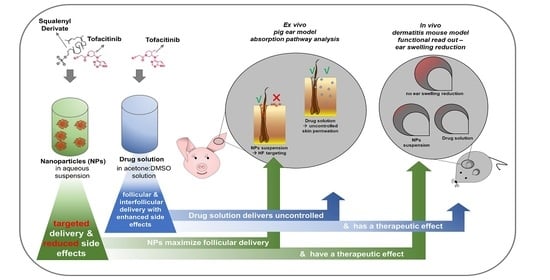
Tofacitinib Loaded Squalenyl Nanoparticles for Targeted Follicular Delivery in Inflammatory Skin Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Procedure and Ethics Statement
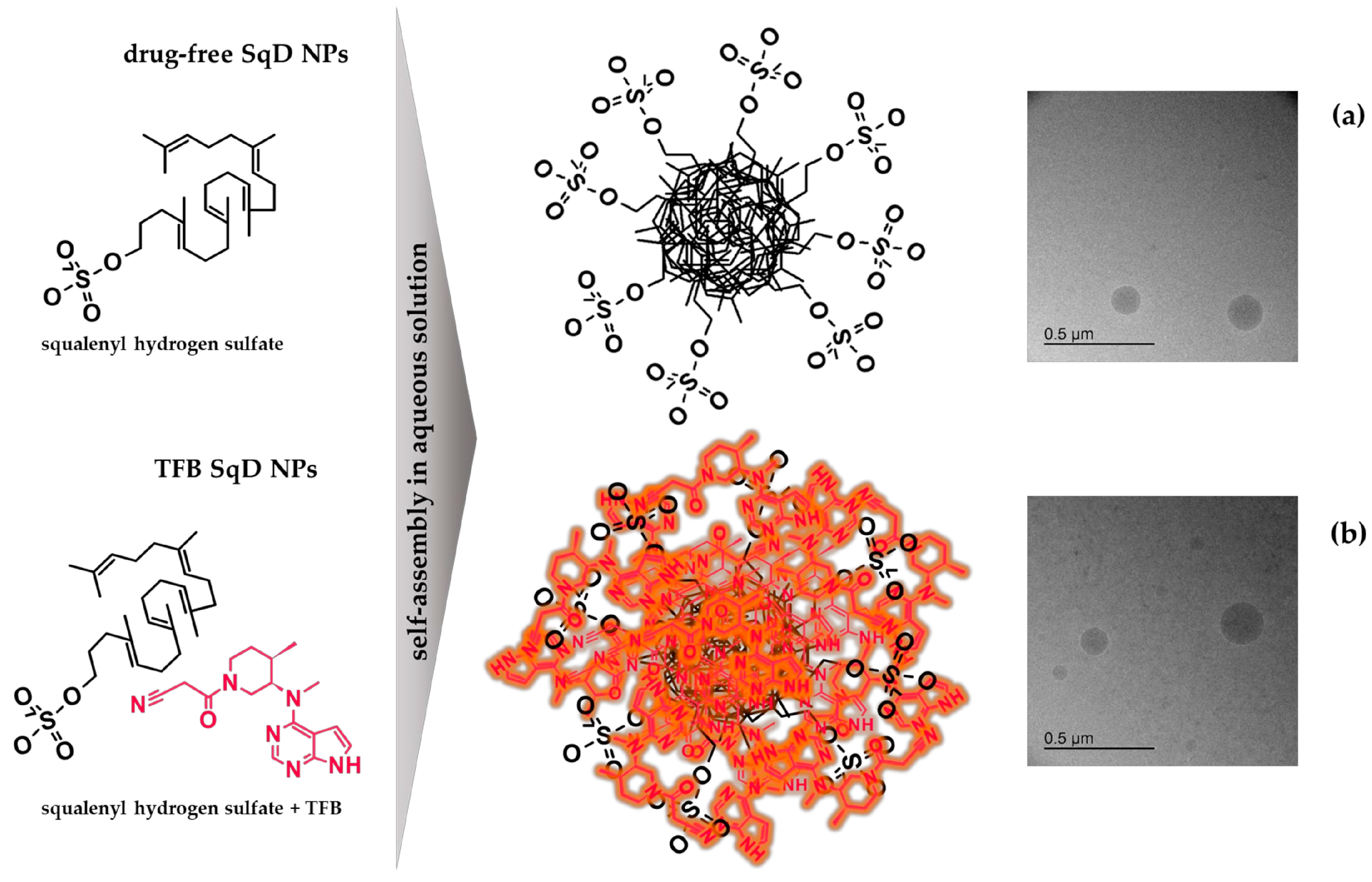
2.3. Preparation of TFB SqD NPs
2.4. Quantification of Drug Loading Capacity and Encapsulation Efficiency
2.5. Size, Polydispersity Index, Zeta-Potential, and pH Value Determination
2.6. Morphology
2.7. In Vitro Release Study
2.8. Ex Vivo and In Vivo Performance of TFB SqD NPs
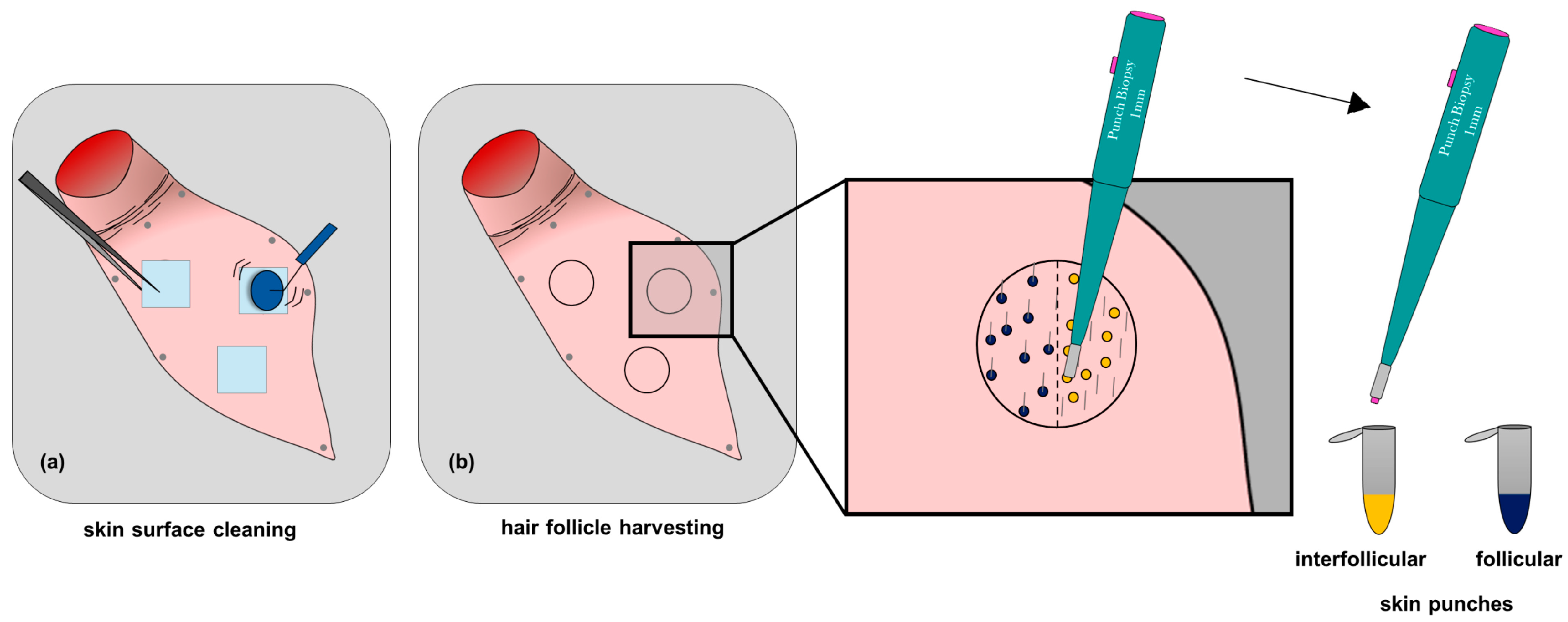
2.8.1. Targeted Follicular Transport of TFB in a Pig Ear Model
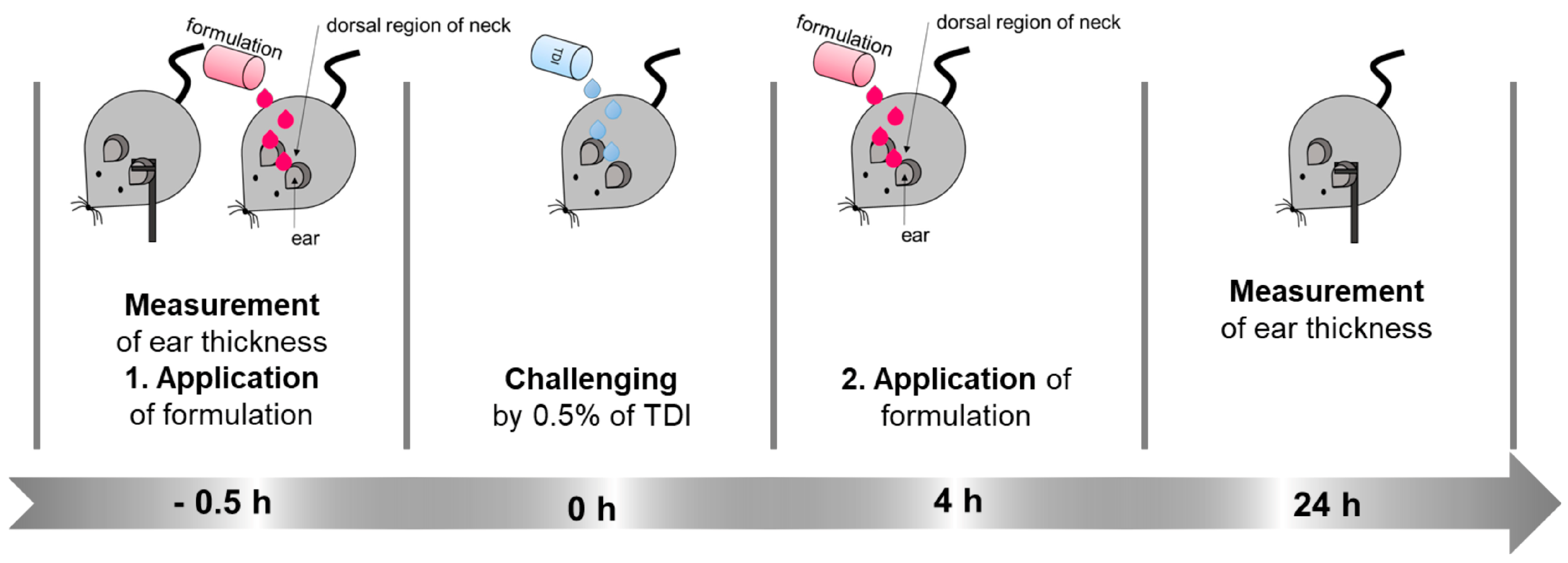
2.8.2. The Therapeutic Efficacy of TFB in an Allergic Dermatitis Mouse Model
