Application of Dominance-Based Rough Set Approach for Optimization of Pellets Tableting Process
Abstract
1. Introduction
2. Materials and Methods
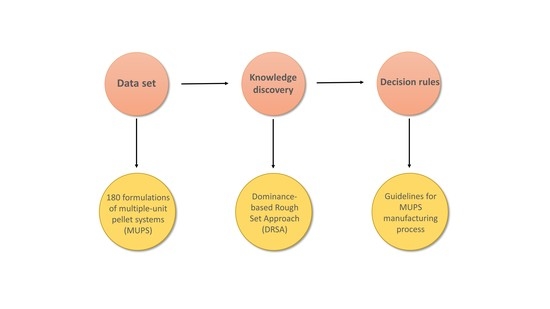
2.1. Data Set
- Tablets with API release profile similar to pellets—f2 ≥ 50—class 1
- Tablets with API release profile different from pellets—f2 < 50—class 2
2.2. Information System
2.3. Knowledge Discovery Technique
2.4. Decision Rules
2.5. Bayesian Confirmation Measures
2.6. Stratified Cross-Validation
3. Results
3.1. Decision Rules
- Rule 1: If absence of ethyl cellulose coating and tablet mass ≤ 553.1 mg and crushing strength ≥ 68.8 (104 N/m2) and amount of Avicel 102 ≥ 12.7%, then class 1;
- Rule 2: If presence of Eudragit NE coating and compression force > 6 kN and tablet mass ≤ 563.3 mg and hardness ≥ 42.4 N and absence of Avicel 101, then class 1;
- Rule 3: If presence of Eudragit NE coating and compression force > 6 kN and hardness ≥ 49.2 N and absence of Vivapur 200, Avicel 101, Povidone K30, then class 1;
- Rule 4: If presence of Eudragit NE coating and compression force > 6 kN and hardness ≥ 42.4 N and absence of Povidone K30 and Mannitol, then class 1;
- Rule 5: If presence of Eudragit NE coating and compression force > 6 kN and crushing strength ≥ 102.5 (104 N/m2) and absence of Vivapur 200 and StarLac, then class 1.
- Rule 1: If rotary tablet press and crushing strength ≤ 97.8 [104 N/m2], then class 2;
- Rule 2: If absence of Eudragit NE coating and compression force < 18 kN and crushing strength ≤ 158.3 [104 N/m2], then class 2;
- Rule 3: If absence of Eudragit NE coating and compression force < 18 kN and absence of Tablettose 80, then class 2;
- Rule 6: If crushing strength ≤ 68.1 [104 N/m2] and Kollidon Cl ≥ 9.5%, then class 2;
- Rule 8: If compression force = 6 kN and crushing strength ≤ 72 [104 N/m2], then class 2;
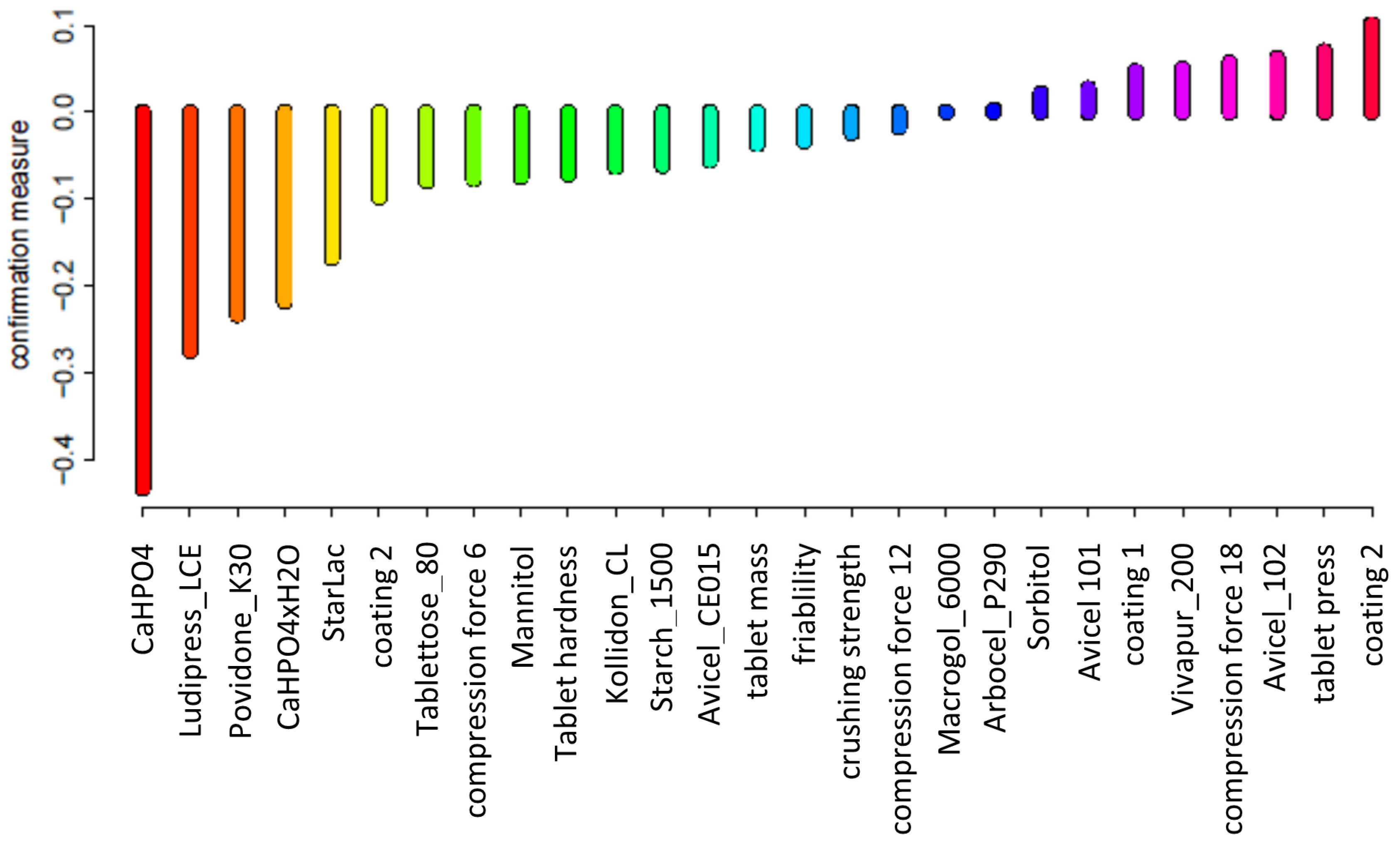
3.2. Predictive Attribute Revelance
3.3. Results of Stratified Cross-Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abdul, S.; Chandewar, A.V.; Jaiswal, S.B. A flexible technology for modified-release drugs: Multiple-unit pellet system (MUPS). J. Control. Release 2010, 147, 2–16. [Google Scholar] [CrossRef]
- Phale, M.D.; Gothoskar, A.V. Multiunit particulate systems: A current drug-delivery technology. Pharm. Technol. 2011, 35, 60–66. [Google Scholar]
- Xu, M.; Heng, P.W.S.; Liew, C.V. Formulation and process strategies to minimize coat damage for compaction of coated pellets in a rotary tablet press: A mechanistic view. Int. J. Pharm. 2016, 499, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Avari, J. Tableting of coated pellets. Int. J. PharmTech Res. 2013, 5, 1355–1359. [Google Scholar]
