Drug-Nutraceutical Co-Crystal and Salts for Making New and Improved Bi-Functional Analgesics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Salts/Co-Crystals of Drugs
Purification of Clonidine-Hydrochloride
2.3. Validation and Characterization Experiments on Synthesized Salts and Co-Crystals
2.3.1. FTIR-ATR Spectroscopy
2.3.2. Powder X-ray Diffraction (PXRD)
2.3.3. Single Crystal X-ray Diffraction (SCXRD)
2.3.4. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
2.3.5. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.4. Formulation of Drugs into Topical Ointments
2.5. Generation of the Rat Model of CRPS
2.6. Mechanical Sensitivity Testing
2.7. Statistics on Animal Behavioral Data
3. Results
3.1. Validation and Characterization of Synthesized Co-Crystal and Salts
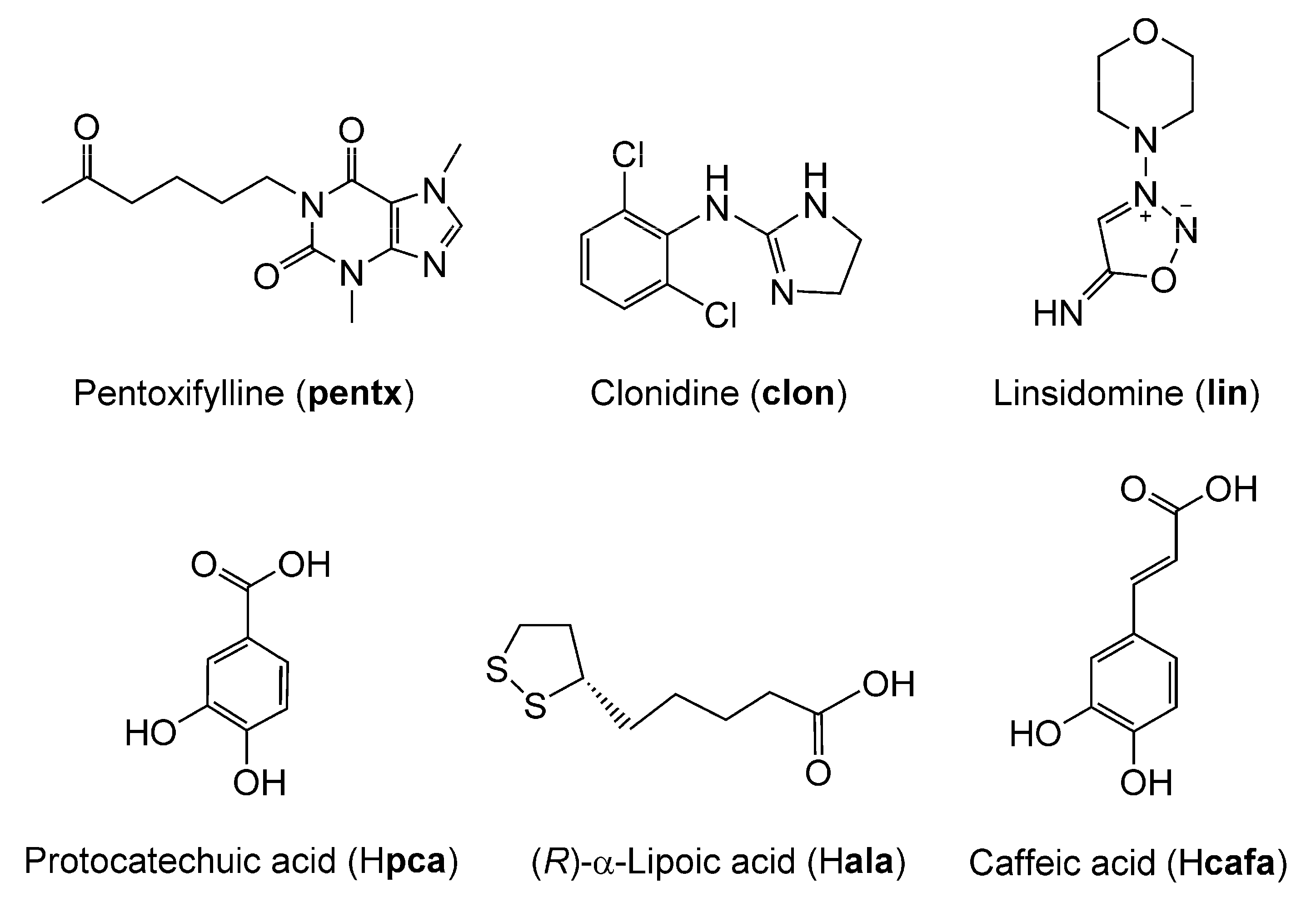
3.1.1. Co-Crystal of Pentoxifylline and Protocatechuic Acid
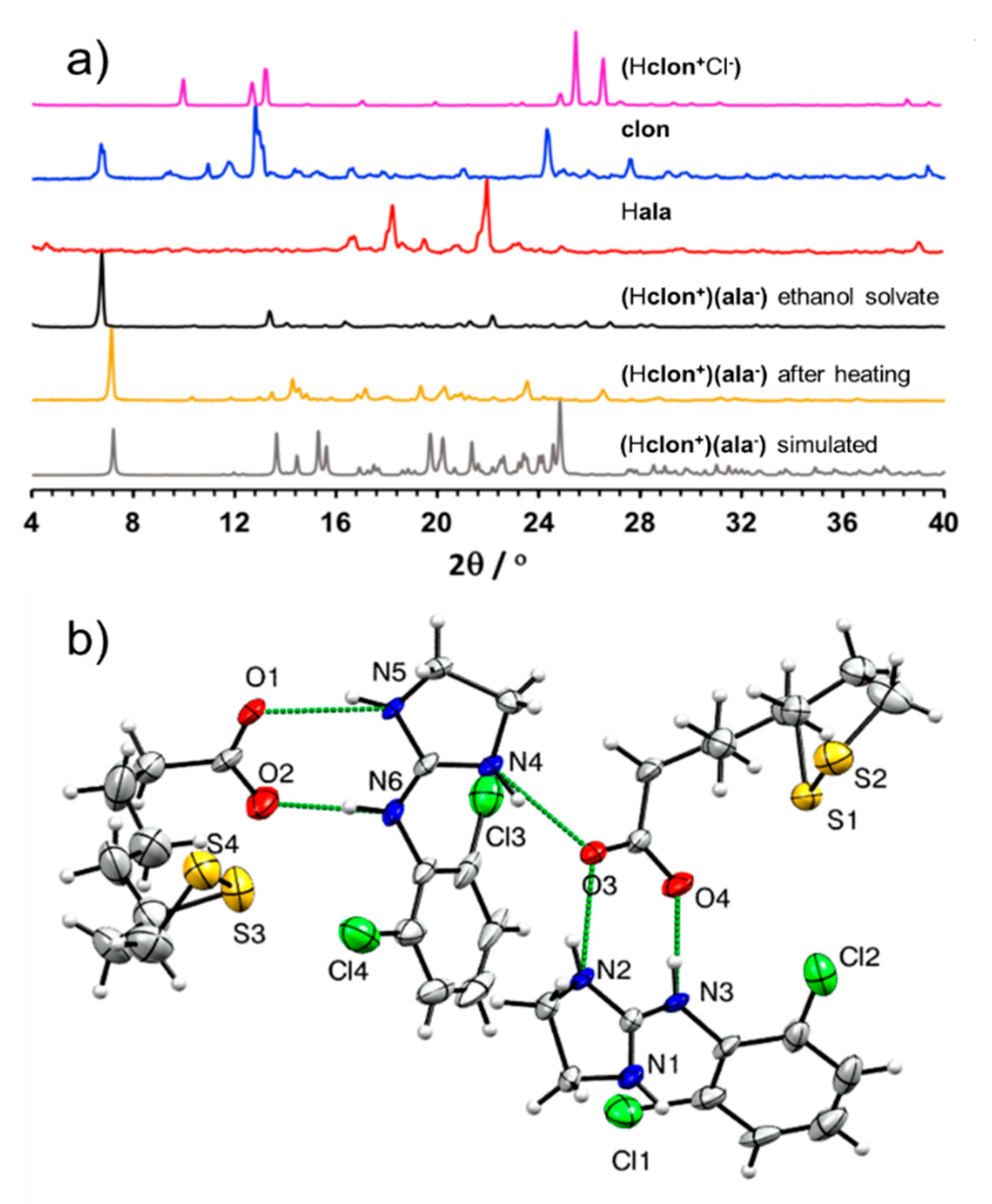
3.1.2. Salt of Clonidine and α-Lipoic Acid (Hclon+)(ala−)
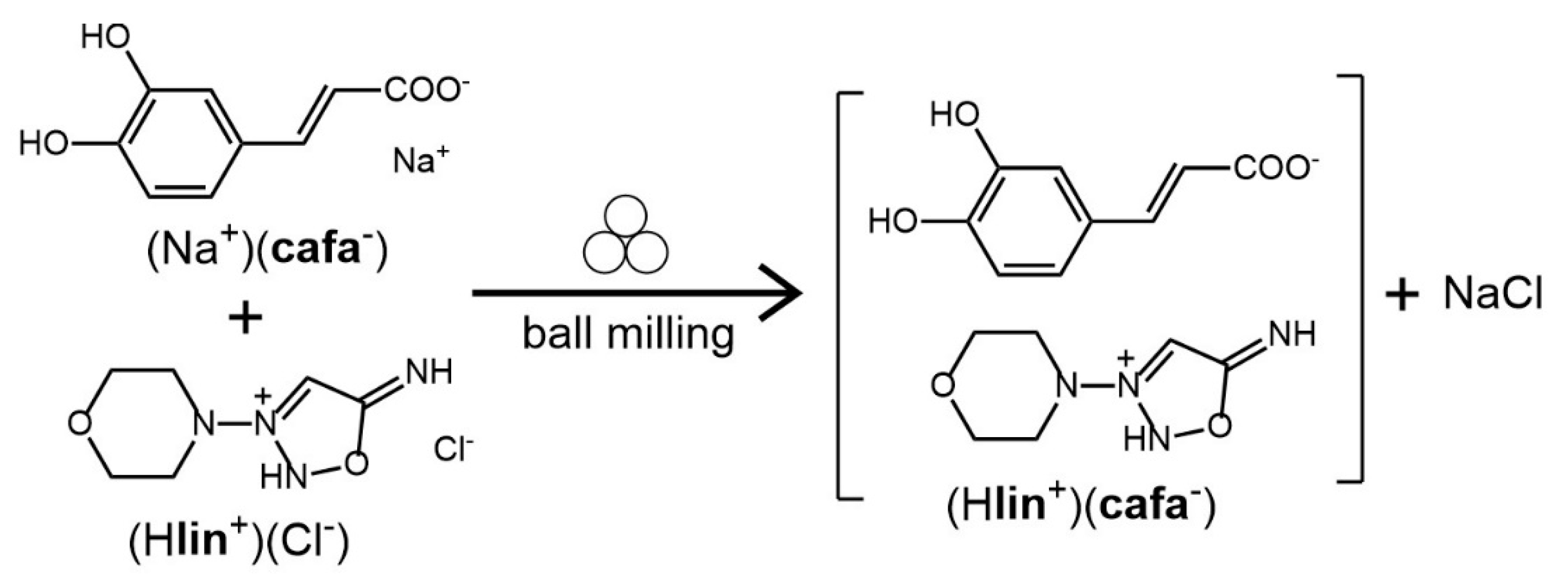
3.1.3. Salt of Linsidomine and Caffeic Acid (Hlin+)(cafa−)
3.2. The Enhanced Analgesic Effects of the Nutraceutical Co-Crystal and Salts of pentx, clon, and lin
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tamburin, S.; Paolucci, S.; Smania, N.; Sandrini, G. The burden of chronic pain and the role of neurorehabilitation: Consensus matters where evidence is lacking. J. Pain Res. 2017, 10, 101–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolf, C.J.; Max, M.B. Mechanism-based Pain Diagnosis: Issues for analgesic drug development. Anesthesiology 2001, 95, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J. Challenges of managing chronic pain. BMJ 2017, 356, j741. [Google Scholar] [CrossRef]
- Woodcock, J.; Witter, J.; Dionne, R.A. Stimulating the development of mechanism-based, individualized pain therapies. Nat. Rev. Drug Discov. 2007, 6, 703–710. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef]
