PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation?
Abstract
1. Introduction
2. Carbon Dioxide as Supercritical Fluid
3. Solubility of Polyesters in SC-CO2
4. SC-CO2 Techniques and Important Formulation Parameters
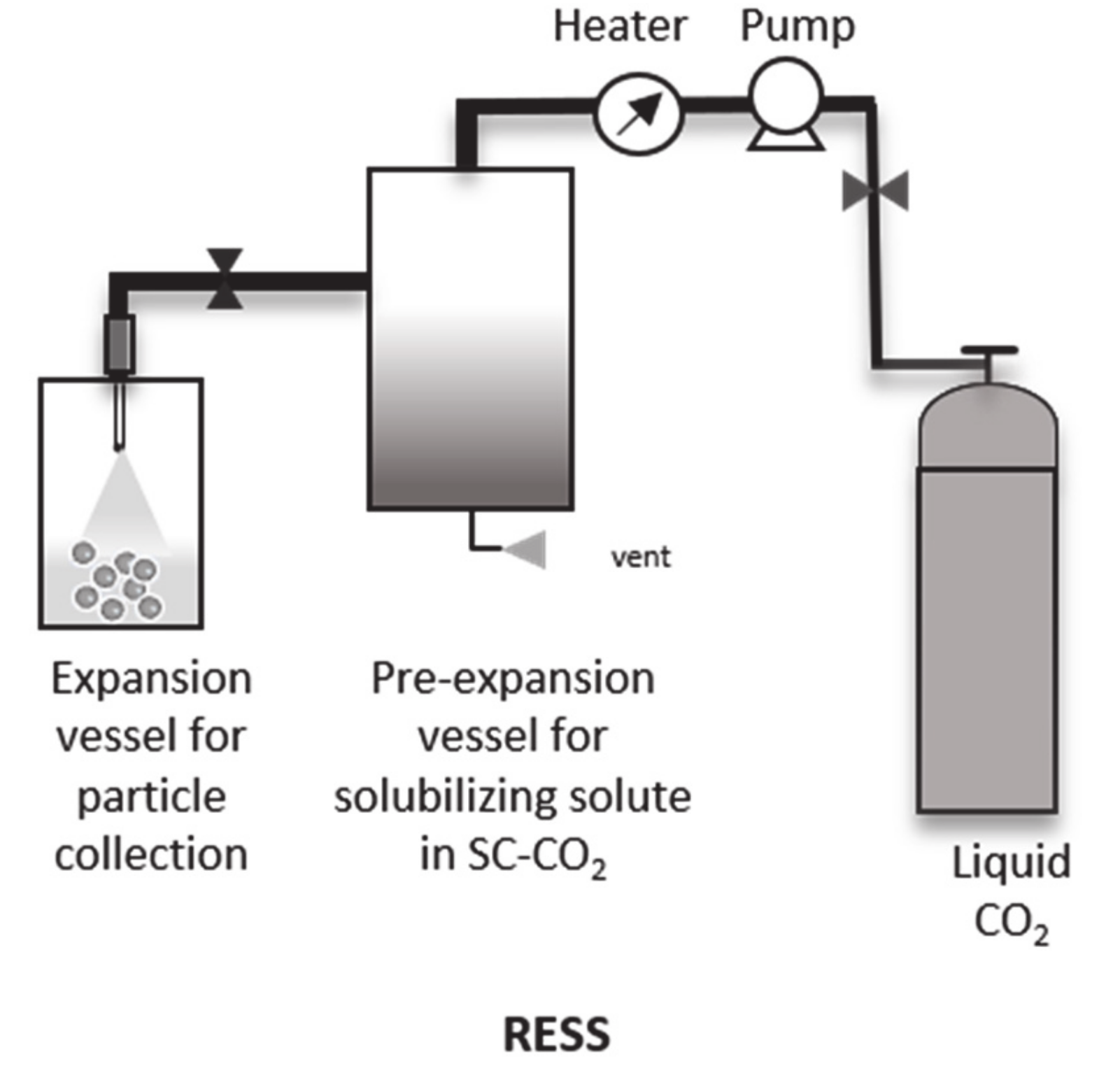
4.1. Rapid Expansion of Supercritical Solution (RESS)
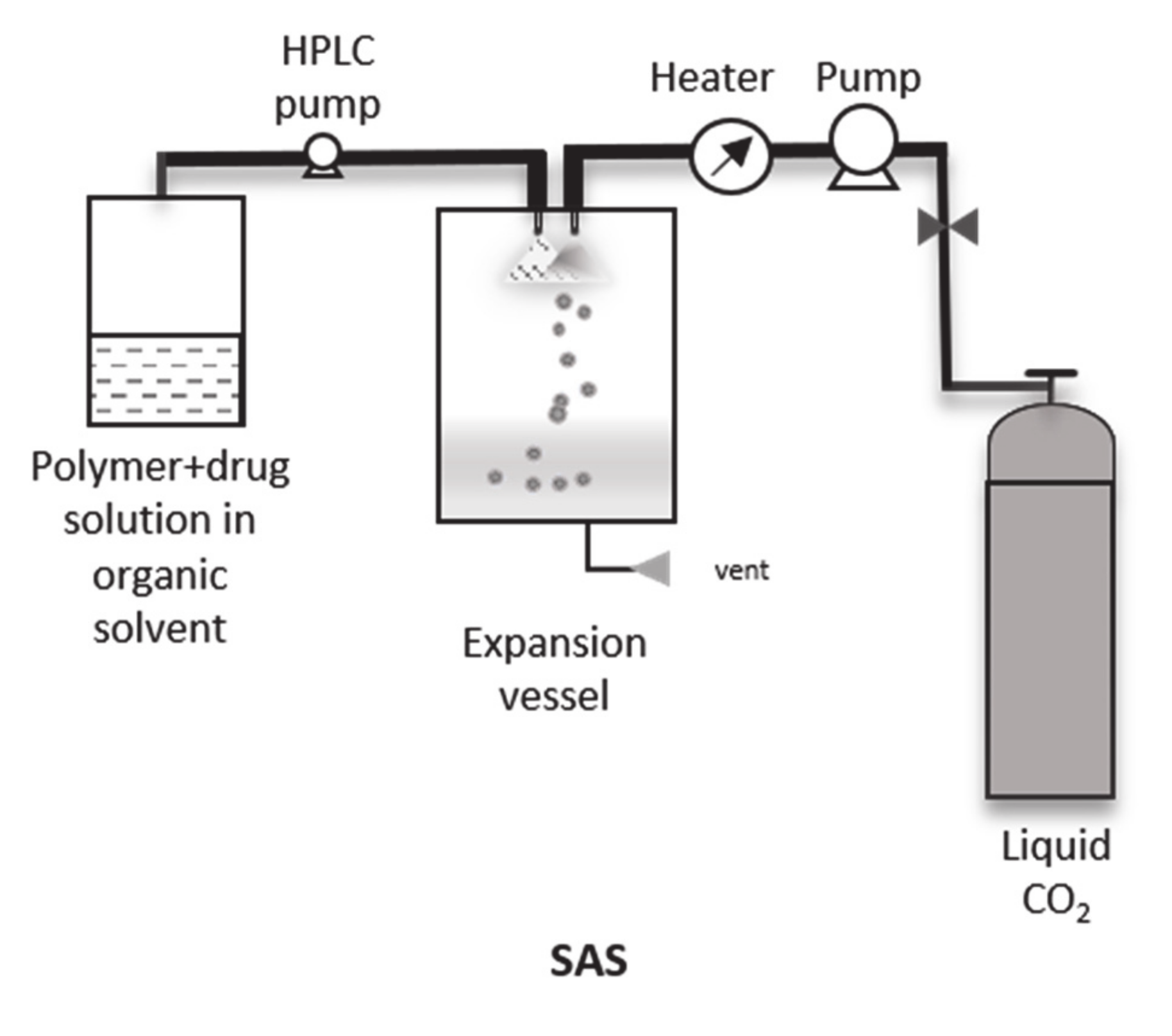
4.2. Supercritical Anti-Solvent (SAS), Gas Anti-Solvent (GAS) and Modifications
4.3. Particles from Gas Saturated Solutions (PGSS)
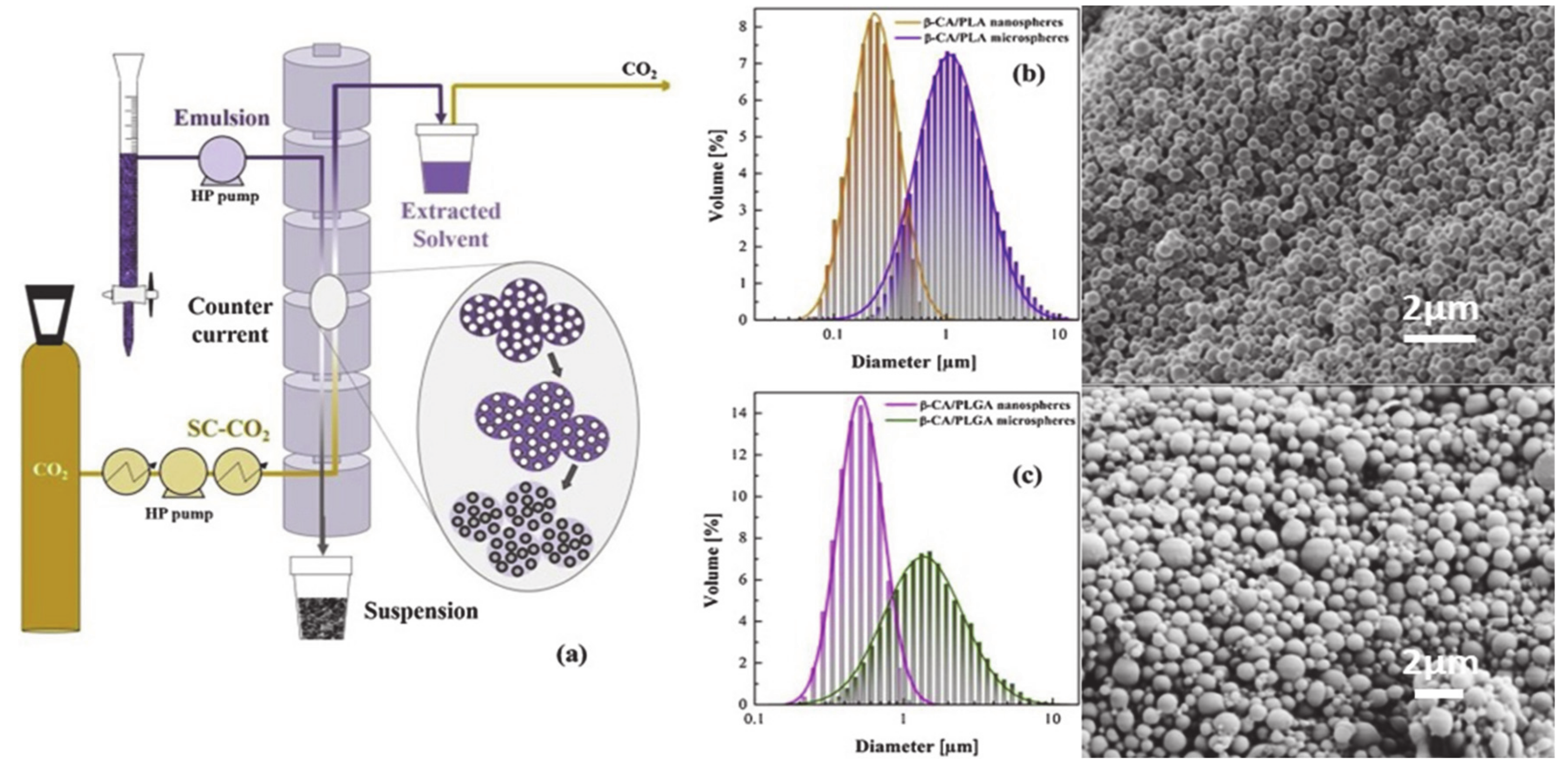
4.4. Supercritical Fluid Extraction Emulsions with SC-CO2 (SFEE)
4.5. Advantages and Limitations of the SCF Techniques
5. Drug-Loaded PLA/PLGA Particles Using SC-CO2
5.1. Drug-Loaded Particles Using SAS
5.2. Drug-Loaded Particles Using Modified SAS
5.3. Drug-Loaded Particles Using Supercritical Fluid Emulsion Extraction (SFEE)
6. Industrial Application of Using SC-CO2 Processes for the Production of Drug-Loaded Particles
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CO2 | Carbon dioxide |
| CH2Cl2 | Dichloromethane |
| DMSO | Dimethyl sulfoxide |
| DSC | Differential scanning calorimetry |
| EE | Encapsulation efficiency |
| FA | Folate |
| FDA | Food and drugs association |
| FTIR | Fourier transform infrared spectroscopy |
| 5-FU | 5-Florouracil |
| GA | Glycolic acid |
| GAS | Gas antisolvent solution |
| G/L | Gas/liquid |
| GMS | Glyceryl monostearate |
| L/D | Length/diameter |
| LA | Lactic acid |
| LC | Loading capacity |
| MP | Microparticle |
| μm | Micrometer |
| Mn | Molar mass |
| NP | Nanoparticle |
| Nm | Nanometer |
| kDa | Kilodalton |
| P | Pressure |
| PCL | Poly(caprolactone) |
| PCA | Pressure compressed antisolvent |
| PEG | Poly (ethylene glycol) |
| PDLA | Poly (D-lactic acid) |
| PLLA | Poly (L-lactic acid) |
| PLGA | Poly (lactic-co-glycolic acid) |
| PC | Critical pressure |
| RESS | Rapid expansion of supercritical solution |
