Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors
Abstract
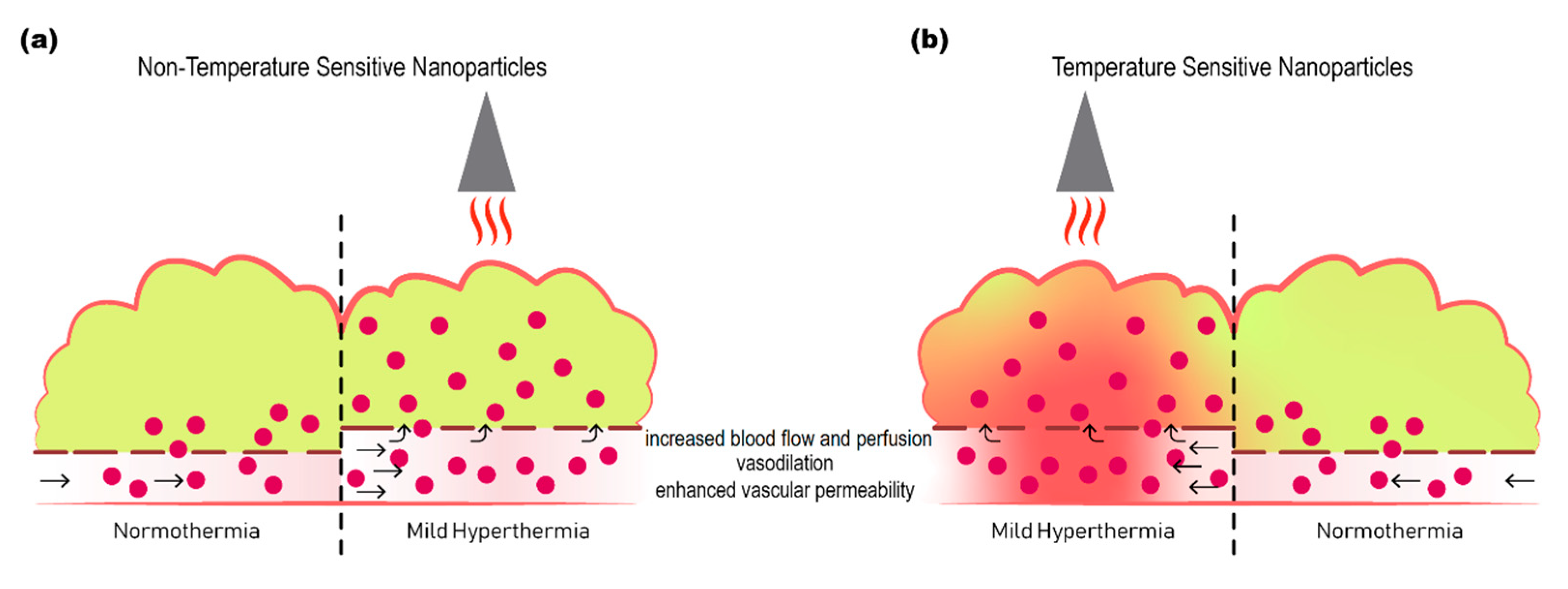
1. Introduction
2. Hyperthermia and Its Clinical Application
3. Thermosensitive Liposomes (TSLs)
4. Temperature Sensitive Polymeric Nanoparticles (TSPN)
5. Temperature Sensitive Liposomes Sensitized by Temperature Sensitive Polymers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kieler-Ferguson, H.M.; Frechet, J.M.; Szoka, F.C., Jr. Clinical developments of chemotherapeutic nanomedicines: Polymers and liposomes for delivery of camptothecins and platinum (ii) drugs. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 130–138. [Google Scholar] [CrossRef]
- Powis, G. Dose-dependent metabolism, therapeutic effect, and toxicity of anticancer drugs in man. Drug Metab. Rev. 1983, 14, 1145–1163. [Google Scholar] [CrossRef]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: Formulation(-)processing aspects and challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Picard, M.; Castells, M.C. Re-visiting hypersensitivity reactions to taxanes: A comprehensive review. Clin. Rev. Allergy Immunol. 2015, 49, 177–191. [Google Scholar] [CrossRef]
- Passero, F.C., Jr.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The safety and efficacy of onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev. Anticancer Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef]
- Lamb, Y.N.; Scott, L.J. Liposomal irinotecan: A review in metastatic pancreatic adenocarcinoma. Drugs 2017, 77, 785–792. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C.; et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via epr: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin: Review of animal and human studies. Clin. Pharm. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil(r)—The first fda-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Northfelt, D.W.; Martin, F.J.; Working, P.; Volberding, P.A.; Russell, J.; Newman, M.; Amantea, M.A.; Kaplan, L.D. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: Pharmacokinetics, tumor localization, and safety in patients with aids-related kaposi’s sarcoma. J. Clin. Pharmacol. 1996, 36, 55–63. [Google Scholar] [CrossRef]
- Udhrain, A.; Skubitz, K.M.; Northfelt, D.W. Pegylated liposomal doxorubicin in the treatment of aids-related kaposi’s sarcoma. Int. J. Nanomed. 2007, 2, 345–352. [Google Scholar]
- Symon, Z.; Peyser, A.; Tzemach, D.; Lyass, O.; Sucher, E.; Shezen, E.; Gabizon, A. Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes. Cancer 1999, 86, 72–78. [Google Scholar] [CrossRef]
- Harrington, K.J.; Mohammadtaghi, S.; Uster, P.S.; Glass, D.; Peters, A.M.; Vile, R.G.; Stewart, J.S. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001, 7, 243–254. [Google Scholar]
- Primeau, A.J.; Rendon, A.; Hedley, D.; Lilge, L.; Tannock, I.F. The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 2005, 11, 8782–8788. [Google Scholar] [CrossRef]
- Teicher, B.A.; Ara, G.; Keyes, S.R.; Herbst, R.S.; Frei, E., 3rd. Acute in vivo resistance in high-dose therapy. Clin. Cancer Res. 1998, 4, 483–491. [Google Scholar]
- Larsen, A.K.; Escargueil, A.E.; Skladanowski, A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol. Ther. 2000, 85, 217–229. [Google Scholar] [CrossRef]
- Gartung, A.; Yang, J.; Sukhatme, V.P.; Bielenberg, D.R.; Fernandes, D.; Chang, J.; Schmidt, B.A.; Hwang, S.H.; Zurakowski, D.; Huang, S.; et al. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual cox-2/seh inhibitor. Proc. Natl. Acad. Sci. USA 2019, 116, 1698–1703. [Google Scholar] [CrossRef]
- Chang, J.; Bhasin, S.S.; Bielenberg, D.R.; Sukhatme, V.P.; Bhasin, M.; Huang, S.; Kieran, M.W.; Panigrahy, D. Chemotherapy-generated cell debris stimulates colon carcinoma tumor growth via osteopontin. FASEB J. 2019, 33, 114–125. [Google Scholar] [CrossRef]
- Seelig, K.J.; Revesz, L. Effect of lethally damaged tumour cells upon the growth of admixed viable cells in diffusion chambers. Br. J. Cancer 1960, 14, 126–138. [Google Scholar] [CrossRef][Green Version]
- Tan, Q.; Saggar, J.K.; Yu, M.; Wang, M.; Tannock, I.F. Mechanisms of drug resistance related to the microenvironment of solid tumors and possible strategies to inhibit them. Cancer J. 2015, 21, 254–262. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Stylianopoulos, T.; Martin, J.D.; Popovic, Z.; Chen, O.; Kamoun, W.S.; Bawendi, M.G.; Fukumura, D.; Jain, R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012, 7, 383–388. [Google Scholar] [CrossRef]
- Tong, R.T.; Boucher, Y.; Kozin, S.V.; Winkler, F.; Hicklin, D.J.; Jain, R.K. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004, 64, 3731–3736. [Google Scholar] [CrossRef]
