A Proposed Methodology for a Risk Assessment-Based Liposome Development Process
Abstract
:1. Introduction
2. Methods
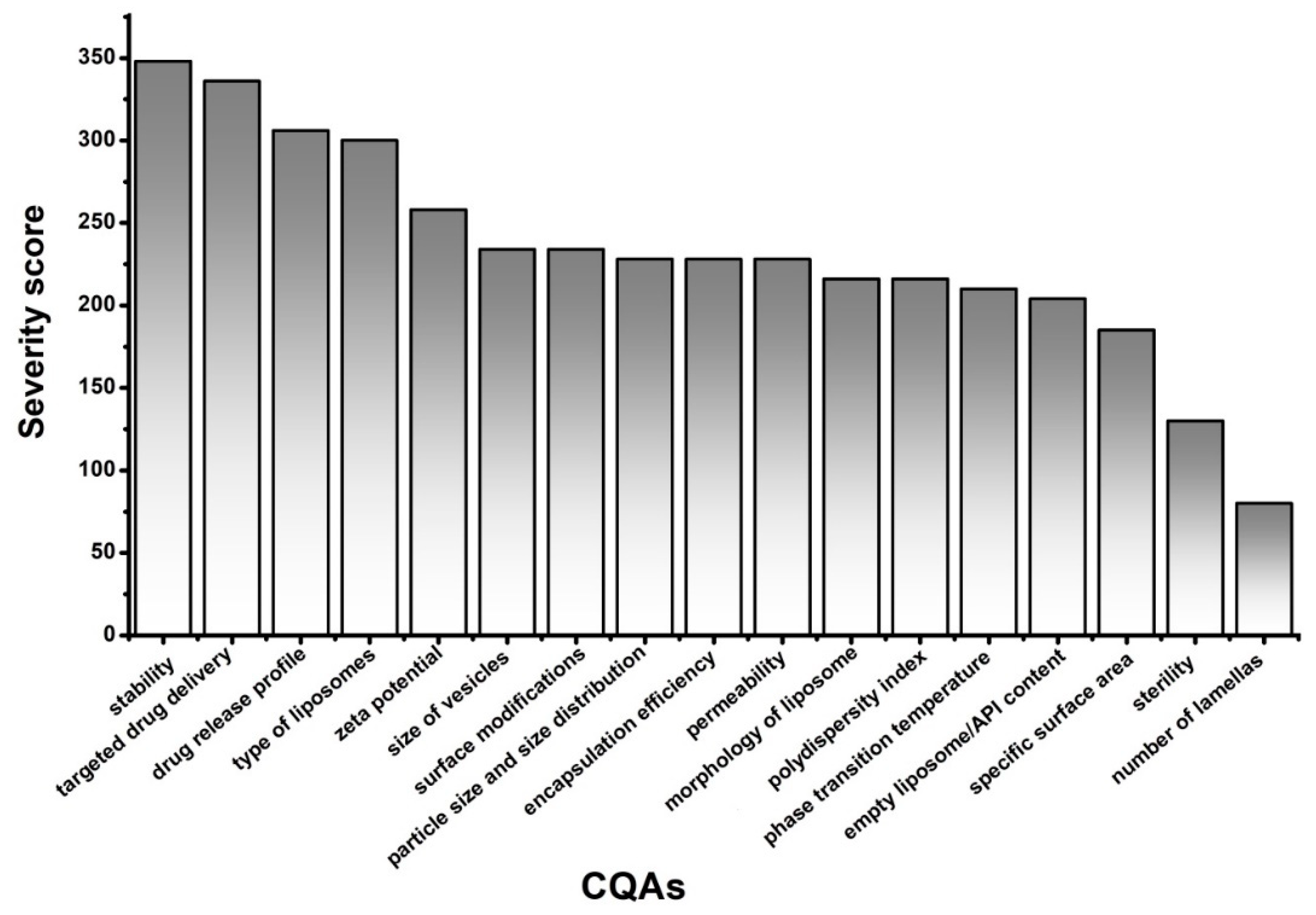
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| API | active pharmaceutical agents |
| CARPA | complement activation-related pseudoallergy |
| CMAs | critical material attributes |
| CPPs | critical process parameters |
| CQAs | critical quality attributes |
| D[3,2] | surface-weighted mean |
| D[4,2] | volume-weighted mean |
| DoE | design of experiments |
| DS | design space |
| EE | encapsulation efficiency |
| GUV | giant unilamellar vesicle |
| ICH | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| IV | intravenous |
| ISO | International Organisation for Standardisation |
| LUV | large unilamellar vesicle |
| MUV | medium-sized unilamellar vesicle |
| MVL | multivesicular liposomes |
| PdI | polydispersity index |
| PE | phosphatidylethanolamine |
| PEG | polyethylene glycol |
| QbD | quality by design |
| QTPP | quality target product profile |
| RA | risk assessment |
| RES | reticuloendothelial system |
| SSA | specific surface area |
| SUV | small unilamellar vesicle |
| Tm | phase transition temperature |
References
- European Medicine Agency. Reflection Paper on the Data Requirements for Intravenous Liposomal Products Developed with Reference to an Innovator Liposomal Product; EMA/ Committee for Human Medicinal Products 806058/2009/Rev. 02; European Medicine Agency: Amsterdam, The Netherlands, 2013; pp. 1–13. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattel, L.; Ceruti, M.; Dosio, F. From conventional to stealth liposomes: A new frontier in cancer chemotherapy. Tumori J. 2003, 89, 237–249. [Google Scholar] [CrossRef]
- Riaz, M.K.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [Green Version]
- Tsermentseli, S.K.; Kontogiannopoulos, K.N.; Papageorgiou, V.P.; Assimopoulou, A.N. Comparative Study of PEGylated and Conventional Liposomes as Carriers for Shikonin. Fluids 2018, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Madni, A.; Sarfraz, M.; Rehman, M.; Ahmad, M.; Akhtar, N.; Ahmad, S.; Tahir, N.; Ijaz, S.; Al-Kassas, R.; Löbenberg, R. Liposomal Drug Delivery: A Versatile Platform for Challenging Clinical Applications. J. Pharm. Pharm. Sci. 2014, 17, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.H.; Mouritsen, O.G.; Arouri, A. Enzymatic action of phospholipase A2 on liposomal drug delivery systems. Int. J. Pharm. 