2.9. Statistics
3. Results and Discussion
3.1. Characteristics of TFB SqD NPs
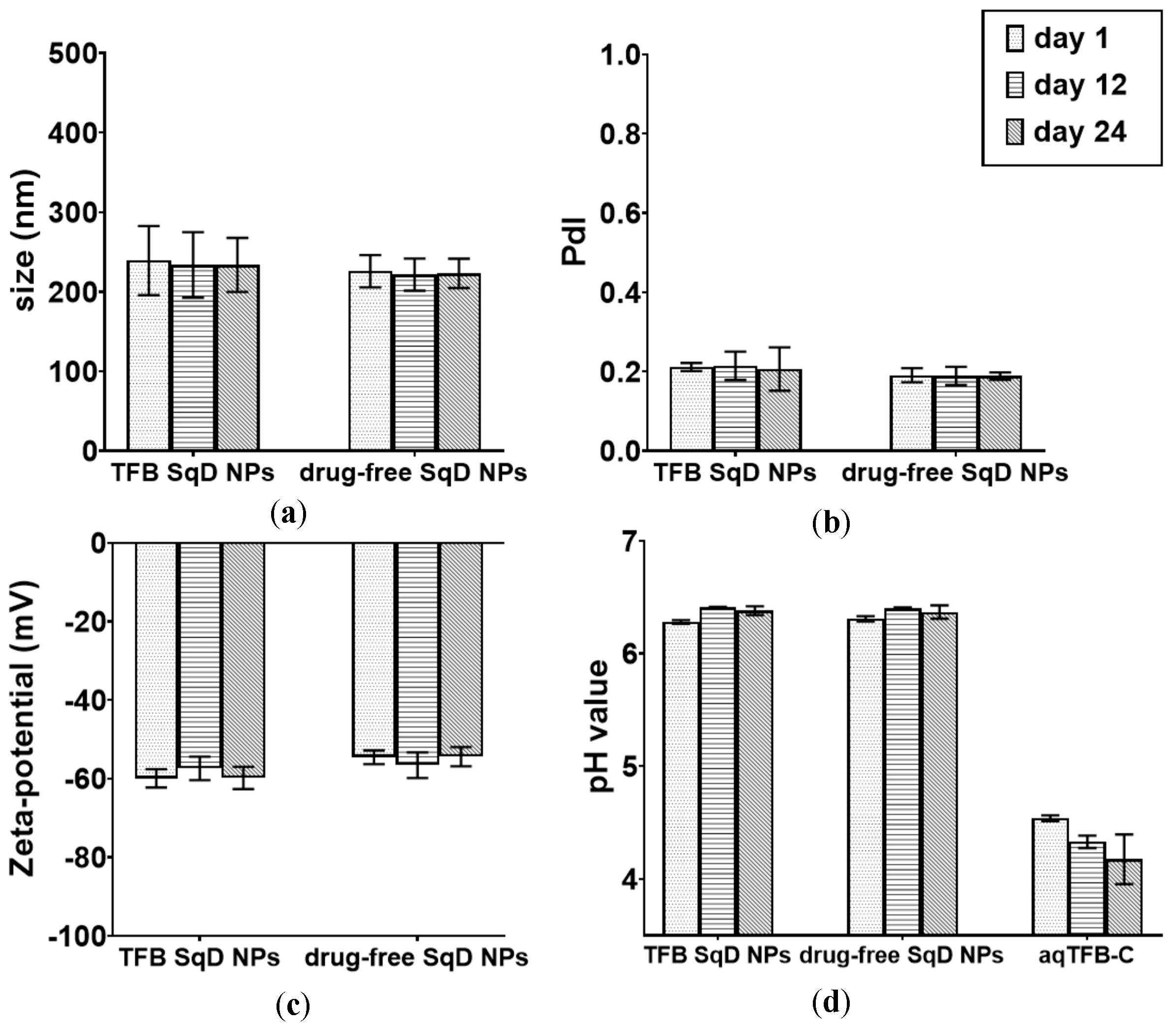
3.2. Stability of TFB SqD NPs
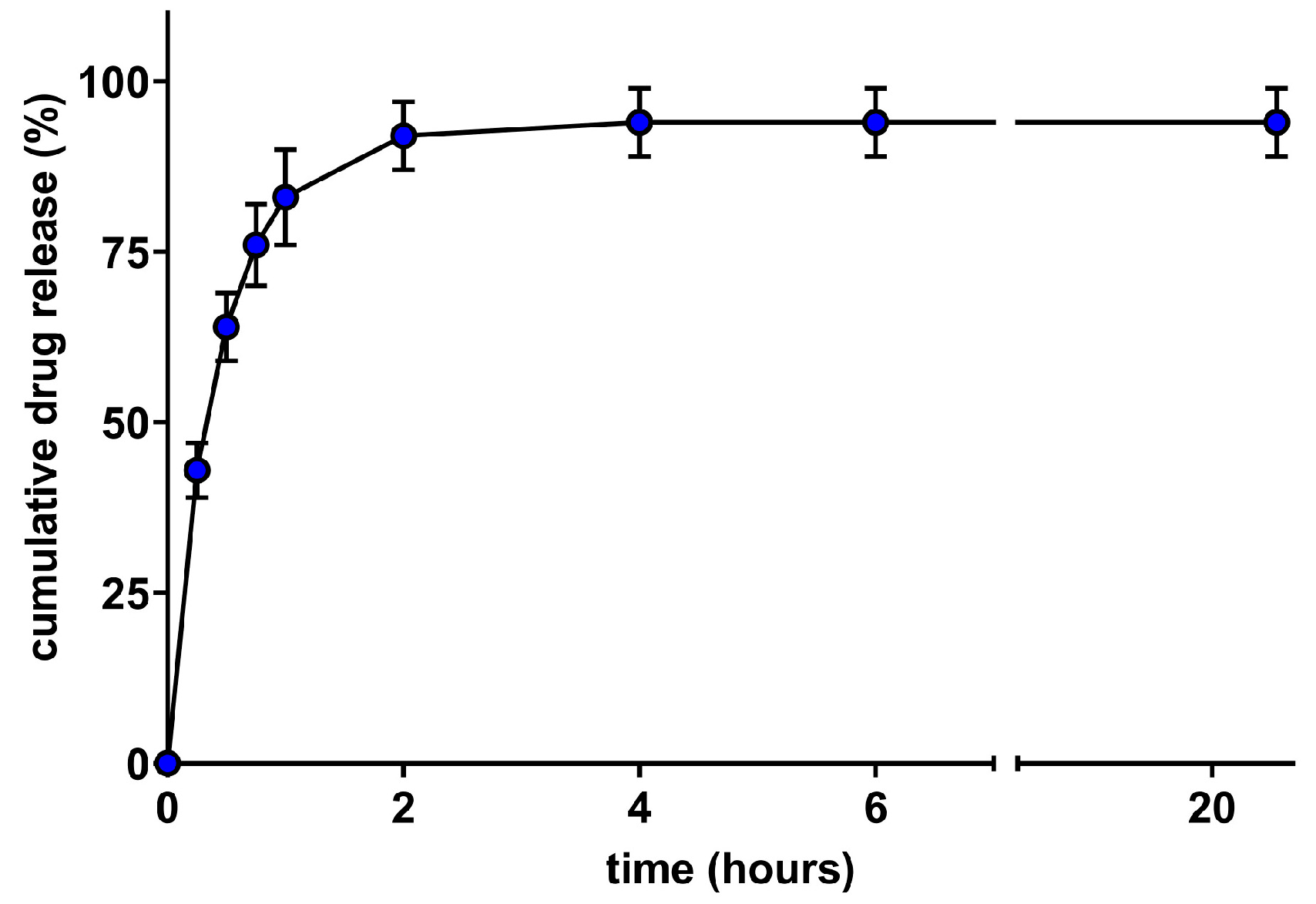
3.3. Release Profile of TFB SqD NPs
3.4. Ex vivo and In vivo Performance of TFB SqD NPs
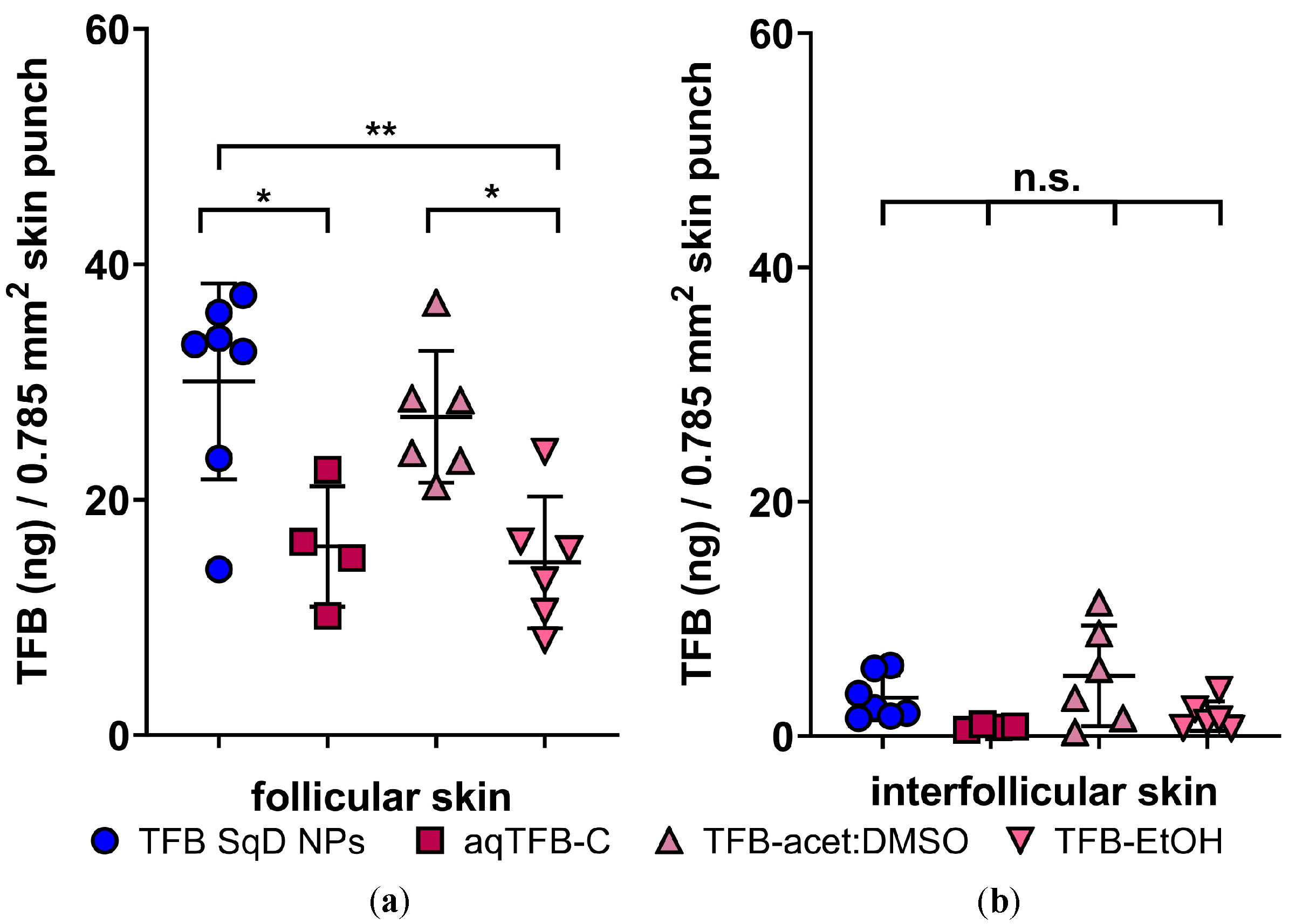
3.4.1. Targeted Follicular Transport of TFB in a Pig Ear Model
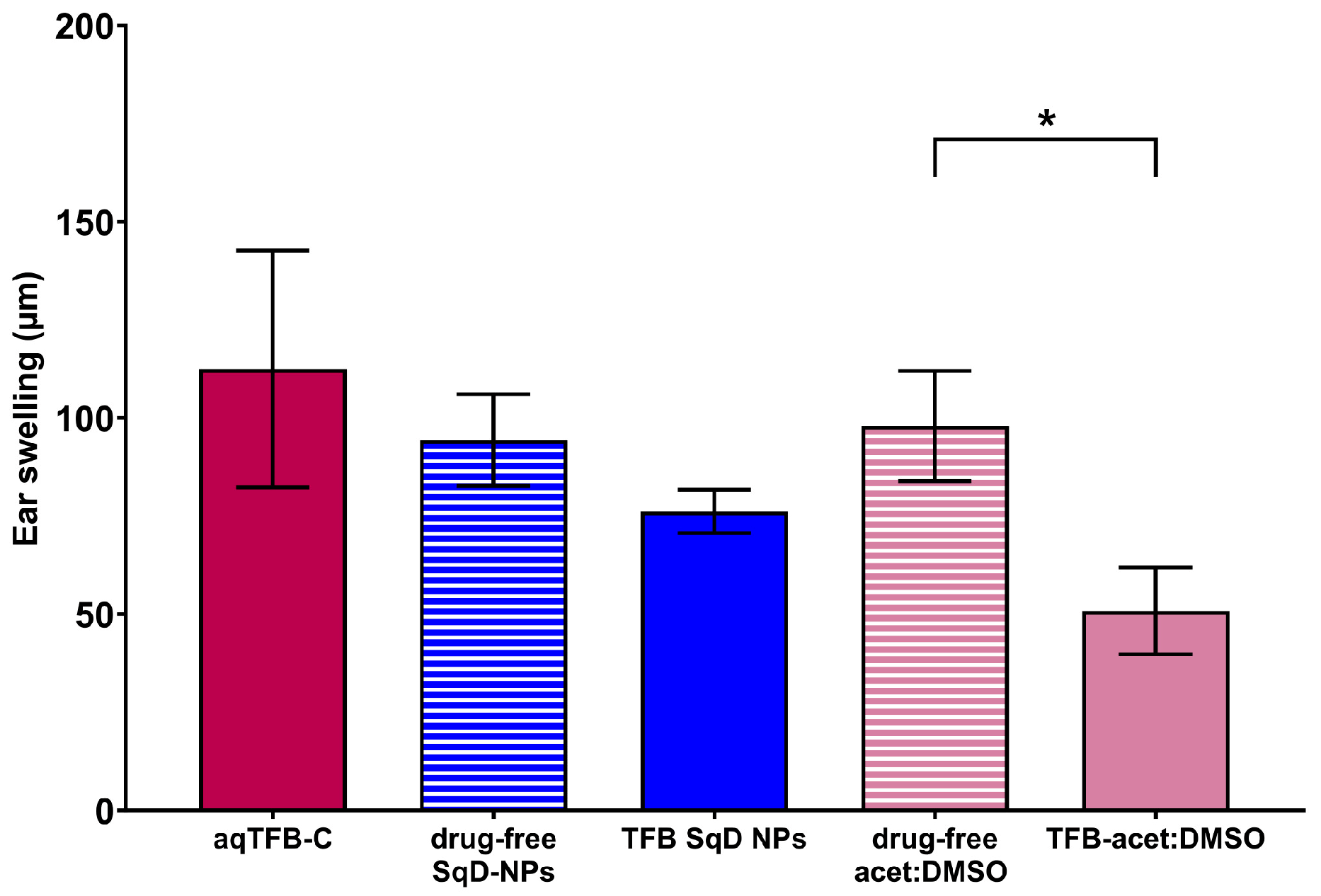
3.4.2. The Therapeutic Efficacy of TFB in an Allergic Dermatitis Mouse Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pratt, C.H.; King, L.E.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mirzoyev, S.A.; Schrum, A.G.; Davis, M.D.P.; Torgerson, R.R. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J. Investig. Dermatol. 