- Mishra, V.; Thakur, S.; Patil, A.; Shukla, A. Quality by design (QbD) approaches in current pharmaceutical set-up. Expert Opin. Drug Deliv. 2018, 15, 737–758. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi-and Megavariate Data Analysis: Basic Principles and Applications, 3rd ed.; MKS Umetrics AB: Malmö, Sweden, 2013. [Google Scholar]
- Greco, S.; Matarazzo, B.; Slowinski, R. Rough sets theory for multicriteria decision analysis. Eur. J. Oper. Res. 2001, 129, 1–47. [Google Scholar] [CrossRef]
- Sawicki, W.; Lunio, R. Compressibility of floating pellets with verapamil hydrochloride coated with dispersion Kollicoat SR 30 D. Eur. J. Pharm. Biopharm. 2005, 60, 153–158. [Google Scholar] [CrossRef]
- Lunio, R.; Sawicki, W. Influence of acrylic esters and methacrylic esters on flotation of pellets and release rate of verapamil hydrochloride. Acta Pol. Pharm. 2006, 63, 69–74. [Google Scholar]
- Sawicki, W.; Lunio, R.; Walentynowicz, O.; Kubasik-Juraniec, J. Influence of the type of cellulose on properties of multi-unit target releasing in stomach dosage form with verapamil hydrochloride. Acta Pol. Pharm. 2007, 64, 81–88. [Google Scholar]
- Lunio, R.; Sawicki, W.; Skoczen, P.; Walentynowicz, O.; Kubasik-Juraniec, J. Compressibility of gastroretentive pellets coated with Eudragit NE using a single-stroke and a rotary tablet press. Pharm. Dev. Technol. 2008, 13, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Lunio, R.; Sawicki, W. Influence of the components of Kollicoat SR film on mechanical properties of floating pellets from the point of view of tableting. Pharmazie 2008, 63, 731–735. [Google Scholar] [CrossRef]
- Sawicki, W.; Lunio, R. Tableting of floating pellets with verapamil hydrochloride: Influence of type of tablet press. Acta Pol. Pharm. 2010, 67, 103–106. [Google Scholar]
- European Medicines Agency. CPMP/EWP/QWP/1401/98Rev.1/Corr-Guideline on the Investigation of Bioequivalence. London, UK, 2010. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 19 October 2020).
- Zhang, Y.; Huo, M.R.; Zhou, J.P.; Zou, A.F.; Li, W.Z.; Yao, C.L.; Xie, S.F. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Blaszczynski, J.; Greco, S.; Matarazzo, B.; Slowinski, R.; Szelag, M. jMAF-Dominance-based Rough Set Data Analysis Framework. Chapter 5. In Rough Sets and Intelligent Systems—Professor Zdzislaw Pawlak in Memoriam; Skowron, A., Suraj, Z., Eds.; Series Intelligent Systems Reference Library; Springer: Berlin/Heidelberg, Germany, 2013; Volume 42, pp. 185–209. [Google Scholar]
