One Step Closer to Clinical Translation: Enhanced Tumor Targeting of [99mTc]Tc-DB4 and [111In]In-SG4 in Mice Treated with Entresto
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Analogs—Inhibitors—Radioligands
2.1.1. Chemicals—Radionuclides
2.1.2. Preparation of Radioligands
2.1.3. Quality Control
2.2. Biological Assays
2.2.1. Cell Culture
2.2.2. Animal Studies
2.2.3. In Vivo Stability Tests
2.2.4. Tumor Induction in Mice
2.2.5. Biodistribution of [99mTc]Tc-DB4 and [111In]In-SG4 in Tumor-Bearing Mice
2.2.6. Statistical Analysis
2.2.7. SPECT/CT Imaging of [111In]In-SG4 in A431-CCK2R(+) Tumor-Bearing Mice
3. Results
3.1. Peptides and Radioligands
3.2. In Vivo Studies
3.2.1. Stability of [99mTc]Tc-DB4 and [111In]In-SG4 in Mice
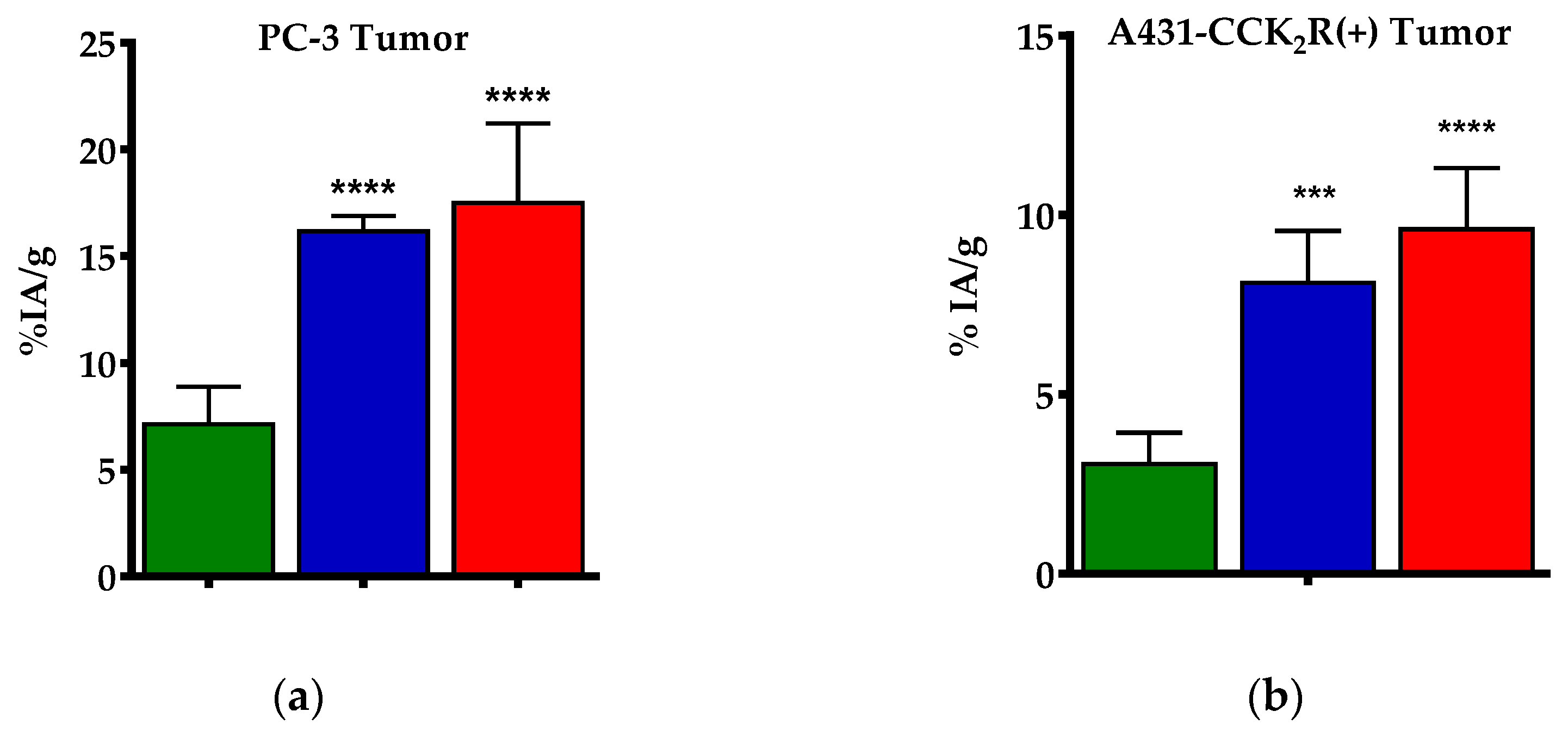
3.2.2. Biodistribution of [99mTc]Tc-DB4 SCID Mice Bearing PC-3 Xenografts
3.2.3. Biodistribution of [111In]In-SG4 in SCID Mice Bearing Twin A431-CCK2R((+/−)) Xenografts
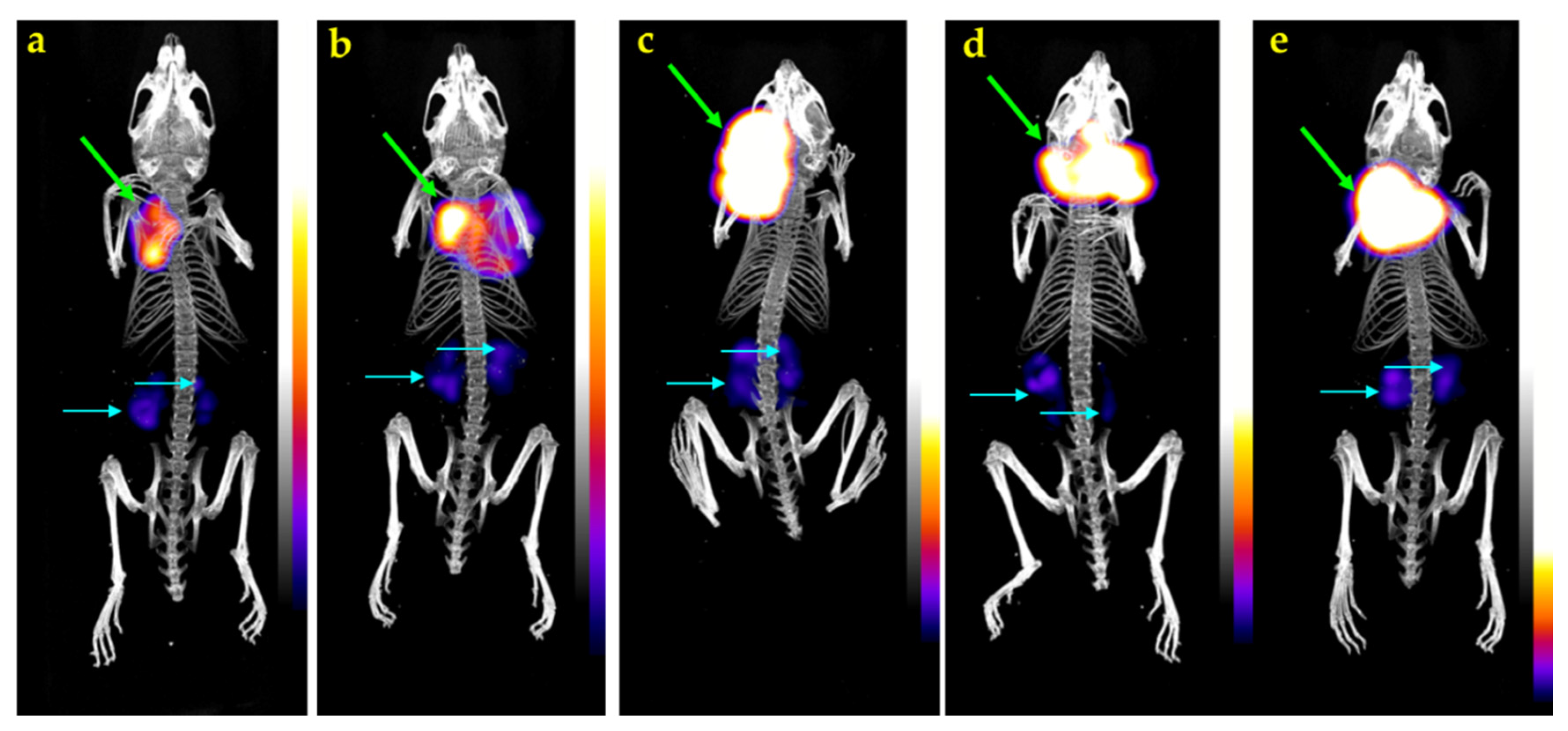
3.2.4. SPECT/CT of [111In]In-SG4 in A431-CCK2R(+) Xenograft-Bearing Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baum, R.P.; Kulkarni, H.R. Theranostics: From molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy-the Bad Berka experience. Theranostics 2012, 2, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Singh, A.; Kulkarni, H.R.; Schuchardt, C.; Müller, D.; Wester, H.J.; Maina, T.; Rösch, F.; van der Meulen, N.P.; Müller, C.; et al. From bench to bedside-the Bad Berka experience with first-in-human studies. Semin. Nucl. Med. 2019, 49, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.; Kwekkeboom, D.J.; de Jong, M. Radiopeptides for imaging and therapy: A radiant future. J. Nucl. Med. 2015, 56, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Kostelnik, T.I.; Orvig, C. Radioactive