Repurposing of Fluvastatin as an Anticancer Agent against Breast Cancer Stem Cells via Encapsulation in a Hyaluronan-Conjugated Liposome
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagent
2.2. Methods
2.2.1. Preparation of Liposomal FLUVA (L-FLUVA) and HA-L-FLUVA
2.2.2. Analysis of the Size Distribution and Zeta Potential of the Liposome
2.2.3. Drug Encapsulation Efficiency
2.2.4. Culture of 2D Bulk Cells (MCF-7 Adherent Cells) and BCSCs (MCF-7 Non-Adherent Cells)
2.2.5. Flow Cytometric Analysis of BCSCs
2.2.6. Inhibition of BCSC Proliferation
2.2.7. Antitumor Effects of HA-L-FLUVA in Xenograft Mouse Models
2.2.8. In Vivo Toxicity Determination
2.2.9. Statistical Analysis
3. Results
3.1. Analysis of the Size Distribution, Zeta Potential, and FLUVA Encapsulation Efficiency (%) of Liposomes
3.2. Cell Culture of 2D Bulk Cells and BCSCs
3.3. Inhibition of BCSC Proliferation
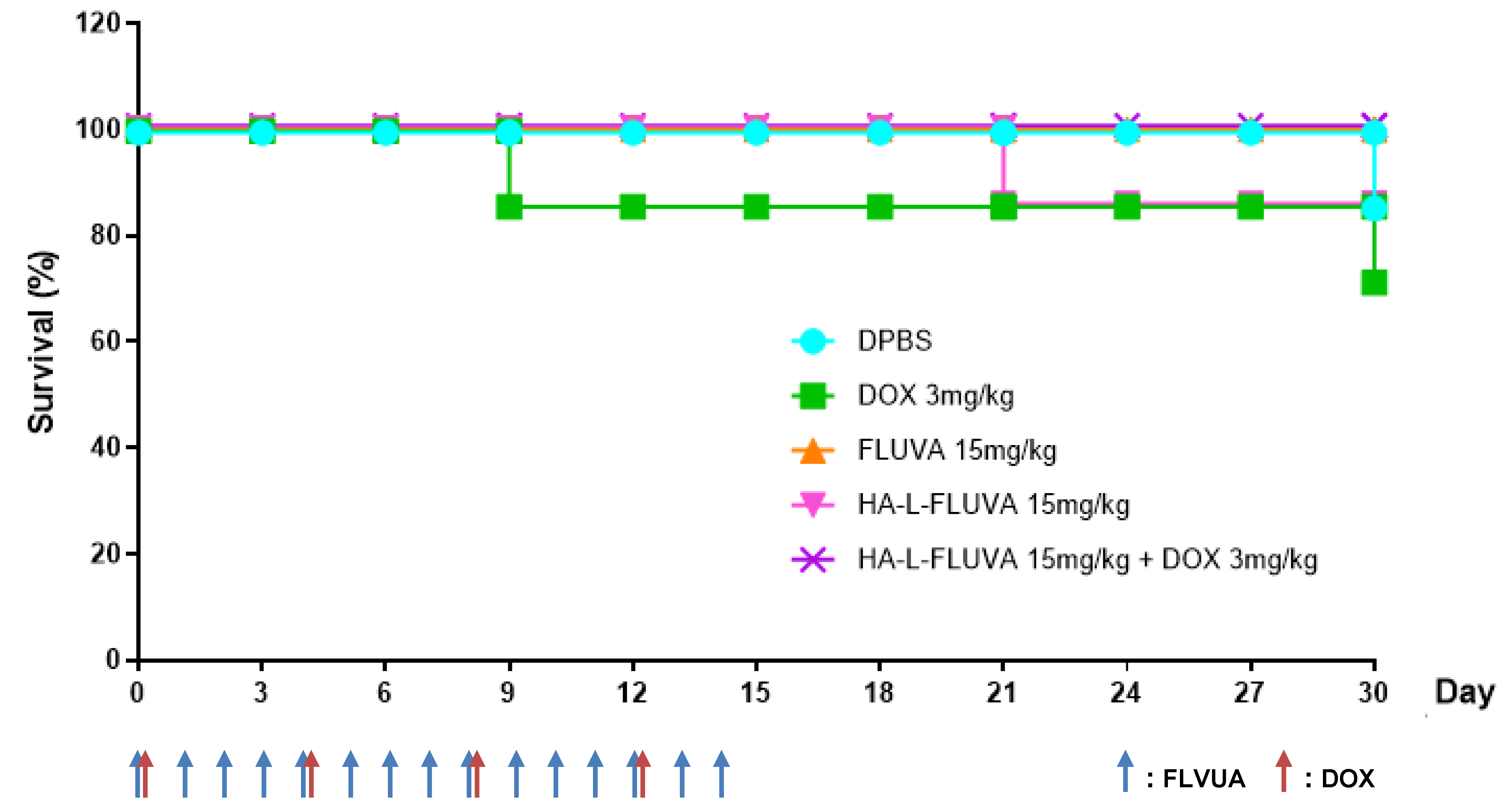
3.4. Antitumor Effects of HA-L-FLUVA in BCSC Xenograft Mouse Models
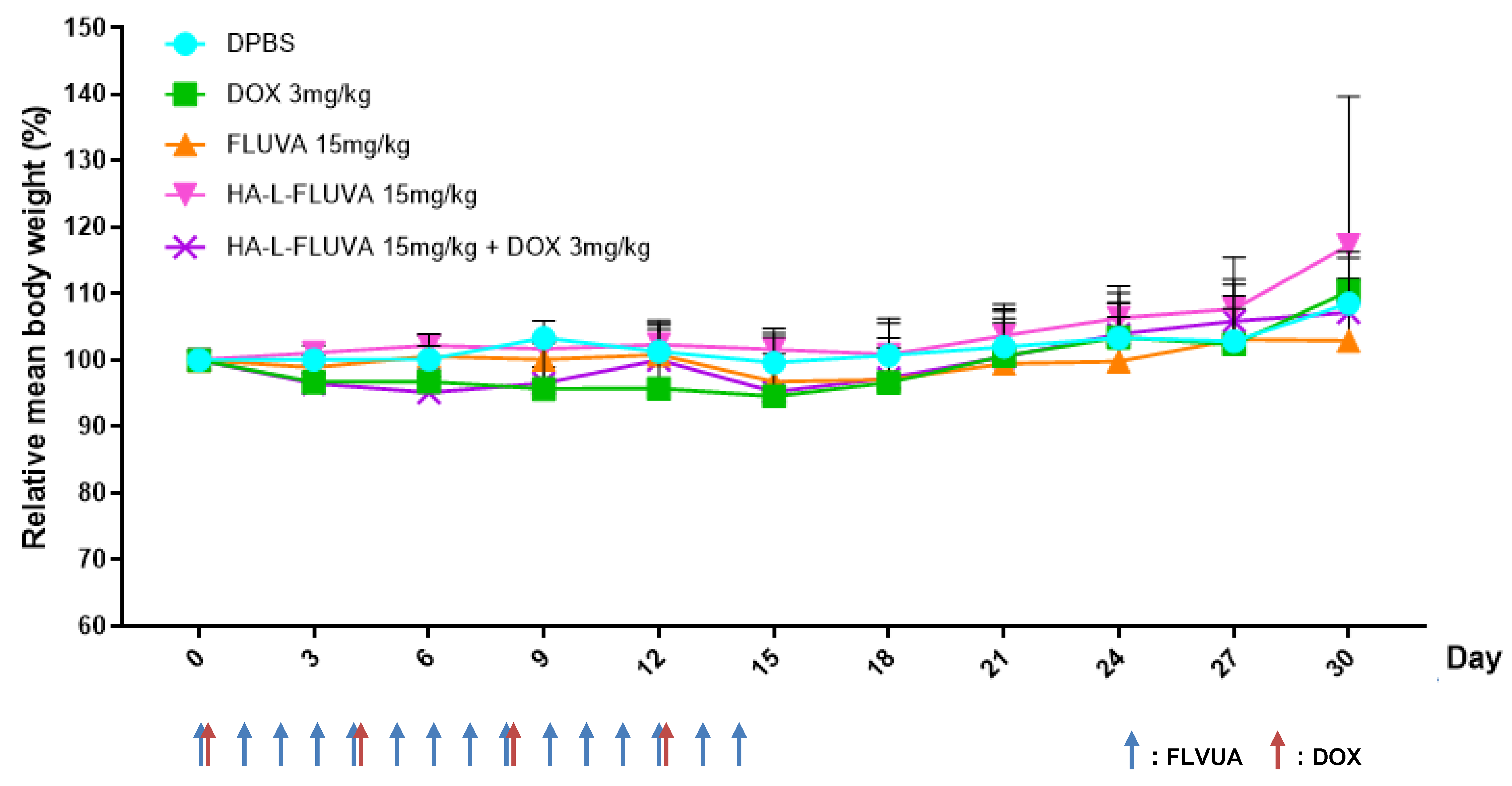
3.5. Determination of the In Vivo Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Comsa, S.; Cimpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Lawson, J.C.; Blatch, G.L.; Edkins, A.L. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res. Treat. 