| RESOLV | Rapid expansion of supercritical solution in liquid |
| SAA | Supercritical assisted atomization |
| SAS | Supercritical antisolvent |
| SAILA | Supercritical antisolvent in liquid |
| SAS-EM | Supercritical antisolvent- enhanced mass transfer |
| SC-CO2 | Supercritical carbon dioxide |
| SCF | Supercritical fluid |
| SDS | Sodium dodecyl sulfate |
| SEDS | Solution-enhanced dispersion solvent |
| SEM | Scanning electron microscopy |
| SFEE | Supercritical fluid emulsion extraction |
| T | Temperature |
| Tg | Glass transition temperature |
| Tm | Melting temperature |
| TAM | Tamoxifen |
| TEM | Transmission electron microscopy |
References
- Farjadian, F.; Ghasemi, A.; Gohar, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine (London) 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef]
- Schubert, S.; Delaney, J.T.; Schubert, U.S. Nanoprecipitation and nanoformulation of polymers: From history to powerful possibilities beyond poly(lactic acid). Soft Matter 2011, 7, 1581–1588. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Shkodra-Pula, B.; Grune, C.; Traeger, A.; Vollrath, A.; Schubert, S.; Fischer, D.; Schubert, U.S. Effect of surfactant on the size and stability of PLGA nanoparticles encapsulating a protein kinase C inhibitor. Int. J. Pharm. 2019, 566, 756–764. [Google Scholar] [CrossRef]
- Konan, Y.N.; Gurny, R.; Allemann, E. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int. J. Pharm. 2002, 233, 239–252. [Google Scholar] [CrossRef]
- Raston, C. Renewables and Green Chemistry. Green Chem. 2005, 7, 57. [Google Scholar] [CrossRef]
- Butler, M.; Olga, K. Introduction. In Green Chemistry Guide; Butler, M., Rivin, J.M., Eds.; National Pollution Prevention Roundtable: Los Angeles, CA, USA, 2015; pp. 1–5. Available online: https://www.lacitysan.org/san/sandocview?docname=cnt022546 (accessed on 19 November 2020).