- Byrn, S.R.; Zografi, G.; Chen, X. Pharmaceutical salts. In Solid State Properties of Pharmaceutical Materials, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 48–59. [Google Scholar]
- Vishweshwar, P.; McMahon, J.A.; Bis, J.A.; Zaworotko, M.J. Pharmaceutical Co-Crystals. J. Pharm. Sci. 2006, 95, 499–516. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Chemey, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, salts, and cocrystals: What’s in a name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Vioglio, P.C.; Chierotti, M.R.; Gobetto, R. Pharmaceutical aspects of salt and cocrystal forms of APIs and characterization challenges. Adv. Drug Deliv. Rev. 2017, 117, 86–110. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Shan, N.; Perry, M.L.; Weyna, D.R.; Zaworotko, M.J. Impact of pharmaceutical co-crystals: The effects on drug pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Gadade, D.D.; Pekamwar, S.S. Pharmaceutical co-crystals: Regulatory and strategic aspects, design and development. Adv. Pharm. Bull. 2016, 6, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Forbes, S.; Desper, J. Using Cocrystals To Systematically Modulate Aqueous Solubility and Melting Behavior of an Anticancer Drug. J. Am. Chem. Soc. 2009, 131, 17048–17049. [Google Scholar] [CrossRef] [Green Version]
- Karki, S.; Friščić, T.; Fábián, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving Mechanical Properties of Crystalline Solids by Cocrystal Formation: New Compressible Forms of Paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Good, D.J.; Rodríguez-Hornedo, N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Žegarac, M.; Lekšić, E.; Šket, P.; Plavec, J.; Devčić Bogdanović, M.; Bučar, D.-K.; Dumić, M.; Meštrović, E. A sildenafil cocrystal based on acetylsalicylic acid exhibits an enhanced intrinsic dissolution rate. CrystEngComm 2014, 16, 32–35. [Google Scholar] [CrossRef]
- McNamara, D.P.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’Donnell, E.; Park, A. Use of a Glutaric Acid Cocrystal to Improve Oral Bioavailability of a Low Solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef]
