeric nanogels as versatile nanoplatforms for biomedical applications. J. Nanomater. 2019, 2019, 1526186. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Manchester, M.; Singh, P. Virus-based nanoparticles (VNPs): Platform technologies for diagnostic imaging. Adv. Drug Deliv. Rev. 2006, 58, 1505–1522. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Leavy, O. Therapeutic antibodies: Past, present and future. Nat. Rev. Immunol. 2010, 10, 297. [Google Scholar] [CrossRef]
- Dutton, R.L.; Scharer, J.M. Advanced Technologies in Biopharmaceutical Processing, 1st ed.; Blackwell Publishing Ltd.: Ames, IA, USA, 2007. [Google Scholar]
- Mellstedt, H. Anti-neoplastic biosimilars--the same rules as for cytotoxic generics cannot be applied. Ann. Oncol. 2013, 24 (Suppl. 5), v23–v28. [Google Scholar] [CrossRef]
- Ecker, D.M.; Jones, S.D.; Levine, H.L. The therapeutic monoclonal antibody market. MAbs 2015, 7, 9–14. [Google Scholar] [CrossRef]
- Rader, R.A. BIOPHARMA: Biopharmaceutical Products in the U.S. and European Markets, U.S. Approvals, 2002-Present. Available online: http://www.biopharma.com/approvals.html (accessed on 20 October 2020).
- Chung, S.W.; Hil-lal, T.A.; Byun, Y. Strategies for non-invasive delivery of biologics. J. Drug Target. 2012, 20, 481–501. [Google Scholar] [CrossRef]
- Mahler, H.C.; Allmendinger, A. Stability, formulation, and delivery of biopharmaceuticals. In Protein Therapeutics, 1st ed.; Vaughan, T., Osbourn, J., Jallal, B., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 469–491. [Google Scholar]
- Daugherty, A.L.; Mrsny, R.J. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliv. Rev. 2006, 58, 686–706. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A. Formulation of biotech products, including biopharmaceutical considerations. In Pharmaceutical Biotechnology: Fundamentals and Applications; Crommelin, D.J.A., Sindelar, R.D., Meibohm, B., Eds.; Springer: New York, NY, USA, 2013; pp. 69–99. [Google Scholar] [CrossRef]
- Lee, W.Y.; Asadujjaman, M.; Jee, J.-P. Long acting injectable formulations: The state of the arts and challenges of poly(lactic-co-glycolic acid) microsphere, hydrogel, organogel and liquid crystal. J. Pharm. Investig. 2019, 49, 459–476. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Min, K.A.; Jang, D.-J.; Ahn, T.Y.; Min, J.H.; Yu, B.E.; Cho, K.H. Practical approaches on the long-acting injections. J. Pharm. Investig. 2020, 50, 147–157. [Google Scholar] [CrossRef]
- Andrews, C.W.; Bennett, L.; Yu, L.X. Predicting human oral bioavailability of a compound: Development of a novel quantitative structure-bioavailability relationship. Pharm. Res. 2000, 17, 639–644. [Google Scholar] [CrossRef]
- Salama, N.N.; Eddington, N.D.; Fasano, A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 15–28. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Khafagy, E.-S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Maftouhian, S.; Aliabadi, A.; Hassanzadeh-Khayyat, M.; Mohammadpour, F.; Khameneh, B.; Hadizadeh, F. Casein-based hydrogel carrying insulin: Preparation, in vitro evaluation and in vivo assessment. J. Pharm. Investig. 2019, 49, 635–641. [Google Scholar] [CrossRef]
- Khan, N.R.; Harun, M.S.; Nawaz, A.; Harjoh, N.; Wong, T.W. Nanocarriers and their actions to improve skin permeability and transdermal drug delivery. Curr. Pharm. Des. 2015, 21, 2848–2866. [Google Scholar] [CrossRef]
- Schuetz, Y.B.; Naik, A.; Guy, R.H.; Kalia, Y.N. Emerging strategies for the transdermal delivery of peptide and protein drugs. Expert Opin. Drug Deliv. 2005, 2, 533–548. [Google Scholar] [CrossRef]
- Cho Lee, A.-R. Microneedle-mediated delivery of cosmeceutically relevant nucleoside and peptides in human skin: Challenges and strategies for dermal delivery. J. Pharm. Investig. 2019, 49, 587–601. [Google Scholar] [CrossRef]
- Emami, F.; Mostafavi Yazdi, S.J.; Na, D.H. Poly(lactic acid)/poly(lactic-co-glycolic acid) particulate carriers for pulmonary drug delivery. J. Pharm. Investig. 2019, 49, 427–442. [Google Scholar] [CrossRef]
- Morales, J.O.; Fathe, K.R.; Brunaugh, A.; Ferrati, S.; Li, S.; Montenegro-Nicolini, M.; Mousavikhamene, Z.; McConville, J.T.; Prausnitz, M.R.; Smyth, H.D.C. Challenges and future prospects for the delivery of biologics: Oral mucosal, pulmonary, and transdermal Routes. Aaps J. 2017, 19, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Douafer, H.; Andrieu, V.; Brunel, J.M. Scope and limitations on aerosol drug delivery for the treatment of infectious respiratory diseases. J. Control. Release 2020, 325, 276–292. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Gao, M.; Shen, X.; Mao, S. Factors influencing drug deposition in thenasal cavity upon delivery via nasal sprays. J. Pharm. Investig. 2020, 50, 251–259. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery—possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for protein delivery: Overview and perspectives. J. Control. Release 2016, 240, 24–37. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Hasan, N.; Rahman, L.; Kim, S.-H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H.; Yoo, J.-W. Recent advances of nanocellulose in drug delivery systems. J. Pharm. Investig. 2020, 50, 553–572. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006, 23, 1417–1450. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.Z.H.; Shah, F.A.; Khan, N.; Muhammad, I.; Ali, K.H.; Ansari, M.M.; Din, F.u.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; et al. Simvastatin-loaded solid lipid nanoparticles for enhanced anti-hyperlipidemic activity in hyperlipidemia animal model. Int. J. Pharm. 2019, 560, 136–143. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle delivery systems in the treatment of diabetes complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Silva, A.C.; Lopes, C.M.; Lobo, J.M.; Amaral, M.H. Delivery systems for biopharmaceuticals. Part II: Liposomes, Micelles, Microemulsions and Dendrimers. Curr. Pharm. Biotechnol. 2015, 16, 955–965. [Google Scholar] [CrossRef]
- Sarmento, B.; Martins, S.; Ferreira, D.; Souto, E.B. Oral insulin delivery by means of solid lipid nanoparticles. Int. J. Nanomed. 2007, 2, 743–749. [Google Scholar]
- Zeb, A.; Cha, J.-H.; Noh, A.R.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; Shin, D.; Shah, F.A.; Majid, A.; Bae, O.-N.; et al. Neuroprotective effects of carnosine-loaded elastic liposomes in cerebral ischemia rat model. J. Pharm. Investig. 2020, 50, 373–381. [Google Scholar] [CrossRef]