- Winkler, F.; Kozin, S.V.; Tong, R.T.; Chae, S.S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by vegfr2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004, 6, 553–563. [Google Scholar]
- Zhang, B.; Shi, W.; Jiang, T.; Wang, L.; Mei, H.; Lu, H.; Hu, Y.; Pang, Z. Optimization of the tumor microenvironment and nanomedicine properties simultaneously to improve tumor therapy. Oncotarget 2016, 7, 62607–62618. [Google Scholar] [CrossRef]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen i synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.; Dicheva, B.M.; Hoving, S.; Koning, G.A.; Ten Hagen, T.L. Intact doxil is taken up intracellularly and released doxorubicin sequesters in the lysosome: Evaluated by in vitro/in vivo live cell imaging. J. Control. Release 2013, 172, 330–340. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.; Hoving, S.; Schipper, D.; Vermeulen, C.E.; de Wiel-Ambagtsheer, G.; van Tiel, S.T.; Eggermont, A.M.; Ten Hagen, T.L. Tumor necrosis factor alpha mediates homogeneous distribution of liposomes in murine melanoma that contributes to a better tumor response. Cancer Res. 2007, 67, 9455–9462. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; Ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- El Emir, E.; Qureshi, U.; Dearling, J.L.; Boxer, G.M.; Clatworthy, I.; Folarin, A.A.; Robson, M.P.; Nagl, S.; Konerding, M.A.; Pedley, R.B. Predicting response to radioimmunotherapy from the tumor microenvironment of colorectal carcinomas. Cancer Res. 2007, 67, 11896–11905. [Google Scholar] [CrossRef][Green Version]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. (64)cu-mm-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef]
- Eberhard, A.; Kahlert, S.; Goede, V.; Hemmerlein, B.; Plate, K.H.; Augustin, H.G. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: Implications for antiangiogenic tumor therapies. Cancer Res. 2000, 60, 1388–1393. [Google Scholar]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Gaber, M.H.; Wu, N.Z.; Hong, K.; Huang, S.K.; Dewhirst, M.W.; Papahadjopoulos, D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 1177–1187. [Google Scholar] [CrossRef]
- Amin, M.; Bagheri, M.; Mansourian, M.; Jaafari, M.R.; Ten Hagen, T.L. Regulation of in vivo behavior of tat-modified liposome by associated protein corona and avidity to tumor cells. Int. J. Nanomed. 2018, 13, 7441–7455. [Google Scholar] [CrossRef]
- Seetharamu, N.; Kim, E.; Hochster, H.; Martin, F.; Muggia, F. Phase ii study of liposomal cisplatin (spi-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Res. 2010, 30, 541–545. [Google Scholar]
- Amin, M.; Badiee, A.; Jaafari, M.R. Improvement of pharmacokinetic and antitumor activity of pegylated liposomal doxorubicin by targeting with n-methylated cyclic rgd peptide in mice bearing c-26 colon carcinomas. Int. J. Pharm. 2013, 458, 324–333. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- van der Meel, R.; Vehmeijer, L.J.; Kok, R.J.; Storm, G.; van Gaal, E.V. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: Current status. Adv. Drug Deliv. Rev. 2013, 65, 1284–1298. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor ph targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Kreutz, W.; Horwitz, B.A.; Shinitzky, M. Ph-sensitive liposomes: Possible clinical implications. Science 1980, 210, 1253–1255. [Google Scholar] [CrossRef]
- Andresen, T.L.; Thompson, D.H.; Kaasgaard, T. Enzyme-triggered nanomedicine: Drug release strategies in cancer therapy. Mol. Membr. Biol. 2010, 27, 353–363. [Google Scholar] [CrossRef]
- Ong, W.; Yang, Y.; Cruciano, A.C.; McCarley, R.L. Redox-triggered contents release from liposomes. J. Am. Chem. Soc. 2008, 130, 14739–14744. [Google Scholar] [CrossRef]
- Wang, C.; Qi, P.; Lu, Y.; Liu, L.; Zhang, Y.; Sheng, Q.; Wang, T.; Zhang, M.; Wang, R.; Song, S. Bicomponent polymeric micelles for ph-controlled delivery of doxorubicin. Drug Deliv. 2020, 27, 344–357. [Google Scholar] [CrossRef]
- Jin, Z.H.; Jin, M.J.; Jiang, C.G.; Yin, X.Z.; Jin, S.X.; Quan, X.Q.; Gao, Z.G. Evaluation of doxorubicin-loaded ph-sensitive polymeric micelle release from tumor blood vessels and anticancer efficacy using a dorsal skin-fold window chamber model. Acta Pharmacol. Sin. 2014, 35, 839–845. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Liu, X.; Huo, P.; Zhang, Y.; Chen, H.; Tian, Q.; Zhang, N. Mmp-2-responsive gelatin nanoparticles for synergistic tumor therapy. Pharm. Dev. Technol. 2019, 24, 1002–1013. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, N.; Ci, T.; Tang, Z.; Gu, Z.; Li, G.; Chen, X. Combretastatin a4 nanodrug-induced mmp9 amplification boosts tumor-selective release of doxorubicin prodrug. Adv. Mater. 2019, 31, e1904278. [Google Scholar] [CrossRef]