- Ciaglia, E.; Montella, F.; Trucillo, P.; Ciardulli, M.C.; Di Pietro, P.; Amodio, G.; Remondelli, P.; Vecchione, C.; Reverchon, E.; Maffulli, N.; et al. A bioavailability study on microbeads and nanoliposomes fabricated by dense carbon dioxide technologies using human-primary monocytes and flow cytometry assay. Int. J. Pharm. 2019, 570, 118686. [Google Scholar] [CrossRef]
- Englert, C.; Pröhl, M.; Czaplewska, J.A.; Fritzsche, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; et al. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Shive, M.S.; Anderson, J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Reul, R.; Tsapis, N.; Hillaireau, H.; Sancey, L.; Mura, S.; Recher, M.; Nicolas, J.; Coll, J.-L.; Fattal, E. Near infrared labeling of PLGA for in vivo imaging of nanoparticles. Polym. Chem. 2012, 3, 694–702. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J.; Hirenkumar, M.; Steven, S. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2012, 3, 1377–1397. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Breton, A.L.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- DeSimone, J.M.; Tumas, W. Green Chemistry Using Liquid and Supercritical Carbon Dioxide; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Berche, B.; Henkel, M.; Kenna, R. Fenômenos críticos: 150 anos desde Cagniard de la Tour. Rev. Bras. de Ensino de Fis. 2009, 31, 2602-1–2602-4. [Google Scholar] [CrossRef]
- Williams, J.R.; Clifford, A.A. Introduction to Supercritical Fluids and Their Applications; Humana Press: Totowa, NJ, USA, 2003; pp. 1–16. [Google Scholar] [CrossRef]
- Girard, E.; Tassaing, T.; Marty, J.-D.; Destarac, M. Structure-Property Relationships in CO2-philic (Co)polymers: Phase Behavior, Self-Assembly, and Stabilization of Water/CO2 Emulsions. Chem. Rev. 2016, 116, 4125–4169. [Google Scholar] [CrossRef]
- Conway, S.E.; Byun, H.S.; McHugh, M.A.; Wang, J.D.; Mandel, F.S. Poly(lactide-co-glycolide) solution behavior in supercritical CO2, CHF3, and CHClF2. J. Appl. Polym. Sci. 2001, 80, 1155–1161. [Google Scholar] [CrossRef]
- Gregorowicz, J.; Bernatowicz, P. Phase behaviour of L-lactic acid based polymers of low molecular weight in supercritical carbon dioxide at high pressures. J. Supercrit. Fluids 2009, 51, 270–277. [Google Scholar] [CrossRef]
- Wilson, R.; George, S.C.; Kumar, S.A.; Thomas, S. Liquid Transport Characteristics in Polymeric Systems. In Transport Properties of Polymeric Membranes; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 3–13. [Google Scholar] [CrossRef]
- Kirby, C.F.; McHugh, M.A. Phase Behavior of Polymers in Supercritical Fluid Solvents. Chem. Rev. 1999, 99, 565–602. [Google Scholar] [CrossRef] [PubMed]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Suttiruengwong, S.; Rolker, J.; Smirnova, I.; Arlt, W.; Seiler, M.; Luderitz, L.; Perez de Diego, Y.; Jansens, P.J. Hyperbranched polymers as drug carriers: Microencapsulation and release kinetics. Pharm. Dev. Technol. 2006, 11, 55–70. [Google Scholar] [CrossRef]
- Gregorowicz, J.; Fras, Z.; Parzuchowski, P.; Rokicki, G.; Kusznerczuk, M.; Dziewulski, S. Phase behaviour of hyperbranched polyesters and polyethers with modified terminal OH groups in supercritical solvents. J. Supercrit. Fluids 2010, 55, 786–796. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Tom, J.W.; Kwauk, X.; Yeo, S.-D. Rapid Expansion of Supercritical Solutions (Ress)—Fundamentals and Applications. Fluid Phase Equilib. 1993, 82, 311–321. [Google Scholar] [CrossRef]
- Türk, M. Formation of small organic particles by RESS: Experimental and theoretical investigations. J. Supercrit. Fluids 1999, 15, 79–89. [Google Scholar] [CrossRef]
- Fahmy, S.M. Solubility of Fluorinated Polymers in Supercritical Carbon Dioxide; Westphalian University of Applied Sciences Aachen: Aachen, Germany, 2005. [Google Scholar]
- Petersen, R.C.; Matson, D.W.; Smith, R.D. Rapid Precipitation of Low Vapor-Pressure Solids from Supercritical Fluid Solutions—The Formation of Thin-Films and Powders. J. Am. Chem. Soc. 1986, 108, 2100–2102. [Google Scholar] [CrossRef]
- Matson, D.W.; Fulton, J.L.; Petersen, R.C.; Smith, R.D. Rapid Expansion of Supercritical Fluid Solutions—Solute Formation of Powders, Thin-Films, and Fibers. Ind. Eng. Chem. Res. 1987, 26, 2298–2306. [Google Scholar] [CrossRef]
- Tom, J.W.; Debenedetti, P.G. Formation of biodegradable PLA microparticles using rapid expansion of SC-CO2. Biotechnol. Progr. 1991, 7, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Paxton, T.E.; Tomasko, D.L. Microencapsulation of naproxen using rapid expansion of supercritical solutions. Biotechnol. Prog. 1996, 12, 650–661. [Google Scholar] [CrossRef]
- Charoenchaitrakool, M.; Dehghani, F.; Foster, N.R.; Chan, H.K. Micronization by rapid expansion of supercritical solutions to enhance the dissolution rates of poorly water-soluble pharmaceuticals. Ind. Eng. Chem. Res. 2000, 39, 4794–4802. [Google Scholar] [CrossRef]
- Hermsdorf, D.; Jauer, S.; Signorell, R. Formation and stabilization of ibuprofen nanoparticles by pulsed rapid expansion of supercritical solutions. Mol. Phys. 2007, 105, 951–959. [Google Scholar] [CrossRef]
- Matsuyama, K.; Donghui, Z.; Urabe, T.; Mishima, K. Formation of L-poly(lactic acid) microspheres by rapid expansion Of CO2 saturated polymer suspensions. J. Supercrit. Fluids 2005, 33, 275–281. [Google Scholar] [CrossRef]
- Yildiz, N.; Tuna, S.; Döker, O.; Calimli, A. Micronization of salicylic acid and taxol (paclitaxel) by rapid expansion of supercritical fluids (RESS). J. Supercrit. Fluids 2007, 41, 440–451. [Google Scholar] [CrossRef]
- Satvati, H.R.; Lotfollahi, M.N. Effects of extraction temperature, extraction pressure and nozzle diameter on micronization of cholesterol by RESS process. Powder Technol. 2011, 210, 109–114. [Google Scholar] [CrossRef]
- Reverchon, E.; Donsi, G. Salicylic-Acid Solubilization in Supercritical Co2 and Its Micronization by Ress. J. Supercrit. Fluids 1993, 6, 241–248. [Google Scholar] [CrossRef]
- Wolff, S.; Beuermann, S.; Türk, M. Impact of rapid expansion of supercritical solution process conditions on the crystallinity of poly(vinylidene fluoride) nanoparticles. J. Supercrit. Fluids 2016, 117, 18–25. [Google Scholar] [CrossRef]
- Helfgen, B.; Turk, M.; Schaber, K. Hydrodynamic and aerosol modelling of the rapid expansion of supercritical solutions (RESS-process). J. Supercrit. Fluids 2003, 26, 225–242. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R. Nanomaterials and supercritical fluids. J. Supercrit. Fluids 2006, 37, 1–22. [Google Scholar] [CrossRef]
- Sane, A.; Limtrakul, J. Formation of retinyl palmitate-loaded poly(L-lactide) nanoparticles using rapid expansion of supercritical solutions into liquid solvents (RESOLV). J. Supercrit. Fluids 2009, 51, 230–237. [Google Scholar] [CrossRef]
- Pathak, P.; Meziani, M.J.; Desai, T.; Sun, Y.-P. Nanosizing drug particles in supercritical fluid processing. J. Am. Chem. Soc. 2004, 126, 10842–10843. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.; Randolph, T.W.; Meyer, J.D.; Kelly, R.M.; Manning, M.C. Controlled release of ionic compounds from poly (L-lactide) microspheres produced by precipitation with a compressed antisolvent. J. Control. Release 1997, 44, 77–85. [Google Scholar] [CrossRef]
- Kalani, M.; Yunus, R. Application of supercritical antisolvent method in drug encapsulation: A review. Int. J. Nanomed. 2011, 6, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E. Supercritical antisolvent precipitation of micro-and nano-particles. J. Supercrit. Fluids 1999, 15, 1–21. [Google Scholar] [CrossRef]
- Byrappa, K.; Ohara, S.; Adschiri, T. Nanoparticles synthesis using supercritical fluid technology—Towards biomedical applications. Adv. Drug Deliv. Rev. 2008, 60, 299–327. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Production of antibiotic micro- and nano-particles by supercritical antisolvent precipitation. Powder Technol. 1999, 106, 23–29. [Google Scholar] [CrossRef]
- Salmaso, S.; Elvassore, N.; Bertucco, A.; Lante, A.; Caliceti, P. Nisin-loaded poly-L-lactide nano-particles produced by CO2 anti-solvent precipitation for sustained antimicrobial activity. Int. J. Pharm. 2004, 287, 163–173. [Google Scholar] [CrossRef]
- Kalani, M.; Yunus, R.; Abdullah, N. Optimizing supercritical antisolvent process parameters to minimize the particle size of paracetamol nanoencapsulated in L-polylactide. Int. J. Nanomed. 2011, 6, 1101–1105. [Google Scholar] [CrossRef]