2009, 118, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Li, X.; Landis, M.; Dixon, J.M.; Neumeister, V.M.; Sjolund, A.; Rimm, D.L.; Wong, H.; Rodriguez, A.; Herschkowitz, J.I.; et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. USA 2009, 106, 13820–13825. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Han, J.S.; Crowe, D.L. Tumor initiating cancer stem cells from human breast cancer cell lines. Int. J. Oncol. 2009, 34, 1449–1453. [Google Scholar]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Khazaei, M.R. New Findings on Breast Cancer Stem Cells: A Review. J. Breast Cancer 2015, 18, 303–312. [Google Scholar] [CrossRef]
- Aktas, B.; Tewes, M.; Fehm, T.; Hauch, S.; Kimmig, R.; Kasimir-Bauer, S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009, 11, R46. [Google Scholar] [CrossRef]
- Holmes, F.A.; Espina, V.; Liotta, L.A.; Nagarwala, Y.M.; Danso, M.; McIntyre, K.J.; Osborne, C.R.; Anderson, T.; Krekow, L.; Blum, J.L.; et al. Pathologic complete response after preoperative anti-HER2 therapy correlates with alterations in PTEN, FOXO, phosphorylated Stat5, and autophagy protein signaling. BMC Res. Notes 2013, 6, 507. [Google Scholar] [CrossRef] [PubMed]