- Nagel, G.; Sousa-Herves, A.; Wedepohl, S.; Calderon, M. Matrix metalloproteinase-sensitive multistage nanogels promote drug transport in 3d tumor model. Theranostics 2020, 10, 91–108. [Google Scholar] [CrossRef]
- Mo, S.; Coussios, C.C.; Seymour, L.; Carlisle, R. Ultrasound-enhanced drug delivery for cancer. Expert Opin. Drug Deliv. 2012, 9, 1525–1538. [Google Scholar] [CrossRef]
- Eggen, S.; Fagerland, S.M.; Morch, Y.; Hansen, R.; Sovik, K.; Berg, S.; Furu, H.; Bohn, A.D.; Lilledahl, M.B.; Angelsen, A.; et al. Ultrasound-enhanced drug delivery in prostate cancer xenografts by nanoparticles stabilizing microbubbles. J. Control. Release 2014, 187, 39–49. [Google Scholar] [CrossRef]
- Huang, S.L. Liposomes in ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1167–1176. [Google Scholar] [CrossRef]
- Leung, S.J.; Romanowski, M. Light-activated content release from liposomes. Theranostics 2012, 2, 1020–1036. [Google Scholar] [CrossRef]
- Bansal, A.; Zhang, Y. Photocontrolled nanoparticle delivery systems for biomedical applications. Acc. Chem. Res. 2014, 47, 3052–3060. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Nittayacharn, P.; Yuan, H.X.; Hernandez, C.; Bielecki, P.; Zhou, H.; Exner, A.A. Enhancing tumor drug distribution with ultrasound-triggered nanobubbles. J. Pharm. Sci. 2019, 108, 3091–3098. [Google Scholar] [CrossRef]
- Rizzitelli, S.; Giustetto, P.; Cutrin, J.C.; Delli Castelli, D.; Boffa, C.; Ruzza, M.; Menchise, V.; Molinari, F.; Aime, S.; Terreno, E. Sonosensitive theranostic liposomes for preclinical in vivo mri-guided visualization of doxorubicin release stimulated by pulsed low intensity non-focused ultrasound. J. Control. Release 2015, 202, 21–30. [Google Scholar] [CrossRef]
- Li, L.; Ten Hagen, T.L.; Bolkestein, M.; Gasselhuber, A.; Yatvin, J.; van Rhoon, G.C.; Eggermont, A.M.; Haemmerich, D.; Koning, G.A. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Control. Release 2013, 167, 130–137. [Google Scholar] [CrossRef]
- Lu, T.; Lokerse, W.J.M.; Seynhaeve, A.L.B.; Koning, G.A.; Ten Hagen, T.L.M. Formulation and optimization of idarubicin thermosensitive liposomes provides ultrafast triggered release at mild hyperthermia and improves tumor response. J. Control. Release 2015, 220, 425–437. [Google Scholar] [CrossRef]
- Gas, P. Essential facts on the history of hyperthermia and their connections with electromedicine. Prz Elektrotechniczn 2011, 87, 37–40. [Google Scholar]
- Lepock, J.R. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperther. 2003, 19, 252–266. [Google Scholar] [CrossRef]
- Roti, J.L.R. Cellular responses to hyperthermia (40–46 °C): Cell killing and molecular events. Int. J. Hyperther. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Overgaard, J.; Grau, C.; Lindegaard, J.C.; Horsman, M.R. The potential of using hyperthermia to eliminate radioresistant hypoxic cells. Radiother. Oncol. 1991, 20, 113–116. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Griffin, R.J.; Ogawa, A.; Williams, B.W.; Song, C.W. Hyperthermic enhancement of tumor radiosensitization strategies. Immunol. Investig. 2005, 34, 343–359. [Google Scholar] [CrossRef]
- Griffin, R.J.; Dings, R.P.; Jamshidi-Parsian, A.; Song, C.W. Mild temperature hyperthermia and radiation therapy: Role of tumour vascular thermotolerance and relevant physiological factors. Int. J. Hyperth. 2010, 26, 256–263. [Google Scholar] [CrossRef]
- Skitzki, J.J.; Repasky, E.A.; Evans, S.S. Hyperthermia as an immunotherapy strategy for cancer. Curr. Opin. Investig. Drugs 2009, 10, 550–558. [Google Scholar]
- Lee, S.; Son, B.; Park, G.; Kim, H.; Kang, H.; Jeon, J.; Youn, H.; Youn, B. Immunogenic effect of hyperthermia on enhancing radiotherapeutic efficacy. Int. J. Mol. Sci. 2018, 19, 2795. [Google Scholar] [CrossRef]
- Toraya-Brown, S.; Sheen, M.R.; Zhang, P.; Chen, L.; Baird, J.R.; Demidenko, E.; Turk, M.J.; Hoopes, P.J.; Conejo-Garcia, J.R.; Fiering, S. Local hyperthermia treatment of tumors induces cd8(+) t cell-mediated resistance against distal and secondary tumors. Nanomedicine 2014, 10, 1273–1285. [Google Scholar] [CrossRef]
- Tsang, Y.W.; Huang, C.C.; Yang, K.L.; Chi, M.S.; Chiang, H.C.; Wang, Y.S.; Andocs, G.; Szasz, A.; Li, W.T.; Chi, K.H. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer 2015, 15, 1–11. [Google Scholar] [CrossRef][Green Version]
- Binder, R.J.; Han, D.K.; Srivastava, P.K. Cd91: A receptor for heat shock protein gp96. Nat. Immunol. 2000, 1, 151–155. [Google Scholar] [CrossRef]
- Bethke, K.; Staib, F.; Distler, M.; Schmitt, U.; Jonuleit, H.; Enk, A.H.; Galle, P.R.; Heike, M. Different efficiency of heat shock proteins (hsp) to activate human monocytes and dendritic cells: Superiority of hsp60. J. Immunol. 2002, 169, 6141–6148. [Google Scholar] [CrossRef]