- Campardelli, R.; Oleandro, E.; Adami, R.; Reverchon, E. Polymethylmethacrylate (PMMA) sub-microparticles produced by Supercritical Assisted Injection in a Liquid Antisolvent. J. Supercrit. Fluids 2014, 92, 93–99. [Google Scholar] [CrossRef]
- Sheth, P.; Sandhu, H.; Singhal, D.; Malick, W.; Shah, N.; Kislalioglu, M.S. Nanoparticles in the pharmaceutical industry and the use of supercritical fluid technologies for nanoparticle production. Curr Drug. Deliv. 2012, 9, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, G.; Wang, Y.; Cui, F.; Zhang, J.; Huang, Q. Preparation and characterization of 5-fluorouracil-loaded PLLA-PEG/PEG nanoparticles by a novel supercritical CO2 technique. Int. J. Pharm. 2012, 436, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, Y.; Yin, G.; Huang, Z.; Yao, Y.; Chen, X.; Liao, X.; Gu, J.; Gao, H. Preparation, characterization and in vitro release properties of morphine-loaded PLLA-PEG-PLLA microparticles via solution enhanced dispersion by supercritical fluids. J. Mater. Sci. Mater. Med 2013, 24, 1693–1705. [Google Scholar] [CrossRef]
- Fraile, M.; Martin, A.; Deodato, D.; Rodriguez-Rojo, S.; Nogueira, I.D.; Simplicio, A.L.; Cocero, M.J.; Duarte, C.M.M. Production of new hybrid systems for drug delivery by PGSS (Particles from Gas Saturated Solutions) process. J. Supercrit. Fluids 2013, 81, 226–235. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef]
- Sekhon, B.S. Supercritical fluid technology: An overview of pharmaceutical applications. Int. J. PharmTech. Res 2010, 2, 810–826. [Google Scholar]
- Chattopadhyay, P.; Huff, R.; Shekunov, B.Y. Drug encapsulation using supercritical fluid extraction of emulsions. J. Pharm. Sci. 2006, 95, 667–679. [Google Scholar] [CrossRef]
- Porta, G.D.; Falco, N.; Giordano, E.; Reverchon, E. PLGA microspheres by Supercritical Emulsion Extraction: A study on insulin release in myoblast culture. J. Biomater. Sci. Polym. Ed. 2013, 24, 1831–1847. [Google Scholar] [CrossRef]
- Tom, J.W.; Debenedetti, P.G.; Jerome, R. Precipitation of Poly(L-Lactic Acid) and Composite Poly(L-Lactic Acid)—Pyrene Particles by Rapid Expansion of Supercritical Solutions. J. Supercrit. Fluids 1994, 7, 9–29. [Google Scholar] [CrossRef]
- Bleich, J.; Muller, B.W. Production of drug loaded microparticles by the use of supercritical gases with the aerosol solvent extraction system (ASES) process. J. Microencapsul. 1996, 13, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, R.; Artursson, P.; Carlfors, J. Preparation of biodegradable microparticles using solution-enhanced dispersion by supercritical fluids (SEDS). Pharm. Res. 1999, 16, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Elvassore, N.; Bertucco, A.; Caliceti, P. Production of insulin-loaded poly(ethylene glycol)/poly(l-lactide) (PEG/PLA) nanoparticles by gas antisolvent techniques. J. Pharm. Sci. 2001, 90, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Taki, S.; Badens, E.; Charbit, G. Controlled release system formed by supercritical anti-solvent coprecipitation of a herbicide and a biodegradable polymer. J. Supercrit. Fluids 2001, 21, 61–70. [Google Scholar] [CrossRef]
- Tu, L.S.; Dehghani, F.; Foster, N.R. Micronisation and microencapsulation of pharmaceuticals using a carbon dioxide antisolvent. Powder Technol. 2002, 126, 134–149. [Google Scholar] [CrossRef]
- Martin, T.M.; Bandi, N.; Shulz, R.; Roberts, C.B.; Kompella, U.B. Preparation of budesonide and budesonide-PLA microparticles using supercritical fluid precipitation technology. AAPS PharmSciTech 2002, 3, 1–11. [Google Scholar] [CrossRef]
- Caliceti, P.; Salmaso, S.; Elvassore, N.; Bertucco, A. Effective protein release from PEG/PLA nano-particles produced by compressed gas anti-solvent precipitation techniques. J. Control. Release 2004, 94, 195–205. [Google Scholar] [CrossRef]
- Koushik, K.; Kompella, U.B. Preparation of large porous deslorelin-PLGA microparticles with reduced residual solvent and cellular uptake using a supercritical carbon dioxide process. Pharm. Res. 2004, 21, 524–535. [Google Scholar] [CrossRef]
- Whitaker, M.J.; Hao, J.; Davies, O.R.; Serhatkulu, G.; Stolnik-Trenkic, S.; Howdle, S.M.; Shakesheff, K.M. The production of protein-loaded microparticles by supercritical fluid enhanced mixing and spraying. J. Control. Release 2005, 101, 85–92. [Google Scholar] [CrossRef]
- Liu, H.; Finn, N.; Yates, M.Z. Encapsulation and sustained release of a model drug, indomethacin, using CO(2)-based microencapsulation. Langmuir 2005, 21, 379–385. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.S.; Kim, J.S.; Park, H.J.; Woo, J.S.; Lee, B.C.; Hwang, S.J. Controlled delivery of a hydrophilic drug from a biodegradable microsphere system by supercritical anti-solvent precipitation technique. J. Microencapsul. 2006, 23, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yin, G.; Ouyang, P.; Huang, Z.; Yao, Y.; Liao, X.; Chen, A.; Pu, X. Preparation of PLLA/PLGA microparticles using solution enhanced dispersion by supercritical fluids (SEDS). J. Colloid Interface Sci. 2008, 322, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Patomchaiviwat, V.; Paeratakul, O.; Kulvanich, P. Formation of inhalable rifampicin-poly(L-lactide) microparticles by supercritical anti-solvent process. AAPS PharmSciTech 2008, 9, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yin, G.; Ouyang, P.; Huang, Z.; Yao, Y.; Liao, X.; Chen, A.; Pu, X. Preparation, characterization and in vitro cytotoxicity of indomethacin-loaded PLLA/PLGA microparticles using supercritical CO2 technique. Eur. J. Pharm. Biopharm. 2008, 70, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.-Z.; Li, Y.; Chen, D.; Hu, J.-Y. Development of core-shell microcapsules by a novel supercritical CO2 process. J. Mater. Sci. Mater. Med. 2009, 20, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Argemí, A.; Vega, A.; Subra-Paternault, P.; Saurina, J. Characterization of azacytidine/poly(L-lactic) acid particles prepared by supercritical antisolvent precipitation. J. Pharm. Biomed. Anal. 2009, 50, 847–852. [Google Scholar] [CrossRef]
- Jacobson, G.B.; Gonzalez-Gonzalez, E.; Spitler, R.; Shinde, R.; Leake, D.; Kaspar, R.L.; Contag, C.H.; Zare, R.N. Biodegradable nanoparticles with sustained release of functional siRNA in skin. J. Pharm. Sci. 2010, 99, 4261–4266. [Google Scholar] [CrossRef]
- Adami, R.; Liparoti, S.; Reverchon, E. A new supercritical assisted atomization configuration, for the micronization of thermolabile compounds. Chem. Eng. J. 2011, 173, 55–61. [Google Scholar] [CrossRef]
- Chen, A.-Z.; Zhao, Z.; Wang, S.-B.; Li, Y.; Zhao, C.; Liu, Y.-G. A continuous RESS process to prepare PLA-PEG-PLA microparticles. J. Supercrit. Fluids 2011, 59, 92–97. [Google Scholar] [CrossRef]
- Adami, R.; Liparoti, S.; Izzo, L.; Pappalardo, D.; Reverchon, E. PLA-PEG copolymers micronization by supercritical assisted atomization. J. Supercrit. Fluids 2012, 72, 15–21. [Google Scholar] [CrossRef]
- Chen, A.-Z.; Li, L.; Wang, S.-B.; Lin, X.-F.; Liu, Y.-G.; Zhao, C.; Wang, G.-Y.; Zhao, Z. Study of Fe3O4-PLLA-PEG-PLLA magnetic microspheres based on supercritical CO2: Preparation, physicochemical characterization, and drug loading investigation. J. Supercrit. Fluids 2012, 67, 139–148. [Google Scholar] [CrossRef]
- Chen, A.-Z.; Pu, X.-M.; Yin, G.-F.; Zhao, C.; Wang, S.-B.; Liu, Y.-G.; Wang, G.-Y.; Kang, Y.-Q. Study of lysozyme-polymer composite microparticles in supercritical CO2. J. Appl. Polym. Sci. 2012, 125, 3175–3183. [Google Scholar] [CrossRef]
- Kalani, M.; Yunus, R. Effect of Supercritical Fluid Density on Nano-encapsulated Drug Particle Size using the Supercritical Anti-solvent Method. Int. J. Nanomed. 2012, 7, 2165–2172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.-Z.; Liao, X.-M.; Yin, G.-F.; Yao, Y.-D.; Chen, X.-C.; Yuan, P.; Huang, Z.-B.; Gu, J.-W. Preparation of water soluble drugs-loaded microparticles using modified solution enhanced dispersion by supercritical CO2. Powder Technol. 2012, 221, 343–350. [Google Scholar] [CrossRef]
- Jung, I.-I.; Haam, S.; Lim, G.; Ryu, J.-H. Preparation of peptide-loaded polymer microparticles using supercritical carbon dioxide. Biotechnol. Bioprocess Eng. 2012, 17, 185–194. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Azad, M.A.; Dave, R. Precipitation and stabilization of ultrafine particles of Fenofibrate in aqueous suspensions by RESOLV. Powder Technol. 2013, 236, 75–84. [Google Scholar] [CrossRef]
- Wang, W.; Liu, G.; Wu, J.; Jiang, Y. Co-precipitation of 10-hydroxycamptothecin and poly (L-lactic acid) by supercritical CO2 anti-solvent process using dichloromethane/ethanol co-solvent. J. Supercrit. Fluids 2013, 74, 137–144. [Google Scholar] [CrossRef]