- Zhang, N.; Ping, Q.; Huang, G.; Xu, W.; Cheng, Y.; Han, X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int. J. Pharm. 2006, 327, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Chen, C.-T.; Liang, H.-F.; Kulkarni, A.R.; Lee, P.-W.; Chen, C.-H.; Sung, H.-W. Novel nanoparticles for oral insulin delivery via the paracellular pathway. Nanotechnology 2007, 18, 105102. [Google Scholar] [CrossRef]
- Wu, Z.M.; Zhou, L.; Guo, X.D.; Jiang, W.; Ling, L.; Qian, Y.; Luo, K.Q.; Zhang, L.J. HP55-coated capsule containing PLGA/RS nanoparticles for oral delivery of insulin. Int. J. Pharm. 2012, 425, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, S.M.; Chen, P.P.; Cheng, L.; Zhou, W.; Tang, W.X.; Chen, Z.W.; Ke, C.M. Controlled release of insulin from PLGA nanoparticles embedded within PVA hydrogels. J. Mater. Sci. Mater. Med. 2007, 18, 2205–2210. [Google Scholar] [CrossRef]
- Jain, S.; Rathi, V.V.; Jain, A.K.; Das, M.; Godugu, C. Folate-decorated PLGA nanoparticles as a rationally designed vehicle for the oral delivery of insulin. Nanomedicine 2012, 7, 1311–1337. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Gryder, B.E.; Austin, L.A.; Tene Defo, B.A.; Hayden, S.C.; Pi, M.; Quarles, L.D.; Oyelere, A.K.; El-Sayed, M.A. Antiandrogen gold nanoparticles dual-target and overcome treatment resistance in hormone-insensitive prostate cancer cells. Bioconjugate Chem. 2012, 23, 1507–1512. [Google Scholar] [CrossRef]
- Park, M.R.; Seo, B.B.; Song, S.C. Dual ionic interaction system based on polyelectrolyte complex and ionic, injectable, and thermosensitive hydrogel for sustained release of human growth hormone. Biomaterials 2013, 34, 1327–1336. [Google Scholar] [CrossRef]
- Naot, D.; Musson, D.S.; Cornish, J. The Activity of Peptides of the Calcitonin Family in Bone. Physiol. Rev. 2019, 99, 781–805. [Google Scholar] [CrossRef]
- Lee, M.-J.; Seo, D.-Y.; Lee, H.-E.; Choi, G.J. Therapeutic effect of chitosan modification on salmon-calcitonin-loaded PLGA nanoparticles. Korean J. Chem. Eng. 2011, 28, 1406–1411. [Google Scholar] [CrossRef]
- Makhlof, A.; Werle, M.; Tozuka, Y.; Takeuchi, H. Nanoparticles of glycol chitosan and its thiolated derivative significantly improved the pulmonary delivery of calcitonin. Int. J. Pharm. 2010, 397, 92–95. [Google Scholar] [CrossRef]
- Si, M.; Sun, Q.; Ding, H.; Cao, C.; Huang, M.; Wang, Q.; Yang, H.; Yao, Y. Melatonin-Loaded Nanoparticles for Enhanced Antidepressant Effects and HPA Hormone Modulation. Adv. Polym. Technol. 2020, 2020, 4789475. [Google Scholar] [CrossRef]
- Rajkumar, L.; Guzman, R.C.; Yang, J.; Thordarson, G.; Talamantes, F.; Nandi, S. Prevention of mammary carcinogenesis by short-term estrogen and progestin treatments. Breast Cancer Res. 2004, 6, R31–R37. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Jiang, X.; Kagan, R. Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int. 2018, 29, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Bhardwaj, V.; Bala, I.; Sitterberg, J.; Bakowsky, U.; Ravi Kumar, M.N. Design of estradiol loaded PLGA nanoparticulate formulations: A potential oral delivery system for hormone therapy. Pharm. Res. 2006, 23, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Watanabe, A.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal permeability of estradiol using combination of PLGA nanoparticles system and iontophoresis. Colloids Surf. B Biointerfaces 2012, 97, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Christian, D.A.; Hunter, C.A. Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy 2012, 4, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, Z.; Su, J.; Wei, L.; Yuan, W.; Jin, T. Development of dextran nanoparticles for stabilizing delicate proteins. Nanoscale Res. Lett. 2013, 8, 197. [Google Scholar] [CrossRef]
- Ribeiro, E.B.; de Marchi, P.G.F.; Honorio-França, A.C.; França, E.L.; Soler, M.A.G. Interferon-gamma carrying nanoemulsion with immunomodulatory and anti-tumor activities. J. Biomed. Mater. Res. Part A 2020, 108, 234–245. [Google Scholar] [CrossRef]
- Fodor-Kardos, A.; Kiss, Á.F.; Monostory, K.; Feczkó, T. Sustained in vitro interferon-beta release and in vivo toxicity of PLGA and PEG-PLGA nanoparticles. Rsc Adv. 2020, 10, 15893–15900. [Google Scholar] [CrossRef]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a drug delivery system based on chitosan nanoparticles for oral administration of interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef]
- Ou, W.; Jiang, L.; Gu, Y.; Soe, Z.C.; Kim, B.K.; Gautam, M.; Poudel, K.; Pham, L.M.; Phung, C.D.; Chang, J.-H.; et al. Regulatory T Cells Tailored with pH-Responsive Liposomes Shape an Immuno-Antitumor Milieu against Tumors. Acs Appl. Mater. Interfaces 2019, 11, 36333–36346. [Google Scholar] [CrossRef]
- Kwon, D.; Cha, B.G.; Cho, Y.; Min, J.; Park, E.-B.; Kang, S.-J.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles for Directing in Vivo M2 Macrophage Polarization by Delivering IL-4. Nano Lett. 2017, 17, 2747–2756. [Google Scholar] [CrossRef]
- Zheng, Y.; Stephan, M.T.; Gai, S.A.; Abraham, W.; Shearer, A.; Irvine, D.J. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Control. Release 2013, 172, 426–435. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Aminabhavi, T.M. Chitosan as a carrier for targeted delivery of small interfering RNA. Int. J. Pharm. 2010, 399, 1–11. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Ganguly, K.; Kulkarni, A.R.; Kulkarni, V.H.; Nadagouda, M.N.; Rudzinski, W.E.; Aminabhavi, T.M. Cyclodextrin-based siRNA delivery nanocarriers: A state-of-the-art review. Expert Opin. Drug Deliv. 2011, 8, 1455–1468. [Google Scholar] [CrossRef]
- Benfer, M.; Kissel, T. Cellular uptake mechanism and knockdown activity of siRNA-loaded biodegradable DEAPA-PVA-g-PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2012, 80, 247–256. [Google Scholar] [CrossRef]
- Frede, A.; Neuhaus, B.; Klopfleisch, R.; Walker, C.; Buer, J.; Müller, W.; Epple, M.; Westendorf, A.M. Colonic gene silencing using siRNA-loaded calcium phosphate/PLGA nanoparticles ameliorates intestinal inflammation in vivo. J. Control. Release 2016, 222, 86–96. [Google Scholar] [CrossRef]
- Canup, B.S.B.; Song, H.; Le Ngo, V.; Meng, X.; Denning, T.L.; Garg, P.; Laroui, H. CD98 siRNA-loaded nanoparticles decrease hepatic steatosis in mice. Dig. Liver Dis. 2017, 49, 188–196. [Google Scholar] [CrossRef]
- Ghalamfarsa, G.; Rastegari, A.; Atyabi, F.; Hassannia, H.; Hojjat-Farsangi, M.; Ghanbari, A.; Anvari, E.; Mohammadi, J.; Azizi, G.; Masjedi, A.; et al. Anti-angiogenic effects of CD73-specific siRNA-loaded nanoparticles in breast cancer-bearing mice. J. Cell. Physiol. 2018, 233, 7165–7177. [Google Scholar] [CrossRef]