- Todryk, S.; Melcher, A.A.; Hardwick, N.; Linardakis, E.; Bateman, A.; Colombo, M.P.; Stoppacciaro, A.; Vile, R.G. Heat shock protein 70 induced during tumor cell killing induces th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J. Immunol. 1999, 163, 1398–1408. [Google Scholar]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: The eortc 62961-esho 95 randomized clinical trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Killock, D. Sarcoma: Local hyperthermia improves survival. Nat. Rev. Clin. Oncol. 2018, 15, 266. [Google Scholar] [CrossRef]
- Huang, S.K.; Stauffer, P.R.; Hong, K.; Guo, J.W.; Phillips, T.L.; Huang, A.; Papahadjopoulos, D. Liposomes and hyperthermia in mice: Increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res. 1994, 54, 2186–2191. [Google Scholar]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001, 61, 3027–3032. [Google Scholar]
- Ponce, A.M.; Viglianti, B.L.; Yu, D.; Yarmolenko, P.S.; Michelich, C.R.; Woo, J.; Bally, M.B.; Dewhirst, M.W. Magnetic resonance imaging of temperature-sensitive liposome release: Drug dose painting and antitumor effects. J. Natl. Cancer Inst. 2007, 99, 53–63. [Google Scholar] [CrossRef]
- Stapleton, S.; Dunne, M.; Milosevic, M.; Tran, C.W.; Gold, M.J.; Vedadi, A.; McKee, T.D.; Ohashi, P.S.; Allen, C.; Jaffray, D.A. Radiation and heat improve the delivery and efficacy of nanotherapeutics by modulating intratumoral fluid dynamics. ACS Nano 2018, 12, 7583–7600. [Google Scholar] [CrossRef]
- Eze, M.O. Phase-transitions in phospholipid-bilayers—Lateral phase separations play vital roles in biomembranes. Biochem. Educ. 1991, 19, 204–208. [Google Scholar] [CrossRef]
- Marsh, D. General features of phospholipid phase-transitions. Chem. Phys. Lipids 1991, 57, 109–120. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Jacobson, K.; Nir, S.; Isac, T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta 1973, 311, 330–348. [Google Scholar] [CrossRef]
- Lu, T.; Ten Hagen, T.L.M. Inhomogeneous crystal grain formation in dppc-dspc based thermosensitive liposomes determines content release kinetics. J. Control. Release 2017, 247, 64–72. [Google Scholar] [CrossRef]
- Phillips, M.C.; Hauser, H.; Paltauf, F. The inter- and intra-molecular mixing of hydrocarbon chains in lecithin-water systems. Chem. Phys. Lipids 1972, 8, 127–133. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Magin, R.L.; Yatvin, M.B.; Zaharko, D.S. Liposomes and local hyperthermia: Selective delivery of methotrexate to heated tumors. Science 1979, 204, 188–191. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Muhlensiepen, H.; Porschen, W.; Weinstein, J.N.; Feinendegen, L.E. Selective delivery of liposome-associated cis-dichlorodiammineplatinum(ii) by heat and its influence on tumor drug uptake and growth. Cancer Res. 1981, 41, 1602–1607. [Google Scholar]
- Iga, K.; Hamaguchi, N.; Igari, Y.; Ogawa, Y.; Gotoh, K.; Ootsu, K.; Toguchi, H.; Shimamoto, T. Enhanced antitumor activity in mice after administration of thermosensitive liposome encapsulating cisplatin with hyperthermia. J. Pharmacol. Exp. Ther. 1991, 257, 1203–1207. [Google Scholar]
- Maruyama, K.; Unezaki, S.; Takahashi, N.; Iwatsuru, M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochim. Biophys. Acta 1993, 1149, 209–216. [Google Scholar] [CrossRef]
- Gaber, M.H.; Hong, K.; Huang, S.K.; Papahadjopoulos, D. Thermosensitive sterically stabilized liposomes: Formulation and in vitro studies on mechanism of doxorubicin release by bovine serum and human plasma. Pharm. Res. 1995, 12, 1407–1416. [Google Scholar] [CrossRef]
- Lokerse, W.J.; Kneepkens, E.C.; ten Hagen, T.L.; Eggermont, A.M.; Grull, H.; Koning, G.A. In depth study on thermosensitive liposomes: Optimizing formulations for tumor specific therapy and in vitro to in vivo relations. Biomaterials 2016, 82, 138–150. [Google Scholar] [CrossRef]
- Li, L.; ten Hagen, T.L.; Hossann, M.; Suss, R.; van Rhoon, G.C.; Eggermont, A.M.; Haemmerich, D.; Koning, G.A. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J. Control. Release 2013, 168, 142–150. [Google Scholar] [CrossRef]
- Li, L.; ten Hagen, T.L.; Schipper, D.; Wijnberg, T.M.; van Rhoon, G.C.; Eggermont, A.M.; Lindner, L.H.; Koning, G.A. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J. Control. Release 2010, 143, 274–279. [Google Scholar] [CrossRef]
- Landon, C.D.; Park, J.Y.; Needham, D.; Dewhirst, M.W. Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed. J. 2011, 3, 38–64. [Google Scholar] [CrossRef]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef]
- Kong, G.; Anyarambhatla, G.; Petros, W.P.; Braun, R.D.; Colvin, O.M.; Needham, D.; Dewhirst, M.W. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res. 2000, 60, 6950–6957. [Google Scholar]