main group and rare earth metals for imaging and therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Wester, H.J. Re-thinking the role of radiometal isotopes: Towards a future concept for theranostic radiopharmaceuticals. J. Labelled Comp. Radiopharm. 2018, 61, 141–153. [Google Scholar] [CrossRef]
- Van der Zwan, W.A.; Brabander, T.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; Krenning, E.P.; de Herder, W.W. Salvage peptide receptor radionuclide therapy with [177Lu-DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2019, 46, 704–717. [Google Scholar] [CrossRef]
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox - transforming natural peptides into peptide therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef]
- Adessi, C.; Soto, C. Converting a peptide into a drug: Strategies to improve stability and bioavailability. Curr. Med. Chem. 2002, 9, 963–978. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.; Maina, T.; Krenning, E.; de Jong, M. In vivo enzyme inhibition—A new promising route toward higher diagnostic sensitivity and therapeutic efficacy of tumor-directed radiopeptides. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Roques, B.P.; Noble, F.; Dauge, V.; Fournie-Zaluski, M.C.; Beaumont, A. Neutral endopeptidase 24.11: Structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 1993, 45, 87–146. [Google Scholar] [PubMed]
- Roques, B.P. Zinc metallopeptidases: Active site structure and design of selective and mixed inhibitors: New approaches in the search for analgesics and anti-hypertensives. Biochem. Soc. Trans. 1993, 21 Pt 3, 678–685. [Google Scholar] [CrossRef]