- Greco, S.; Pawlak, Z.; Slowinski, R. Can Bayesian confirmation measures be useful for rough set decision rules? Eng. Appl. Artif. Intell. 2004, 17, 345–361. [Google Scholar] [CrossRef]
- Blaszczynski, J.; Slowinski, R.; Susmaga, R. Rule-based estimation of attribute relevance. In RSKT 2011, LNCS 6954; Yao, J.T., Ramanna, S., Wang, G., Suraj, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 36–44. [Google Scholar]
- Blaszczynski, J.; Slowinski, R.; Stefanowski, J. Ordinal classification with monotonicity constraints by variable consistency bagging. In RSCTC 2010, LNAI 6086; Szczuka, M., Kryszkiewicz, M., Ramanna, S., Jensen, R., Hu, Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 392–401. [Google Scholar]
- Frank, E.; Hall, M.A.; Witten, I.H. The WEKA Workbench. Online Appendix for Data Mining: Practical Machine Learning Tools and Techniques, 4th ed.; Morgan Kaufmann: Burlington, VT, USA, 2016. [Google Scholar]
- Chen, T.; Li, J.; Chen, T.; Sun, C.C.; Zheng, Y. Tablets of Multi-Unit Pellet System for Controlled Drug Delivery. J. Control. Release 2017, 262, 222–231. [Google Scholar] [CrossRef]
- Damiati, S.A. Digital Pharmaceutical Sciences. AAPS PharmSciTech 2020, 21, 206. [Google Scholar] [CrossRef]
- Lecomte, F.; Siepmann, J.; Walther, M.; MacRae, R.J.; Bodmeier, R. Polymer blends used for the aqueous coating of solid dosage forms: Importance of the type of plasticizer. J. Control. Release. 2004, 99, 1–13. [Google Scholar] [CrossRef]
- Dashevsky, A.; Kolter, K.; Bodmeier, R. Compression of pellets coated with various aqueous polymer dispersions. Int. J. Pharm. 2004, 279, 19–26. [Google Scholar] [CrossRef]
- Jasti, B.R.; Leopold, C.S.; Li, X.; Penning, M. Shellac. In Handbook of Pharmaceutical Excipients, 8th ed.; Sheskey, P.J., Cook, W.G., Cable, C.G., Eds.; Pharmaceutical Press: London, UK, 2017. [Google Scholar]
- Farag, Y.; Leopold, C.S. Investigation of drug release from pellets coated with different shellac types. Drug Dev. Ind. Pharm. 2011, 37, 193–200. [Google Scholar] [CrossRef]
- Torrado, J.J.; Augsburger, L.L. Effect of different excipients on the tableting of coated particles. Int. J. Pharm. 1994, 106, 149–155. [Google Scholar] [CrossRef]