- Chen, Q.; Krol, A.; Wright, A.; Needham, D.; Dewhirst, M.W.; Yuan, F. Tumor microvascular permeability is a key determinant for antivascular effects of doxorubicin encapsulated in a temperature sensitive liposome. Int. J. Hyperth. 2008, 24, 475–482. [Google Scholar] [CrossRef]
- Chen, Q.; Tong, S.; Dewhirst, M.W.; Yuan, F. Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Mol. Cancer Ther. 2004, 3, 1311–1317. [Google Scholar]
- Anyarambhatla, G.R.; Needham, D. Enhancement of the phase transition permeability of dppc liposomes by incorporation of mppc: A new temperature-sensitive liposome for use with mild hyperthermia. J. Liposome Res. 1999, 9, 491–506. [Google Scholar] [CrossRef]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar]
- Mills, J.K.; Needham, D. Lysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transition. Biochim. Biophys. Acta 2005, 1716, 77–96. [Google Scholar] [CrossRef]
- Zagar, T.M.; Vujaskovic, Z.; Formenti, S.; Rugo, H.; Muggia, F.; O’Connor, B.; Myerson, R.; Stauffer, P.; Hsu, I.C.; Diederich, C.; et al. Two phase i dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (ltld) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int. J. Hyperth. 2014, 30, 285–294. [Google Scholar] [CrossRef]
- Tagami, T.; Ernsting, M.J.; Li, S.D. Optimization of a novel and improved thermosensitive liposome formulated with dppc and a brij surfactant using a robust in vitro system. J. Control. Release 2011, 154, 290–297. [Google Scholar] [CrossRef]
- Tagami, T.; Ernsting, M.J.; Li, S.D. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J. Control. Release 2011, 152, 303–309. [Google Scholar] [CrossRef]
- Tagami, T.; May, J.P.; Ernsting, M.J.; Li, S.D. A thermosensitive liposome prepared with a cu(2)(+) gradient demonstrates improved pharmacokinetics, drug delivery and antitumor efficacy. J. Control. Release 2012, 161, 142–149. [Google Scholar] [CrossRef]
- Lindner, L.H.; Eichhorn, M.E.; Eibl, H.; Teichert, N.; Schmitt-Sody, M.; Issels, R.D.; Dellian, M. Novel temperature-sensitive liposomes with prolonged circulation time. Clin. Cancer Res. 2004, 10, 2168–2178. [Google Scholar] [CrossRef]
- Hossann, M.; Wiggenhorn, M.; Schwerdt, A.; Wachholz, K.; Teichert, N.; Eibl, H.; Issels, R.D.; Lindner, L.H. In vitro stability and content release properties of phosphatidylglyceroglycerol containing thermosensitive liposomes. Biochim. Biophys. Acta 2007, 1768, 2491–2499. [Google Scholar] [CrossRef]
- Zimmermann, K.; Hossann, M.; Hirschberger, J.; Troedson, K.; Peller, M.; Schneider, M.; Bruhschwein, A.; Meyer-Lindenberg, A.; Wess, G.; Wergin, M.; et al. A pilot trial of doxorubicin containing phosphatidyldiglycerol based thermosensitive liposomes in spontaneous feline soft tissue sarcoma. Int. J. Hyperth. 2017, 33, 178–190. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and ph-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(n-isopropylacrylamide). J. Macromol. Sci. Part A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Tu, X.Y.; Meng, C.; Wang, Y.F.; Ma, L.W.; Wang, B.Y.; He, J.L.; Ni, P.H.; Ji, X.L.; Liu, M.Z.; Wei, H. Fabrication of thermosensitive cyclic brush copolymer with enhanced therapeutic efficacy for anticancer drug delivery. Macromol. Rapid Commun. 2018, 39, 1700744. [Google Scholar] [CrossRef]
- Cheng, C.C.; Liang, M.C.; Liao, Z.S.; Huang, J.J.; Lee, D.J. Self-assembled supramolecular nanogels as a safe and effective drug delivery vector for cancer therapy. Macromol. Biosci. 2017, 17, 1600370. [Google Scholar] [CrossRef]
- Mackay, J.A.; Chilkoti, A. Temperature sensitive peptides: Engineering hyperthermia-directed therapeutics. Int. J. Hyperther. 2008, 24, 483–495. [Google Scholar] [CrossRef]
- McDaniel, J.R.; Radford, D.C.; Chilkoti, A. A unified model for de novo design of elastin-like polypeptides with tunable inverse transition temperatures. Biomacromolecules 2013, 14, 2866–2872. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.H. Globule-to-coil transition of a single homopolymer chain in solution. Phys. Rev. Lett. 1998, 80, 4092–4094. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-ionic thermoresponsive polymers in water. Adv. Polym. Sci. 2011, 242, 29–89. [Google Scholar]
- Zhang, J.; Qian, Z.; Gu, Y. In vivo anti-tumor efficacy of docetaxel-loaded thermally responsive nanohydrogel. Nanotechnology 2009, 20, 325102. [Google Scholar] [CrossRef]
- Ku, B.; Seo, H.I.; Chung, B.G. Synthesis and characterization of thermoresponsive polymeric nanoparticles. BioChip J. 2014, 8, 8–14. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Wan, Z.; Quan, Z.; Tan, Q. Investigation of thermo-sensitive amphiphilic micelles as drug carriers for chemotherapy in cholangiocarcinoma in vitro and in vivo. Int. J. Pharm. 2014, 463, 81–88. [Google Scholar] [CrossRef]
