Optimal Design of THEDES Based on Perillyl Alcohol and Ibuprofen
Abstract
:1. Introduction
2. Materials and Methods
3. Results
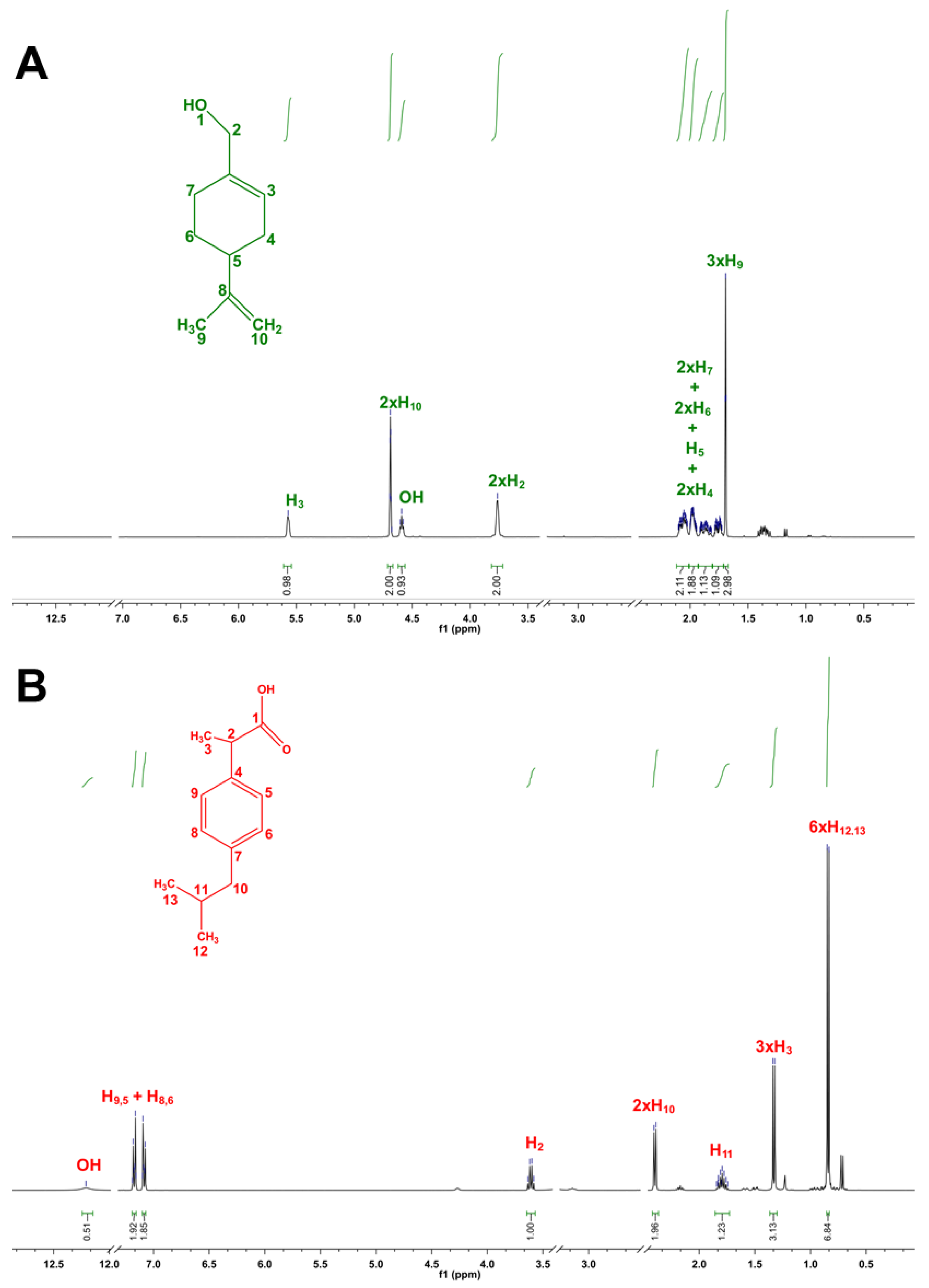
3.1. Design and Characterization of THEDES
3.2. Solubility of Ibuprofen in Physiologically Simulated Media
3.3. Antimicrobial Potential of the Formulated THEDES
3.4. THEDES Anticancer Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Natl. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural product drug discovery in the next millennium. Pharm. Biol. 2001, 39, 8–17. [Google Scholar] [PubMed] [Green Version]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Jachak, S.M.; Saklani, A. Challenges and opportunities in drug discovery from plants. Curr. Sci. 2007, 1251–1257. [Google Scholar]
- Lee, K.-H. Research and future trends in the pharmaceutical development of medicinal herbs from Chinese medicine. Public Health Nutr. 2000, 3, 515–522. [Google Scholar] [CrossRef]
- Patwardhan, B.; Vaidya, A.D.; Chorghade, M. Ayurveda and natural products drug discovery. Curr. Sci. 2004, 86, 789–799. [Google Scholar]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Jansen, D.J.; Shenvi, R.A. Synthesis of medicinally relevant terpenes: Reducing the cost and time of drug discovery. Future Med. Chem. 2014, 6, 1127–1148. [Google Scholar] [CrossRef] [Green Version]
- Maione, F.; Cicala, C.; Musciacco, G.; De Feo, V.; Amat, A.G.; Ialenti, A.; Mascolo, N. Phenols, alkaloids and terpenes from medicinal plants with antihypertensive and vasorelaxant activities. A review of natural products as leads to potential therapeutic agents. Natl. Prod. Commun. 2013, 8, 1934578X1300800434. [Google Scholar] [CrossRef] [Green Version]
- Ramawat, K.G.; Mérillon, J.-M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Cham, Switzerland, 2013. [Google Scholar]
- Saito, A.Y.; Rodriguez, A.A.M.; Vega, D.S.M.; Sussmann, R.A.; Kimura, E.A.; Katzin, A.M. Antimalarial activity of the terpene nerolidol. Int. J. Antimicrob. Agents 2016, 48, 641–646. [Google Scholar] [CrossRef]