- Suda, H.; Aoyagi, T.; Takeuchi, T.; Umezawa, H. Letter: A thermolysin inhibitor produced by actinomycetes: Phosphoramidon. J. Antibiot. (Tokyo) 1973, 26, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Oefner, C.; D’Arcy, A.; Hennig, M.; Winkler, F.K.; Dale, G.E. Structure of human neutral endopeptidase (neprilysin) complexed with phosphoramidon. J. Mol. Biol. 2000, 296, 341–349. [Google Scholar] [CrossRef]
- Chatalic, K.L.; Konijnenberg, M.; Nonnekens, J.; de Blois, E.; Hoeben, S.; de Ridder, C.; Brunel, L.; Fehrentz, J.A.; Martinez, J.; van Gent, D.C.; et al. In vivo stabilization of a gastrin-releasing peptide receptor antagonist enhances PET imaging and radionuclide therapy of prostate cancer in preclinical studies. Theranostics 2016, 6, 104–117. [Google Scholar] [CrossRef]
- Kaloudi, A.; Lymperis, E.; Kanellopoulos, P.; Waser, B.; de Jong, M.; Krenning, E.P.; Reubi, J.C.; Nock, B.A.; Maina, T. Localization of 99mTc-GRP analogs in GRPR-expressing tumors: Effects of peptide length and neprilysin inhibition on biological responses. Pharmaceuticals 2019, 12, 42. [Google Scholar] [CrossRef]
- Lymperis, E.; Kaloudi, A.; Sallegger, W.; Bakker, I.L.; Krenning, E.P.; de Jong, M.; Maina, T.; Nock, B.A. Radiometal-dependent biological profile of the radiolabeled gastrin-releasing peptide receptor antagonist SB3 in cancer theranostics: Metabolic and biodistribution patterns defined by neprilysin. Bioconjug. Chem. 2018, 29, 1774–1784. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Sallegger, W.; Krenning, E.P.; de Jong, M.; Maina, T. In vivo inhibition of neutral endopeptidase enhances the diagnostic potential of truncated gastrin 111In-radioligands. Nucl. Med. Biol. 2015, 42, 824–832. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Valkema, R.; Krenning, E.P.; de Jong, M.; Maina, T. Impact of clinically tested nep/ace inhibitors on tumor uptake of [111In-DOTA]MG11-first estimates for clinical translation. EJNMMI Res. 2016, 6, 15. [Google Scholar] [CrossRef]