- Cammas, S.; Suzuki, K.; Sone, C.; Sakurai, Y.; Kataoka, K.; Okano, T. Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J. Control. Release 1997, 48, 157–164. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Yamato, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(n-isopropylacrylamide) and poly(butylmethacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, X.Z.; Zhou, Y.; Cheng, S.X.; Zhuo, R.X. Self-assembled thermoresponsive micelles of poly(n-isopropylacrylamide-b-methyl methacrylate). Biomaterials 2006, 27, 2028–2034. [Google Scholar] [CrossRef]
- Sun, X.L.; Tsai, P.C.; Bhat, R.; Bonder, E.M.; Michniak-Kohn, B.; Pietrangelo, A. Thermoresponsive block copolymer micelles with tunable pyrrolidone-based polymer cores: Structure/property correlations and application as drug carriers. J. Mater. Chem. B 2015, 3, 814–823. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chang, F.C.; Kao, W.Y.; Hwang, S.M.; Liao, L.C.; Chang, Y.J.; Liang, M.C.; Chen, J.K.; Lee, D.J. Highly efficient drug delivery systems based on functional supramolecular polymers: In vitro evaluation. Acta Biomater. 2016, 33, 194–202. [Google Scholar] [CrossRef]
- van Elk, M.; Murphy, B.P.; Eufrasio-da-Silva, T.; O’Reilly, D.P.; Vermonden, T.; Hennink, W.E.; Duffy, G.P.; Ruiz-Hernandez, E. Nanomedicines for advanced cancer treatments: Transitioning towards responsive systems. Int. J. Pharm. 2016, 515, 132–164. [Google Scholar] [CrossRef]
- Akimoto, J.; Nakayama, M.; Sakai, K.; Okano, T. Thermally controlled intracellular uptake system of polymeric micelles possessing poly(n-isopropylacrylamide)-based outer coronas. Mol. Pharm. 2010, 7, 926–935. [Google Scholar] [CrossRef]
- Raucher, D.; Chilkoti, A. Enhanced uptake of a thermally responsive polypeptide by tumor cells in response to its hyperthermia-mediated phase transition. Cancer Res. 2001, 61, 7163–7170. [Google Scholar]
- Akimoto, J.; Nakayama, M.; Sakai, K.; Okano, T. Temperature-induced intracellular uptake of thermoresponsive polymeric micelles. Biomacromolecules 2009, 10, 1331–1336. [Google Scholar] [CrossRef]
- McDaniel, J.R.; MacEwan, S.R.; Dewhirst, M.; Chilkoti, A. Doxorubicin-conjugated chimeric polypeptide nanoparticles that respond to mild hyperthermia. J. Control. Release 2012, 159, 362–367. [Google Scholar] [CrossRef]
- Kohori, F.; Sakai, K.; Aoyagi, T.; Yokoyama, M.; Sakurai, Y.; Okano, T. Preparation and characterization of thermally responsive block copolymer micelles comprising poly(n-isopropylacrylamide-b-dl-lactide). J. Control. Release 1998, 55, 87–98. [Google Scholar] [CrossRef]
- Yang, M.; Ding, Y.; Zhang, L.; Qian, X.; Jiang, X.; Liu, B. Novel thermosensitive polymeric micelles for docetaxel delivery. J. Biomed. Mater. Res. A 2007, 81, 847–857. [Google Scholar] [CrossRef]
- Sun, F.L.; Wang, Y.X.; Wei, Y.; Cheng, G.; Ma, G.H. Thermo-triggered drug delivery from polymeric micelles of poly(n-isopropylacrylamide-co-acrylamide)-b-poly(n-butyl methacrylate) for tumor targeting. J. Bioact. Compat. Polym. 2014, 29, 301–317. [Google Scholar] [CrossRef]
- Panja, S.; Dey, G.; Bharti, R.; Kumari, K.; Maiti, T.K.; Mandal, M.; Chattopadhyay, S. Tailor-made temperature-sensitive micelle for targeted and on-demand release of anticancer drugs. ACS Appl. Mater. Interfaces 2016, 8, 12063–12074. [Google Scholar] [CrossRef]
- Ringsdorf, H.; Venzmer, J.; Winnik, F.M. Interaction of hydrophobically-modified poly-n-isopropylacrylamides with model membranes—Or playing a molecular accordion. Angew. Chem. Int. Ed. 1991, 30, 315–318. [Google Scholar] [CrossRef]
- Wu, X.S.; Hoffman, A.S.; Yager, P. Conjugation of phosphatidylethanolamine to poly(n-isopropylacrylamide) for potential use in liposomal drug delivery systems. Polymer 1992, 33, 4659–4662. [Google Scholar] [CrossRef]
- Kono, K.; Hayashi, H.; Takagishi, T. Temperature-sensitive liposomes—Liposomes bearing poly(n-isopropylacrylamide). J. Control. Release 1994, 30, 69–75. [Google Scholar] [CrossRef]
- van Elk, M.; Deckers, R.; Oerlemans, C.; Shi, Y.; Storm, G.; Vermonden, T.; Hennink, W.E. Triggered release of doxorubicin from temperature-sensitive poly(n-(2-hydroxypropyl)-methacrylamide mono/dilactate) grafted liposomes. Biomacromolecules 2014, 15, 1002–1009. [Google Scholar] [CrossRef]
- Paasonen, L.; Romberg, B.; Storm, G.; Yliperttula, M.; Urtti, A.; Hennink, W.E. Temperature-sensitive poly(n-(2-hydroxypropyl)methacrylamide mono/dilactate)-coated liposomes for triggered contents release. Bioconjug. Chem. 2007, 18, 2131–2136. [Google Scholar] [CrossRef]
- Mo, Y.L.; Du, H.L.; Chen, B.L.; Liu, D.C.; Yin, Q.Q.; Yan, Y.; Wang, Z.H.; Wan, F.J.; Qi, T.; Wang, Y.Q.; et al. Quick-responsive polymer-based thermosensitive liposomes for controlled doxorubicin release and chemotherapy. ACS Biomater. Sci. Eng. 2019, 5, 2316–2329. [Google Scholar] [CrossRef]