2014, 134, 1141–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolini, M.; McElwee, K.; Gilhar, A.; Bulfone-Paus, S.; Paus, R. Hair follicle immune privilege and its collapse in alopecia areata. Exp. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Keren, A.; Paus, R. JAK inhibitors and alopecia areata. Lancet 2019, 393, 318–319. [Google Scholar] [CrossRef]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: An appraisal of new treatment approaches and overview of current therapies. J. Am. Acad. Dermatol. 2018, 78, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Carviel, J.L.; Abramovits, W. Efficacy of tofacitinib in treatment of alopecia universalis in two patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1373–1378. [Google Scholar] [CrossRef]
- Craiglow, B.G.; King, B.A. Killing two birds with one stone: Oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J. Investig. Dermatol. 2014, 134, 2988–2990. [Google Scholar] [CrossRef] [Green Version]
- FDA. XELJANZ (Tofacitinib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf (accessed on 7 August 2020).
- Divito, S.J.; Kupper, T.S. Inhibiting Janus kinases to treat alopecia areata. Nat. Med. 2014, 20, 989–990. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Tegtmeyer, K.; Zhao, J.; Maloney, N.J.; Atassi, G.; Beestrum, M.; Lio, P.A. Off-label studies on tofacitinib in dermatology: A review. J. Dermatol. Treat. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shreberk-Hassidim, R.; Ramot, Y.; Zlotogorski, A. Janus kinase inhibitors in dermatology: A systematic review. J. Am. Acad. Dermatol. 2017, 76, 745–753.e19. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.A.; Kawabata, T.T.; Krishnaswami, S.; Clark, J.D.; Telliez, J.-B.; Dowty, M.E.; Menon, S.; Lamba, M.; Zwillich, S. The mechanism of action of tofacitinib—An oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 318–328. [Google Scholar]
- FDA Drug Safety Communication. FDA Approves Boxed Warning about Increased Risk of Blood Clots and Death with Higher Dose of Arthritis and Ulcerative Colitis Medicine Tofacitinib (Xeljanz, Xeljanz XR). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (accessed on 7 August 2020).