- Ning, N.; Pan, Q.; Zheng, F.; Teitz-Tennenbaum, S.; Egenti, M.; Yet, J.; Li, M.; Ginestier, C.; Wicha, M.S.; Moyer, J.S.; et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012, 72, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, S.; Nandy, A.; Hor, P.; Mukhopadhyay, A. Breast cancer stem cells: A novel therapeutic target. Clin. Breast Cancer 2013, 13, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Gentile, P.; Fabbri, G.; Cervelli, V.; Orlandi, A. The Role of Breast Cancer Stem Cells as a Prognostic Marker and a Target to Improve the Efficacy of Breast Cancer Therapy. Cancers 2019, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.L.; Hillaireau, H.; Vergnaud, J.; Fattal, E. Lipid-based nanosystems for CD44 targeting in cancer treatment: Recent significant advances, ongoing challenges and unmet needs. Nanomedicine 2016, 11, 1865–1887. [Google Scholar] [CrossRef] [PubMed]
- Van Wyhe, R.D.; Rahal, O.M.; Woodward, W.A. Effect of statins on breast cancer recurrence and mortality: A review. Breast Cancer 2017, 9, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Esserman, L.J.; Zhou, Y.; Shoemaker, M.; Lobo, M.; Borman, E.; Baehner, F.; Kumar, A.S.; Adduci, K.; Marx, C.; et al. Breast cancer growth prevention by statins. Cancer Res. 2006, 66, 8707–8714. [Google Scholar] [CrossRef]