- Pan, X.; Chen, M.W.; Han, K.; Peng, X.S.; Wen, X.G.; Chen, B.; Wang, J.; Li, G.; Wu, C.B. Novel compaction techniques with pellet-containing granules. Eur. J. Pharm. Biopharm. 2010, 75, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Chaerunisaa, A.Y.; Sriwidodo, S.; Abdassah, M. Microcrystalline Cellulose as Pharmaceutical Excipient. In Pharmaceutical Formulation Design—Recent Practices; Ahmad, U., Akhtar, J., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Beckert, T.E.; Lehmann, K.; Schmidt, P.C. Compression of enteric-coated pellets to disintegrating tablets. Int. J. Pharm. 1996, 143, 13–23. [Google Scholar] [CrossRef]
- El-Mahdi, I.M.; Deasy, P.B. Tableting of coated ketoprofen pellets. J. Microencapsul. 2000, 17, 133–144. [Google Scholar] [CrossRef]
- Palkowski, L.; Karolak, M.; Kubiak, B.; Blaszczynski, J.; Slowinski, R.; Thommes, M.; Kleinebudde, P.; Krysinski, J. Optimization of pellets manufacturing process using rough set theory. Eur. J. Pharm. Sci. 2018, 124, 295–303. [Google Scholar] [CrossRef]
| Condition Attribute | Domain |
|---|---|
| Type of coating | 1—Eudragit NE, 2—ethyl cellulose, 3—shellac |
| Tablet press | 1—single punch, 2—rotary |
| Compression force (kN) | 6, 12, 18 |
| Tablet mass (mg) | 516.0–568.0 |
| Tablet hardness (N) | 11.0–297.0 |
| Crushing strength (104 N/m2) | 11.0–619.0 |
| Friability (%) | 0.00–10.1 |
| Avicel PH 101 (%) | 0, 12.7 |
| Avicel PH 102 (%) | 0, 12.7, 47.0 |
| Mannitol (%) | 0, 34.3 |
| Tablettose 80 (%) | 0, 34.3 |
| Ludipress_LCE (%) | 0, 34.3 |
| Sorbitol (%) | 0, 34.3 |
| Povidone_K30 (%) | 0, 34.3 |
| StarLac (%) | 0, 34.3 |
| CaHPO4xH2O (%) | 0, 34.3 |
| CaHPO4 (%) | 0, 34.3 |
| Vivapur_200 (%) | 0, 34.3 |
| Avicel_CE015 (%) | 0, 34.3 |
| Macrogol_6000 (%) | 0, 34.3 |
| Starch_1500 (%) | 0, 43.8 |
| Arbocel_P290 (%) | 0, 47 |
| Kollidon_CL (%) | 0, 9.5 |
| No. of Formulation | Coating | Tablet Press | Compression Force (kN) | Tablet Mass (mg) | Tablet Hardness (N) | Crushing Strength (104 N/m2) | Friability (%) | Avicel_102 (%) | Avicel_101 (%) | Mannitol (%) | Tablettose_80 (%) | Ludipress_LCE (%) | Arbocel_P290 (%) | Sorbitol (%) | Povidone_K30 (%) | StarLac (%) | Starch_1500 (%) | CaHPO4xH2O (%) | CaHPO4 (%) | Vivapur_200 (%) | Avicel_CE015 (%) | Macrogol_6000 (%) | Kollidon_CL (%) | f2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 6 | 559 | 63 | 92 | 2.30 | 12.7 | 0 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 59.4 |
| 2 | 1 | 1 | 6 | 548 | 66 | 96 | 3.10 | 0 | 12.7 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 56.3 |
| 3 | 1 | 1 | 6 | 557 | 111 | 163 | 4.20 | 0 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 9.5 | 35.7 |
| 4 | 1 | 1 | 6 | 558 | 96 | 140 | 0.50 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 37.5 |