- Blume-Peytavi, U.; Vogt, A. Translational Positioning of Janus Kinase (JAK) Inhibitors in Alopecia Areata. EBioMedicine 2015, 2, 282–283. [Google Scholar] [CrossRef] [Green Version]
- Kandekar, S.G.; Del Río-Sancho, S.; Lapteva, M.; Kalia, Y.N. Selective delivery of adapalene to the human hair follicle under finite dose conditions using polymeric micelle nanocarriers. Nanoscale 2018, 10, 1099–1110. [Google Scholar] [CrossRef]
- Mittal, A.; Schulze, K.; Ebensen, T.; Weißmann, S.; Hansen, S.; Lehr, C.M.; Guzmán, C.A. Efficient nanoparticle-mediated needle-free transcutaneous vaccination via hair follicles requires adjuvantation. Nanomedicine 2015, 11, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R.; et al. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Schaefer, U.F.; Blume-Peytavi, U.; Teichmann, A.; Otberg, N.; Sterry, W. Hair follicles—A long-term reservoir for drug delivery. Skin Pharmacol. Physiol. 2006, 19, 232–236. [Google Scholar] [CrossRef]
- Mathes, C.; Melero, A.; Conrad, P.; Vogt, T.; Rigo, L.; Selzer, D.; Prado, W.A.; de Rossi, C.; Garrigues, T.M.; Hansen, S.; et al. Nanocarriers for optimizing the balance between interfollicular permeation and follicular uptake of topically applied clobetasol to minimize adverse effects. J. Control. Release 2016, 223, 207–214. [Google Scholar] [CrossRef]
- Melero, A.; Ferreira Ourique, A.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Lehr, C.-M.; Ruver Beck, R.C.; Schaefer, U. Nanoencapsulation in lipid-core nanocapsules controls mometasone furoate skin permeability rate and its penetration to the deeper skin layers. Skin Pharmacol. Physiol. 2014, 27, 217. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Mak, W.C.; Jung, S.; Knorr, F.; Meinke, M.C.; Richter, H.; Rühl, E.; Cheung, K.Y.; Tran, N.B.N.N.; Lademann, J. Do nanoparticles have a future in dermal drug delivery? J. Control. Release 2017, 246, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Radtke, M.; Patzelt, A.; Knorr, F.; Lademann, J.; Netz, R.R. Ratchet effect for nanoparticle transport in hair follicles. Eur. J. Pharm. Biopharm. 2017, 116, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christmann, R.; Thomas, C.; Jager, N.; Raber, A.S.; Loretz, B.; Schaefer, U.F.; Tschernig, T.; Vogt, T.; Lehr, C.-M. Nanoparticle Targeting to Scalp Hair Follicles: New Perspectives for a Topical Therapy for Alopecia Areata. J. Investig. Dermatol. 2020, 140, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [Green Version]
- Lademann, J.; Richter, H.; Knorr, F.; Patzelt, A.; Darvin, M.E.; Rühl, E.; Cheung, K.Y.; Lai, K.K.; Renneberg, R.; Mak, W.C. Triggered release of model drug from AuNP-doped BSA nanocarriers in hair follicles using IRA radiation. Acta Biomater. 2016, 30, 388–396. [Google Scholar] [CrossRef]
- Paris, J.L.; Vallet-Regí, M. Ultrasound-Activated Nanomaterials for Therapeutics. BCSJ 2020, 93, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.-K.; Nichols, B.L.B.; Edgar, K.J.; Murgia, X.; Loretz, B.; Lehr, C.-M. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur. J. Pharm. Biopharm. 2019, 144, 110–124. [Google Scholar] [CrossRef]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Yichuan, Z. Lipid Nanoparticle-Mediated Delivery of Enhanced Costimulation Blockade to Prevent Type 1 Diabetes. Master of Science, Master’s Thesis, Johns Hopkins University, Baltimore, MD, USA, 2019. [Google Scholar]
- Bashir, S.; Aamir, M.; Sarfaraz, R.M.; Hussain, Z.; Sarwer, M.U.; Mahmood, A.; Akram, M.R.; Qaisar, M.N. Fabrication, characterization and in vitro release kinetics of tofacitinib-encapsulated polymeric nanoparticles: A promising implication in the treatment of rheumatoid arthritis. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–10. [Google Scholar] [CrossRef]
- Smith, K.R.; Thiboutot, D.M. Thematic review series: Skin lipids. Sebaceous gland lipids: Friend or foe? J. Lipid Res. 2008, 49, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Stella, B.; Reddy, L.H.; Hillaireau, H.; Dubernet, C.; Desmaële, D.; Lepêtre-Mouelhi, S.; Rocco, F.; Dereuddre-Bosquet, N.; Clayette, P.; et al. Squalenoyl nanomedicines as potential therapeutics. Nano Lett. 2006, 6, 2544–2548. [Google Scholar] [CrossRef] [PubMed]
- Desmaële, D.; Gref, R.; Couvreur, P. Squalenoylation: A generic platform for nanoparticular drug delivery. J. Control. Release 2012, 161, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, J.; Zhao, G.; Galdamez, J.; Lele, S.M.; Wang, X.; Liu, Y.; Soni, D.M.; Purdue, P.E.; Mikuls, T.R.; et al. Development of a Janus Kinase Inhibitor Prodrug for the Treatment of Rheumatoid Arthritis. Mol. Pharm. 2018, 15, 3456–3467. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, X.; Wang, L.; Hang, T.; Song, M. Identification of related substances in tofacitinib citrate by LC-MS techniques for synthetic process optimization. J. Pharm. Biomed. Anal. 2017, 143, 17–25. [Google Scholar] [CrossRef]
- Younis, U.S.; Vallorz, E.; Addison, K.J.; Ledford, J.G.; Myrdal, P.B. Preformulation and Evaluation of Tofacitinib as a Therapeutic Treatment for Asthma. AAPS PharmSciTech 2019, 20, 167. [Google Scholar] [CrossRef]