- Guesmi, F.; Prasad, S.; Tyagi, A.K.; Landoulsi, A. Antinflammatory and anticancer effects of terpenes from oily fractions of Teucruim alopecurus, blocker of IκBα kinase, through downregulation of NF-κB activation, potentiation of apoptosis and suppression of NF-κB-regulated gene expression. Biomed. Pharmacother. 2017, 95, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Ginkgo terpene component has an anti-inflammatory effect on Candida albicans-caused arthritic inflammation. Int. Immunopharmacol. 2005, 5, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Solís, C.; Becerra, J.; Flores, C.; Robledo, J.; Silva, M. Antibacterial and antifungal terpenes from Pilgerodendron uviferum (D. Don) Florin. J. Chil. Chem. Soc. 2004, 49, 157–161. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Cara, P.D.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259. [Google Scholar] [PubMed]
- Foti, C.; Zambonin, C.G.; Conserva, A.; Casulli, C.; D’Accolti, L.; Angelini, G. Occupational contact dermatitis to a limonene-based solvent in a histopathology technician. Contact Dermat. 2007, 56, 109–112. [Google Scholar] [CrossRef]
- Hebeish, A.; Fouda, M.M.; Hamdy, I.; El-Sawy, S.; Abdel-Mohdy, F. Preparation of durable insect repellent cotton fabric: Limonene as insecticide. Carbohydr. Polym. 2008, 74, 268–273. [Google Scholar] [CrossRef]
- Matta, G.B. D-Limonene Based Aqueous Cleaning Compositions. U.S. Patent 1,222,425A, 2 June 1987. [Google Scholar]
- D’Alessio, P.A.; Ostan, R.; Bisson, J.-F.; Schulzke, J.D.; Ursini, M.V.; Béné, M.C. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. [Google Scholar] [CrossRef]
- Del Toro-Arreola, S.; Flores-Torales, E.; Torres-Lozano, C.; Del Toro-Arreola, A.; Tostado-Pelayo, K.; Ramirez-Dueñas, M.G.; Daneri-Navarro, A. Effect of D-limonene on immune response in BALB/c mice with lymphoma. Int. Immunopharmacol. 2005, 5, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Hirota, R.; Roger, N.N.; Nakamura, H.; Song, H.S.; Sawamura, M.; Suganuma, N. Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J. Food Sci. 2010, 75, H87–H92. [Google Scholar] [CrossRef] [PubMed]