- Giacalone, G.; Bochot, A.; Fattal, E.; Hillaireau, H. Drug-Induced Nanocarrier Assembly as a Strategy for the Cellular Delivery of Nucleotides and Nucleotide Analogues. Biomacromolecules 2013, 14, 737–742. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Q.; Li, H.; Kang, C.; Liu, Y.; Guo, T.; Shang, K.; Yan, C.; Cheng, G.; Lee, R.J. Lipid nanoparticles loaded with an antisense oligonucleotide gapmer against Bcl-2 for treatment of lung cancer. Pharm. Res. 2017, 34, 310–320. [Google Scholar] [CrossRef]
- Brito, L.A.; Chan, M.; Shaw, C.A.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.R.; Beard, C.W.; et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef]
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, J.; Cantley, W.; Qin, L.L.; Racie, T.; et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 1864–1869. [Google Scholar] [CrossRef]
- Akinc, A.; Goldberg, M.; Qin, J.; Dorkin, J.R.; Gamba-Vitalo, C.; Maier, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Manoharan, M.; et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol. Ther. 2009, 17, 872–879. [Google Scholar] [CrossRef]
- Khan, O.F.; Kowalski, P.S.; Doloff, J.C.; Tsosie, J.K.; Bakthavatchalu, V.; Winn, C.B.; Haupt, J.; Jamiel, M.; Langer, R.; Anderson, D.G. Endothelial siRNA delivery in nonhuman primates using ionizable low–molecular weight polymeric nanoparticles. Sci. Adv. 2018, 4, eaar8409. [Google Scholar] [CrossRef]
- Shim, G.; Han, S.-E.; Yu, Y.-H.; Lee, S.; Lee, H.Y.; Kim, K.; Kwon, I.C.; Park, T.G.; Kim, Y.B.; Choi, Y.S.; et al. Trilysinoyl oleylamide-based cationic liposomes for systemic co-delivery of siRNA and an anticancer drug. J. Control. Release 2011, 155, 60–66. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Chen, Y.; Chen, L.; Lin, L.; Li, Y. The characteristics and performance of a multifunctional nanoassembly system for the co-delivery of docetaxel and iSur-pDNA in a mouse hepatocellular carcinoma model. Biomaterials 2010, 31, 916–922. [Google Scholar] [CrossRef]
- Lee, S.J.; Yook, S.; Yhee, J.Y.; Yoon, H.Y.; Kim, M.-G.; Ku, S.H.; Kim, S.H.; Park, J.H.; Jeong, J.H.; Kwon, I.C.; et al. Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J. Control. Release 2015, 220, 631–641. [Google Scholar] [CrossRef]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. Acs Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef]
- Köping-Höggård, M.; Sánchez, A.; Alonso, M.J. Nanoparticles as carriers for nasal vaccine delivery. Expert Rev. Vaccines 2005, 4, 185–196. [Google Scholar] [CrossRef]
- Bastola, R.; Lee, S. Physicochemical properties of particulate vaccine adjuvants: Their pivotal role in modulating immune responses. J. Pharm. Investig. 2019, 49, 279–285. [Google Scholar] [CrossRef]
- Gregory, A.; Williamson, D.; Titball, R. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Lundstrom, K. Replicon RNA viral vectors as vaccines. Vaccines 2016, 4, 39. [Google Scholar] [CrossRef]
- Chong, C.S.W.; Cao, M.; Wong, W.W.; Fischer, K.P.; Addison, W.R.; Kwon, G.S.; Tyrrell, D.L.; Samuel, J. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J. Control. Release 2005, 102, 85–99. [Google Scholar] [CrossRef]
- Bazzill, J.D.; Stronsky, S.M.; Kalinyak, L.C.; Ochyl, L.J.; Steffens, J.T.; van Tongeren, S.A.; Cooper, C.L.; Moon, J.J. Vaccine nanoparticles displaying recombinant Ebola virus glycoprotein for induction of potent antibody and polyfunctional T cell responses. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 414–425. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, W.; Liu, H.; Li, C.; Zhang, Y.; Meng, X.; Tang, T.; Xi, T.; Xing, Y. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur. J. Pharm. Biopharm. 2017, 111, 33–43. [Google Scholar] [CrossRef]
- Tao, P.; Mahalingam, M.; Zhu, J.; Moayeri, M.; Sha, J.; Lawrence, W.S.; Leppla, S.H.; Chopra, A.K.; Rao, V.B. A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague. mBio 2018, 9, e01926-18. [Google Scholar] [CrossRef]
- Gao, Y.; Wijewardhana, C.; Mann, J.F.S. Virus-Like Particle, Liposome, and Polymeric Particle-Based Vaccines against HIV-1. Front. Immunol. 2018, 9, 345. [Google Scholar] [CrossRef]
- Visciano, M.L.; Diomede, L.; Tagliamonte, M.; Tornesello, M.L.; Asti, V.; Bomsel, M.; Buonaguro, F.M.; Lopalco, L.; Buonaguro, L. Generation of HIV-1 Virus-Like Particles expressing different HIV-1 glycoproteins. Vaccine 2011, 29, 4903–4912. [Google Scholar] [CrossRef]
- Fan, Y.-N.; Li, M.; Luo, Y.-L.; Chen, Q.; Wang, L.; Zhang, H.-B.; Shen, S.; Gu, Z.; Wang, J. Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater. Sci. 2018, 6, 3009–3018. [Google Scholar] [CrossRef]
- Coolen, A.-L.; Lacroix, C.; Mercier-Gouy, P.; Delaune, E.; Monge, C.; Exposito, J.-Y.; Verrier, B. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials 2019, 195, 23–37. [Google Scholar] [CrossRef]
- Maynard, J.; Georgiou, G. Antibody engineering. Annu. Rev. Biomed. Eng. 2000, 2, 339–376. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Liao, C.; Sun, Q.; Liang, B.; Shen, J.; Shuai, X. Targeting EGFR-overexpressing tumor cells using Cetuximab-immunomicelles loaded with doxorubicin and superparamagnetic iron oxide. Eur. J. Radiol. 2011, 80, 699–705. [Google Scholar] [CrossRef]
- Zhang, R.; Qian, J.; Li, X.; Yuan, Y. Treatment of experimental autoimmune uveoretinitis with intravitreal injection of infliximab encapsulated in liposomes. Br. J. Ophthalmol. 2017, 101, 1731–1738. [Google Scholar] [CrossRef]
- Pabari, R.M.; Mattu, C.; Partheeban, S.; Almarhoon, A.; Boffito, M.; Ciardelli, G.; Ramtoola, Z. Novel polyurethane-based nanoparticles of infliximab to reduce inflammation in an in-vitro intestinal epithelial barrier model. Int. J. Pharm. 2019, 565, 533–542. [Google Scholar] [CrossRef]
- Vongchan, P.; Wutti-In, Y.; Sajomsang, W.; Gonil, P.; Kothan, S.; Linhardt, R.J. N,N,N-Trimethyl chitosan nanoparticles for the delivery of monoclonal antibodies against hepatocellular carcinoma cells. Carbohydr. Polym. 2011, 85, 215–220. [Google Scholar] [CrossRef]
- Cheng, Y.A.; Chen, I.J.; Su, Y.C.; Cheng, K.W.; Lu, Y.C.; Lin, W.W.; Hsieh, Y.C.; Kao, C.H.; Chen, F.M.; Roffler, S.R.; et al. Enhanced drug internalization and therapeutic efficacy of PEGylated nanoparticles by one-step formulation with anti-mPEG bispecific antibody in intrinsic drug-resistant breast cancer. Biomater. Sci. 2019, 7, 3404–3417. [Google Scholar] [CrossRef]
- Kouchakzadeh, H.; Shojaosadati, S.A.; Tahmasebi, F.; Shokri, F. Optimization of an anti-HER2 monoclonal antibody targeted delivery system using PEGylated human serum albumin nanoparticles. Int. J. Pharm. 2013, 447, 62–69. [Google Scholar] [CrossRef]