- Steed, J.W. The role of co-crystals in pharmaceutical design. Trends Pharmacol. Sci. 2013, 34, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef]
- Grobelny, P.; Mukherjee, A.; Desiraju, G.R. Drug-drug co-crystals: Temperature-dependent proton mobility in the molecular complex of isoniazid with 4-aminosalicylic acid. CrystEngComm 2011, 13, 4358–4364. [Google Scholar] [CrossRef]
- Thakuria, R.; Sarma, B. Drug-Drug and Drug-Nutraceutical Cocrystal/Salt as Alternative Medicine for Combination Therapy: A Crystal Engineering Approach. Crystals 2018, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Thipparaboina, R.; Kumar, D.; Chavan, R.B.; Shastri, N.R. Multidrug co-crystals: Towards the development of effective therapeutic hybrids. Drug Discov. Today 2016, 21, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S. Drug-drug co-crystals. DARU J. Pharm. Sci. 2012, 20, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, A.; Rizvi, S. Utility of novel dual functionalized co-crystallized and ionic liquid-based drugs for the pain management. IJPRBS 2016, 5, 97–109. [Google Scholar]
- Stepanovs, D.; Jure, M.R.; Kuleshova, L.N.; Hofmann, D.W.; Mishnev, A. Cocrystals of Pentoxifylline: In Silico and Experimental Screening. Cryst. Growth Des. 2015, 15, 3652–3660. [Google Scholar] [CrossRef]
- Lim, T.K.; Shi, X.Q.; Johnson, J.M.; Rone, M.B.; Antel, J.P.; David, S.; Zhang, J. Peripheral Nerve Injury Induces Persistent Vascular Dysfunction and Endoneurial Hypoxia, Contributing to the Genesis of Neuropathic Pain. J. Neurosci. 2015, 35, 3346–3359. [Google Scholar] [CrossRef] [Green Version]