- Ahern, T.P.; Lash, T.L.; Damkier, P.; Christiansen, P.M.; Cronin-Fenton, D.P. Statins and breast cancer prognosis: Evidence and opportunities. Lancet. Oncol. 2014, 15, e461–e468. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Ciofu, C. The statins as anticancer agents. Maedica 2012, 7, 377. [Google Scholar]
- Hindler, K.; Cleeland, C.S.; Rivera, E.; Collard, C.D. The role of statins in cancer therapy. Oncologist 2006, 11, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Beckwitt, C.H.; Brufsky, A.; Oltvai, Z.N.; Wells, A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Kmietowicz, Z. Statins could be new treatment option in women with oestrogen receptor positive breast cancer. BMJ 2016, 353, i3108. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J.; Wojcicka, G.; Jamroz-Wisniewska, A. Adverse effects of statins—mechanisms and consequences. Curr. Drug Saf. 2009, 4, 209–228. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- He, L.; Gu, J.; Lim, L.Y.; Yuan, Z.X.; Mo, J. Nanomedicine-Mediated Therapies to Target Breast Cancer Stem Cells. Front. Pharmacol. 2016, 7, 313. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Peach, R.; Aruffo, A.; Stamenkovic, I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J. Exp. Med. 1994, 180, 53–66. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Ponta, H. Perspectives of CD44 targeting therapies. Arch. Toxicol. 2015, 89, 3–14. [Google Scholar] [CrossRef]