- Nock, B.A.; Nikolopoulou, A.; Galanis, A.; Cordopatis, P.; Waser, B.; Reubi, J.C.; Maina, T. Potent bombesin-like peptides for GRP-receptor targeting of tumors with 99mTc: A preclinical study. J. Med. Chem. 2005, 48, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Mather, S.J.; Nock, B.A.; Maina, T.; Gibson, V.; Ellison, D.; Murray, I.; Sobnack, R.; Colebrook, S.; Wan, S.; Halberrt, G.; et al. GRP receptor imaging of prostate cancer using [99mTc]demobesin 4: A first-in-man study. Mol. Imaging Biol. 2014, 16, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Kaloudi, A.; Lymperis, E.; Krenning, E.P.; Nock, B.A.; de Jong, M.; Maina, T. Phosphoramidon enhances tumor uptake of truncated [111In-DOTA]gastrins. Nucl. Med. Biol. 2014, 41, 637. [Google Scholar] [CrossRef]
- Valkema, R.; Froberg, A.; Maina, T.; Nock, B.; de Blois, E.; Melis, M.; Konijnenberg, M.; Koolen, S.; Peeters, R.; de Herder, W.; et al. Clinical translation of the pepprotect concept: Improved detection of cancer and metastases, applied in medullary thyroid cancer patients with [111In]In-MG11 scanning during neprilysin inhibition. Eur. J. Nucl. Med. Mol. Imaging 2019, 46 (Suppl. 1), S701–S702. [Google Scholar]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Committees, Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Solomon, S.D. Neprilysin inhibition for heart failure. N. Engl. J. Med. 2014, 371, 2336–2337. [Google Scholar] [CrossRef]
- Gu, J.; Noe, A.; Chandra, P.; Al-Fayoumi, S.; Ligueros-Saylan, M.; Sarangapani, R.; Maahs, S.; Ksander, G.; Rigel, D.F.; Jeng, A.Y.; et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNI). J. Clin. Pharmacol. 2010, 50, 401–414. [Google Scholar] [CrossRef]