- Raphael, T.; Kuttan, G. Immunomodulatory activity of naturally occurring monoterpenes carvone, limonene, and perillic acid. Immunopharmacol. Immunotoxicol. 2003, 25, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Sundrarajan, M.; Rukmani, A. Durable antibacterial finishing on cotton by impregnation of limonene microcapsules. Adv. Chem. Lett. 2013, 1, 40–43. [Google Scholar] [CrossRef]
- Raphael, T.; Kuttan, G. Effect of naturally occurring monoterpenes carvone, limonene and perillic acid in the inhibition of experimental lung metastasis induced by B16F-10 melanoma cells. J. Exp. Clin. Cancer Res. CR 2003, 22, 419–424. [Google Scholar]
- Belanger, J.T. Perillyl alcohol: Applications in oncology. Altern. Med. Rev. A J. Clin. Ther. 1998, 3, 448–457. [Google Scholar]
- Ansari, M.A.; Fatima, Z.; Ahmad, K.; Hameed, S. Monoterpenoid perillyl alcohol impairs metabolic flexibility of Candida albicans by inhibiting glyoxylate cycle. Biochem. Biophys. Res. Commun. 2018, 495, 560–566. [Google Scholar] [CrossRef]
- Ansari, M.A.; Fatima, Z.; Hameed, S. Anticandidal effect and mechanisms of monoterpenoid, perillyl alcohol against Candida albicans. PLoS ONE 2016, 11, e0162465. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, R.D.A.; Ortega, A.C.; Gonzalez Maldonado, L.A.; De Castro, R.D.; Avila-Campos, M.J.; Rossa, C., Jr.; De Aquino, S.G. Perillyl alcohol has antibacterial effects and reduces ROS production in macrophages. J. Appl. Oral Sci. 2020, 28. [Google Scholar] [CrossRef]
- Ripple, G.H.; Gould, M.N.; Stewart, J.A.; Tutsch, K.D.; Arzoomanian, R.Z.; Alberti, D.; Feierabend, C.; Pomplun, M.; Wilding, G.; Bailey, H.H. Phase I clinical trial of perillyl alcohol administered daily. Clin. Cancer Res. 1998, 4, 1159–1164. [Google Scholar]
- Chastain, D.E.; Sanders, W.E., Jr.; Sanders, C.C. Using Perillyl Alcohol to Kill Bacteria and Yeasts. U.S. Patent 5,110,832A, 5 May 1992. [Google Scholar]