- Kang, Y.-Q.; Zhao, C.; Chen, A.-Z.; Wang, S.-B.; Liu, Y.-G.; Wu, W.-G.; Su, X.-Q. Study of Lysozyme-Loaded Poly-L-Lactide (PLLA) Porous Microparticles in a Compressed CO(2) Antisolvent Process. Materials 2013, 6, 3571–3583. [Google Scholar] [CrossRef]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; de la Ossa, E.J.M. Supercritical CO2 precipitation of poly(L-lactic acid) in a wide range miscibility. J. Supercrit. Fluids 2013, 81, 236–244. [Google Scholar] [CrossRef]
- Yandrapu, S.K.; Upadhyay, A.K.; Petrash, J.M.; Kompella, U.B. Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab. Mol. Pharm. 2013, 10, 4676–4686. [Google Scholar] [CrossRef]
- Zhan, S.; Chen, C.; Zhao, Q.; Wang, W.; Liu, Z. Preparation of 5-Fu-Loaded PLLA Microparticles by Supercritical Fluid Technology. Ind. Eng. Chem. Res. 2013, 52, 2852–2857. [Google Scholar] [CrossRef]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; De los Santos, D.M.; de la Ossa, E.J.M. Ibuprofen-polymer precipitation using supercritical CO2 at low temperature. J. Supercrit. Fluids 2014, 94, 91–101. [Google Scholar] [CrossRef]
- Montes, A.; Kin, N.; Gordillo, M.D.; Pereyra, C.; Ossa, E.J.M.d.l. Polymer-naproxen precipitation by supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2014, 89, 58–67. [Google Scholar] [CrossRef]
- Zabihi, F.; Xin, N.; Jia, J.; Chen, T.; Zhao, Y. High Yield and High Loading Preparation of Curcumin-PLGA Nanoparticles Using a Modified Supercritical Antisolvent Technique. Ind. Eng. Chem. Res. 2014, 53, 6569–6574. [Google Scholar] [CrossRef]
- Zabihi, F.; Xin, N.; Li, S.; Jia, J.; Cheng, T.; Zhao, Y. Polymeric coating of fluidizing nano-curcumin via anti-solvent supercritical method for sustained release. J. Supercrit. Fluids 2014, 89, 99–105. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Yin, G.; Pu, X.; Liao, X.; Huang, Z.; Chen, X.; Yao, Y. Tumor-targeted paclitaxel-loaded folate conjugated poly(ethylene glycol)-poly(L-lactide) microparticles produced by supercritical fluid technology. J. Mater. Sci. Mater. Med. 2015, 26, 95. [Google Scholar] [CrossRef]
- Yoshida, V.M.H.; Balcao, V.M.; Vila, M.M.; Oliveira Junior, J.M.; Aranha, N.; Chaud, M.V.; Gremiao, M.P. Zidovudine-poly(L-lactic acid) solid dispersions with improved intestinal permeability prepared by supercritical antisolvent process. J. Pharm. Sci. 2015, 104, 1691–1700. [Google Scholar] [CrossRef]
- Chen, A.-Z.; Tang, N.; Wang, S.-B.; Kang, Y.-Q.; Song, H.-F. Insulin-loaded poly-L-lactide porous microspheres prepared in supercritical CO2 for pulmonary drug delivery. J. Supercrit. Fluids 2015, 101, 117–123. [Google Scholar] [CrossRef]
- Sacchetin, P.S.C.; Setti, R.F.; e Rosa, P.D.; Moraes, Â.M. Properties of PLA/PCL particles as vehicles for oral delivery of the androgen hormone 17alpha-methyltestosterone. Mater. Sci. Eng. C 2016, 58, 870–881. [Google Scholar] [CrossRef]
- Campardelli, R.; Oleandro, E.; Reverchon, E. Supercritical assisted injection in a liquid antisolvent for PLGA and PLA microparticle production. Powder Technol. 2016, 287, 12–19. [Google Scholar] [CrossRef]
- Songtipya, L.; Thies, M.C.; Sane, A. Effect of rapid expansion of subcritical solutions processing conditions on loading capacity of tetrahydrocurcumin encapsulated in poly(L-lactide) particles. J. Supercrit. Fluids 2016, 113, 119–127. [Google Scholar] [CrossRef]
- Alias, D.; Yunus, R.; Chong, G.H.; Abdullah, C.A.C. Single step encapsulation process of tamoxifen in biodegradable polymer using supercritical anti-solvent (SAS) process. Powder Technol. 2017, 309, 89–94. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E. Instantaneous coprecipitation of polymer/drug microparticles using the supercritical assisted injection in a liquid antisolvent. J. Supercrit. Fluids 2017, 120, 151–160. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, G.; Zhao, Z.; Wei, D.; Pang, J.; Jiang, Y. Design of gefitinib-loaded poly (l-lactic acid) microspheres via a supercritical anti-solvent process for dry powder inhalation. Int. J. Pharm. 2017, 532, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-F.; Kankala, R.K.; Tang, N.; Xu, P.-Y.; Hao, L.-Z.; Yang, D.-Y.; Wang, S.-B.; Zhang, Y.S.; Chen, A.-Z. Supercritical Fluid-Assisted Porous Microspheres for Efficient Delivery of Insulin and Inhalation Therapy of Diabetes. Adv. Healthc. Mater. 2019, 8, 1800910. [Google Scholar] [CrossRef] [PubMed]
- Cuadra, I.A.; Zahran, F.; Martín, D.; Cabañas, A.; Pando, C. Preparation of 5-fluorouracil microparticles and 5-fluorouracil/poly(L-lactide) composites by a supercritical CO2 antisolvent process. J. Supercrit. Fluids 2019, 143, 64–71. [Google Scholar] [CrossRef]
- Lin, C.; Ng, K.M.; Wibowo, C. Producing nanoparticles using precipitation with compressed antisolvent. Ind. Eng. Chem. Res. 2007, 46, 3580–3589. [Google Scholar] [CrossRef]
- Gimenez-Rota, C.; Palazzo, I.; Scognamiglio, M.R.; Mainar, A.; Reverchon, E.; Della Porta, G. β-Carotene, α-tocoferol and rosmarinic acid encapsulated within PLA/PLGA microcarriers by supercritical emulsion extraction: Encapsulation efficiency, drugs shelf-life and antioxidant activity. J. Supercrit. Fluids 2019, 146, 199–207. [Google Scholar] [CrossRef]
- Kluge, J.; Fusaro, F.; Mazzotti, M.; Muhrer, G. Production of PLGA micro- and nanocomposites by supercritical fluid extraction of emulsions: I. Encapsulation of lysozyme. J. Supercrit. Fluids 2009, 50, 327–335. [Google Scholar] [CrossRef]
- Kluge, J.; Fusaro, F.; Mazzotti, M.; Muhrer, G. Production of PLGA micro- and nanocomposites by supercritical fluid extraction of emulsions: II. Encapsulation of Ketoprofen. J. Supercrit. Fluids 2009, 50, 336–343. [Google Scholar] [CrossRef]
- Della Porta, G.; Falco, N.; Reverchon, E. NSAID drugs release from injectable microspheres produced by supercritical fluid emulsion extraction. J. Pharm. Sci. 2010, 99, 1484–1499. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, G.; Campardelli, R.; Falco, N.; Reverchon, E. PLGA microdevices for retinoids sustained release produced by supercritical emulsion extraction: Continuous versus batch operation layouts. J. Pharm. Sci. 2011, 100, 4357–4367. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, G.; Campardelli, R.; Reverchon, E. Monodisperse biopolymer nanoparticles by continuous supercritical emulsion extraction. J. Supercrit. Fluids 2013, 76, 67–73. [Google Scholar] [CrossRef]
- Dhanda, D.S.; Tyagi, P.; Mirvish, S.S.; Kompella, U.B. Supercritical fluid technology based large porous celecoxib–PLGA microparticles do not induce pulmonary fibrosis and sustain drug delivery and efficacy for several weeks following a single dose. J. Control. Release 2013, 168, 239–250. [Google Scholar] [CrossRef]
- Falco, N.; Reverchon, E.; Porta, G.D. Injectable PLGA/hydrocortisone formulation produced by continuous supercritical emulsion extraction. Int. J. Pharm. 2013, 441, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Perrut, M.; Clavier, J.-Y. Supercritical fluid formulation: Process choice and scale-up. Ind. Eng. Chem. Res. 2003, 42, 6375–6383. [Google Scholar] [CrossRef]
- Corazza, M.L.; Cardozo Filho, L.; Dariva, C. Modeling and simulation of rapid expansion of supercritical solutions. Braz. J. Chem. Eng. 2006, 23, 417–425. [Google Scholar] [CrossRef][Green Version]
- Sievers, R.E.; Karst, U. Methods for Fine Particle Formation. US Patents 5,639,441, 17 June 1997. [Google Scholar]
- Shekunov, B.Y.; Chattopadhyay, P.; Huff, R.W. Method for Producing Solid-Lipid Composite Drug Particles. US Patents US8642091 B2, 4 February 2014. [Google Scholar]
- Tabernero, A.; del Valle, E.M.M.; Galán, M.A. Supercritical fluids for pharmaceutical particle engineering: Methods, basic fundamentals and modelling. Chem. Eng. Process. 2012, 60, 9–25. [Google Scholar] [CrossRef]
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using Supercritical Fluid Technology as a Green Alternative During the Preparation of Drug Delivery Systems. Pharmaceutics 2019, 11, 629. [Google Scholar] [CrossRef]





| SCF Technique | Advantages | Limitations |
|---|---|---|
| RESS, RESOLV | Single-step particle production. No/low amount of organic solvent required. Final product properties can be controlled by controlling the process parameters. The final product is free of residual solvent. | Solute should be solid or amorphous. The solubility of solute is selective for low molecular weight polymer and small molecules. |