2015, 491, 49–57. [Google Scholar] [CrossRef]
- Samoshin, V.V. Fliposomes: Stimuli-triggered conformational flip of novel amphiphiles causes an instant cargo release from liposomes. Biomol. Concepts 2014, 5, 131–141. [Google Scholar] [CrossRef]
- Perez-Soler, R. Liposomes as carriers of anticancer agents. Drug News Perspect 1990, 3, 287–291. [Google Scholar]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Tansi, F.L.; Rüger, R.; Kollmeier, A.M.; Teichgräber, U.; Steiniger, F.; Kontermann, R.E.; Teichgräber, U.K.; Fahr, A.; Hilger, I. Targeting the Tumor Microenvironment with Fluorescence-Activatable Bispecific Endoglin/Fibroblast Activation Protein Targeting Liposomes. Pharmaceutics 2020, 12, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biosca, A.; Dirscherl, L.; Moles, E.; Imperial, S.; Fernàndez-Busquets, X. An ImmunoPEGliposome for Targeted Antimalarial Combination Therapy at the Nanoscale. Pharmaceutics 2019, 11, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnet, T.; Groo, A.-C.; Picard, C.; Davis, A.; Corvaisier, S.; Since, M.; Bounoure, F.; Rochais, C.; Le Pluart, L.; Dallemagne, P.; et al. Pharmacotechnical Development of a Nasal Drug Delivery Composite Nanosystem Intended for Alzheimer’s Disease Treatment. Pharmaceutics 2020, 12, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepicć, I.; Hafner, A.; Sainz, V.; Lovrić, J.; Lakos, G.P. Nanotherapeutics in the EU: An overview on current state and future directions. Int. J. Nanomed. 2014, 9, 1005–1023. [Google Scholar] [CrossRef] [Green Version]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupanǒiǒ, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR 18401:2017 Nanotechnologies—Plain Lang Explan Sel Terms from ISO/IEC 80004 Series. 2017. Available online: https://www.iso.org/standard/62384.html (accessed on 30 September 2020).