- Akrami, H.; Aminzadeh, S.; Fallahi, H. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumor Biol. 2015, 36, 3237–3243. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the anticancer potential of limomene based therapeutic deep eutectic solvents. Sci. Rep. 2019, 9, 14926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, A.P.; Capper, G.; Gray, S. Design of improved deep eutectic solvents using hole theory. Chem. A Eur. J. Chem. Phys. Phys. Chem. 2006, 7, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroso, I.M.; Craveiro, R.; Rocha, Â.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES—Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutierrez, M.C.; Ferrer, M.L. Deep eutectic solvents in polymerizations: A greener alternative to conventional syntheses. Chem. Sus. Chem. 2014, 7, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Morais, E.S.; Mendonça, P.V.; Coelho, J.F.; Freire, M.G.; Freire, C.S.; Coutinho, J.A.; Silvestre, A.J. Deep eutectic solvent aqueous solutions as efficient media for the solubilization of hardwood xylans. Chem. Sus. Chem. 2018, 11, 753–762. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef]
- Di Marino, D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M. Electrochemical depolymerisation of lignin in a deep eutectic solvent. Green Chem. 2016, 18, 6021–6028. [Google Scholar] [CrossRef]
- Jahan, M.S.; Islam, M.J.; Begum, R.; Kayesh, R.; Rahman, A. A study of method development, validation, and forced degradation for simultaneous quantification of Paracetamol and Ibuprofen in pharmaceutical dosage form by RP-HPLC method. Alytic Chem. Insights 2014, 9, 75. [Google Scholar]
- Silva, J.M.; Akkache, S.; Araújo, A.C.; Masmoudi, Y.; Reis, R.L.; Badens, E.; Duarte, A.R.C. Development of innovative medical devices by dispersing fatty acid eutectic blend on gauzes using supercritical particle generation processes. Mater. Sci. Eng. C 2019, 99, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of functional therapeutic deep eutectic solvents based on choline chloride and ascorbic acid. ACS Sustain. Chem. Eng. 2018, 6, 10355–10363. [Google Scholar] [CrossRef]