- Ralay-Ranaivo, B.; Desmaële, D.; Bianchini, E.P.; Lepeltier, E.; Bourgaux, C.; Borgel, D.; Pouget, T.; Tranchant, J.F.; Couvreur, P.; Gref, R. Novel self assembling nanoparticles for the oral administration of fondaparinux: Synthesis, characterization and in vivo evaluation. J. Control. Release 2014, 194, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.-K.; Murgia, X.; de Rossi, C.; Christmann, R.; Hüfner de Mello Martins, A.G.; Koch, M.; Andreas, A.; Herrmann, J.; Müller, R.; Empting, M.; et al. Squalenyl Hydrogen Sulfate Nanoparticles for Simultaneous Delivery of Tobramycin and an Alkylquinolone Quorum Sensing Inhibitor Enable the Eradication of P. aeruginosa Biofilm Infections. Angew. Chem. Int. Ed. Engl. 2020, 59, 10292–10296. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.-K.; Christmann, R.; Murgia, X.; de Rossi, C.; Frisch, S.; Koch, M.; Schaefer, U.F.; Loretz, B.; Desmaële, D.; Couvreur, P.; et al. Synthesis and biopharmaceutical characterization of amphiphilic squalenyl derivatives based versatile drug delivery platform. Front. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Raber, A.S.; Mittal, A.; Schäfer, J.; Bakowsky, U.; Reichrath, J.; Vogt, T.; Schaefer, U.F.; Hansen, S.; Lehr, C.-M. Quantification of nanoparticle uptake into hair follicles in pig ear and human forearm. J. Control. Release 2014, 179, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Möller, M.; Gurny, R.; Kalia, Y.N. Self-assembled polymeric nanocarriers for the targeted delivery of retinoic acid to the hair follicle. Nanoscale 2015, 7, 18651–18662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuyama, T.; Ehling, S.; Cook, E.; Bäumer, W. Topically Administered Janus-Kinase Inhibitors Tofacitinib and Oclacitinib Display Impressive Antipruritic and Anti-Inflammatory Responses in a Model of Allergic Dermatitis. J. Pharmacol. Exp. Ther. 2015, 354, 394–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrakchi, S.; Maibach, H.I. Biophysical parameters of skin: Map of human face, regional, and age-related differences. Contact Dermat. 2007, 57, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Patzelt, A.; Richter, H.; Schanzer, S.; Sterry, W.; Filbry, A.; Bohnsack, K.; Rippke, F.; Meinke, M. Comparison of two in vitro models for the analysis of follicular penetration and its prevention by barrier emulsions. Eur. J. Pharm. Biopharm. 2009, 72, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Richter, H.; Buettemeyer, R.; Huber, H.J.R.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. Differential stripping demonstrates a significant reduction of the hair follicle reservoir in vitro compared to in vivo. Eur. J. Pharm. Biopharm. 2008, 70, 234–238. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Meinke, M.; Sterry, W.; Patzelt, A. Which skin model is the most appropriate for the investigation of topically applied substances into the hair follicles? Skin Pharmacol. Physiol. 2010, 23, 47–52. [Google Scholar] [CrossRef]
- Buist, H.; Craig, P.; Dewhurst, I.; Hougaard Bennekou, S.; Kneuer, C.; Machera, K.; Pieper, C.; Court Marques, D.; Guillot, G.; Ruffo, F.; et al. Guidance on dermal absorption. EFSA J. 2017, 15, e04873. [Google Scholar]
- Teichmann, A.; Jacobi, U.; Ossadnik, M.; Richter, H.; Koch, S.; Sterry, W.; Lademann, J. Differential stripping: Determination of the amount of topically applied substances penetrated into the hair follicles. J. Investig. Dermatol. 2005, 125, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Li, B.S.; Cary, J.H.; Maibach, H.I. Should we instruct patients to rub topical agents into skin? The evidence. J. Dermatol. Treat. 2019, 30, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Schanzer, S.; Richter, H.; Meinke, M.C.; Weigmann, H.-J.; Patzelt, A. Stripping Procedures for Penetration Measurements of Topically Applied Substances. In Percutaneous Penetration Enhancers Drug Penetration Into/Through the Skin: Methodology and General Considerations; Dragicevic, N.I., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 205–214. ISBN 978-3-662-53270-6. [Google Scholar]
- FDA/CDER. Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 7 August 2020).
- Bäumer, W.; Seegers, U.; Braun, M.; Tschernig, T.; Kietzmann, M. TARC and RANTES, but not CTACK, are induced in two models of allergic contact dermatitis. Effects of cilomilast and diflorasone diacetate on T-cell-attracting chemokines. Br. J. Dermatol. 2004, 151, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, W.; Tschernig, T.; Sülzle, B.; Seegers, U.; Lührmann, A.; Kietzmann, M. Effects of cilomilast on dendritic cell function in contact sensitivity and dendritic cell migration through skin. Eur. J. Pharmacol. 2003, 481, 271–279. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Committee for Medicinal Products for Human Use. Assessment Report: Xeljanz; INN-Tofacitinib. Available online: https://www.ema.europa.eu/en/documents/assessment-report/xeljanz-epar-public-assessment-report_en-0.pdf (accessed on 15 September 2020).