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, M.R. Chapter 2—Nanoliposomes: Preparation and Analysis. In Liposomes—Methods and Protocols Volume 1: Pharm Nanocarriers; Humana Press: New York, USA, 2010; pp. 41–62. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.G.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 369–400. [Google Scholar]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.X. Pharmaceutical Quality by Design: Product and Process Development, Understanding, and Control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- Csóka, I.; Pallagi, E.; Paál, T.L. Extension of quality-by-design concept to the early development phase of pharmaceutical R&D processes. Drug Discov. Today 2018, 23, 1340–1343. [Google Scholar] [CrossRef]
- Gieszinger, P.; Csóka, I.; Pallagi, E.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Ambrus, R. Preliminary study of nanonized lamotrigine containing products for nasal powder formulation. Drug Des. Dev. Ther. 2017, 11, 2453–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallagi, E.; Ambrus, R.; Szaborevesz, P.; Csóka, I. Adaptation of the quality by design concept in early pharmaceutical development of an intranasal nanosized formulation. Int. J. Pharm. 2015, 491, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Pallagi, E.; Jójárt-Laczkovich, O.; Németh, Z.; Szabó-Révész, P.; Csóka, I. Application of the QbD-based approach in the early development of liposomes for nasal administration. Int. J. Pharm. 2019, 562, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Sipos, B.; Szabó-Révész, P.; Csóka, I.; Pallagi, E.; Dobó, D.G.; Bélteky, P.; Kónya, Z.; Deák, Á.; Janovák, L.; Katona, G. Quality by Design Based Formulation Study of Meloxicam-Loaded Polymeric Micelles for Intranasal Administration. Pharmaceutics 2020, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Katona, G.; Balogh, G.T.; Dargó, G.; Gáspár, R.; Márki, Á.; Ducza, E.; Sztojkov-Ivanov, A.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; et al. Development of Meloxicam-Human Serum Albumin Nanoparticles for Nose-to-Brain Delivery via Application of a Quality by Design Approach. Pharmaceutics 2020, 12, 97. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, M.; Pallagi, E.; Csóka, I.; Benke, E.; Farkas, Á.; Zeeshan, M.; Burian, K.; Kokai, D.; Ambrus, R. Aerodynamic properties and in silico deposition of isoniazid loaded chitosan/thiolated chitosan and hyaluronic acid hybrid nanoplex DPIs as a potential TB treatment. Int. J. Biol. Macromol. 2020, 165, 3007–3019. [Google Scholar] [CrossRef]
- Porfire, A.; Achim, M.; Barbalata, C.; Rus, I.; Tomuta, I.; Cristea, C. Pharmaceutical Development of Liposomes Using the QbD Approach. Liposomes Adv. Perspect. 2019, 2019, 1–20. [Google Scholar] [CrossRef] [Green Version]
- ICH. Pharmaceutical Development Q8; ICH Harmonised Tripartite Guideline; ICH: Geneva, Switzerland, 2009; pp. 1–28. [Google Scholar]
- ICH. Quality Risk Management Q9; ICH Harmonised Tripartite Guideline; ICH: Geneva, Switzerland, 2005. [Google Scholar]
- ICH. ICH Q10 Pharmaceutical Quality Systems; ICH: Geneva, Switzerland, 2008. [Google Scholar]
- Bangham, A.; Standish, M.; Watkins, J. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238-IN27. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. Struct. Genom. Drug Discov. 2017, 1522, 17–22. [Google Scholar] [CrossRef]
- Powell, T.; Sammut-Bonnici, T. Pareto analysis. In Wiley Encyclopedia of Management; Wiley: West Sussex, UK, 2015; pp. 1–2. [Google Scholar]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Maja, L.; Knez, Ž.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Storm, G.; Crommelin, D.J. Liposomes: Quo vadis? Pharm. Sci. Technol. Today 1998, 1, 19–31. [Google Scholar] [CrossRef]
- Shashidhar, G.M.; Manohar, B. Nanocharacterization of liposomes for the encapsulation of water soluble compounds from Cordyceps sinensis CS1197 by a supercritical gas anti-solvent technique. RSC Adv. 2018, 8, 34634–34649. [Google Scholar] [CrossRef] [Green Version]
- Wang, W. Tolerability of hypertonic injectables. Int. J. Pharm. 2015, 490, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E. The permeability of liposomes to nonelectrolytes. J. Membr. Biol. 1975, 20, 235–268. [Google Scholar] [CrossRef]
- Szebeni, J.; Muggia, F.; Gabizon, A.; Barenholz, Y. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: Prediction and prevention. Adv. Drug Deliv. Rev. 2011, 63, 1020–1030. [Google Scholar] [CrossRef]