- Chen, T.C.; Da Fonseca, C.O.; Schönthal, A.H. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am. J. Cancer Res. 2015, 5, 1580. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Pereira, C.V.; Leonardo, I.C.; Fernández, N.; Gaspar, F.B.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Terpene-Based Natural Deep Eutectic Systems as Efficient Solvents To Recover Astaxanthin from Brown Crab Shell Residues. ACS Sustain. Chem. Eng. 2020, 8, 2246–2259. [Google Scholar] [CrossRef]
- Davies, N.M. Clinical pharmacokinetics of ibuprofen. Clin. Pharm. 1998, 34, 101–154. [Google Scholar] [CrossRef]
- Harris, R.E.; Beebe-Donk, J.; Doss, H.; Doss, D.B. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: A critical review of non-selective COX-2 blockade. Oncol. Rep. 2005, 13, 559–583. [Google Scholar] [CrossRef]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of terpene alcohols as highly potent, reversible, and low toxic skin penetration enhancers. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Organ. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Mainberger, S.; Kindlein, M.; Bezold, F.; Elts, E.; Minceva, M.; Briesen, H. Deep eutectic solvent formation: A structural view using molecular dynamics simulations with classical force fields. Mol. Phys. 2017, 115, 1309–1321. [Google Scholar] [CrossRef]
- Geoghegan, K.; Evans, P. Synthesis of (+)-perillyl alcohol from (+)-limonene. Tetrahedron Lett. 2014, 55, 1431–1433. [Google Scholar] [CrossRef]
- Johnson, C.H.; Wilson, I.D.; Harding, J.R.; Stachulski, A.V.; Iddon, L.; Nicholson, J.K.; Lindon, J.C. NMR spectroscopic studies on the in vitro acyl glucuronide migration kinetics of ibuprofen ((±)-(R, S)-2-(4-isobutylphenyl) propanoic acid), its metabolites, and analogues. Anal. Chem. 2007, 79, 8720–8727. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017, 448, 2–8. [Google Scholar] [CrossRef]