| 5 | 1 | 1 | 6 | 562 | 41 | 58 | 0.00 | 12.7 | 0 | 0 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 38.7 |
| 6 | 1 | 1 | 6 | 557 | 45 | 67 | 2.40 | 12.7 | 0 | 0 | 0 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 35.9 |
| 7 | 1 | 1 | 6 | 562 | 105 | 153 | 0.60 | 0 | 0 | 0 | 0 | 0 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 40.9 |
| 8 | 1 | 1 | 6 | 559 | 76 | 111 | 0.40 | 12.7 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 59.4 |
| 9 | 1 | 1 | 6 | 555 | 55 | 80 | 0.70 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 56.3 |
| 10 | 1 | 1 | 6 | 565 | 36 | 52 | 2.30 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 35.7 |
| 11 | 1 | 1 | 6 | 566 | 26 | 38 | 3.40 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43.8 | 0 | 0 | 0 | 0 | 0 | 0 | 37.5 |
| 12 | 1 | 1 | 6 | 559 | 40 | 58 | 1.20 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 0 | 0 | 0 | 9.5 | 38.7 |
| 13 | 1 | 1 | 6 | 560 | 39 | 57 | 0.90 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 0 | 0 | 9.5 | 35.9 |
| 14 | 1 | 1 | 6 | 562 | 40 | 59 | 0.70 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 0 | 9.5 | 40.9 |
| 15 | 1 | 1 | 6 | 550 | 40 | 58 | 0.40 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 9.5 | 59.4 |
| 16 | 1 | 1 | 6 | 555 | 40 | 58 | 0.60 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 9.5 | 55.9 |
| 17 | 1 | 1 | 12 | 549 | 91 | 134 | 1.10 | 12.7 | 0 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 50.7 |
| 18 | 1 | 1 | 12 | 545 | 79 | 115 | 0.90 | 0 | 12.7 | 34.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 63.5 |
| 19 | 1 | 1 | 12 | 554 | 248 | 363 | 0.90 | 0 | 12.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 34.3 | 0 | 9.5 | 48.3 |
| 20 | 1 | 1 | 12 | 566 | 229 | 335 | 0.40 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.5 | 58.7 |
| No. of Rule | Coating_1 | Coating_2 | Tablet Press | Compression Force (kN) | Tablet Mass (mg) | Hardness (N) | Crushing Strength (104 N/m2) | Friability (%) | Avicel_102 (%) | Vivapur_200 (%) | Avicel_101 (%) | Povidone_K30 (%) | StarLac (%) | Tablettose_80 (%) | Mannitol (%) | Rule Support | Rule Strength | Confirmation Measure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | 0 | - | - | ≤553.1 | - | ≥68.8 | ≥12.7 | - | - | - | - | - | - | 32 | 0.1777 | 0.75 | |
| 2 | 1 | - | - | >6 | ≤563.3 | ≥42.4 | - | - | - | - | 0 | - | - | - | - | 46 | 0.2555 | 0.75 |
| 3 | 1 | - | - | >6 | - | ≥49.2 | - | - | - | 0 | 0 | 0 | - | - | - | 43 | 0.2388 | 0.73 |
| 4 | 1 | - | - | >6 | - | ≥42.4 | - | - | - | - | - | 0 | - | - | 0 | 47 | 0.2611 | 0.73 |