- Elgharbawy, H.; Morsy, R. Preparation and Physicochemical Evaluation of Magnetoliposomes as Drug Carriers for 5-Fluorouracile. J. Biophys. Biomed. Sci. March 2016, 9, 901–906. [Google Scholar]
- Li, N.; Shi, A.; Wang, Q.; Zhang, G. Multivesicular Liposomes for the Sustained Release of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Peanuts: Design, Characterization, and In Vitro Evaluation. Molecules 2019, 24, 1746. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Costa, A.; Khan, M.A.; Burgess, D.J. Application of quality by design to formulation and processing of protein liposomes. Int. J. Pharm. 2012, 434, 349–359. [Google Scholar] [CrossRef] [PubMed]



| Preparation Methods | Subtypes | Comments |
|---|---|---|
| Mechanical dispersion methods | probe or bath sonication | − the critical parameters vary on the basis of the selected preparation method; therefore, the definition of the production technique has to be the first step of every liposome formulation process − the properties of the liposomes (e.g., number of lamellas, size, and distribution of vesicles) |
| French pressure cells—extrusion | ||
| freeze-thawed liposomes | ||
| membrane extrusion | ||
| lipid film hydration techniques | ||
| hydration of proliposomes | ||
| micro emulsification, coalescence of small vesicles | ||
| dual asymmetric centrifugation | ||
| heating method, Mozafari method | ||
| electro-formation | ||
| Solvent dispersion methods | ether injection | |
| ethanol injection | ||
| reverse-phase evaporation | ||
| solvent spherule method | ||
| Detergent removal methods | dialysis | |
| detergent removal of mixed micelles | ||
| gel-permeation chromatography | ||
| Novel methods | microfluidisation | |
| supercritical-assisted method | ||
| freeze-drying of double emulsions | ||
| membrane contractor method | ||
| curvature-tuning | ||
| biometric reaction for vesicular self-assembly |
| QTPP Factors | Details | Comments |
|---|---|---|
| Indication/therapeutic effect | based on the API | its characteristics may necessitate the use of liposomes |
| not important for empty liposomes | empty liposomes are used, e.g., in cosmetology | |
| Target patient population | based on the indication | applicable for each age group in the suitable dosage form |
| Route of administration | the composition may differ on the basis of the target | can be determined by the API and the target patient population |
| Site of activity/target | based on the indication | targeted delivery |
| based on the API | ||
| Dosage strength | based on the API | differs even in the same pharmaceutical subgroup |
| based on the target patient population | needed dose changes with age and health condition | |
| based on the indication | appear in the case of preparation with a wide range of indications | |
| based on the administration route | e.g., in the case of nasal application, the needed dose is less than per os | |
| Dosage form/appearance | liposomes in aqueous solution | transparent, light scattering liquid (vesicles in colloid size) |
| lyophilised powder | solid powder; colour based on the API and the excipients | |
| Viscosity | based on the administration route | sign of stability; maintains efficient drug release; higher viscosity indicates a smaller size, a narrow PdI, slower drug release, and lower clearance rate |
| Osmolarity | based on the administration route | be tolerable, ideally 300 ± 30 mOsm/kg |
| Physical attributes of the liposomes | morphology, particle size, and zeta potential | change with the adjustment of the composition |
| Pharmacokinetics | liberation, adsorption, distribution, metabolism, elimination | necessary mostly for API-loaded liposomes |
| Safety | complement activation-related pseudoallergy (CARPA) | all types of intravenous liposomes can cause CARPA; enhanced by increasing size in the 70–300 nm range; more than 71 mol% cholesterol; PEG-PE insertion |
| chemical/biological decomposition | needs to be investigated | |
| degradation products | concentration must be under the legal limit | |
| Sterility | based on the administration route | sterile and pyrogen-free or aseptic preparation is not needed |
| Stability | in aqueous solution | needs to be stable; duration of stability is decisive |
| in freeze-dried powder form | ||