- Coderre, T.J. Complex Regional Pain Syndrome: What’s in a Name? J. Pain 2011, 12, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Coderre, T.J.; Xanthos, D.N.; Francis, L.; Bennett, G.J. Chronic post-ischemia pain (CPIP): A novel animal model of complex regional pain syndrome-Type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain 2004, 112, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Laferrière, A.; Millecamps, M.; Xanthos, D.N.; Xiao, W.H.; Siau, C.; De Mos, M.; Sachot, C.; Ragavendran, J.V.; Huygen, F.J.P.M.; Bennett, G.J.; et al. Cutaneous tactile allodynia associated with microvascular dysfunction in muscle. Mol. Pain 2008, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Etter, M.C.; Macdonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef]
- Apperley, D.C.; Harris, R.K.; Hodgkinson, P. Solid-State NMR: Basic Principles and Practice; Momentum Press, LLC: New York, NY, USA, 2012. [Google Scholar]
- Rosenkranz, B.; Winkelmann, B.R.; Rosenkranz, B.; Parnham, M.J. Clinical Pharmacokinetics of Molsidomine. Clin. Pharmacokinet. 1996, 30, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Coderre, T.J.; Bennett, G.J. A hypothesis for the cause of complex regional pain syndrome-type I (reflex sympathetic dystrophy): Pain due to deep-tissue microvascular pathology. Pain Med. 2010, 11, 1224–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragavendran, J.V.; Laferrière, A.; Xiao, W.H.; Bennett, G.J.; Padi, S.S.; Zhang, J.; Coderre, T.J. Topical Combinations Aimed at Treating Microvascular Dysfunction Reduce Allodynia in Rat Models of CRPS-I and Neuropathic Pain. J. Pain 2013, 14, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Kirchmair, R.; Tietz, A.B.; Panagiotou, E.; Walter, D.H.; Silver, M.; Yoon, Y.-S.; Schratzberger, P.; Weber, A.; Kusano, K.; Weinberg, D.H.; et al. Therapeutic Angiogenesis Inhibits or Rescues Chemotherapy-induced Peripheral Neuropathy: Taxol- and Thalidomide-induced Injury of Vasa Nervorum is Ameliorated by VEGF. Mol. Ther. 2007, 15, 69–75. [Google Scholar] [CrossRef]
- Kirchmair, R.; Walter, D.H.; Ii, M.; Rittig, K.; Tietz, A.B.; Murayama, T.; Emanueli, C.; Silver, M.; Wecker, A.; Amant, C.; et al. Anti-angiogenesis mediates cisplatin-induced peripheral neuropathy: Attenuation or reversal by local vascular endothelial growth factor gene therapy without augmenting tumor growth. Circulation 2005, 111, 2662–2670. [Google Scholar] [CrossRef]