- Karra, N.; Nassar, T.; Ripin, A.N.; Schwob, O.; Borlak, J.; Benita, S. Antibody conjugated PLGA nanoparticles for targeted delivery of paclitaxel palmitate: Efficacy and biofate in a lung cancer mouse model. Small 2013, 9, 4221–4236. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Jhaveri, A.M.; Koshkaryev, A.; Qureshi, F.; Torchilin, V.P. The effect of dual ligand-targeted micelles on the delivery and efficacy of poorly soluble drug for cancer therapy. J. Drug Target. 2013, 21, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Marega, R.; Karmani, L.; Flamant, L.; Nageswaran, P.G.; Valembois, V.; Masereel, B.; Feron, O.; Borght, T.V.; Lucas, S.; Michiels, C.; et al. Antibody-functionalized polymer-coated gold nanoparticles targeting cancer cells: An in vitro and in vivo study. J. Mater. Chem. 2012, 22, 21305–21312. [Google Scholar] [CrossRef]
- Taheri, A.; Dinarvand, R.; Atyabi, F.; Ghahremani, M.H.; Ostad, S.N. Trastuzumab decorated methotrexate-human serum albumin conjugated nanoparticles for targeted delivery to HER2 positive tumor cells. Eur. J. Pharm. Sci. 2012, 47, 331–340. [Google Scholar] [CrossRef]
- Qian, C.; Wang, Y.; Chen, Y.; Zeng, L.; Zhang, Q.; Shuai, X.; Huang, K. Suppression of pancreatic tumor growth by targeted arsenic delivery with anti-CD44v6 single chain antibody conjugated nanoparticles. Biomaterials 2013, 34, 6175–6184. [Google Scholar] [CrossRef]
- Lu, Y.-M.; Huang, J.-Y.; Wang, H.; Lou, X.-F.; Liao, M.-H.; Hong, L.-J.; Tao, R.-R.; Ahmed, M.M.; Shan, C.-l.; Wang, X.-L.; et al. Targeted therapy of brain ischaemia using Fas ligand antibody conjugated PEG-lipid nanoparticles. Biomaterials 2014, 35, 530–537. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Coelho, M.A.N.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B Biointerfaces 2016, 145, 8–13. [Google Scholar] [CrossRef]
- Baklaushev, V.P.; Nukolova, N.N.; Khalansky, A.S.; Gurina, O.I.; Yusubalieva, G.M.; Grinenko, N.P.; Gubskiy, I.L.; Melnikov, P.A.; Kardashova, K.; Kabanov, A.V.; et al. Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT1. Drug Deliv. 2015, 22, 276–285. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, H.H.; Sohn, Y.; Ryu, J.; Park, J.H.; Rhee, W.J.; Park, T.H. α-Galactosidase delivery using 30Kc19-human serum albumin nanoparticles for effective treatment of Fabry disease. Appl. Microbiol. Biotechnol. 2016, 100, 10395–10402. [Google Scholar] [CrossRef]
- Sheng, Y.; He, H.; Zou, H. Poly(lactic acid) nanoparticles coated with combined WGA and water-soluble chitosan for mucosal delivery of β-galactosidase. Drug Deliv. 2014, 21, 370–378. [Google Scholar] [CrossRef]
- Doroud, D.; Zahedifard, F.; Vatanara, A.; Najafabadi, A.R.; Rafati, S. Cysteine proteinase type I, encapsulated in solid lipid nanoparticles induces substantial protection against Leishmania major infection in C57BL/6 mice. Parasite Immunol. 2011, 33, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Baek, S.-H.; Park, J.-S.; Yang, H.K.; Kim, J.-Y.; Kim, C.-K.; Hwang, J.-M. Effect of subconjunctivally injected liposome-encapsulated tissue plasminogen activator on the absorption rate of subconjunctival hemorrhages in rabbits. Cornea 2011, 30, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.T.; Moody, M.R.; Kim, H.; Smulevitz, B.; Huang, S.L.; Holland, C.K.; McPherson, D.D.; Klegerman, M.E. Thrombolytic efficacy of tissue plasminogen activator-loaded echogenic liposomes in a rabbit thrombus model. Thromb. Res. 2012, 130, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Baharifar, H.; Tavoosidana, G.; Karimi, R.; Bidgoli, S.A.; Ghanbari, H.; Faramarzi, M.A.; Amani, A. Optimization of self-assembled chitosan/streptokinase nanoparticles and evaluation of their cytotoxicity and thrombolytic activity. J. Nanosci. Nanotechnol. 2015, 15, 10127–10133. [Google Scholar] [CrossRef]
- Vaidya, B.; Agrawal, G.P.; Vyas, S.P. Platelets directed liposomes for the delivery of streptokinase: Development and characterization. Eur. J. Pharm. Sci. 2011, 44, 589–594. [Google Scholar] [CrossRef]
- Green, J.J.; Zhou, B.Y.; Mitalipova, M.M.; Beard, C.; Langer, R.; Jaenisch, R.; Anderson, D.G. Nanoparticles for Gene Transfer to Human Embryonic Stem Cell Colonies. Nano Lett. 2008, 8, 3126–3130. [Google Scholar] [CrossRef]
- Liang, H.; Huang, K.; Su, T.; Li, Z.; Hu, S.; Dinh, P.-U.; Wrona, E.A.; Shao, C.; Qiao, L.; Vandergriff, A.C.; et al. Mesenchymal Stem Cell/Red Blood Cell-Inspired Nanoparticle Therapy in Mice with Carbon Tetrachloride-Induced Acute Liver Failure. Acs Nano 2018, 12, 6536–6544. [Google Scholar] [CrossRef]
- Jian, W.-H.; Wang, H.-C.; Kuan, C.-H.; Chen, M.-H.; Wu, H.-C.; Sun, J.-S.; Wang, T.-W. Glycosaminoglycan-based hybrid hydrogel encapsulated with polyelectrolyte complex nanoparticles for endogenous stem cell regulation in central nervous system regeneration. Biomaterials 2018, 174, 17–30. [Google Scholar] [CrossRef]
- Ni, M.; Xiong, M.; Zhang, X.; Cai, G.; Chen, H.; Zeng, Q.; Yu, Z. Poly (lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. Int. J. Nanomed. 2015, 10, 2537–2554. [Google Scholar]
- Muntimadugu, E.; Kumar, R.; Saladi, S.; Rafeeqi, T.A.; Khan, W. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf. B Biointerfaces 2016, 143, 532–546. [Google Scholar] [CrossRef]
- Swaminathan, S.K.; Roger, E.; Toti, U.; Niu, L.; Ohlfest, J.R.; Panyam, J. CD133-targeted paclitaxel delivery inhibits local tumor recurrence in a mouse model of breast cancer. J. Control. Release 2013, 171, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Du, X.-J.; Liu, J.; Sun, R.; Zhu, Y.-H.; Wang, J. Delivery of bortezomib with nanoparticles for basal-like triple-negative breast cancer therapy. J. Control. Release 2015, 208, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Jiang, Y.; Zhang, H.; Sun, B.; Hou, C.; Zheng, J.; Liu, Y.; Zuo, P. Active targeting docetaxel-PLA nanoparticles eradicate circulating lung cancer stem-like cells and inhibit liver metastasis. Mol. Pharm. 2015, 12, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, S.; Ming, Y.; Wang, L.; Li, C.; Luo, M.; Li, Z.; Li, B.; Chen, J. Specific cancer stem cell-therapy by albumin nanoparticles functionalized with CD44-mediated targeting. J. Nanobiotechnology 2018, 16, 99. [Google Scholar] [CrossRef]
- Binsalamah, Z.M.; Paul, A.; Khan, A.A.; Prakash, S.; Shum-Tim, D. Intramyocardial sustained delivery of placental growth factor using nanoparticles as a vehicle for delivery in the rat infarct model. Int. J. Nanomed. 2011, 6, 2667. [Google Scholar]
- Zhu, K.; Wu, M.; Lai, H.; Guo, C.; Li, J.; Wang, Y.; Chen, Y.; Wang, C.; Shi, J. Nanoparticle-enhanced generation of gene-transfected mesenchymal stem cells for in vivo cardiac repair. Biomaterials 2016, 74, 188–199. [Google Scholar] [CrossRef]
- Ishii, M.; Shibata, R.; Numaguchi, Y.; Kito, T.; Suzuki, H.; Shimizu, K.; Ito, A.; Honda, H.; Murohara, T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2210–2215. [Google Scholar] [CrossRef]