| Solubility/dissolution | in aqueous solution | media: non-toxic, non-irritable |
| in freeze-dried powder | immediate release | |
| Homogeneity | homogenous formulation | sign of stability |
| Drug release | based on the treatment | site and timing can be modified |
| CQAs | Details | Comments |
|---|---|---|
| Type of liposomes | conventional liposomes | neutral or negative phospholipids |
| immune liposomes | antibodies, antibody fragments | |
| cationic liposomes | positive phospholipids | |
| magnetic liposomes | metal particles | |
| bioresponsive liposomes | thermosensitive (37 °C < Tm) | |
| pH-sensitive (acidic milieu) | ||
| LiPlasome (secretory phospholipase A2) | ||
| Number of lamellas | more layers | multilamellar (>0.5 µm) |
| oligolamellar (0.1–1.0 µm) | ||
| one layer | unilamellar | |
| Size of vesicle | small unilamellar vesicle (SUV) | 20–100 nm |
| medium-sized unilamellar vesicle (MUV) | between SUV and LUV, >100 nm | |
| large unilamellar vesicle (LUV) | >100 nm | |
| giant unilamellar vesicle (GUV) | >1 µm | |
| Surface modifications | no modification | rapid elimination |
| polyethylene glycol (PEG) chains (stealth liposomes) (quality and quantity of the chains) | steric exclusion (decreased opsonisation and phagocytosis); prolonged circulation | |
| monoclonal antibodies, antibody fragments, peptides, nucleic acids, carbohydrates, small molecules | provide targeted delivery by biding to the targeted receptors | |
| Morphology of liposomes | spherical vesicles | self-organised structure |
| concentric layers | multi-layered vesicles | |
| spherical with multiple non-concentric lipid vesicles inside | multivesicular liposome (MVL) | |
| Particle size and size distribution | d(0.1), d(0.5), d(0.9), span, surface weighted mean (D[3,2]), volume weighted mean (D[4,2]) | mean particle size should be under 200 nm; ideal around 100 nm |
| Polydispersity index (PdI) | indicating polydispersity of the system | below 0.5 is acceptable |
| Specific surface area (SSA) | influences drug release | smaller vesicles maintain higher surface area-to-volume ratio than the larger particles |
| Zeta potential | indicating stability | stable formulation around ±30 mV |
| Phase transition temperature (Tm) | influences drug release | determined by the composition of the liposome; cholesterols reduce the value |
| Empty liposomes/API content | modifies the physical attributes of the liposomes | the characteristics of the API determine its position |
| Position of the API | hydrophilic API | in the hydrophilic aqueous centre |
| lipophilic API | in the lipophilic double membrane | |
| surface-bounded | monoclonal antibodies, antibody fragments, peptides, nucleic acids, carbohydrates, small molecules | |
| Encapsulation efficiency (EE) | higher EE% is the goal to increase the drug concentration in the final formulation | manufacturing costs can be reduced, and more flexible dosing can be provided by higher EE% |
| Permeability | semi-permeable membrane | the highest permeability is at Tm; |
| targeted drug delivery | target specificity | increases effectiveness |
| Drug release profile | maintains therapeutic activity | site and timing can be modified |
| Sterility | if necessary | even for the materials |
| in the case of aseptic preparation | ||
| Stability | chemical, biological, microbiological | characteristic values must remain |
| in the recommended ranges until use |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, Z.; Pallagi, E.; Dobó, D.G.; Csóka, I. A Proposed Methodology for a Risk Assessment-Based Liposome Development Process. Pharmaceutics 2020, 12, 1164. https://doi.org/10.3390/pharmaceutics12121164
Németh Z, Pallagi E, Dobó DG, Csóka I. A Proposed Methodology for a Risk Assessment-Based Liposome Development Process. Pharmaceutics. 2020; 12(12):1164. https://doi.org/10.3390/pharmaceutics12121164
Chicago/Turabian StyleNémeth, Zsófia, Edina Pallagi, Dorina Gabriella Dobó, and Ildikó Csóka. 2020. "A Proposed Methodology for a Risk Assessment-Based Liposome Development Process" Pharmaceutics 12, no. 12: 1164. https://doi.org/10.3390/pharmaceutics12121164
APA StyleNémeth, Z., Pallagi, E., Dobó, D. G., & Csóka, I. (2020). A Proposed Methodology for a Risk Assessment-Based Liposome Development Process. Pharmaceutics, 12(12), 1164. https://doi.org/10.3390/pharmaceutics12121164