- Kono, K.; Henmi, A.; Takagishi, T. Temperature-controlled interaction of thermosensitive polymer-modified cationic liposomes with negatively charged phospholipid membranes. Bba-Biomembranes 1999, 1421, 183–197. [Google Scholar] [CrossRef][Green Version]
- Kono, K.; Yoshino, K.; Takagishi, T. Effect of poly(ethylene glycol) grafts on temperature-sensitivity of thermosensitive polymer-modified liposomes. J. Control. Release 2002, 80, 321–332. [Google Scholar] [CrossRef]
- Kono, K.; Murakami, T.; Yoshida, T.; Haba, Y.; Kanaoka, S.; Takagishi, T.; Aoshima, S. Temperature sensitization of liposomes by use of thermosensitive block copolymers synthesized by living cationic polymerization: Effect of copolymer chain length. Bioconjug. Chem. 2005, 16, 1367–1374. [Google Scholar] [CrossRef]
- Kono, K.; Ozawa, T.; Yoshida, T.; Ozaki, F.; Ishizaka, Y.; Maruyama, K.; Kojima, C.; Harada, A.; Aoshima, S. Highly temperature-sensitive liposomes based on a thermosensitive block copolymer for tumor-specific chemotherapy. Biomaterials 2010, 31, 7096–7105. [Google Scholar] [CrossRef]
- Kono, K.; Nakashima, S.; Kokuryo, D.; Aoki, I.; Shimomoto, H.; Aoshima, S.; Maruyama, K.; Yuba, E.; Kojima, C.; Harada, A.; et al. Multi-functional liposomes having temperature-triggered release and magnetic resonance imaging for tumor-specific chemotherapy. Biomaterials 2011, 32, 1387–1395. [Google Scholar] [CrossRef]
- Kokuryo, D.; Nakashima, S.; Ozaki, F.; Yuba, E.; Chuang, K.H.; Aoshima, S.; Ishizaka, Y.; Saga, T.; Kono, K.; Aoki, I. Evaluation of thermo-triggered drug release in intramuscular-transplanted tumors using thermosensitive polymer-modified liposomes and mri. Nanomedicine 2015, 11, 229–238. [Google Scholar] [CrossRef]
- Na, K.; Lee, S.A.; Jung, S.H.; Hyun, J.; Shin, B.C. Elastin-like polypeptide modified liposomes for enhancing cellular uptake into tumor cells. Colloid Surf. B 2012, 91, 130–136. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, M.S.; Park, S.J.; Park, E.S.; Choi, K.S.; Kim, Y.S.; Kim, H.R. Novel temperature-triggered liposome with high stability: Formulation, in vitro evaluation, and in vivo study combined with high-intensity focused ultrasound (hifu). J. Control. Release 2013, 170, 373–379. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.S.; Al-Jamal, W.T.; Bossche, J.V.; Bui, T.T.; Drake, A.F.; Mason, A.J.; Kostarelos, K. Lipid-peptide vesicle nanoscale hybrids for triggered drug release by mild hyperthermia in vitro and in vivo. ACS Nano 2012, 6, 9335–9346. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Singh, S.K.; Reja, R.M.; Gopi, H.N. Gamma-amino acid mutated alpha-coiled coils as mild thermal triggers for liposome delivery. Chem. Commun. 2013, 49, 11065–11067. [Google Scholar] [CrossRef]
- Wang, J.; Ayano, E.; Maitani, Y.; Kanazawa, H. Tunable surface properties of temperature-responsive polymer-modified liposomes induce faster cellular uptake. ACS Omega 2017, 2, 316–325. [Google Scholar] [CrossRef]
- Hayashi, H.; Kono, K.; Takagishi, T. Temperature-controlled release property of phospholipid vesicles bearing a thermo-sensitive polymer. Bba-Biomembranes 1996, 1280, 127–134. [Google Scholar] [CrossRef]
- Kim, J.C.; Bae, S.K.; Kim, J.D. Temperature-sensitivity of liposomal lipid bilayers mixed with poly(n-isopropylacrylamide-co-acrylic acid). J. Biochem.-Tokyo 1997, 121, 15–19. [Google Scholar] [CrossRef]
- Kono, K.; Nakai, R.; Morimoto, K.; Takagishi, T. Thermosensitive polymer-modified liposomes that release contents around physiological temperature. Biochim. Biophys. Acta 1999, 1416, 239–250. [Google Scholar] [CrossRef]
- Kono, K.; Henmi, A.; Yamashita, H.; Hayashi, H.; Takagishi, T. Improvement of temperature-sensitivity of poly(n-isopropylacrylamide)-modified liposomes. J. Control. Release 1999, 59, 63–75. [Google Scholar] [CrossRef]
- Ta, T.; Convertine, A.J.; Reyes, C.R.; Stayton, P.S.; Porter, T.M. Thermosensitive liposomes modified with poly(n-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin. Biomacromolecules 2010, 11, 1915–1920. [Google Scholar] [CrossRef]
- Kono, K.; Nakai, R.; Morimoto, K.; Takagishi, T. Temperature-dependent interaction of thermo-sensitive polymer-modified liposomes with cv1 cells. FEBS Lett. 1999, 456, 306–310. [Google Scholar] [CrossRef]
- Yoshino, K.; Kadowaki, A.; Takagishi, T.; Kono, K. Temperature sensitization of liposomes by use of n-isopropylacrylamide copolymers with varying transition endotherms. Bioconjug. Chem. 2004, 15, 1102–1109. [Google Scholar] [CrossRef]