- Yuan, W.; Hu, Z.; Su, J.; Wu, F.; Liu, Z.; Jin, T. Preparation and characterization of recombinant human growth hormone–Zn2+-dextran nanoparticles using aqueous phase–aqueous phase emulsion. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 424–427. [Google Scholar] [CrossRef]
- Takeuchi, I.; Fukuda, K.; Kobayashi, S.; Makino, K. Transdermal delivery of estradiol-loaded PLGA nanoparticles using iontophoresis for treatment of osteoporosis. Biomed. Mater. Eng. 2016, 27, 475–483. [Google Scholar] [CrossRef]
- Frick, S.U.; Domogalla, M.P.; Baier, G.; Wurm, F.R.; Mailänder, V.; Landfester, K.; Steinbrink, K. Interleukin-2 Functionalized Nanocapsules for T Cell-Based Immunotherapy. Acs Nano 2016, 10, 9216–9226. [Google Scholar] [CrossRef]
- McHugh, M.D.; Park, J.; Uhrich, R.; Gao, W.; Horwitz, D.A.; Fahmy, T.M. Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials 2015, 59, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zheng, Y.; Melo, M.B.; Mabardi, L.; Castaño, A.P.; Xie, Y.Q.; Li, N.; Kudchodkar, S.B.; Wong, H.C.; Jeng, E.K.; et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 2018, 36, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Paul, A.; Prakash, S. Investigation of siRNA-loaded polyethylenimine-coated human serum albumin nanoparticle complexes for the treatment of breast cancer. Cell Biochem. Biophys. 2011, 61, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Han, F.Y.; Grøndahl, L.; Xu, Z.P.; Li, L. Enhanced oral vaccine efficacy of polysaccharide-coated calcium phosphate nanoparticles. Acs Omega 2020, 5, 18185–18197. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Henry, B.; Carter, K.D.; Roni, M.A.; Kouzi, S.S. A Novel Formulation Strategy to Deliver Combined DNA and VLP Based HPV Vaccine. J. Pharm. Pharm. Sci. 2019, 22, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-large pore mesoporous silica nanoparticles enabling co-delivery of high amounts of protein antigen and toll-like receptor 9 agonist for enhanced cancer vaccine efficacy. Acs Cent. Sci. 2018, 4, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Gracia, R.; Marradi, M.; Salerno, G.; Pérez-Nicado, R.; Pérez-San Vicente, A.; Dupin, D.; Rodriguez, J.; Loinaz, I.; Chiodo, F.; Nativi, C. Biocompatible single-chain polymer nanoparticles loaded with an antigen mimetic as potential anticancer vaccine. Acs Macro Lett. 2018, 7, 196–200. [Google Scholar] [CrossRef]
- Voltan, R.; Secchiero, P.; Ruozi, B.; Forni, F.; Agostinis, C.; Caruso, L.; Vandelli, M.A.; Zauli, G. Nanoparticles engineered with rituximab and loaded with Nutlin-3 show promising therapeutic activity in B-leukemic xenografts. Clin. Cancer Res. 2013, 19, 3871–3880. [Google Scholar] [CrossRef]
- Duan, D.; Wang, A.; Ni, L.; Zhang, L.; Yan, X.; Jiang, Y.; Mu, H.; Wu, Z.; Sun, K.; Li, Y. Trastuzumab- and Fab’ fragment-modified curcumin PEG-PLGA nanoparticles: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2018, 13, 1831–1840. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Sievers, E.L.; Senter, P.D. Antibody-drug conjugates in cancer therapy. Annu. Rev. Med. 2013, 64, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.C.; Dawson, M.; Lai, S.K.; Wang, Y.-Y.; Suk, J.S.; Yang, M.; Zeitlin, P.; Boyle, M.P.; Fu, J.; Hanes, J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc. Natl. Acad. Sci. USA 2009, 106, 19268–19273. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.C.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef]
- Hafner, A.; Lovrić, J.; Lakoš, G.P.; Pepić, I. Nanotherapeutics in the EU: An overview on current state and future directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar]
- Teli, M.K.; Mutalik, S.; Rajanikant, G.K. Nanotechnology and nanomedicine: Going small means aiming big. Curr. Pharm. Des. 2010, 16, 1882–1892. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. A chemical engineering perspective of nanoparticle-based targeted drug delivery: A unit process approach. Aiche J. 2016, 62, 966–974. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive imaging of nanomedicines and nanotheranostics: Principles, progress, and prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef] [PubMed]
- Storm, G.; Oussoren, C.; Peeters, P.; Barenholz, Y. Tolerability of liposomes in vivo. Liposome Technol. 1993, 3, 345–383. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Rösslein, M.; Liptrott, N.J.; Owen, A.; Boisseau, P.; Wick, P.; Herrmann, I.K. Sound understanding of environmental, health and safety, clinical, and market aspects is imperative to clinical translation of nanomedicines. Nanotoxicology 2017, 11, 147–149. [Google Scholar] [CrossRef]
- Li, M.; Zou, P.; Tyner, K.; Lee, S. Physiologically based pharmacokinetic (PBPK) modeling of pharmaceutical nanoparticles. Aaps J. 2017, 19, 26–42. [Google Scholar] [CrossRef]
- Yuan, D.; He, H.; Wu, Y.; Fan, J.; Cao, Y. Physiologically based pharmacokinetic modeling of nanoparticles. J. Pharm. Sci. 2019, 108, 58–72. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
| Brand Name | Generic Name | Target | Class | FDA Approved Indications | Company/Developer |
|---|---|---|---|---|---|
| Biopharmaceuticals approved in 2020 | |||||
| Tacartus | Brexucabtagene autoleucel | TNF | mAb | Mantle cell lymphoma | Kite Pharma |
| Hulio | Adalimumab | TNF | mAb | Rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis | Mylan and Fujifilm Kyowa Kirin Biopharmaceuticals |
| Tepezza | Teprotumumab | IGF-1R | mAb | Thyroid eye disease | Horizon Therapeutics |
| Phesgo | Pertuzumab, transtuzumab, and hyaluronidase | HER + hyaluronidase | mAb | Early HER-2-positive breast cancer | Genentech/Roche |
| Lyumjev | Insulin lispro | Beta-cells | rDNA | Type I and type II diabetes | Eli Lilly & Co. |
| Semglee | Insulin glargine | Beta-cells | rDNA | Type I and type II diabetes | Biocon |
| Uplizna | Inebilizumab | Aquaporin-4 | mAb | Neuromyelitis optica spectrum disorder | Viela Bio |
| Nyvepria | Pegfilgrastim | Filgrastim | rDNA | Neutropenia | Neulasta |
| Trodelvy | Sacituzumab | Trop-2 | mAb | Metastatic triple negative breast cancer | Immunomedics |
| Sarclisa | Isatuximab | CD38 | mAb | Multiple myeloma | Sanofi-Aventis |
| Influenza vaccine | H1n1 influenza vaccine | Virus | Vaccine | Prevention of seasonal influenza | Seqirus |
| Vyepti | Eptinezumab | CGRP | mAb | Migraine | Lundbeck |
| Tepezza | Teprotumumab | IGF-IR | mAb | Thyroid eye disease | Horizon Therapeutics Ireland |
| Biopharmaceuticals approved in 2019 | |||||
| Cutaquig | Human immunoglobulin | Immune cells | Ab | Primary humoral immunodeficiency | Octapharma Pharmazeutika |
| Ubrelvy | Ubrogepant | Calcitonin | rDNA | Migraine | Allergen USA |