- Schiering, N.; D’Arcy, A.; Villard, F.; Ramage, P.; Logel, C.; Cumin, F.; Ksander, G.M.; Wiesmann, C.; Karki, R.G.; Mogi, M. Structure of neprilysin in complex with the active metabolite of sacubitril. Sci. Rep. 2016, 6, 27909. [Google Scholar] [CrossRef]
- Reile, H.; Armatis, P.E.; Schally, A.V. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: Internalization of receptor bound 125I-(Tyr4) bombesin by tumor cells. Prostate 1994, 25, 29–38. [Google Scholar] [CrossRef]
- Aloj, L.; Caracó, C.; Panico, M.; Zannetti, A.; Del Vecchio, S.; Tesauro, D.; De Luca, S.; Arra, C.; Pedone, C.; Morelli, G.; et al. In vitro and in vivo evaluation of 111In-DTPAGLU-G-CCK8 for cholecystokinin-B receptor imaging. J. Nucl. Med. 2004, 45, 485–494. [Google Scholar] [PubMed]
- Ayalasomayajula, S.; Langenickel, T.; Pal, P.; Boggarapu, S.; Sunkara, G. Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): A novel angiotensin receptor-neprilysin inhibitor. Clin. Pharmacokinet. 2017, 56, 1461–1478. [Google Scholar] [CrossRef] [PubMed]
- Laverman, P.; Joosten, L.; Eek, A.; Roosenburg, S.; Peitl, P.K.; Maina, T.; Mäcke, H.; Aloj, L.; von Guggenberg, E.; Sosabowski, J.K.; et al. Comparative biodistribution of 12 111In-labelled gastrin/CCK2 receptor-targeting peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Van Holen, R.; Vandeghinste, B.; Deprez, K.; Vandenberghe, S. Design and performance of a compact and stationary microspect system. Med. Phys. 2013, 40, 112501. [Google Scholar] [CrossRef] [PubMed]
- Lymperis, E.; Kaloudi, A.; Kanellopoulos, P.; de Jong, M.; Krenning, E.P.; Nock, B.A.; Maina, T. Comparing Gly11/DAla11-replacement vs. the in-situ neprilysin-inhibition approach on the tumor-targeting efficacy of the 111In-SB3/111In-SB4 radiotracer pair. Molecules 2019, 24, 1015. [Google Scholar] [CrossRef] [PubMed]