- Arabi, L.; Badiee, A.; Mosaffa, F.; Jaafari, M.R. Targeting CD44 expressing cancer cells with anti-CD44 monoclonal antibody improves cellular uptake and antitumor efficacy of liposomal doxorubicin. J. Control. Release Off. J. Control. Release Soc. 2015, 220, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qi, H.; Xu, J.; Guo, P.; Chen, F.; Li, F.; Yang, X.; Sheng, N.; Wu, Y.; Pan, W. Hyaluronan-based nanocarriers with CD44-overexpressed cancer cell targeting. Pharm. Res. 2014, 31, 2988–3005. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C.; et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.; Yoshizawa, Y.; Un, K.; Araki, T.; Kimura, T.; Higaki, K. Nanoparticle-based passive drug targeting to tumors: Considerations and implications for optimization. Biol. Pharm. Bull. 2013, 36, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release Off. J. Control. Release Soc. 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef]
- Trapani, G.; Denora, N.; Trapani, A.; Laquintana, V. Recent advances in ligand targeted therapy. J. Drug Target. 2012, 20, 1–22. [Google Scholar] [CrossRef]
- Peer, D.; Margalit, R. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int. J. Cancer 2004, 108, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Yerushalmi, N.; Margalit, R. Hyaluronic acid-modified bioadhesive liposomes as local drug depots: Effects of cellular and fluid dynamics on liposome retention at target sites. Arch. Biochem. Biophys. 1998, 349, 21–26. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Kang, M.R.; Han, S.B.; Yoon, W.K.; Kim, J.H.; Lee, T.C.; Lee, C.W.; Lee, K.H.; Lee, K.; Park, S.K.; et al. Low dose estrogen supplementation reduces mortality of mice in estrogen-dependent human tumor xenograft model. Biol. Pharm. Bull. 2009, 32, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Dall, G.; Vieusseux, J.; Unsworth, A.; Anderson, R.; Britt, K. Low Dose, Low Cost Estradiol Pellets Can Support MCF-7 Tumour Growth in Nude Mice without Bladder Symptoms. J. Cancer 2015, 6, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shin, D.H.; Kim, J.S. Dual-targeting immunoliposomes using angiopep-2 and CD133 antibody for glioblastoma stem cells. J. Control. Release Off. J. Control. Release Soc. 2018, 269, 245–257. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as cancer stem cell markers: An enduring ambiguity. Clin. Dev. Immunol. 2012, 2012, 708036. [Google Scholar] [CrossRef]