- Filippa, M.A.; Gasull, E.I. Ibuprofen solubility in pure organic solvents and aqueous mixtures of cosolvents: Interactions and thermodynamic parameters relating to the solvation process. Fluid Phase Equilib. 2013, 354, 185–190. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Mycol. Med. 2014, 24, e51–e56. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Mehdizadeh, L. Chemistry of Essential Oils and Factors Influencing Their Constituents. In Soft Chemistry and Food Fermentation; Elsevier: New York, NY, USA, 2017; pp. 379–419. [Google Scholar]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glee, P.M.; Sundstrom, P.; Hazen, K.C. Expression of surface hydrophobic proteins by Candida albicans in vivo. Infect. Immun. 1995, 63, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- Vaara, M.; Nurminen, M. Outer membrane permeability barrier in Escherichia coli mutants that are defective in the late acyltransferases of lipid A biosynthesis. Antimicrob. Agents Chemother. 1999, 43, 1459–1462. [Google Scholar] [CrossRef] [Green Version]
- Wiener, M.C.; Horanyi, P.S. How hydrophobic molecules traverse the outer membranes of Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 10929–10930. [Google Scholar] [CrossRef] [Green Version]
- Campos-García, J. Metabolism of acyclic terpenes by Pseudomonas. In Pseudomonas; Springer: Cham, Switzerland, 2010; pp. 235–253. [Google Scholar]
- Förster-Fromme, K.; Höschle, B.; Mack, C.; Bott, M.; Armbruster, W.; Jendrossek, D. Identification of genes and proteins necessary for catabolism of acyclic terpenes and leucine/isovalerate in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2006, 72, 4819–4828. [Google Scholar] [CrossRef] [Green Version]
- Molina, G.; Pimentel, M.R.; Pastore, G.M. Pseudomonas: A promising biocatalyst for the bioconversion of terpenes. Appl. Microbiol. Biotechnol. 2013, 97, 1851–1864. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Ahmed, E.F.; El-Baky, R.M.A.; Ahmed, A.B.F.; Waly, N.G.; Gad, G.F.M. Antibacterial activity of some non-steroidal anti-inflammatory drugs against bacteria causing urinary tract infection. Am. J. Infect. Dis. Microbiol. 2017, 5, 66–73. [Google Scholar]