| Enhertu | Trastuzumab | HER-2 | mAb | Breast cancer | Astra Zeneca and Daiichi Sankyo Co. Ltd. |
| Ervebo | Ebola Zaire vaccine | Glycoprotein | Vaccine | Ebola disease | Merck & Co |
| Padcev | EnfortumAb-vedotin | Nectin-4 | mAb | Urothelial cancer | Seattle Genetics |
| Vyondys 53 | Golodersin | Dystrophin antisense | Oligonucleotide | Duchenne muscular dystrophy | Sarepta Therapeutics |
| Avsola | Infliximab | TNF | mAb | Autoimmune disorders | Amgen |
| Givlaari | Givosiran | ALN-AS1 mRNA | RNAi | Acute hepatic porphyria | Alnylam Pharmaceuticals |
| Adakveo | Crizanlizumab | P-selectin | mAb | Vaso-occlusive crisis | Novartis |
| Abrilada | Adalimumab | TNF | mAb | Rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis | Pfizer |
| Reblozyl | Luspatercept | Activin receptor-igg1 | Fusion protein | Anemia with beta thalassemia | Celgene |
| Ziextenzo | Pegfilgrastim | G-CSF | rDNA | Neutropenia | Sandoz/Novartis |
| Beovu | Brolucizumab | VEGF | mAb | Neovascular (wet) age-related macular degeneration | Novartis |
| Bonsity-teriperatide | Parathyroid hormone | PTH | Protein | Osteoporosis | Pfenex Inc. |
| Jynneos | Smallpox and monkeypox vaccine | Viral proteins | Protein | Smallpox and monkeypox vaccine | Bavarian Nordic |
| Rybelsus | Semaglutide | Glucagon like peptide 1 | Protein | Type 2 diabetes | Novo Nordisk |
| Hadlima | Adalimumab | TNF | mAb | Rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis | Samsung Bioepis |
| Ruxience | Rituximab | CD20 | mAb | Cancer | Pfizer |
| Myxredlin | Insulin, human | Beta cells | Glycoprotein | Diabetes | Baxter |
| Baqsimi nasal powder | Glucagon | - | rDNA | Hypoglycemia | Eli Lilly & Co. |
| Xembify | Immunoglobulin subcutaneous | Immune cells | Ab | Primary immunodeficiency | Grifols |
| Zirabev | Bevacizumab | VEGF | mAb | Colorectal cancer, nonsquamous nonsmall cell lung cancer, glioblastoma, metastatic renal cell carcinoma, and cervical cancer | Pfizer |
| Kanjinti | Trastuzumab | HER-2 | mAb | HER2-positive breast cancer and gastric cancer | Amgen |
| Polivy | Polatuzumab | CD79b | mAb | Diffuse large B-cell lymphoma | Genentech/Roche |
| Zolgensma | Onasemnogene-abeparvovec | Survival motor neuron 1 | Gene therapy | Spinal muscular atrophy | AveXis |
| Dengvaxia | Dengue tetravalent vaccine | Viral protein | Vaccine | Dengue disease | Sanofi Pasteur |
| Enticovo | Etanercept | Tnfr-Fc | Fusion protein | Rheumatoid arthritis, ankylosing spondylitis, plague psoriasis, psoriatic arthritis, and polyarticular juvenile idiopathic arthritis | Samsung Bioepis |
| Skyrizi | Risankizumab | IL-23 | mAb | Plaque psoriasis | AbbVie |
| Evenity | Romosozumab | Sclerostin | mAb | Osteoporotic fracture | Amgen |
| Asceniv | Immunoglobulin | IVIG | Ab | Primary humoral immunodeficiency disease | ADMA Biopharmaceuticals |
| Trazimera | Trastuzumab | HER receptor | mAb | Breast cancer | Pfizer |
| Herceptin hylecta | Trastuzumab and hyaluronidase | MAb plus hyaluronidase | mAb | Breast cancer | Genentech/Roche |
| Esperoct | Turoctocog alfa pegol | Factor VIII | Glycoprotein | Hemophilia | Novo Nordisk |
| Cablivi | Caplacizumab | Von Willebrand’s factor | mAb | Thrombotic thrombocytopenic purpura | Ablynx |
| Jeuveau | Prabotulinumtoxin toxin type A | Botulinum toxin A | Protein | Glabellar lines | Evolus Inc. |
| Ontruzant | Trastuzumab | HER receptor | mAb | Breast cancer | Samsung Biopharmaceuticals |
| Biopharmaceuticals approved in 2018 | |||||
| Aimovig | Erenumab | CGRP | mAb | Migraine prevention | Amgen |
| Retacrit | Epoeitin alfa | EPO | Glycoprotein | Anemia related indication | Hospira/Pfizer |
| Crysvita | Trastzumab | FGF | mAb | X-linked phosphatemia | Ultragenyx Pharmaceutical Inc, |
| Ilumya | Tildrakizumab | IL-23 | mAb | Plaque psoriasis | Sun pharmaceutical Industries LTD. |
| Trogarz | Ibalizumab | Cd4 | mAb | HIV infection | TaiMed Biopharmaceuticals |
| Vaxelis | DTaP-Hb, rDNA | Protein | Hexavalent vaccine | Diphtheria, tetanus, acellular pertussis, polio virus, Hemophilus b conjugate, andhepatitis B | Sanofi Pasteur |
| Ultomiris | Ravulizumab | C5 | mAb | Paroxysmal nocturnal hemoglobinuria | Alexion Pharmaceutical |
| Elzonris | Tagraxofusp-erzs | CD 123 | mAb | Blastic plasmacytoid dendritic cell neoplasm | Stemline Therapeutics |
| Asparlas | Calaspargase | Asparaginase | Enzyme | Acute lymphoblastic leukemia | Servier Pharmaceuticals LLC |
| Herzuma | Transtuzumab | HER receptor | mAb | Breast cancer | Celltrion and Teva |
| Cutaquig | Immunoglobulin subcutaneous | Immunoglobulin | Ab | Primary humoral immunodeficiency | Octapharma |
| Truxima | Rituximab | CD20 | mAb | Non-Hodgkin lymphoma | Celltrion |
| Gamifant | Emapalumab | Interferon gamma | mAb | Hemophagocytic lymphohistiocytosis | Novimmune SA |
| Udenyca | Pegfligrastim | G-CSF | rDNA | Neutropenia from cancer treatment | KBI Biopharma |
| Hyrimoz | Adalimumab | TNF | mAb | Rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis | Sandoz/Novartis |
| Revcovi | Elapegademase | Adenosine deaminase | rDNA | Adenosine deaminase-severe combined immunodeficiency | Leadiant Biosciences |
| Libtayo | Cemiplimab | PD-1 | mAb | Cutaneous squamous cell carcinoma | Regeneron Pharmaceuticals |
| Emgality | Galcanezumab | CGRP | mAb | Migraine | Eli Lilly & Co. |
| Ajovy | Fremanezumab | CGRP | mAb | Migraine | Teva |
| Lumoxiti | Moxetumomab | CD22 | mAb | Hairy cell leukemia | Astra Zeneca |
| Jivi | Anti-hemophilic factor | Factor VIII | RNAi | Hemophilia A | Bayer Corp |
| Takhzyro | Lanadelumab | Kallikrein | mAb | Type I and II hereditary angioedema | Dyax Corp. Shire plc |
| Oxervate | Cenegermin | Transthyretin | RNAi | Neurotrophic keratitis | Alnylam Pharmaceuticals |
| Onpattro | Patisiran | Transthyretin mRNA | RNAi | Polyneuropathy | Alnylam Pharmaceuticals |
| Poteligeo | Mogamulizumab | CCR-4 | mAb | Resistant mycosis fungoides or Sezary syndrome | Kyowa Kirin |
| Panzyga | Immunoglobulin intravenous | Immune cells | Ab | Immune thrombocytopenic purpura | Octapharma |
| Nivestym | Filgrastim | G-CSF | rDNA | Neutropenia | Pfizer |
| Human albumin solution | Albumin | - | Albumin | Hypovolemia, ascites, hypoalbuminemia, acute nephritis, and cardiopulmonary bypass | Bio Products Library |
| Fulphila | Pegfilgrastim | G-CSF | rDNA | Neutropenia | Mylan GmbH |
| Palynziq | Pegvaliase | Phenylalanine ammonia lyase | rDNA | Phenylketonuria | BioMarin |