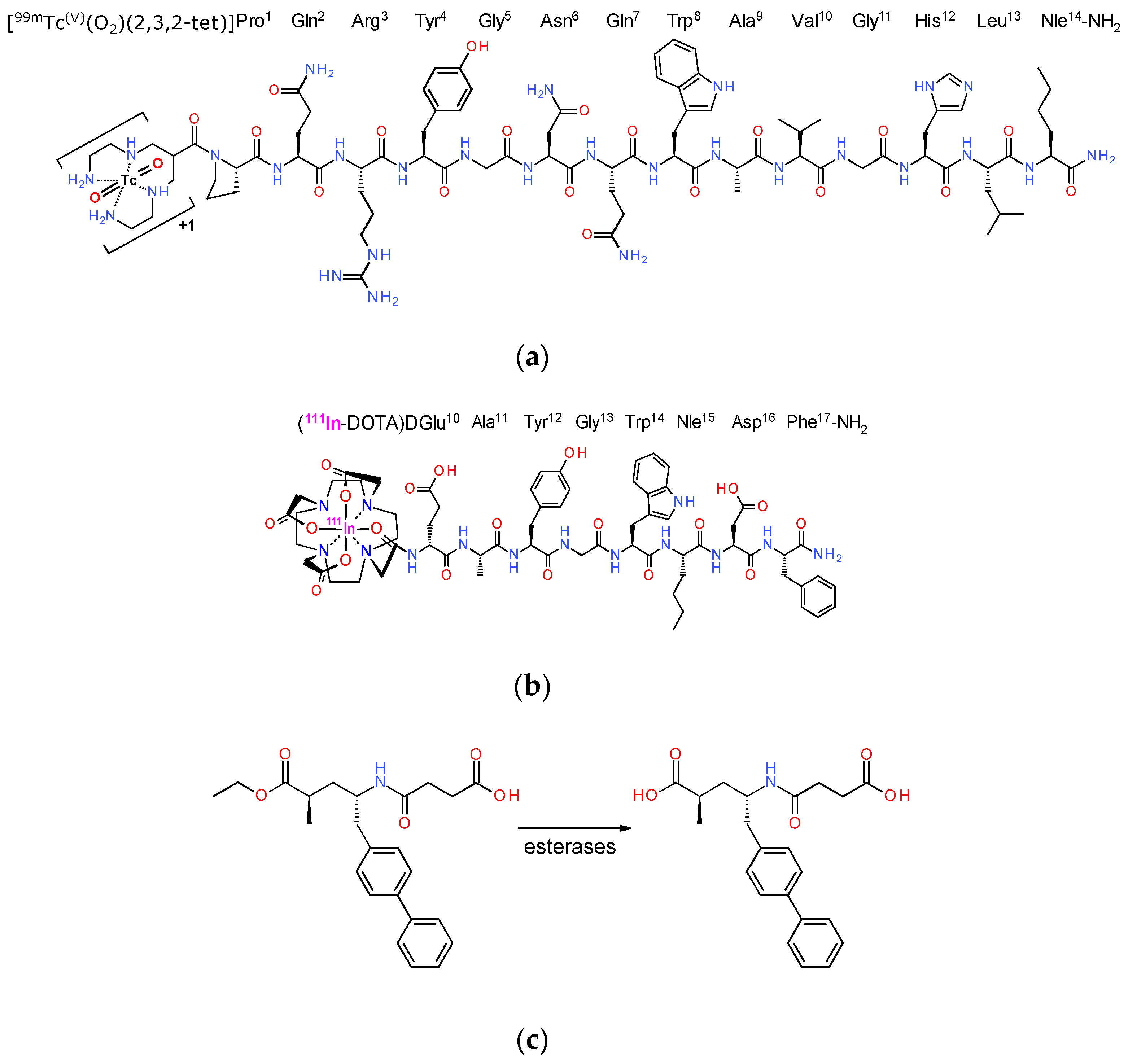

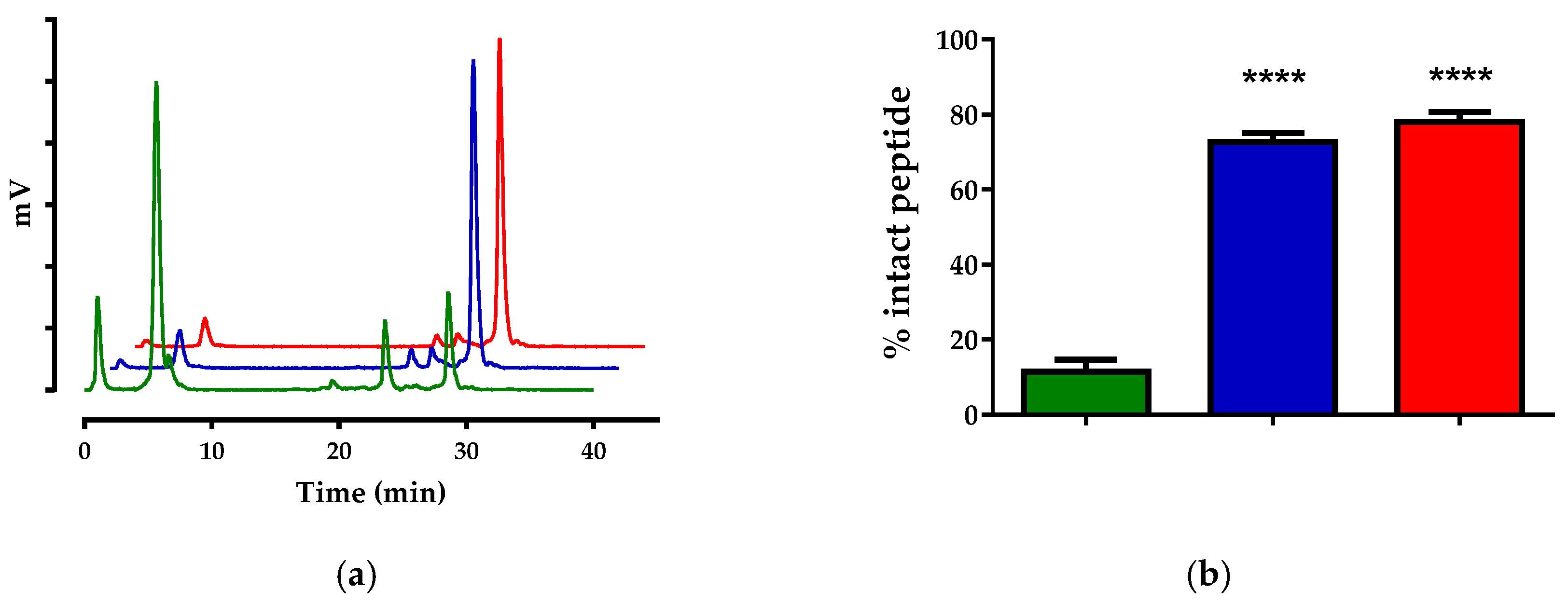
| Treatment | [99mTc]Tc-DB4 | [111In]In-SG4 |
|---|---|---|
| Control | 26.9 ± 3.7 (n = 3) | 11.5 ± 3.2 (n = 4) |
| LBQ | 74.4 ± 8.6 (n = 3) | 72.7 ± 2.4 (n = 3) |
| Entresto | 71.5 ± 1.8 (n = 4) | 78.0 ± 2.7 (n = 3) |
| Tissue | [99mTc]Tc-DB4—4 h pi | |||
|---|---|---|---|---|
| Controls 1 | LBQ657 2 | Entresto 3 | Block 4 | |
| Blood | 0.12 ± 0.11 | 0.14 ± 0.03 | 0.17 ± 0.03 | 0.08 ± 0.01 |
| Liver | 1.76 ± 0.74 | 1.61 ± 0.19 | 1.27 ± 0.20 | 0.90 ± 0.10 |
| Heart | 0.24 ± 0.13 | 0.26 ± 0.03 | 0.23 ± 0.07 | 0.13 ± 0.00 |
| Kidneys | 9.56 ± 4.35 | 4.67 ± 1.03 | 7.86 ± 2.46 | 4.72 ± 0.72 |
| Stomach | 1.50 ± 0.71 | 1.12 ± 0.20 | 1.66 ± 0.30 | 0.20 ± 0.04 |
| Intestines | 7.99 ± 0.08 | 9.00 ± 0.64 | 11.36 ± 0.95 | 1.23 ± 0.21 |
| Spleen | 1.92 ± 0.75 | 2.46 ± 0.95 | 1.93 ± 0.44 | 2.60 ± 0.48 |
| Muscle | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.10 ± 0.09 | 0.03 ± 0.00 |
| Lungs | 0.40 ± 0.21 | 0.52 ± 0.18 | 0.66 ± 0.11 | 0.72 ± 0.26 |
| Pancreas | 27.71 ± 6.36 | 56.06 ± 4.24 | 60.98 ± 6.41 | 0.68 ± 0.04 |
| Tumor | 7.13 ± 1.76 | 16.17 ± 0.71 | 17.50 ± 3.70 | 0.55 ± 0.04 |
| Tissue | [111In]In-SG4—4 h pi | ||
|---|---|---|---|
| Controls 1 | LBQ657 2 | Entresto 3 | |
| Blood | 0.03 ± 0.02 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| Liver | 0.15 ± 0.02 | 0.24 ± 0.02 | 0.23 ± 0.03 |
| Heart | 0.05 ± 0.02 | 0.07 ± 0.01 | 0.10 ± 0.01 |
| Kidneys | 2.10 ± 0.49 | 2.39 ± 0.11 | 2.40 ± 0.61 |
| Stomach | 1.29 ± 0.25 | 3.10 ± 0.41 | 2.86 ± 0.53 |
| Intestines | 0.46 ± 0.05 | 0.49 ± 0.12 | 0.34 ± 0.07 |
| Spleen | 0.11 ± 0.04 | 0.23 ± 0.06 | 0.22 ± 0.05 |