- Sheridan, C.; Kishimoto, H.; Fuchs, R.K.; Mehrotra, S.; Bhat-Nakshatri, P.; Turner, C.H.; Goulet, R., Jr.; Badve, S.; Nakshatri, H. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: An early step necessary for metastasis. Breast Cancer Res. 2006, 8, R59. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef]
- Tirino, V.; Desiderio, V.; Paino, F.; Papaccio, G.; De Rosa, M. Methods for cancer stem cell detection and isolation. Methods Mol. Biol. 2012, 879, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, H.J.; Yoon, H.Y.; Yoon, I.S.; Ko, S.H.; Shim, J.S.; Cho, J.H.; Park, J.H.; Kim, K.; Kwon, I.C.; et al. Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control. Release Off. J. Control. Release Soc. 2014, 174, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Margalit, R. Tumor-targeted hyaluronan nanoliposomes increase the antitumor activity of liposomal Doxorubicin in syngeneic and human xenograft mouse tumor models. Neoplasia 2004, 6, 343–353. [Google Scholar] [CrossRef] [PubMed]
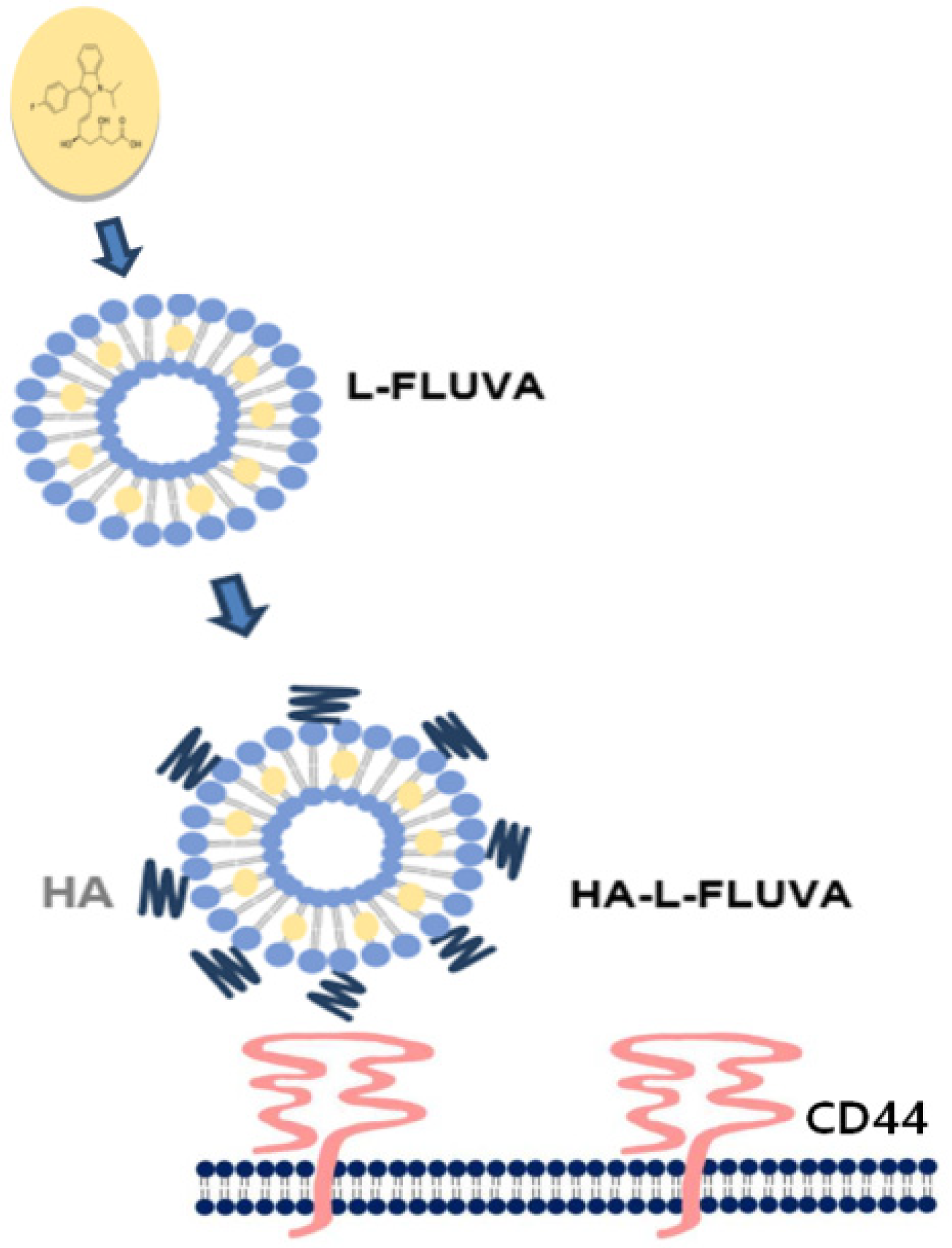
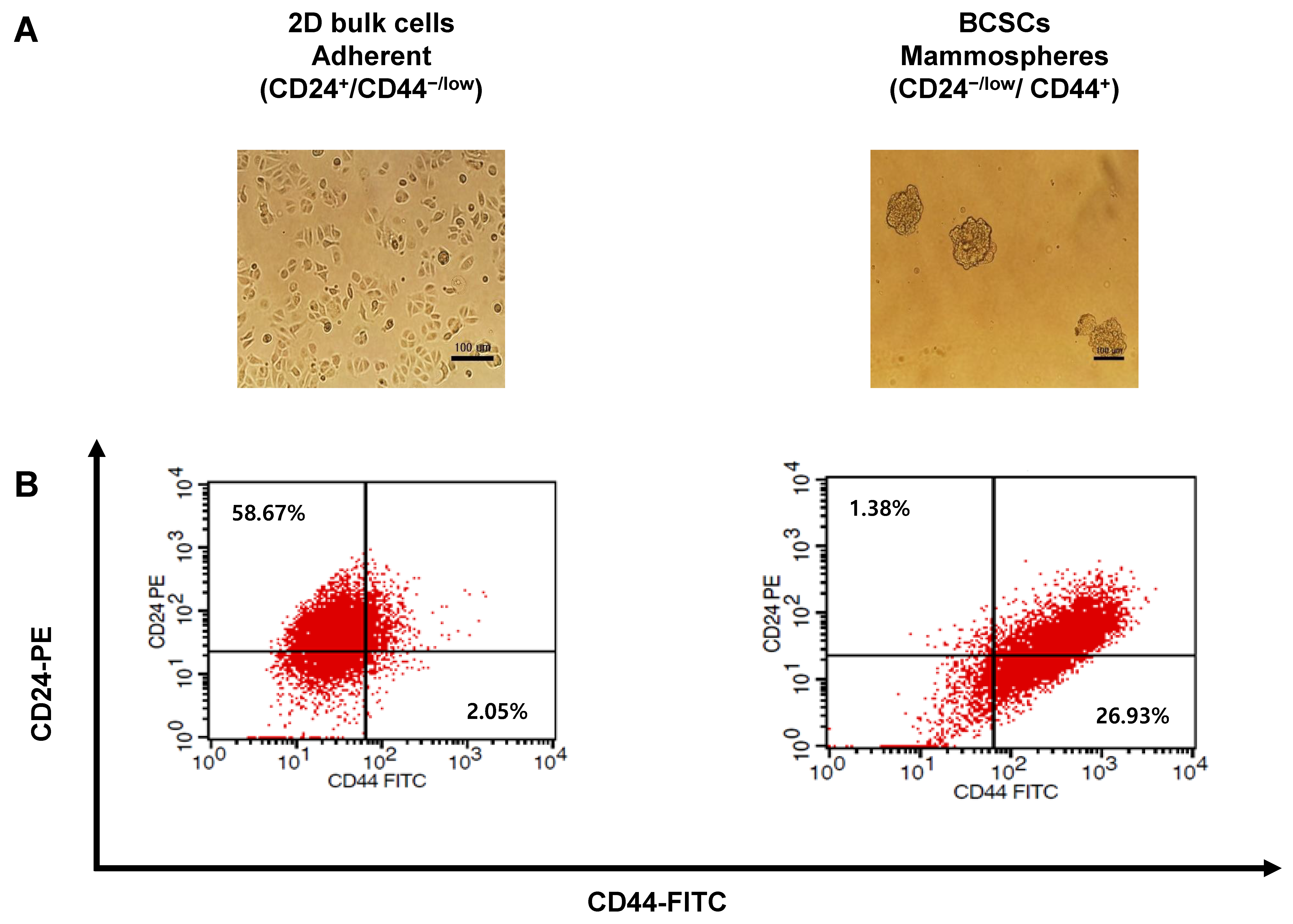
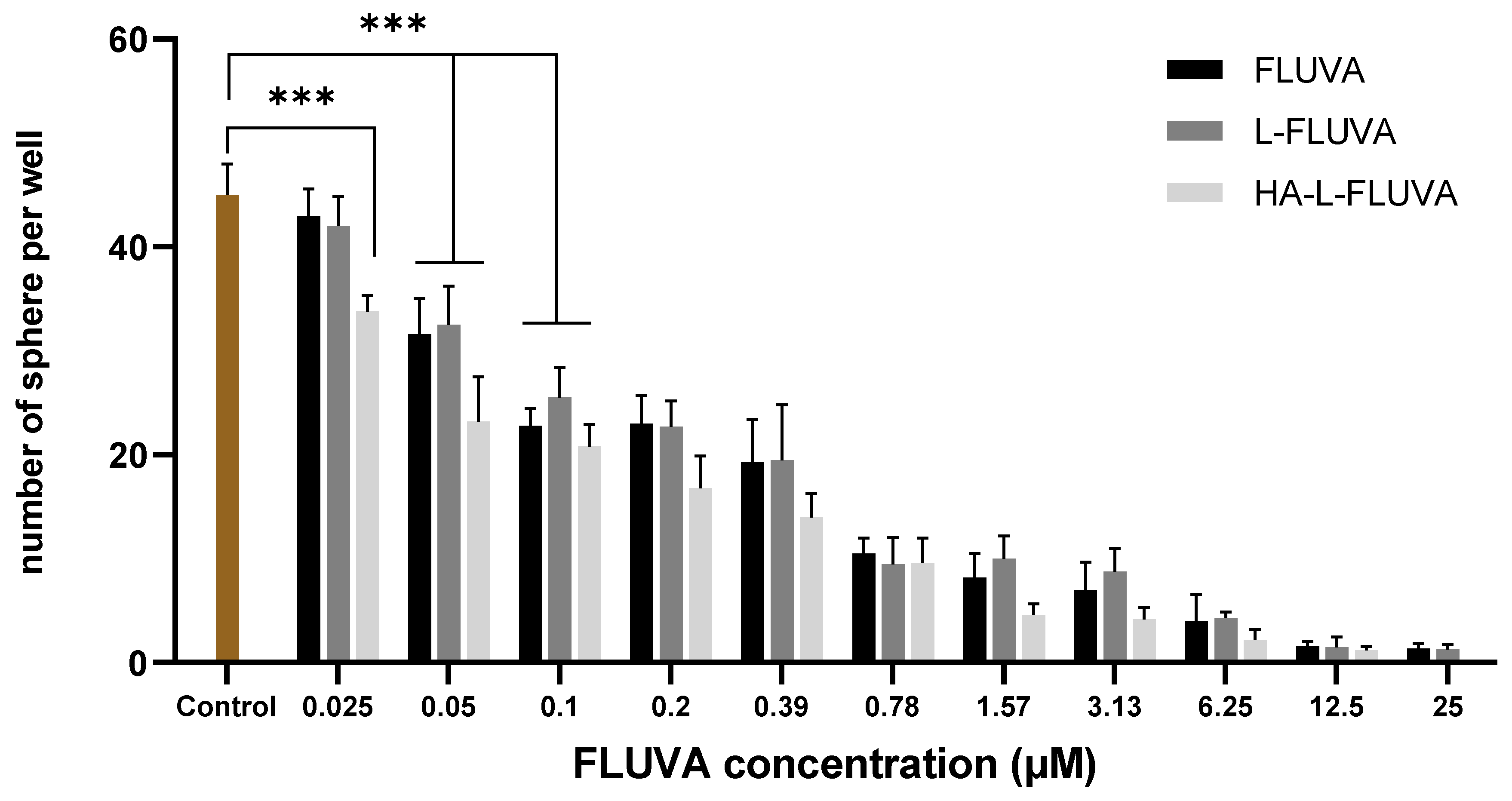
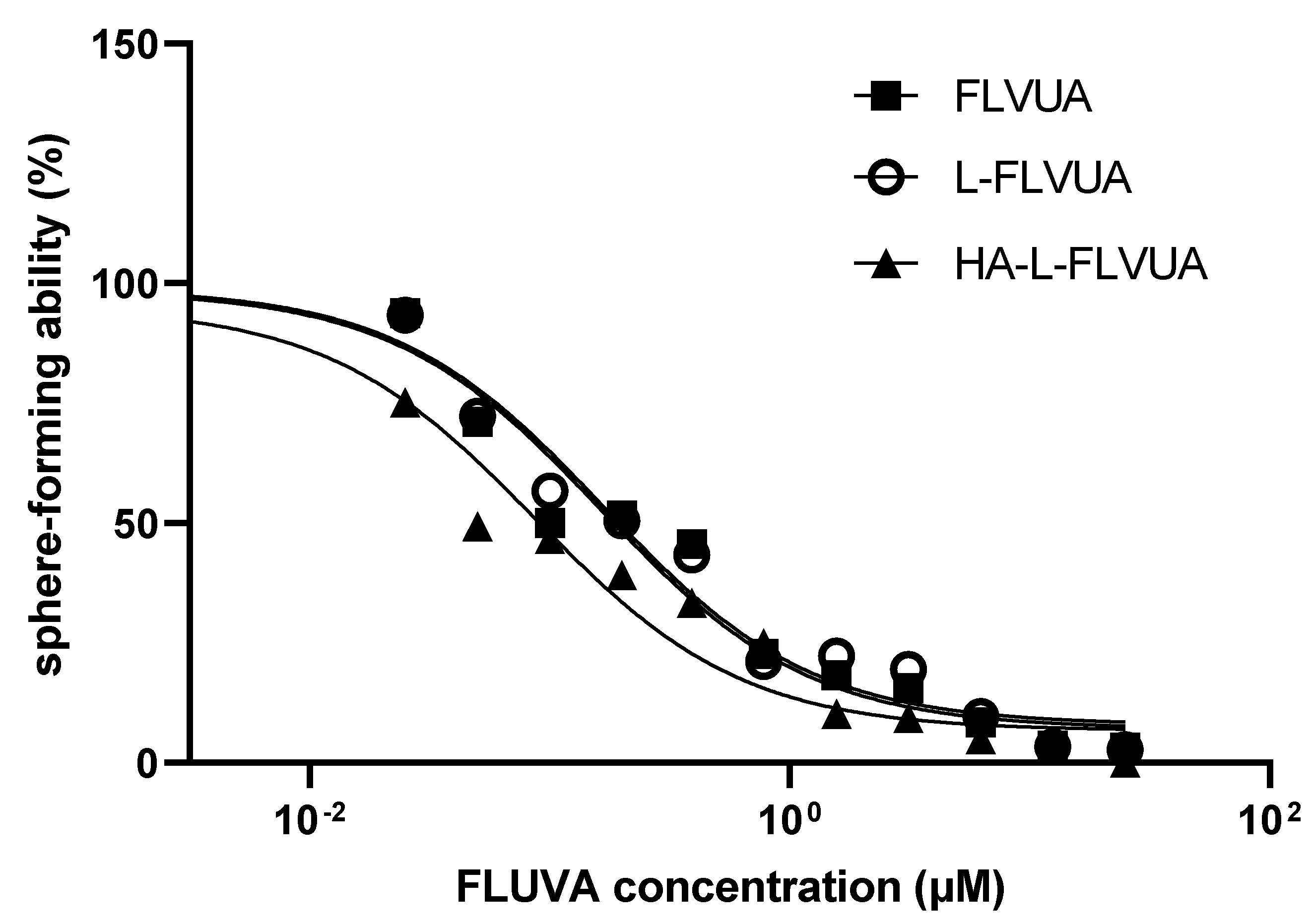


| Formulation | L-FLUVA | HA-L-FLUVA |
|---|---|---|
| Mean diameter (nm) | 115.7 ± 2.00 | 158.4 ± 1.78 |