- AL-Janabi, A.A.H.S. In vitro antibacterial activity of ibuprofen and acetaminophen. J. Glob. Infect. Dis. 2010, 2, 105. [Google Scholar]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Evaluation of toxicity and biodegradability for cholinium-based deep eutectic solvents. RSC Adv. 2015, 5, 83636–83647. [Google Scholar] [CrossRef]
- Radošević, K.; Bubalo, M.C.; Srček, V.G.; Grgas, D.; Dragičević, T.L.; Redovniković, I.R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Abidin, M.H.Z.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J. Controll. Release 2019, 316, 168–195. [Google Scholar] [CrossRef]
- Hayyan, M.; Looi, C.Y.; Hayyan, A.; Wong, W.F.; Hashim, M.A. In vitro and in vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS ONE 2015, 10, e0117934. [Google Scholar] [CrossRef] [Green Version]
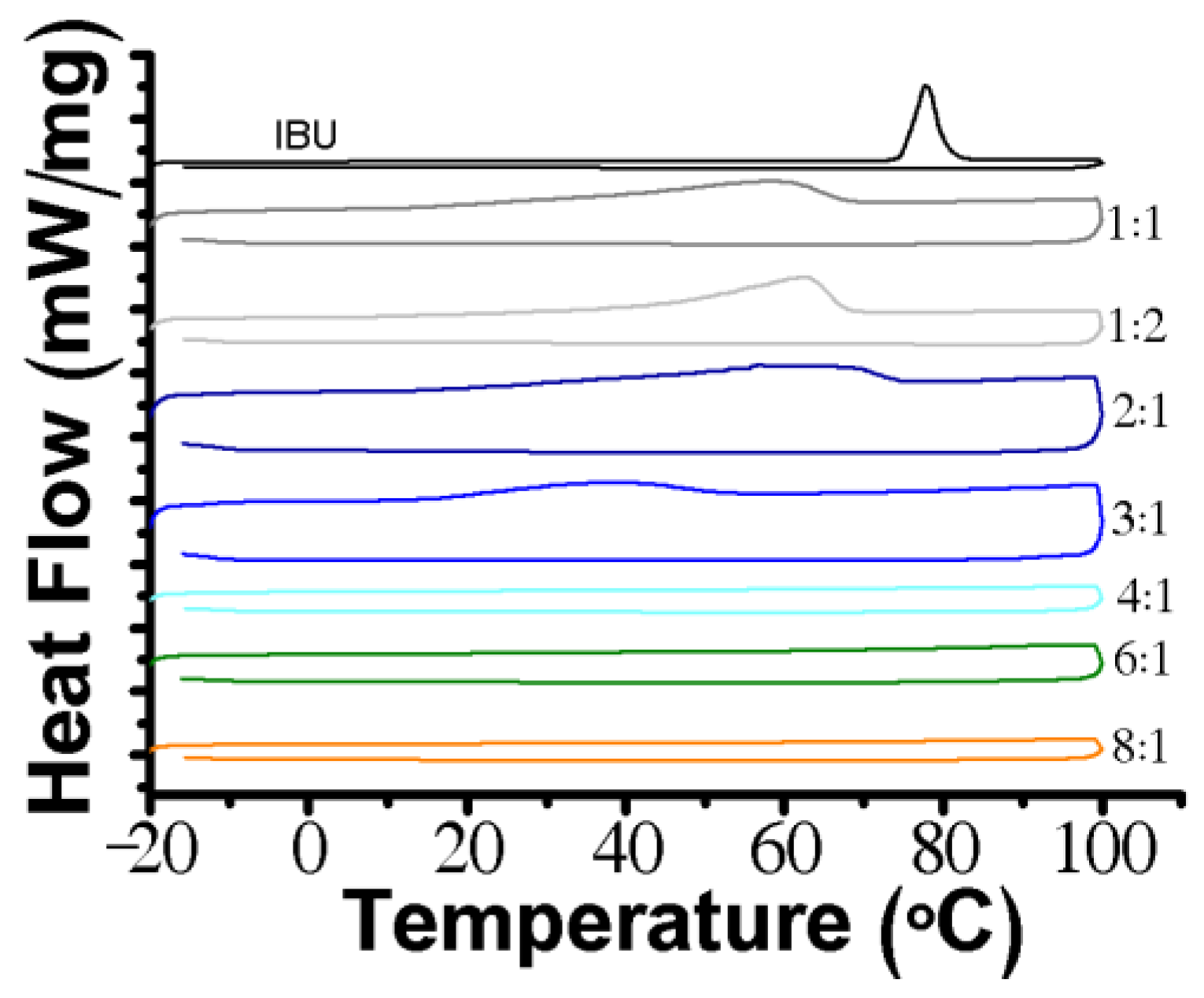
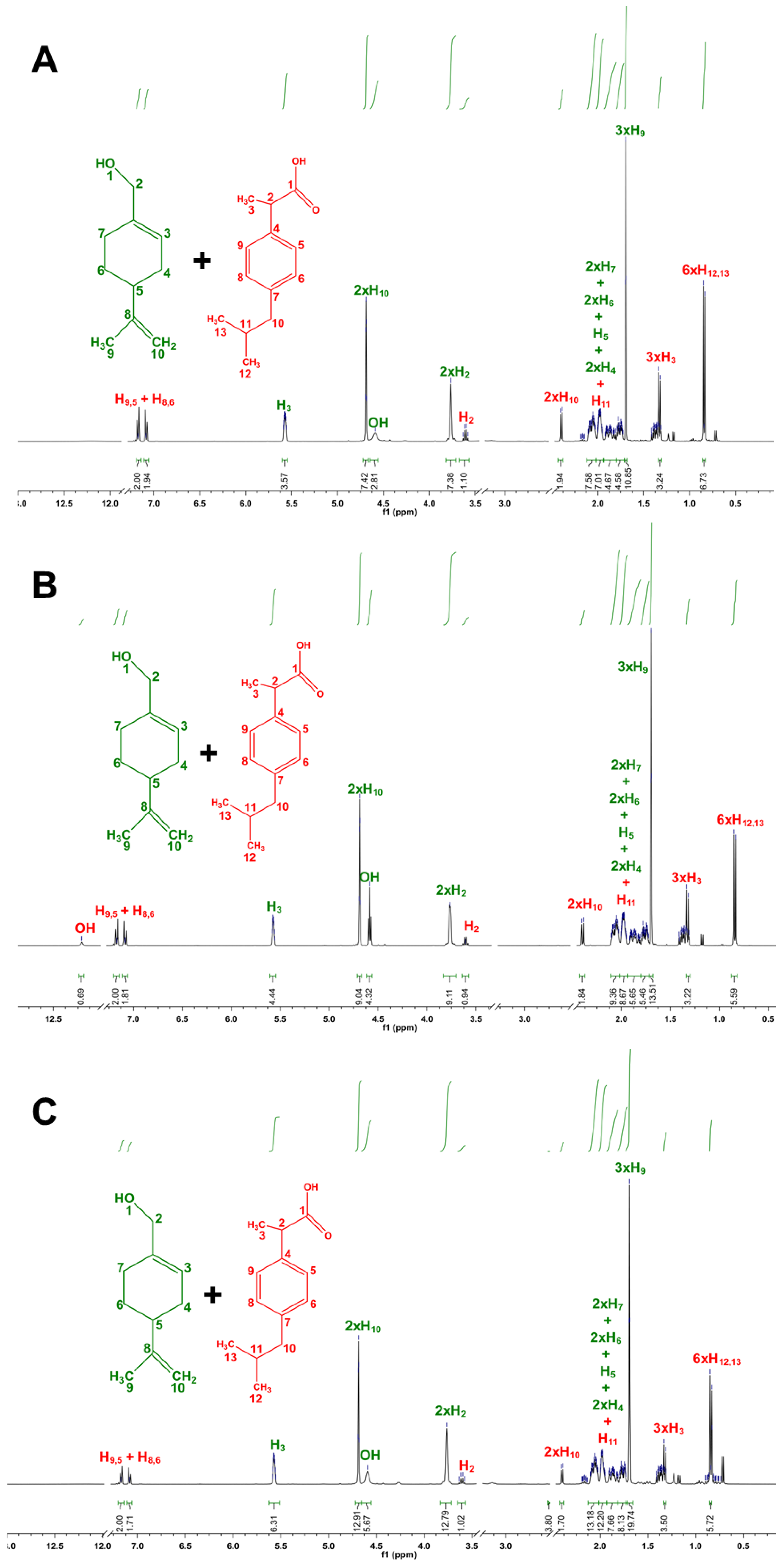
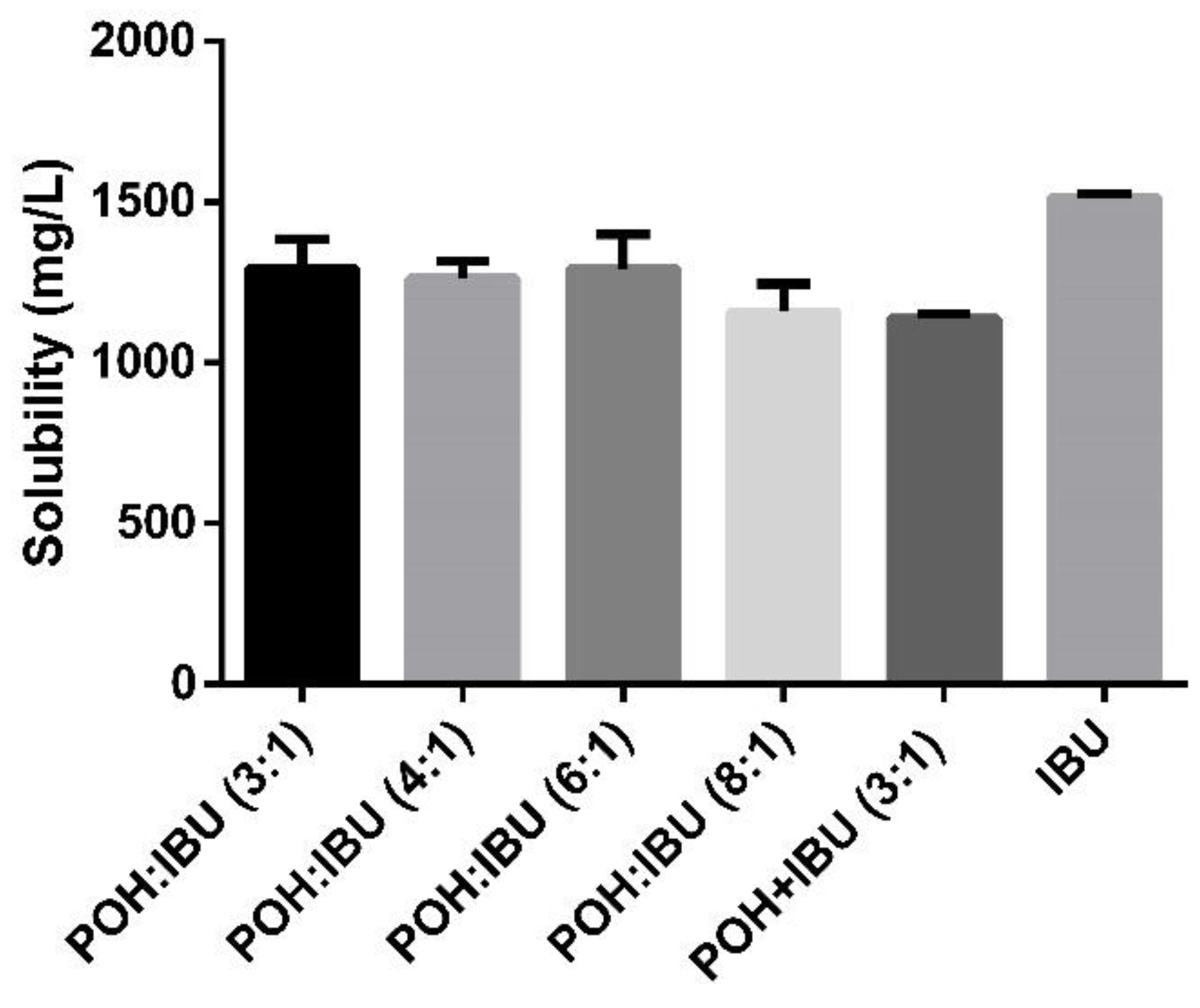
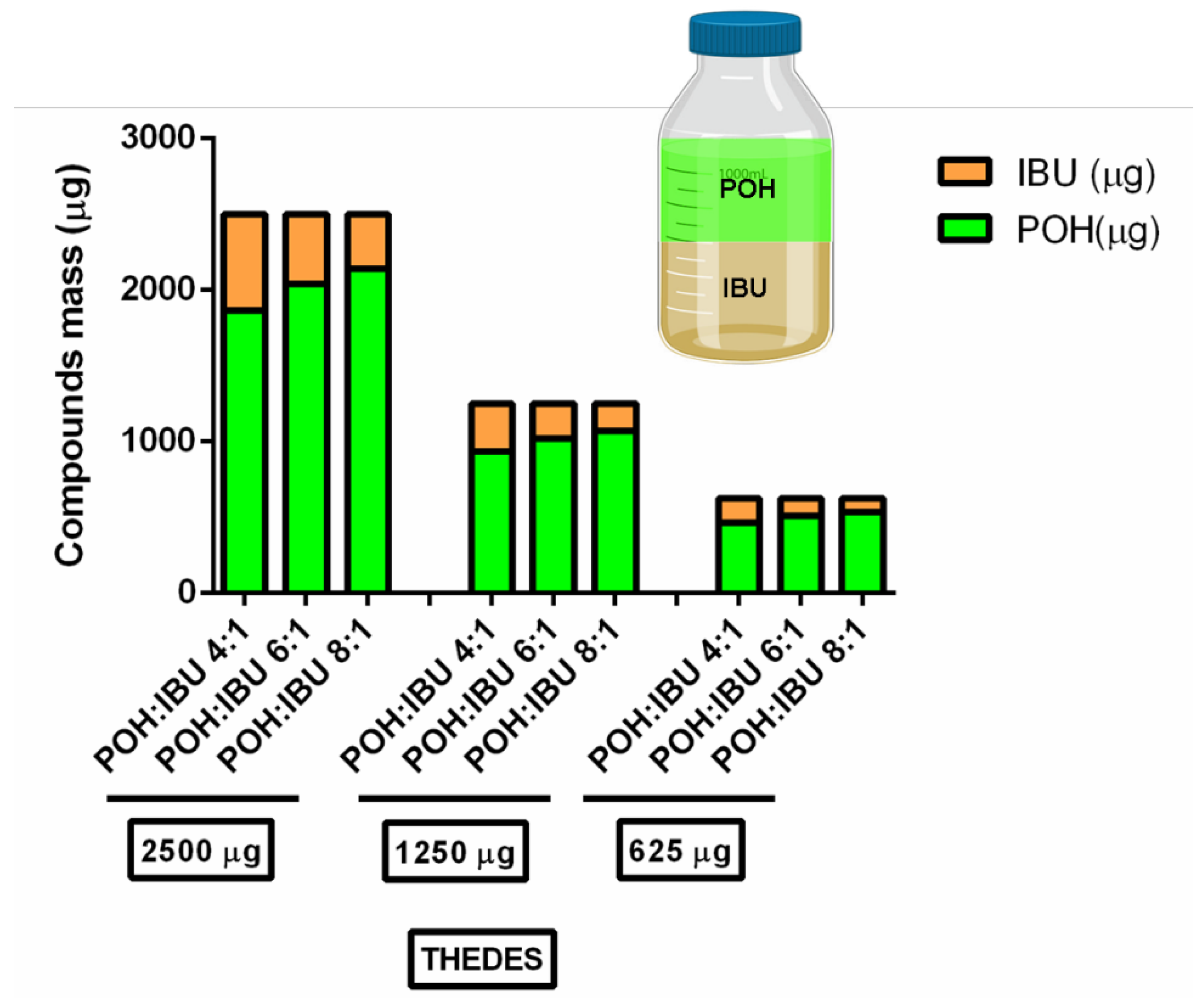

| Molar Ratio | Visual Aspect at RT | Melting Point (°C) |
|---|---|---|
| 1:1 | Solid | ≈57.9 |
| 1:2 | Solid | ≈62.2 |
| 2:1 | Solid | ≈57.9 |
| 3:1 | Liquid with a few crystals | ≈38.3 |
| 4:1 | Liquid | - |
| 6:1 | Liquid | - |
| 8:1 | Liquid | - |
| THEDES/Compound | E. coli | P. aeruginosa | S. aureus | MRSA | MRSE | C. albicans |
|---|---|---|---|---|---|---|
| POH | 17.50 ± 0.41 | NI | 20.33 ± 0.47 | 19.17 ± 0.24 | 21.50 ± 0.70 | 31.33 ± 1.70 |
| IBU | NI | NI | NI | NI | NI | NI |
| POH:IBU 4:1 | 16.67 ± 0.94 | NI | 21.83 ± 0.24 | 22.50 ± 0.71 * | 24.33 ± 0.85 * | 25.60 ± 0.94 * |
| POH:IBU 6:1 | 17.33 ± 0.82 | NI | 19.33 ± 0.41 | 22.33 ± 2.94 * | 24.50 ± 1.97 * | 30.67 ± 0.81 |
| POH:IBU 8:1 | 15.33 ± 0.94 | NI | 20.33 ± 0.47 | 21.00 ± 0.41 | 20.83 ± 0.24 | 32.00 ± 1.67 |
| Sterile water | NI | NI | NI | NI | NI | NI |
| MIC (μg/mL) | |||||
|---|---|---|---|---|---|