| SAS, GAS, SEDS | Milder process parameters (temperature and pressure required) compared to RESS. Overcomes the limitation of solute solubility in SC-CO2. Encapsulation of labile active substances is possible. | Use of organic solvent. Some biopolymers tend to plasticize in presence of SC-CO2. |
| PGSS | Organic solvent free process. Homogeneous product obtained. Encapsulation of labile active substances is possible. | Particle aggregation can occur during the product formation. Nozzle blockage can occur. |
| SFEE | Wider range of biopolymers can be processed including PCL and PMMA. Encapsulation of hydrophobic drugs, proteins and essential oils is possible. Monodisperse particle production. | Multiple steps required. Organic solvent is used. |
| SCF Technique | Polymer; Molar Mass (kDa) | Cargo/ Drug | Overview | Ref/Year |
|---|---|---|---|---|
| RESS, SAS | PLLA; 5.5 PDLLA; 5.3 | - | Production of MPs of PLLA, PDLLA and PGA via successful processing of polyhydroxy polymers. | [36]/ 1991 |
| RESS | PLLA; 10 | Pyrene | Production of composite particles using RESS: variation of production parameters and use of pyrene as a model compound for coprecipitation with PLLA. | [65]/ 1994 |
| RESS | PLLA; 2 | Naproxen | Microencapsulation of Naproxen in PLLA. Application of specific equation of the state theory for a prediction of the parameters: EOS modeling of the process parameters was successfully correlated with the experimental results. | [37]/ 1996 |
| ASES | PLLA; 102 | Hyoscine butyl bromide, indomethacin, piroxicam and thymopentin | The Aerosol solvent extraction system (ASES) technique was used to produce drug/polymer particles using various model drugs. Drug-loaded PLLA MPs with sizes below 50 µm were obtained. | [66]/ 1996 |
| PCA | PLLA; 100 | Gentamycin, rifamycin and naltrexone | The PCA method with ion pairing as a preliminary stage was used to encapsulate drugs into PLLA. Their release profile was revealed indicating matrix-controlled diffusion for particles produced by PCA. | [49]/ 1997 |
| SEDS | PDLLA; n/d PLLA; n/d PLGA; n/d | - | Successful preparation of PDLLA, PLLA, PLGA and PCL MPs using SEDS technique. | [67]/ 1999 |
| GAS | PLLA; 102 | Insulin | Preparation of insulin-loaded PEG-PLLA NPs with varying content and molar mass of PEG. The low molar mass PEG improved the morphology of the co-precipitation product and drug release kinetics. | [68]/ 2001 |
| SAS | PLLA; 29 | Diuron | Production of diuron loaded PLLA MPs ranging from 1–5 µm by single-step SAS process. A different concentration of diuron and PLLA in the system affected the final morphology of the product. | [69]/ 2001 |
| ASES | PLLA; 50 | 4-Hydroxy-benzoic acid | Drug-loaded PLLA MPs with sizes between 2 to 3 µm and loading capacities of 9.2% and 15.6% were achieved (for p-hydroxybenzoic acid and lysozyme). | [70]/ 2002 |
| PCA | PLLA; 100 | Budesonide | Preparation of budesonide loaded PLLA MPs was successful. The obtained product particles showed ~80% encapsulation efficiency (EE) and were studied in vitro prior to in vitro drug release analysis. | [71]/ 2002 |
| GAS | PLLA; 102 | Insulin | Production and in vitro investigation of insulin-loaded PEG-PLLA particles with sizes <1 µm. The PEG content was varied observing that the loading capacity of insulin was inversely proportional to the molar mass of PEG. | [72]/ 2004 |
| GAS | PLLA; 102 | Nissin | Formulation of nissin loaded PLLA particles with antibacterial activity via GAS technique proving its potential for protein encapsulation. | [54]/ 2004 |
| Pressure quenching | PLGA (50/50); 96 (65/35); 10 (75/25); 97 PLLA; 160 | Deslorelin | Drug encapsulation into PLGA polymer particles of different compositions. PLGA (50/50) was found to work the best; The sizes of the drug-loaded particles ranged from 2.2 to 13.8 µm. PLLA was not used further. The technique proved to maintain the integrity of the active substance, low residual solvent and sustained release of the final product. | [73]/ 2004 |
| SC-CO2 assisted plasticization and spraying | PDLLA; 8 | Ribonuclease-A, lysozyme, insulin, and calcitonin | Successful encapsulation of proteins in PDLLA MPs. PDLLA was first plasticized using SC-CO2, then the drug was mixed with it and the molten mixture was sprayed to obtain dry particles. The particles retained the enzymatic activity of insulin and calcitonin which was confirmed by biological assays. | [74]/ 2005 |
| SSI | PDLLA; 9 | Indomethacin | Indomethacin was impregnated on PDLLA NP carriers maintaining the size between 190 to 290 nm and spherical morphology of the particles with up to 6.6 wt% of the drug | [75]/ 2005 |
| SAS | PLLA; 2 PLLA; 50 PLGA (50:50); 48 | Bupivacaine hydrochloride | Encapsulation of bupivacaine hydrochloride in PLLA (MW = 2 kDa) MPs. Varying the ratios of the PLGA/PLLA polymer content, two different levels of bupivacaine HCl (5 and 10%) were used and particles of 4 to 10 µm in geometric mean diameter were obtained. A controlled drug release was observed for up to 7 days. | [76]/ 2006 |
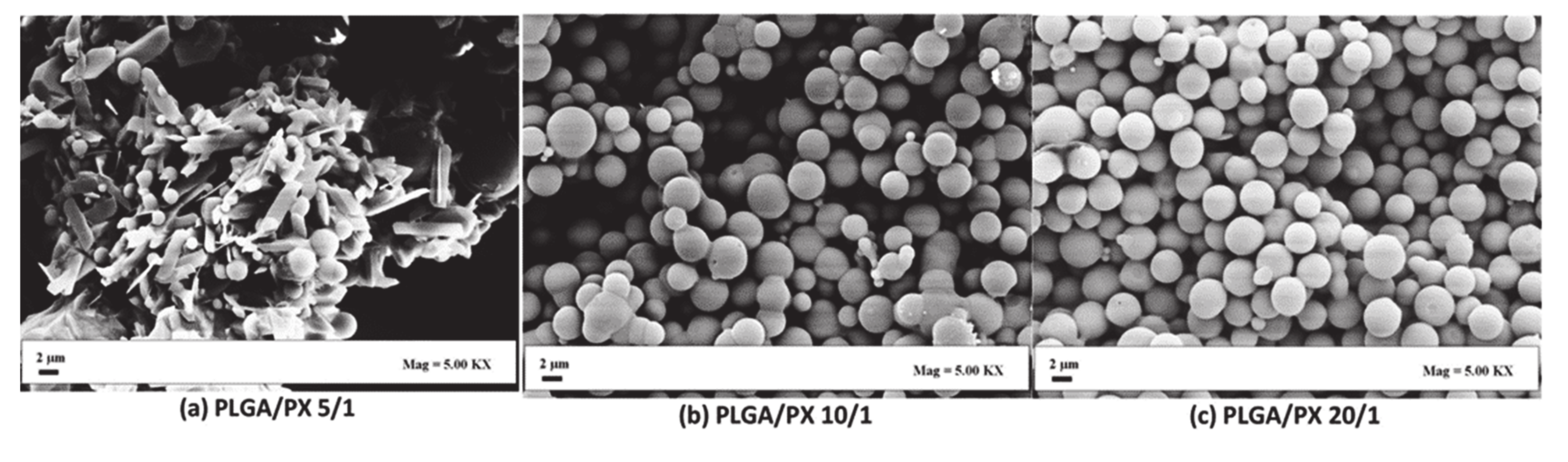
| SEDS | PLLA; 100 PLGA; 100 | Paclitaxel | PLLA, PLGA(50/50) and mixtures of both (PLLA/PLGA(50/50)) were co-precipitated with paclitaxel to obtain MPs. The size of the MPs was influenced by the feed ratio. Final drug loading was between 14.1 and 16.3%. The release rate depended on the ratio of PLLA/PLGA in the final product. | [77]/ 2008 |
| SAS | PLLA; 85–160 | Rifampicin | Formulation of rifampicin loaded PLLA NPs with varying PLLA:drug ratios. The average particle size was <5 µm. A lower polymer content resulted in more irregular shaped particles, but a higher polymer content decreased the drug loading. 7 to 80% of polymer content particles were found to show a more controlled release of the drug without an initial burst. | [78]/ 2008 |
| SEDS | PLLA; 100 PLGA; 100 | Indomethacin | Indomethacin loaded PLLA/PLGA(50/50) MPs with 2.35 µm size were produced. Release studies revealed diffusion-controlled release in early stage and bioerosion in later. In vitro cell studies revealed the activity of IDMC-PLLA/PLGA particles on the non-small cell lung cancer cell line. | [79]/ 2008 |
| SEDS | PLLA;100 | SiO2 NP 5-Fluorouracil | 5-Fluorouracil was adsorbed on SiO2 NPs which were then co-precipitated with PLLA. Spherical smooth particles with a size of ~530 nm were obtained. The EE was claimed to be 80.53%. The technique proved to be efficient for the preparation of drug-loaded MPs with controlled release of the drug. | [80]/ 2009 |
| SAS | PLLA; 100 | 5-Azacytidine | The drug was encapsulated in PLLA MPs final sizes were ~2 µm. Optimal conditions of 40 °C and 11 MPa, and a CO2 flow rate of 30 mL/min were used to obtain 5-Azacytidine loaded MPs with 95% drug entrapment. The drug release profiles in acid and basic medium were reported as well as the stability of the entrapped drug. | [81]/ 2009 |
| RESOLV | PLLA; 11 | Retinyl palmitate | Retinyl palmitate was encapsulated in PLLA yielding particle sizes in the range of 40 to 110 nm and an average loading of 0.9 to 6.2%. The entrapment efficiency increased with increasing the pre-expansion temperature and the concentration of drug, but the size of the NPs was affected by the degree of saturation. | [47]/ 2009 |
| SEDS | PLLA; 50 PLLA-PEG; 70-5 | siRNA | siRNA loaded L-PLA and PEG-PLA particles with a size range between 100 to 300 nm and 600 to 700 nm respectively were obtained. Furthermore, the drug release was found to be dependent on the polymer used. The in vivo sustained release was successfully studied without affecting the biological activity of the siRNA. | [82]/ 2010 |
| SAA | PLLA; 85–140 | - | Spherical MPs of PLLA were produced in the size range of 1 to 1.5 µm. BSA was also processed and spherical particles with retained biological activity were obtained. The process was hence claimed to be suitable for thermolabile compounds. | [83]/ 2011 |
| RESS + SEDS | PLA-PEG-PLA; 29 | - | The study revealed the advantages of combined SEDS and RESS technique for processing partially soluble polymers obtaining particles with sizes of ~2 µm. The process parameters controlled the morphology and size of the final product during the process. | [84]/ 2011 |
| Reverse emulsion SEDS | PLLA-PEG; 25:5; 50:5; 100:5 | 5-Fluorouracil | Drug/copolymer emulsion with varying ratios of PLLA:PEG (organic phase) were formed with the subsequent SC-CO2 spraying. The drug-loaded particles revealed a size ≤ 1 µm and were efficient to inhibit the growth of tumor in animal studies by 51.92%, which is higher than the free 5-Fluorouracil. This technique proved to be efficacious for the encapsulation of hydrophilic compounds. | [58]/ 2012 |