| 5 | 1 | - | - | >6 | - | - | ≥102.5 | - | - | 0 | - | - | 0 | - | - | 48 | 0.2666 | 0.73 |
| 6 | 1 | - | - | - | - | ≥105.8 | - | - | - | - | 0 | - | - | - | - | 33 | 0.1833 | 0.72 |
| 7 | 1 | - | - | >6 | - | ≥105 | - | - | - | - | - | - | - | - | - | 36 | 0.2000 | 0.72 |
| 8 | - | - | - | - | - | >111.9 | >163.6 | - | - | - | - | - | - | - | - | 36 | 0.2000 | 0.72 |
| 9 | - | 0 | - | - | - | - | ≥180.8 | - | - | - | - | - | - | - | - | 39 | 0.2166 | 0.72 |
| 10 | - | 0 | - | - | - | ≥107 | - | - | - | - | - | - | - | - | - | 41 | 0.2277 | 0.72 |
| 11 | 1 | - | - | - | ≤556.4 | - | - | 1.6 | - | - | - | - | - | - | - | 50 | 0.2777 | 0.72 |
| 12 | 1 | - | - | - | ≤554.1 | - | - | 1.1 | - | - | - | - | - | - | - | 34 | 0.1888 | 0.70 |
| 13 | 1 | - | 2 | - | - | ≥49.2 | - | - | - | - | - | - | - | - | - | 37 | 0.2055 | 0.70 |
| 14 | 1 | - | - | >6 | ≤561.5 | ≥49.2 | - | - | - | 0 | 0 | - | - | - | - | 41 | 0.2277 | 0.70 |
| 15 | 1 | - | - | >6 | - | >42.4 | - | - | - | - | - | 0 | - | - | - | 53 | 0.2944 | 0.70 |
| 16 | - | - | - | - | - | ≥148.9 | - | - | - | 0 | - | - | - | - | - | 21 | 0.1166 | 0.69 |
| 17 | 1 | - | - | - | ≤554.1 | - | - | - | ≥12.7 | - | - | - | - | - | - | 32 | 0.1777 | 0.69 |
| 18 | 1 | - | - | >6 | - | - | - | - | - | 0 | 0 | 0 | 0 | - | - | 43 | 0.2388 | 0.69 |
| 19 | 1 | - | - | >6 | - | ≥49.2 | - | - | - | 0 | - | - | 0 | - | 0 | 43 | 0.2388 | 0.69 |
| 20 | - | 0 | - | - | - | - | ≥163.6 | - | - | - | - | - | - | - | - | 47 | 0.2611 | 0.69 |
| No. of Rule | Coating_1 | Coating_2 | Tablet Press | Compression Force (kN) | Tablet Mass (mg) | Hardness (N) | Crushing Strength (104 N/m2) | Avicel_CE015 (%) | Kollidon_Cl (%) | Tablettose_80 (%) | Macrogol 6000 (%) | Arbocel_P290 (%) | Rule Support | Rule Strength | Confirmation Measure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | - | 2 | - | - | - | ≤97.8 | - | - | - | - | - | 30 | 0.1666 | 0.70 |
| 2 | 0 | - | - | <18 | - | - | ≤158.3 | - | - | - | - | - | 48 | 0.2666 | 0.70 |
| 3 | 0 | - | - | <18 | - | - | - | - | - | 0 | - | - | 42 | 0.2333 | 0.70 |
| 4 | 0 | - | - | <18 | - | - | ≤133.1 | - | - | - | - | - | 43 | 0.2388 | 0.70 |
| 5 | 0 | - | 2 | - | - | - | - | - | - | - | - | 0 | 31 | 0.1722 | 0.70 |
| 6 | - | - | - | - | - | - | ≤68.1 | - | 9.5 | - | - | - | 37 | 0.2055 | 0.69 |
| 7 | 0 | - | - | <18 | - | - | - | 0 | - | - | - | - | 42 | 0.2333 | 0.67 |
| 8 | - | - | - | 6 | - | - | ≤72 | - | - | - | - | - | 31 | 0.1722 | 0.67 |
| 9 | 0 | - | - | 12 | - | - | - | - | - | - | - | - | 23 | 0.1277 | 0.67 |