- Han, H.D.; Shin, B.C.; Choi, H.S. Doxorubicin-encapsulated thermosensitive liposomes modified with poly(n-isopropylacrylamide-co-acrylamide): Drug release behavior and stability in the presence of serum. Eur. J. Pharm. Biopharm. 2006, 62, 110–116. [Google Scholar] [CrossRef]
- Han, H.D.; Choi, M.S.; Hwang, T.; Song, C.K.; Seong, H.; Kim, T.W.; Choi, H.S.; Shin, B.C. Hyperthermia-induced antitumor activity of thermosensitive polymer modified temperature-sensitive liposomes. J. Pharm. Sci. 2006, 95, 1909–1917. [Google Scholar] [CrossRef]
- Klemetsrud, T.; Hiorth, M.; Smistad, G.; Kjoniksen, A.L. Characterization of temperature induced changes in liposomes coated with poly(n-isopropylacrylamide-co-methacrylic acid). J. Colloid Interface Sci. 2015, 450, 7–16. [Google Scholar] [CrossRef]
- Ta, T.; Bartolak-Suki, E.; Park, E.J.; Karrobi, K.; McDannold, N.J.; Porter, T.M. Localized delivery of doxorubicin in vivo from polymer-modified thermosensitive liposomes with mr-guided focused ultrasound-mediated heating. J. Control. Release 2014, 194, 71–81. [Google Scholar] [CrossRef]
- Soga, O.; van Nostrum, C.F.; Hennink, W.E. Poly(n-(2-hydroxypropyl) methacrylamide mono/di lactate): A new class of biodegradable polymers with tuneable thermosensitivity. Biomacromolecules 2004, 5, 818–821. [Google Scholar] [CrossRef]
- Chilkoti, A.; Dreher, M.R.; Meyer, D.E.; Raucher, D. Targeted drug delivery by thermally responsive polymers. Adv. Drug Deliv. Rev. 2002, 54, 613–630. [Google Scholar] [CrossRef]

| Copolymer | Drug | Size (nm) | LCST | Release Rate | Ref |
|---|---|---|---|---|---|
| PNIPAAm-PBMA poly(N-isopropylacrylamide-b-butylmethacrylate) | Doxorubicin | 338 ± 23 | 32.5 °C | 57% after 5 h at 37 °C 80% after 5 h at 40 °C | [130] |
| P-(NIPAAm-co-DMAAm)-b-P-(d,l-lactide) poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(d,l-lactide) | Doxorubicin | 69.2 | 40 °C | 2.5% after 4 days at 37 °C 11% after 4 days at 42.5 °C | [139] |
| PNIPAAm-b-PMMA poly(N-isopropylacrylamide-b-methyl methacrylate) | Prednisone acetate | 190 | 33 °C | 22.5% after 5 h at 27 °C 37 °C na 50% after 5 h at 40 °C | [131] |
| P-(NIPAAm-co-AAm)-b-PDLLA poly(N-isopropylacrylamide-co-acrylamide)-b-poly(d,l-lactide) | Docetaxel | 80 | 40 °C | 30% after 10 h at 37 °C 36% after 10 h at 43 °C | [140] |
| P-(NIPAm-co-AAm) poly(N-isopropylacrylamide-co-acrylamide) | Docetaxel | 50 | 40 °C | 80% after 50 h at 37 °C 40% after 50 h at 43 °C | [126] |
| P-(NIPAAm-co-AAm)-b-PBMA poly(N-isopropylacrylamide-co-acrylamide)-b-poly(n-butyl methacrylate) | Methotrexate | 175 ± 15 | 40 °C | 21% after 5 h at 37 °C 53% after 5 h at 42 °C | [141] |
| P-(NIPAAm-co-NHMAAm)-b-PCL P-(N,N-isopropylacrylamide-co-N-hydroxymethylacrylamide)-b-caprolactone | Doxorubicin | 97.2 ± 20.4 | 38 °C | 35% after 25 h at 38 °C Na at 37 57% after 25 h at 43 °C | [128] |
| PNI-U-DPy poly(N-isopropylacrylamide) containing pendant U-DPy | Doxorubicin | 164 | 34 °C | 63% 2.5 h 37 | [133] |
| Cy-functionalized supramolecular polymer, Cy-PPG | Doxorubicin | 76 | 45 °C | 5% after 2.5 h at 25 °C 37 °C na 85% after 2.5 h at 40 °C | [121] |
| NIPAM-co-AAc poly(NIPAM-co-acrylic acid) | Doxorubicin retinoic acid | 400 at 25 °C 100 at 37 °C | 37.2 °C | 40% after 4 h at 37 °C 80% after 48 h at 37 °C | [127] |
| Pentaerythritol polycaprolactone-b-poly(N-isopropylacrylamide)-folic acid (four star-arm PE-PCL-b-PNIPAM-FA) | Doxorubicin | 85 | 31 °C | 5% after 5 h at 37 °C 45% after 5 h at 40 °C | [142] |
| Pentaerythritol polycaprolactone-b-poly(N-vinylcaprolactam)-Folic acid (four star-arm PE-PCL-b-PNVCL-FA) | Doxorubicin | 185 | 39 °C | 5% after 5 h at 37 °C 50% after 5 h at 40 °C | [142] |
| (cb-P-(HEMA-g-P-(NIPAAm-st-HEAAm))) cyclic brush poly(2-hydroxyethyl methacrylate-gpoly(N-isopropylacrylamide-st-N-hydroxyethylacrylamide)) (cb-P-(HEMA-g-P-(NIPAAm-st-HEAAm))) | Doxorubicin | 28 | 38 °C | 28% after 40 h at 25 °C 32% after 40 h at 37 °C 52.5% after 40 h at 40 °C | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.; Huang, W.; Seynhaeve, A.L.B.; ten Hagen, T.L.M. Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors. Pharmaceutics 2020, 12, 1007. https://doi.org/10.3390/pharmaceutics12111007
Amin M, Huang W, Seynhaeve ALB, ten Hagen TLM. Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors. Pharmaceutics. 2020; 12(11):1007. https://doi.org/10.3390/pharmaceutics12111007
Chicago/Turabian StyleAmin, Mohamadreza, Wenqiu Huang, Ann L. B. Seynhaeve, and Timo L. M. ten Hagen. 2020. "Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors" Pharmaceutics 12, no. 11: 1007. https://doi.org/10.3390/pharmaceutics12111007
APA StyleAmin, M., Huang, W., Seynhaeve, A. L. B., & ten Hagen, T. L. M. (2020). Hyperthermia and Temperature-Sensitive Nanomaterials for Spatiotemporal Drug Delivery to Solid Tumors. Pharmaceutics, 12(11), 1007. https://doi.org/10.3390/pharmaceutics12111007