- Schratzberger, P.; Walter, D.H.; Rittig, K.; Bahlmann, F.H.; Pola, R.; Curry, C.; Silver, M.; Krainin, J.G.; Weinberg, D.H.; Ropper, A.H.; et al. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J. Clin. Investig. 2001, 107, 1083–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulas, O.A.; Laferriere, A.; Stein, R.S.; Bohle, D.S.; Coderre, T.J. Topical combination of meldonium and N-acetyl cysteine relieves allodynia in rat models of CRPS-1 and peripheral neuropathic pain by enhancing NO-mediated tissue oxygenation. J. Neurochem. 2020, 152, 570–584. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulas, O.A.; Laferrière, A.; Ayoub, G.; Gandrath, D.; Mottillo, C.; Titi, H.M.; Stein, R.S.; Friščić, T.; Coderre, T.J. Drug-Nutraceutical Co-Crystal and Salts for Making New and Improved Bi-Functional Analgesics. Pharmaceutics 2020, 12, 1144. https://doi.org/10.3390/pharmaceutics12121144
Fulas OA, Laferrière A, Ayoub G, Gandrath D, Mottillo C, Titi HM, Stein RS, Friščić T, Coderre TJ. Drug-Nutraceutical Co-Crystal and Salts for Making New and Improved Bi-Functional Analgesics. Pharmaceutics. 2020; 12(12):1144. https://doi.org/10.3390/pharmaceutics12121144
Chicago/Turabian StyleFulas, Oli Abate, André Laferrière, Ghada Ayoub, Dayaker Gandrath, Cristina Mottillo, Hatem M. Titi, Robin S. Stein, Tomislav Friščić, and Terence J. Coderre. 2020. "Drug-Nutraceutical Co-Crystal and Salts for Making New and Improved Bi-Functional Analgesics" Pharmaceutics 12, no. 12: 1144. https://doi.org/10.3390/pharmaceutics12121144
APA StyleFulas, O. A., Laferrière, A., Ayoub, G., Gandrath, D., Mottillo, C., Titi, H. M., Stein, R. S., Friščić, T., & Coderre, T. J. (2020). Drug-Nutraceutical Co-Crystal and Salts for Making New and Improved Bi-Functional Analgesics. Pharmaceutics, 12(12), 1144. https://doi.org/10.3390/pharmaceutics12121144