| 10 | - | - | 2 | - | - | ≤48.8 | - | - | - | - | - | - | 23 | 0.1277 | 0.67 |
| 11 | - | - | 2 | - | - | ≤60.1 | - | - | - | - | - | - | 31 | 0.1722 | 0.67 |
| 12 | 0 | - | - | <18 | <558 | - | - | - | - | - | - | - | 41 | 0.2277 | 0.66 |
| 13 | 0 | - | 2 | - | - | ≤84.5 | - | - | - | - | - | - | 30 | 0.1666 | 0.66 |
| 14 | 0 | - | - | <18 | - | - | ≤90.9 | - | - | - | - | - | 37 | 0.2055 | 0.66 |
| 15 | - | - | - | <18 | - | - | ≤67.3 | - | - | - | - | - | 35 | 0.1944 | 0.66 |
| 16 | - | 1 | - | - | - | - | - | - | - | - | - | - | 42 | 0.2333 | 0.65 |
| 17 | 0 | - | 2 | - | - | - | ≤158.3 | - | - | - | - | - | 36 | 0.2000 | 0.65 |
| 18 | 0 | - | 2 | - | - | - | ≤138 | - | - | - | - | - | 33 | 0.1833 | 0.65 |
| 19 | - | - | - | <18 | - | - | ≤68.1 | - | - | - | - | - | 36 | 0.2000 | 0.65 |
| 20 | 0 | - | 2 | - | - | - | ≤148.5 | - | - | - | - | - | 34 | 0.1888 | 0.65 |
| VC-Bagging | Random Forest | Logistic Regression | ||||
|---|---|---|---|---|---|---|
| (Avg. No.) | (Avg. %) | (Avg. No.) | (Avg. %) | (Avg. No.) | (Avg. %) | |
| Correctly Classified Instances | 148.91 | 82.72 | 149.11 | 80.24 | 141.02 | 78.34 |
| Incorrectly Classified Instances | 31.09 | 17.27 | 30.89 | 19.76 | 38.98 | 21.65 |
| Average Classification Accuracy [%] | 82.72 | 82.74 | 78.32 | |||
| Average Precision [%] | 82.75 | 83.12 | 78.40 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karolak, M.; Pałkowski, Ł.; Kubiak, B.; Błaszczyński, J.; Łunio, R.; Sawicki, W.; Słowiński, R.; Krysiński, J. Application of Dominance-Based Rough Set Approach for Optimization of Pellets Tableting Process. Pharmaceutics 2020, 12, 1024. https://doi.org/10.3390/pharmaceutics12111024
Karolak M, Pałkowski Ł, Kubiak B, Błaszczyński J, Łunio R, Sawicki W, Słowiński R, Krysiński J. Application of Dominance-Based Rough Set Approach for Optimization of Pellets Tableting Process. Pharmaceutics. 2020; 12(11):1024. https://doi.org/10.3390/pharmaceutics12111024
Chicago/Turabian StyleKarolak, Maciej, Łukasz Pałkowski, Bartłomiej Kubiak, Jerzy Błaszczyński, Rafał Łunio, Wiesław Sawicki, Roman Słowiński, and Jerzy Krysiński. 2020. "Application of Dominance-Based Rough Set Approach for Optimization of Pellets Tableting Process" Pharmaceutics 12, no. 11: 1024. https://doi.org/10.3390/pharmaceutics12111024
APA StyleKarolak, M., Pałkowski, Ł., Kubiak, B., Błaszczyński, J., Łunio, R., Sawicki, W., Słowiński, R., & Krysiński, J. (2020). Application of Dominance-Based Rough Set Approach for Optimization of Pellets Tableting Process. Pharmaceutics, 12(11), 1024. https://doi.org/10.3390/pharmaceutics12111024