| SAA | PEG-PLA; 7.7–9.7; 8.1–23.1 | - | Spherical MPs of PLLA were produced in the size range of 1 to 1.5 µm. BSA was also processed and spherical particles with retained biological activity were obtained. The process was therefore claimed suitable for thermolabile compounds. | [85]/ 2012 |
| SpEDS | PLLA; 77 | Fe3O4 NPs Methotrexate | Production of methotrexate loaded PLLA MPs via drug and polymer co-precipitation on Fe3O4 NPs was presented. Particle size decreased with increase in Fe3O4 content. The EE was 60.8% and a sustained released was observed. The SpEDS process was successful in the production of magnetic particles. | [86]/ 2012 |
| SEDS | PLLA; 10 PLLA-PEG; 50, 100, 200 | Lysozyme | Lysozyme loaded PLLA/PLLA-PEG MPs with varying sizes were produced. The particles size and drug loading were affected by the PEG content and by the MW of the PLLA. However, the structure of the initial polymer did not change during the SEDS processing confirming that protein or labile compounds can be processed using this technique. | [87]/ 2012 |
| SAS | PLLA; n/d | Paracetamol | The process parameters for the encapsulation of paracetamol into PLLA MPs were investigated. Final particle sizes varied with increasing pressure or temperature. A sustained drug release was observed over 4 weeks proving that SAS can be promising for the production of drug-loaded vehicles. | [88]/ 2012 |
| SEDS | PLLA; 100 | Morphine | Morphine loaded PLLA (MF-PLLA) spherical MPs with a mean diameter of 2.45 μm were prepared using the optimum conditions of 17.5/2.5 aqueous solution, ethanol and DCM ratio. The calculated drug loading was 4.73 ± 0.34%. The release profile revealed a burst release in first 4 h and slow-release until 168 h. | [89]/ 2012 |
| ASES | PLLA; 50 and 100; mPEG-PLLA various ratios | Leuprolide acetate | Leuprolide acetate loaded MPs using PLLA and mPEG-PLLA were studied regarding the polymer molar mass, the block length and the drug:polymer ratio. The increase of the PEG length in the copolymer increased the particles size from 2.86 to 5.63 µm. The study showed the influence of the varying factors on the final product morphology and size. | [90]/ 2012 |
| SEDS | PLLA; 100 PLLA-PEG-PLLA; n/d | Morphine | Morphine was encapsulated in PLLA-PEG-PLLA particles with particle size range of 2.04–5.73 µm. The influence of the process parameters and the content of PEG on the final product was studied. The highest EE of ~87% was obtained at drug:polymer ratio of 1:10 with 3% PEG and at 120 bar and morphine concentration of 8 mg/mL. The release rate was faster by increasing PEG content in the final product. | [59]/ 2013 |
| RESOLV | PLGA (85/25); n/d | Fenofibrate | PLGA was co-precipitated with fenofibrate obtaining particles with sizes of ~3 µm. The most suitable stabilizer was SDS driving to a particles size of ~0.89 µm. Furthermore, coprecipitation of PLGA and fenofibrate was attempted with SDS as a stabilizer, where the structures obtained were comparatively non agglomerated and in the size range below 1 µm. Increasing the number of depressurization cycles resulted in the agglomeration of the final product. | [91]/ 2013 |
| SAS | PLLA; 100 | 10-Hydroxycamptothecin | Encapsulation of 10-hydroxycamptothecin into PLLA NPs with subsequent in vitro release studies is shown. The loading capacity of the particles was influenced by the solvent combination used. The loaded particles produced with suitable conditions were spherical in shape with 794 nm (mass median diameter) size with a loading capacity of 13.3%. The in vitro release investigation proved that the increase in the concentration of PLLA influences the controlled release and loading capacity of the drug. | [92]/ 2013 |
| PCA | PLLA; 50 | Lysozyme | Lysozyme loaded PLLA MPs were produced with irregular shape, in a size range of 16.9 ro 18.8 µm and with a porosity of 78.2–86.3%. Further characterization also revealed no changes in the structure or activity of the active compound during the process, proving the suitable for processing and encapsulation of labile materials. | [93]/ 2013 |
| RESS + SAS | PLLA; n/d | - | RESS and SAS techniques were applied to PLLA for the production of MPs and revealed that the technique had a major influence on the morphology and size of the particles. Spherical particles were obtained with SAS process, which was then chosen for further investigation. The size of the particles was largely dependent on the initial concentration, flow rate, process temperature and pressure in the SAS process. | [94]/ 2013 |
| SC-CO2 infusion pressure quench technique | PLLA; n/d PLGA; n/d | Bevacizumab | Bevacizumab was coated on PLLA NPs that were further encapsulated into porous PLGA microparticles by exposing the mixture to SC-CO2. The Bevacizumab loaded PLLA particles (265 nm) were infused in porous PLGA of 1.67 µm. The sustained release proved to be effective for 4 months with retained signaling protein activity (Vascular endothelial growth factor). | [95]/ 2013 |
| SSI | PLLA; 100 | 5-Fluorouracil | PLLA MPs were prepared and impregnated with 5-fluorouracil. The resulting size was 0.68 µm. The impregnation efficiency was influenced by the pressure, temperature, and co-solvent concentration. The highest impregnation efficiency observed was 12 µg/mg at 60 °C and 100 bar without cosolvent and 60 µg/mg with 4 mol % of cosolvent. | [96]/ 2013 |
| SAS | PLLA; n/d | Ibuprofen | Co-precipitation of ibuprofen and PLLA resulting in MPs with sizes between 0.9 to 1.8 µm and up to 10.1% drug loading. It was observed that increasing the pressure and decreasing the temperature decreased the particles size. The maximum amount of drug remained on the surface of the particles, which was confirmed by release rates and FTIR, XRD and DSC analysis. | [97]/ 2014 |
| SAS | PLLA; n/v | Naproxen | Co-precipitation of naproxen and PLLA yielded MPs with a size of 1.2 µm and 13.2% drug loading. A slow controlled release was observed compared to free drug. The pressure had an inverse effect on the final particle size. The drug:polymer ratios did not influence the release rate of the profiles and the pH value of the external medium. | [98]/ 2014 |
| SAS-EM | PLGA; 50 | Curcumin | Production of curcumin loaded PLGA particles with modified SAS whereby the polymer-drug solution was ultrasonicated before spraying to enhance the mixing which resulted in a higher drug loading and improved yield. | [99]/ 2014 |
| SAS-EM | PLGA; 50 | Curcumin | Optimization of the process parameters to obtain curcumin coated PLGA (75:25) NPs. The influence of the solvent type, the initial concentration of solute, the CO2 flow rates, the curcumin:PLGA ratio and the ultra-sonication power was investigated. Loading, size distribution and yield of the final curcumin coated PLGA NPs improved with increasing the ultrasonic power and high solvent flow rates. | [100]/ 2014 |
| SEDS | PEG-PLLA; 27 | Paclitaxel | Encapsulation of paclitaxel into a folic acid (FA)-PEG-PLLA copolymer for tumor targeting yielded MPs with sizes between 2.4 and 2.9 µm and a drug loading of 7 to 9%. The FA content influenced the EE obtaining the highest (23%) for a FA:polymer ratio of 0.6:1. The PTX-FA-PEG-PLLA particles showed higher uptake in tumor cell lines while the distribution of the drug was found to be specifically higher in tumor tissues. In vivo studies revealed that FA conjugated particles were more effective compared to the unconjugated particles. | [101]/ 2015 |
| SAS | PLLA; n/d | Zidovudine | Zidovudine-loaded PLLA particles (dry dispersions) were formulated. The nine possible combinations from three variables of the process parameters (drug ratio, pressure, temperature) were tested. The batch with 1:2 (Zidovudine–PLLA) ratio, at 45 °C and 85 bar gave 91.5% yield, with 40% drug content. Intestinal permeability studies revealed drug permeability of approximately 9.9%, which was higher than that of pure Zidovudine (3.8%). | [102]/ 2015 |
| PCA | PLLA; 50 | Insulin | Insulin-loaded PLLA porous MPs with sizes of ~4 µm were produced. The insulin encapsulation was found to be the highest with the lowest concentration of drug (5 wt. %). The physicochemical characterization revealed no major chemical changes in insulin but only minor changes in the secondary structure. The hypoglycemic activity was retained and sustained released was observed. | [103]/ 2015 |