| Muscle | 0.03 ± 0.02 | 0.07 ± 0.02 | 0.05 ± 0.01 |
| Lungs | 0.06 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 |
| Pancreas | 0.08 ± 0.09 | 0.31 ± 0.01 | 0.34 ± 0.14 |
| Femur | 0.10 ± 0.03 | 0.13 ± 0.01 | 0.14 ± 0.03 |
| Tumor (+) | 3.07 ± 0.87 | 8.11 ± 1.45 | 9.61 ± 1.70 |
| Tumor (–) | 0.28 ± 0.21 | 0.28 ± 0.01 | 0.40 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanellopoulos, P.; Kaloudi, A.; Rouchota, M.; Loudos, G.; de Jong, M.; Krenning, E.P.; Nock, B.A.; Maina, T. One Step Closer to Clinical Translation: Enhanced Tumor Targeting of [99mTc]Tc-DB4 and [111In]In-SG4 in Mice Treated with Entresto. Pharmaceutics 2020, 12, 1145. https://doi.org/10.3390/pharmaceutics12121145
Kanellopoulos P, Kaloudi A, Rouchota M, Loudos G, de Jong M, Krenning EP, Nock BA, Maina T. One Step Closer to Clinical Translation: Enhanced Tumor Targeting of [99mTc]Tc-DB4 and [111In]In-SG4 in Mice Treated with Entresto. Pharmaceutics. 2020; 12(12):1145. https://doi.org/10.3390/pharmaceutics12121145
Chicago/Turabian StyleKanellopoulos, Panagiotis, Aikaterini Kaloudi, Maritina Rouchota, George Loudos, Marion de Jong, Eric P. Krenning, Berthold A. Nock, and Theodosia Maina. 2020. "One Step Closer to Clinical Translation: Enhanced Tumor Targeting of [99mTc]Tc-DB4 and [111In]In-SG4 in Mice Treated with Entresto" Pharmaceutics 12, no. 12: 1145. https://doi.org/10.3390/pharmaceutics12121145
APA StyleKanellopoulos, P., Kaloudi, A., Rouchota, M., Loudos, G., de Jong, M., Krenning, E. P., Nock, B. A., & Maina, T. (2020). One Step Closer to Clinical Translation: Enhanced Tumor Targeting of [99mTc]Tc-DB4 and [111In]In-SG4 in Mice Treated with Entresto. Pharmaceutics, 12(12), 1145. https://doi.org/10.3390/pharmaceutics12121145