| Biopharmaceuticals | Therapeutic Class | Target Disease | Nanocarrier | Route | Purpose of the Study | Characteristics of Nanocarriers | Key Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Insulin | Hormone | Diabetes mellitus | FA-PEG-PLGA NPs | Oral | Improving oral delivery of insulin | PS: ~260 nm, PDI: 0.14 ± 0.04, EE: 87.0 ± 1.92% | Twofold increase in insulin bioavailability following NP administration, along with maintenance of blood glucose levels for 24 h. | [69] |
| hGH | Hormone | Hormone deficiency | Thermosensitive hydrogel | Subcutaneous | To enhance the bioavailability and sustained release of hGH | PS: 500 nm, ZP: +8 mV | Sustained release of hGH for 7 days, with a 13-fold extended half-life in hypophysectomized rats. | [71] |
| rhGH | Hormone | Hormone deficiency | Dextran NPs | In vitro assay on rat Nb2-11 lymphoma cells | Efficient and stable rhGH delivery | PS: ~25 nm | 99% bioactivity of rhGH was preserved and analyzed by Nb2-11 cell proliferation assay. | [152] |
| Melatonin | Hormone | Depression | PLA-NPs | Subcutaneous | Enhancing the antidepressant activity and HPA hormone modulation of melatonin | PS: 96.1 ± 13.5 nm, PDI: 0.203 ± 0.01 EE: 33.82 ± 0.53% | Pharmacodynamic models, sucrose preference test, FST, and TST demonstrated efficient antidepressant activity, and HPA axis hormone secretion in pinealectomized rats also improved. | [75] |
| Estradiol | Hormone | Osteoporosis | PLGA-NPs | Transdermal | Increasing skin permeability of estradiol using a nanocarrier and iontophoresis | PS: 165 ± 13.1 nm, EE: 63.4 ± 3.09% | Bone mineral density was significantly increased after iontophoresis; permeation of estradiol also increased, with an effective concentration in blood. | [153] |
| IFNα-2b | Cytokines | Cancers and viral infections | Chitosan NPs | Oral | To improve oral delivery of IFN | PS: 36 ± 8 nm, ZP: +30 mV EE: ~100% | Antiviral activity of NPs in vitro and IFN gene expression were comparable to commercial IFNα; remarkable plasma levels of IFNα were observed following oral administration in mice. | [84] |
| IL-2 | Cytokines | Immune therapy | Nanocapsules | Intravenous | To enhance T cell-based immune therapy by IL-2 | PS: 215 nm, ZP: −7 mV | In vitro T cell targeting and in vivo IL-2 receptor-mediated internalization were enhanced. | [154] |
| TGF-b and IL-2 | Cytokines | Cancer and autoimmune diseases | PLGA NPs | Intraperitoneal | Induction and maintenance of Treg cells by CD4 targeted nanoparticles | PS: 168 nm | In vitro induction and in vivo expansion of CD4+ Treg cells was observed. | [155] |
| IL-4 | Cytokines | Immune therapy | MSNs | Intraperitoneal | Macrophage polarization by cytokine delivery | PS: <200 nm | Targeted delivery of cytokines to phagocytic myeloid cells triggering macrophage polarization and the induction of an immune response. | [86] |
| IL-15 | Cytokines | ACT in metastatic tumors | Nanogels | Intravenous | To enhance T cell therapy through TCR signaling | PS: 80–130 nm, EE: >90% | A 16-fold increase in T cell expansion was observed in tumor cells; increased tumor cell clearance in mice. | [156] |
| siRNA | Nucleotide | Gene therapy in cancers | HAS-NPs | In vitro assay in MCF-7 cells | To prevent degradation and low transfection of siRNA | PS: ~90 nm, ZP: +26 mV, PDI: <0.25 | High transfection (61.66 ± 6.8%) and cytotoxicity were observed. | [157] |
| siRNA | Nucleotide | Intestinal inflammation | PLGA-PEI-NPs | Intrarectal | To prevent intestinal inflammation by colonic gene silencing | PS: 151.52 nm, PDI: 0.38, ZP: 22.08 mV | Excellent gene silencing with no toxicity in cell culture; in vivo application resulted in significant decrease in the target genes in colonic biopsies and mesenteric lymph nodes. | [92] |
| CD98 siRNA | Nucleotide | Nonalcoholic fatty liver disease | PLA-NPs | Parenteral | To reduce hepatic steatosis in mice | PS: 280 nm, ZP: −12.84 mV | Significant downregulation of CD98 and pro-inflammatory cytokines was observed, along with a reduction in blood markers, lipid accumulation, and fibrosis in vivo. | [93] |
| CD73-specific siRNA | Nucleotide | Breast cancer | Chitosan lactate NPs | Intravenous | To evaluate anti-angiogenic effects of CD73 suppression | PS: 70–126 nm, PDI: ~0.3, ZP: ~19 mV, EE: 50–90% | Downregulation of angiogenesis-related molecules and pro-inflammatory cytokines, along with tumor regression due to CD73 gene silencing. | [94] |
| HBcAg antigen | Vaccine | Hepatitis B | PLGA-NPs | Subcutaneous | To enhance the immune response against hepatitis B virus | PS: 279 nm, PDI: 0.17, EE: ~50% | Cellular immune response with high TNF-γ. | [109] |
| Recombinant Ebola virus antigen | Vaccine | Ebola virus disease | Lipid NPs | Subcutaneous | To induce potent antibody and polyfunctional T cell responses | PS: 117.5 ± 17.6 nm, PDI: 0.18 ± 0.01, ZP: −21.7 ± 1.3 mV, EE: ~60% | Germinal center B cells and polyfunctional T cells were produced, along with elicited antibody response. | [110] |
| H. pylori recombinant antigen | Vaccine | Peptic ulcer | PLGA-NPs | Oral | Increasing immune protection in Helicobacter pylori infections | PS: ~200 nm, PDI: 0.228 ± 0.030, EE: 79.07% | 43% of the immunized mice showed a protective effect from infection, along with high levels of urease-specific antibodies and memory T cell responses. | [111] |
| Ovalbumin | Vaccine | Immune therapy | Calcium phosphate NPs | Oral | Enhancing oral vaccine efficacy | PS: 22 nm, ZP: −9.6 mV | Sufficient GI stability, along with effective Caco-2 permeability and enhanced IgA and IgG responses. | [158] |
| HPV antigen | Vaccine | Cervical cancer | VLPs | Oral for systemic and vaginal for local action | Combining the effects of VLP- and DNA-based vaccines | – | Induction of antibody and T cell response. | [159] |
| mRNA-based vaccines | Vaccine | Immune therapy | Lipid NPs | Intravenous | Efficient transport of mRNA-based cancer vaccines | PS: 110 nm, ZP: 25 mV, EE: 80% | Strong and specific T cell response and reduced tumor growth in lymphoma model. | [115] |
| mRNA-based vaccines | Vaccine | HIV | PLA-NPs | In vitro and ex vivo assay | Targeting dendritic cells for effective immune responses | PS: ~275 nm, PDI: 0.13, ZP: 30 mV | Effective phagocytic uptake with strong induction of dendritic cells. | [116] |
| Cancer antigens | Vaccine | Tumor | MSNs | Subcutaneous | To deliver large amounts of protein antigen and Toll-like receptor 9 agonist for enhanced cancer vaccine efficacy | PS: 100–200 nm, ZP: −10.5 mV | Efficient delivery of TLR9 agonist to draining lymph nodes, induction of antigen-specific cytotoxic T lymphocytes, and suppression of tumor growth. | [160] |
| Tn antigen | Vaccine | Tumor | Dextran-based NPs | Ex vivo assay | To conjugate synthetic Tn-antigen mimetic to dextran-based single-chain nanoparticles | PS: ~70 nm, PDI: 0.4, ZP: −18.8 mV | Specific innate tumor modulation, as demonstrated by analysis of IL production. | [161] |