| SAS | PLLA; n/d. | 17α-Methyltestosterone | Drug-loaded particles were produced and the in vitro drug release behavior was investigated. The study shows the use of SAS for the encapsulation of a hormone in biodegradable systems. The investigation leads to the conclusion that increasing the drug ratio concerning the polymer the drug entrapment increased but also led to aggregation in the final product. | [104]/ 2016 |
| SAILA | PLLA; 28 PLGA; 20 | Piroxicam | The drug encapsulation capacity of PLLA and PLGA was compared using piroxicam as the model drug. Particles of 1.53 μm ± 0.53 size were obtained from PLGA and broader distribution was obtained from PLA with 1.76 μm ± 1.05 size particles. Preliminary encapsulation studies revealed an EE of 60% and controlled release of up to 5 days. | [105]/ 2016 |
| Rapid expansion of subcritical solution | PLLA; 4.7 | Tetrahydro curcumin | Various drug:PLLA ratios on the particle morphology and stability of drug after processing were investigated. The average particle size was ∼80 to 110 nm and the loading capacity varied between ∼13 and 25%. It was shown that the loading capacity was directly proportional to the expansion temperature and the drug:polymer weight ratio, but inversely proportional to the expansion temperature. While the size of the particles had no major effect of process conditions. | [106]/ 2016 |
| SAS | PLLA; n/d | Tamoxifen citrate | Encapsulation of tamoxifen citrate into PLLA particles was studied resulting in 280 nm size particles and in a high EE of 94%. Compared to the conventional encapsulation process, the SAS process provided the advantage of single-step processing. | [107]/ 2017 |
| SAILA | PLGA (75:25); 20 | Piroxicam; indomethacin; diclofenac | Various drug-loaded PLGA particles were produced with narrow distributions and sizes ranging from 0.3 to 2.5 µm. A higher drug ratio, temperature and pressure resulted in co-precipitation of the drug and polymer. A low EE was observed for the drugs that had more solubility in anti-solvent medium and also for higher process temperature. | [108]/ 2017 |
| SAS | PLLA; 100 | Gefitinib | The production of gefitinib (GFB) loaded PLLA particles resulted in being influenced by the solution flow rate affecting the loading of the GFB. The highest drug loading obtained with optimized conditions was 15.8% with an EE of 94% in spherical particles with D50 of 2.48 µm. GFB loaded MPs revealed a lower crystallinity compared to raw GFB. A sustained release over the time was measured which then was complemented with the in vitro anti-tumor experiments, where the encapsulated particles were more effective than raw GFB. | [109]/ 2017 |
| PCA | PLLA; 50 | Insulin | Preparation of insulin-loaded and -unloaded porous PLLA NPs at 80 bar and 35 °C (INS) with a solution flow rate of 4 mL min−1. The INS loading was determined to be 4.85% and the EE 97%. The particles revealed a porous, rough surface and sizes of 16 ± 3.14 µm for PLLA PM and 19.03 ± 3.05 µm for INS-PLLA PM as mean geometric diameter. The authors showed in vivo hypoglycemic effects for pulmonary delivery of INS-PLLA PMs compared to an untreated group supported with sustained release. | [110]/ 2019 |
| SAS | PLLA; n/d | 5-Fluorouracil | The production of pure drug particles and drug-loaded PLLA particles yielded particles with sizes from 220 to 670 nm depending on the process conditions. The highest drug loading capacity obtained was of 42% at 50 °C and 120 bar pressure. The organic solution mixture also influenced the particles sizes. | [111]/ 2019 |
| Technique | Polymer; Molar Mass (kDa) | Cargo | Overview | Ref/Year |
|---|---|---|---|---|
| SFEE | PLGA (50:50); 42–65 | Lysozyme | The influence of various emulsion technique parameters on the morphology of lysozyme loaded PLGA(50/50) particles was studied. The particle sizes ranged from 0.1 to 1.7 µm. Among three different encapsulation methods, the in situ suspension emulsion was found to be most efficient with an EE of 48.5%. | [114]/ 2009 |
| SFEE | PLGA (50/50); 42–65 | Ketoprofen | Encapsulation of ketoprofen into PLGA particles applying first a conventional emulsion technique and subsequently SFEE was examined. Particles revealed a final particle size of 100–200 nm. The higher ketoprofen content reduced the stability and EE over time. Suspended co-formulation was speculated to be metastable and provided important information for the limit of the overloaded formulations. | [115]/ 2009 |
| SFEE | PLGA (75/25); 60–120 | Piroxicam; diclophenac sodium | Single and double emulsion techniques were compared with varying the emulsion process parameters in the encapsulation of piroxicam and diclophenac sodium into PLGA particles. Drug-loaded particles with sizes between 1 to 3 µm were obtained with EEs of 88% and 97% for diclofenac sodium and piroxicam, respectively. The SFEE proved to be an efficient technique for the production of drug-loaded particles. | [116]/ 2010 |
| SFEE | PLGA (75/25); 20 | Retinyl acetate | The production of retinyl encapsulated PLGA (75:25) NPs via batch and continuous mode of operation was tested. The continuous mode was more efficient and yielded 3.3 to 4.5 µm sized particles with high EEs of 80 to 90%. In this study, the mass transfer phenomenon of the process was exploited. | [117]/ 2011 |
| SFEE-C | PLLA; 28 PLGA (75/25); 20 | - | PLLA, PLGA (75/25) and PCL particles were prepared by the application of various process parameters. Operating conditions of 38 °C, 80 bar and L/G ratio of 0.1 resulted in particles of PLA, PCL and PLGA with a mean size of 233 nm, 342 nm and 212 nm, respectively. The influence of various polymer concentrations on the final particle sizes and distributions were studied. | [118] 2013 |
| SFEE-C | PLGA (50/50); 44 | Insulin | Insulin-loaded PLGA MPs were produced using double emulsion and tested on rat embryonic cell for viability and drug release studies. Particles sizes resulted between 2 to 3 µm and revealed no degradation of the insulin which maintained further the activity in the cells. | [64]/ 2013 |
| SFEE | PLGA (75/25); n/d | Celecoxib | Celecoxib loaded PLGA (75/25) MPs of 10.5 µm size were produced with a drug loading of 10.9%. These particles were found to be more stable and had higher accumulation levels of celecoxib to the targeted area compared to the conventional particles. | [119]/ 2013 |
| SAA and SFEE-C | PLGA (50/50); 38–54 | Hydrocortisone acetate | The rapid recrystallization of hydrocortisone acetate while encapsulation into PLGA and continuous emulsion processing were resolved and particles of ~3 µm with an EE of 75% were obtained. | [120]/ 2013 |
| SFEE | PLLA; 60 PLGA (75/25); 20 | β-Carotene, α-tocoferol, rosmarinic acid | Drugs were encapsulated in the polymeric carriers to improve their shelf life and stability. The solvent was removed at 80 bar and 37 °C. The encapsulation of β-carotene in PLGA, as well as co-encapsulation of α-tocoferol and β-carotene, was possible resulting in range of sizes 0.3 to 4.3 µm (for PLLA and PLGA) with improved shelf life of the drugs. Rosmarinic acid encapsulation was limited to low efficiencies. | [113]/ 2019 |
| SFEE | PLLA; n/d | Rhodamine B | Rhodamine B loaded emulsion microbeads were prepared and exposed to SFEE. The size of the microbeads was 1 ± 0.2 µm. Human monocytes showed uptake of loaded microbeads. The technique provided an excellent alternative to the conventional technique with improved product quality and reduced residual solvent. | [11]/ 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangapurwala, G.; Vollrath, A.; De San Luis, A.; Schubert, U.S. PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation? Pharmaceutics 2020, 12, 1118. https://doi.org/10.3390/pharmaceutics12111118
Gangapurwala G, Vollrath A, De San Luis A, Schubert US. PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation? Pharmaceutics. 2020; 12(11):1118. https://doi.org/10.3390/pharmaceutics12111118
Chicago/Turabian StyleGangapurwala, Gauri, Antje Vollrath, Alicia De San Luis, and Ulrich S. Schubert. 2020. "PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation?" Pharmaceutics 12, no. 11: 1118. https://doi.org/10.3390/pharmaceutics12111118
APA StyleGangapurwala, G., Vollrath, A., De San Luis, A., & Schubert, U. S. (2020). PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation? Pharmaceutics, 12(11), 1118. https://doi.org/10.3390/pharmaceutics12111118