| Zeta potential (mV) | −8.13 ± 7.72 | −24.85 ± 6.26 |
| Encapsulation efficiency (%) | 33 ± 2.8 | 35 ± 3.4 |
| Formulation | FLUVA | L-FLVUA | HA-L-FLUVA |
|---|---|---|---|
| IC50 (μM) | 0.16 | 0.17 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.S.; Shin, D.H.; Kim, J.-S. Repurposing of Fluvastatin as an Anticancer Agent against Breast Cancer Stem Cells via Encapsulation in a Hyaluronan-Conjugated Liposome. Pharmaceutics 2020, 12, 1133. https://doi.org/10.3390/pharmaceutics12121133
Yu JS, Shin DH, Kim J-S. Repurposing of Fluvastatin as an Anticancer Agent against Breast Cancer Stem Cells via Encapsulation in a Hyaluronan-Conjugated Liposome. Pharmaceutics. 2020; 12(12):1133. https://doi.org/10.3390/pharmaceutics12121133
Chicago/Turabian StyleYu, Ji Su, Dae Hwan Shin, and Jin-Seok Kim. 2020. "Repurposing of Fluvastatin as an Anticancer Agent against Breast Cancer Stem Cells via Encapsulation in a Hyaluronan-Conjugated Liposome" Pharmaceutics 12, no. 12: 1133. https://doi.org/10.3390/pharmaceutics12121133
APA StyleYu, J. S., Shin, D. H., & Kim, J.-S. (2020). Repurposing of Fluvastatin as an Anticancer Agent against Breast Cancer Stem Cells via Encapsulation in a Hyaluronan-Conjugated Liposome. Pharmaceutics, 12(12), 1133. https://doi.org/10.3390/pharmaceutics12121133