| Compound/THEDES | E. coli | S. aureus | MRSA | MRSE | C. albicans |
| POH | 1250 | 625 | 625 | 1250 | 625 |
| IBU | ND | ND | ND | ND | ND |
| POH:IBU 4:1 | 1250 | 1250 | 1250 | 1250 | 625 |
| POH:IBU 6:1 | 1250 | 1250 | 1250 | 1250 | 625 |
| POH:IBU 8:1 | 1250 | 625 | 625 | 625 | 625 |
| MBC/MFC (μg/mL) | |||||
|---|---|---|---|---|---|
| Compound/THEDES | E. coli | S. aureus | MRSA | MRSE | C. albicans |
| POH | 2500 | 1250 | 1250 | 2500 | 1250 |
| IBU | ND | ND | ND | ND | ND |
| POH:IBU 4:1 | 2500 | 2500 | 2500 | 2500 | 1250 |
| POH:IBU 6:1 | 2500 | 2500 | 2500 | 2500 | 1250 |
| POH:IBU 8:1 | 2500 | 1250 | 1250 | 1250 | 1250 |
| System/Compound | EC50 Values (mM) | Selectivity Index | |
|---|---|---|---|
| Cytotoxicity Assay | Antiproliferative Assay | ||
| POH | 4.86 ± 1.58 1 | 2.37 ± 0.20 1 | 2.06 |
| IBU | 2.89 ± 0.06 2 | 2.346 ± 0.09 2 | 1.23 |
| POH:IBU 3:1 | 8.46 ± 1.13 | 1.316 ± 0.07 | 5.89 |
| POH:IBU 8:1 | 4.35 ± 0.29 | 1.37 ± 0.09 | 2.60 |
| POH + IBU 3:1 | - | 4.51± 0.26 | - |
| POH + IBU 8:1 | - | 4.43 ± 0.23 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, E.; Oliveira, F.; Silva, J.M.; Matias, A.; Reis, R.L.; Duarte, A.R.C. Optimal Design of THEDES Based on Perillyl Alcohol and Ibuprofen. Pharmaceutics 2020, 12, 1121. https://doi.org/10.3390/pharmaceutics12111121
Silva E, Oliveira F, Silva JM, Matias A, Reis RL, Duarte ARC. Optimal Design of THEDES Based on Perillyl Alcohol and Ibuprofen. Pharmaceutics. 2020; 12(11):1121. https://doi.org/10.3390/pharmaceutics12111121
Chicago/Turabian StyleSilva, Eduardo, Filipe Oliveira, Joana M. Silva, Ana Matias, Rui L. Reis, and Ana Rita C. Duarte. 2020. "Optimal Design of THEDES Based on Perillyl Alcohol and Ibuprofen" Pharmaceutics 12, no. 11: 1121. https://doi.org/10.3390/pharmaceutics12111121
APA StyleSilva, E., Oliveira, F., Silva, J. M., Matias, A., Reis, R. L., & Duarte, A. R. C. (2020). Optimal Design of THEDES Based on Perillyl Alcohol and Ibuprofen. Pharmaceutics, 12(11), 1121. https://doi.org/10.3390/pharmaceutics12111121