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar]
- Dimde, M.; Sahle, F.F.; Wycisk, V.; Steinhilber, D.; Camacho, L.C.; Licha, K.; Lademann, J.; Haag, R. Synthesis and Validation of Functional Nanogels as pH-Sensors in the Hair Follicle. Macromol. Biosci. 2017, 17, 1600505. [Google Scholar] [CrossRef] [Green Version]
- Kaden, D.; Dähne, L.; Knorr, F.; Richter, H.; Lademann, J.; Meinke, M.C.; Patzelt, A.; Darvin, M.E.; Jung, S. Determination of the pH Gradient in Hair Follicles of Human Volunteers Using pH-Sensitive Melamine Formaldehyde-Pyranine Nile Blue Microparticles. Sensors 2020, 20, 5243. [Google Scholar] [CrossRef]
- Otberg, N.; Patzelt, A.; Rasulev, U.; Hagemeister, T.; Linscheid, M.; Sinkgraven, R.; Sterry, W.; Lademann, J. The role of hair follicles in the percutaneous absorption of caffeine. Br. J. Clin. Pharmacol. 2008, 65, 488–492. [Google Scholar] [CrossRef] [Green Version]
- Scientific Committee on Consumer Safety SCCS. The Sccs Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 10th Revision. Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_224.pdf (accessed on 8 July 2020).
- Bäumer, W.; Gorr, G.; Hoppmann, J.; Ehinger, A.M.; Rundfeldt, C.; Kietzmann, M. AWD 12-281, a highly selective phosphodiesterase 4 inhibitor, is effective in the prevention and treatment of inflammatory reactions in a model of allergic dermatitis. J. Pharm. Pharmacol. 2003, 55, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Zou, Y.; Celli, A.; Zhu, H.; Elmahdy, A.; Cao, Y.; Hui, X.; Maibach, H. Confocal laser scanning microscopy to estimate nanoparticles’ human skin penetration in vitro. Int. J. Nanomed. 2017, 12, 8035–8041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Group | Abbreviation | Components | Formulation Type | No. Ear | No. Area |
|---|---|---|---|---|---|
| Treatment (TFB 2 mg/mL) | TFB SqD NPs | TFB 1, SqD 2, phos-buffer 3 | suspension | 4 | 7 |
| aqTFB-C | TFB-C 4, phos-buffer 3 | solution | 2 | 4 | |
| TFB-EtOH | TFB 1, EtOH 5:H2O (50:50 v/v) | solution | 3 | 6 | |
| TFB-acet:DMSO | TFB 1, acetone:DMSO 6 (7:1 v/v) | solution | 3 | 6 | |
| vehicle control | phos-buffer | phos-buffer 3 | solution | 4 | 4 |
| EtOH:H2O | EtOH 5:H2O (50:50 v/v) | solution | 3 | 3 | |
| acet:DMSO | acetone:DMSO 6 (7:1 v/v) | solution | 3 | 3 |
| Group | Abbreviation | Components | Formulation Type | No. Mice |
|---|---|---|---|---|
| Treatment TFB 2 mg/mL | TFB SqD NPs | TFB 1, SqD 2, phos-buffer 3 | suspension | 7 |
| aqTFB-C | TFB-C 4, phos-buffer 3 | solution | 7 | |
| TFB-acet:DMSO | TFB 1, acetone:DMSO 5 (7:1 v/v) | solution | 7 | |
| vehicle control | Drug-free SqD NPs | SqD 2, phos-buffer 3 | suspension | 7 |
| Drug-free acet:DMSO | acetone:DMSO 5 (7:1 v/v) | solution | 7 |
| Physicochemical Characteristic | Drug-Free SqD NPs | TFB SqD NPs |
|---|---|---|
| size (nm) | 225.7 ± 20.3 | 239.1 ± 43.5 |
| PdI | 0.19 ± 0.02 | 0.21 ± 0.01 |
| zeta-potential (mV) | −54.5 ± 1.7 | −59.9 ± 2.3 |
| pH | 6.28 ± 0.02 | 6.31 ± 0.02 |
| LC (%) | N.A. | 20.1 ± 2.1 |
| EE (%) | N.A. | 19.8 ± 3.6 |
| Treatment Group | Mass Balance (%) |
|---|---|
| TFB SqD NPs | 90.4 ± 6.4 |
| aqTFB-C | 87.4 ± 1.9 |
| TFB-acet:DMSO | 69.7 ± 10.7 |
| TFB-EtOH | 95.7 ± 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christmann, R.; Ho, D.-K.; Wilzopolski, J.; Lee, S.; Koch, M.; Loretz, B.; Vogt, T.; Bäumer, W.; Schaefer, U.F.; Lehr, C.-M. Tofacitinib Loaded Squalenyl Nanoparticles for Targeted Follicular Delivery in Inflammatory Skin Diseases. Pharmaceutics 2020, 12, 1131. https://doi.org/10.3390/pharmaceutics12121131
Christmann R, Ho D-K, Wilzopolski J, Lee S, Koch M, Loretz B, Vogt T, Bäumer W, Schaefer UF, Lehr C-M. Tofacitinib Loaded Squalenyl Nanoparticles for Targeted Follicular Delivery in Inflammatory Skin Diseases. Pharmaceutics. 2020; 12(12):1131. https://doi.org/10.3390/pharmaceutics12121131
Chicago/Turabian StyleChristmann, Rebekka, Duy-Khiet Ho, Jenny Wilzopolski, Sangeun Lee, Marcus Koch, Brigitta Loretz, Thomas Vogt, Wolfgang Bäumer, Ulrich F. Schaefer, and Claus-Michael Lehr. 2020. "Tofacitinib Loaded Squalenyl Nanoparticles for Targeted Follicular Delivery in Inflammatory Skin Diseases" Pharmaceutics 12, no. 12: 1131. https://doi.org/10.3390/pharmaceutics12121131
APA StyleChristmann, R., Ho, D.-K., Wilzopolski, J., Lee, S., Koch, M., Loretz, B., Vogt, T., Bäumer, W., Schaefer, U. F., & Lehr, C.-M. (2020). Tofacitinib Loaded Squalenyl Nanoparticles for Targeted Follicular Delivery in Inflammatory Skin Diseases. Pharmaceutics, 12(12), 1131. https://doi.org/10.3390/pharmaceutics12121131