| Infliximab | Antibody | Autoimmune uveoretinitis | Liposomes | Intravitreal | To evaluate the effectiveness of intravitreal injection of liposomes encapsulating infliximab. | PS: 351.3 ± 58 nm, EE: 90.65 ± 2.68%, PDI: 0.386 ZP: −20.8 ± 9.8 mV | Decreased inflammation in eyes with lower toxicity and side effects in autoimmune uveoretinitis rats. | [120] |
| 1E4-1C2 mAb | Antibody | Hepatocellular carcinoma | Chitosan NPs | In vitro mouse monocyte models | Improving the delivery of mAbs against hepatocellular carcinoma | PS: 11.2 ± 0.09 nm, ZP: 16.5 ± 0.5 mV | Sufficient cellular uptake by mononuclear cells and reduced cytotoxicity in monolayer cells. | [122] |
| Anti-HER2 mAb | Antibody | Cancers | PEGylated HSA NPs | In vitro assays | Improving the delivery of anti-HER2 mAbs to cancers | PS: 203 ± 15 nm, PDI: 0.07 ± 0.02, ZP: −14.2 ± 2.1 mV | High interaction with HER2 receptors on the surface of BT474 cells, with no noted toxicity. | [124] |
| Cetuximab | Antibody conjugation | Nonsmall cell lung cancer | PLGA-NPs | Intravenous | Bioconjugation of cetuximab with paclitaxel to enhance its efficacy | PS: 80 nm, ZP: −50 mV, EE: 85–100% | High binding affinity toward overexpressed EGFR cells in tumors; in mice, high inhibition of tumor growth and increased survival rate. | [125] |
| Rituximab | Antibody conjugation | Leukemia | PLGA-NPs | Subcutaneous | Targeted delivery of Nutlin-3 toward CD20 malignant cells using antibody conjugated nanocarriers | – | Increase in the activation of the p53 pathway and enhanced tumor suppression. | [162] |
| Transferrin and 2C5 mAb | Antibody conjugation | Ovarian cancer | Micelles | Subcutaneous | To increase cytotoxicity and targeting efficiency of poorly water-soluble anticancer drug | PS: ~16 nm | In vitro cytotoxicity against ovarian cancer cells was optimal, along with targeted and profound in vivo antitumor activity due to antibody conjugation. | [126] |
| EGFR-targeted mAb | Antibody conjugation | Epidermoid carcinoma tumor | Au-NPs | Intravenous | To enhance tumor targeting and biodistribution | PS: ~5 nm Antibody loading: 1.7 nmol/mg | Enhanced biodistribution profile in both in vitro and in vivo carcinoma models. | [127] |
| Trastuzumab- and Fab′ fragment | Antibody conjugation | Breast cancer | PEG-PLGA NPs | Intravenous | Targeted delivery of curcumin nanoparticles to HER2 in breast cancer cells | PS: 128.5 ± 1.3 nm and 142.5 ± 4.6 PDI: 0.125 ± 0.012 and 0.137 ± 0.023 ZP: 79.5 ± 1.56 and 77.1 ± 5.64 mV | Enhanced cytotoxicity against HER2 cells in vitro and enhanced biodistribution in vivo. | [163] |
| Cysteine proteinase type-I | Enzyme | Leishmania major infection | SLNs | Intraperitoneal | To develop safe, immunogenic vaccine against Leishmania with potent immune response | PS: 380 nm, PDI: 0.4, ZP: −12·4 ± 0·3 mV EE: 48 ± 3% | Following vaccination, the occurrence of parasite decreased, and the cytokine response increased, indicating the necessary immune response. | [135] |
| Tissue plasminogen activator | Enzyme | Subconjunctival hemorrhages | Liposomes | Intravenous | Enhancing the thrombolytic activity of tissue plasminogen activator | PS: 600 nm, EE: 50% | Thrombolytic activity was sufficient and comparable to other clinical regimens. | [137] |
| Streptokinase | Enzyme | Deep vein thrombosis | Chitosan NPs | In vitro assay | Developing streptokinase-loaded nanocarriers for efficient thrombolytic activity | PS: 526 ± 121 nm, PDI: 0.3 ± 0.2, EE: 43 ± 10% | Thrombolytic activity was sufficient in vitro, along with lack of cytotoxic activity. | [138] |
| Streptokinase | Enzyme | Thrombosis | Liposomes | Intraarterial | To estimate the effect of RGD peptide conjugation on the biodistribution behavior of liposomes | PS: 115 ± 12 nm, PDI: 0.158 ± 0.043 EE: 18.0 ± 1.3% | Thrombolytic activity was sufficient, with increased accumulation in the thrombus. | [139] |
| Mesenchymal stem cells | Gene- and cell-based therapy | Acute liver failure | PLGA-NPs | Intravenous | To enhance therapeutic efficacy and increase tolerability | PS: 200 nm, ZP: −10 mV | Increased internalization and growth of liver cells. | [141] |
| Salinomycin | Gene- and cell-based therapy | Osteosarcoma | PLGA-NPs | Subcutaneous | Increasing aqueous solubility and tumor targeting | PS: 150 nm, EE: 50% | CD133+ osteosarcoma was resolved both in vitro and in vivo. | [143] |
| Bortezomib | Gene- and cell-based therapy | Breast cancer | PLA-NPs | Intravenous | To enhance therapeutic effectiveness of bortezomib | PS: 112.8 ± 2.3 nm PDI: 0.13 ± 0.1, EE: 72.8% | Increased targeting and tumor suppression. | [146] |
| Placental growth factor | Gene- and cell-based therapy | Myocardial infarction | Chitosan alginate NPs | Intramyocardial | Sustained release and prolonged effect of placental growth factor | PS: 100–200 nm, ZP: 7.2 ± 0.5 mV, EE: 38.4% ± 3.4% | Significant increase in cardiac functioning, with decreased incidence of inflammation and negligible toxicity. | [149] |
| Mesenchymal stem cells | Gene- and cell-based therapy | Myocardial infarction | MSNs | Intramyocardial | To overcome toxicity and insufficient gene transfection. | PS: 514 nm | Decrease in apoptotic cardiac myocytes, reduced infarct and fibrosis, increased angiogenesis. | [150] |
| Mesenchymal stem cells | Gene- and cell-based therapy | Ischemia | Magnetite NPs in liposomes | Parenteral | To enhance the targeting of ischemic tissues | PS: 10 nm | Enhanced therapeutic activity in ischemia-induced angiogenesis. | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeb, A.; Rana, I.; Choi, H.-I.; Lee, C.-H.; Baek, S.-W.; Lim, C.-W.; Khan, N.; Arif, S.T.; Sahar, N.u.; Alvi, A.M.; et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics 2020, 12, 1184. https://doi.org/10.3390/pharmaceutics12121184
Zeb A, Rana I, Choi H-I, Lee C-H, Baek S-W, Lim C-W, Khan N, Arif ST, Sahar Nu, Alvi AM, et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics. 2020; 12(12):1184. https://doi.org/10.3390/pharmaceutics12121184
Chicago/Turabian StyleZeb, Alam, Isra Rana, Ho-Ik Choi, Cheol-Ho Lee, Seong-Woong Baek, Chang-Wan Lim, Namrah Khan, Sadia Tabassam Arif, Najam us Sahar, Arooj Mohsin Alvi, and et al. 2020. "Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals" Pharmaceutics 12, no. 12: 1184. https://doi.org/10.3390/pharmaceutics12121184
APA StyleZeb, A., Rana, I., Choi, H.-I., Lee, C.-H., Baek, S.-W., Lim, C.-W., Khan, N., Arif, S. T., Sahar, N. u., Alvi, A. M., Shah, F. A., Din, F. u., Bae, O.-N., Park, J.-S., & Kim, J.-K. (2020). Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics, 12(12), 1184. https://doi.org/10.3390/pharmaceutics12121184







