Abstract
Triple negative breast cancer (TNBC) is one of the most aggressive types of breast cancer. Owing to the absenteeism of hormonal receptors expressed at the cancerous breast cells, hormonal therapies and other medications targeting human epidermal growth factor receptor 2 (HER2) are ineffective in TNBC patients, making traditional chemotherapeutic agents the only current appropriate regimen. Patients’ predisposition to relapse and metastasis, chemotherapeutics’ cytotoxicity and resistance and poor prognosis of TNBC necessitates researchers to investigate different novel-targeted therapeutics. The role of small interfering RNA (siRNA) in silencing the genes/proteins that are aberrantly overexpressed in carcinoma cells showed great potential as part of TNBC therapeutic regimen. However, targeting specificity, siRNA stability, and delivery efficiency cause challenges in the progression of this application clinically. Nanotechnology was highlighted as a promising approach for encapsulating and transporting siRNA with high efficiency-low toxicity profile. Advances in preclinical and clinical studies utilizing engineered siRNA-loaded nanotherapeutics for treatment of TNBC were discussed. Specific and selective targeting of diverse signaling molecules/pathways at the level of tumor proliferation and cell cycle, tumor invasion and metastasis, angiogenesis and tumor microenvironment, and chemotherapeutics’ resistance demonstrated greater activity via integration of siRNA-complexed nanoparticles.
1. Introduction
Cancer is heterogeneous complex disease characterized by rapid and uncontrollable cell proliferation resulting from genetic and epigenetic mutations [1,2]. Cancer burden is greatly significant; it is the second leading cause of morbidity and mortality in the word and is the first in low- and middle-income countries [3,4]. Breast cancer is one of the most common cancers in women [5]. Globally, breast cancer is the leading cause of mortality among females [6]. The overall breast cancer-related mortality rate increased in the past decade, although the overall death rate has declined significantly from 1990 to 2017. The early detection of breast cancer and the advances in cancer treatments and diagnostic tools are the major contributor to decreasing the overall cancer-related mortality [7].
From the molecular level and according to the expression and/or lack expression of hormone receptors (HR); estrogen receptors (ER) and progesterone receptors (PR) and HER-2 on breast cancerous cells, breast cancer is categorized into four subtypes: Luminal A and B characterized by expression of hormonal receptors with or without HER-2, basal-like cancerous cells where the three hormonal receptors are deficient, so they are commonly known as triple negative breast cancer (TNBC), and HER-2 enriched cancer that is enriched with HER-2 only [6,8]. Among the different subtypes of breast cancer, TNBC is clinically deliberated to be the most aggressive form with poorer prognosis that attributes for patients’ death within five years post-diagnosis [9]. TNBC has higher recurrence rate that occur usually within shorter average time [10]. To date, there are no FDA-approved targeted therapies for treating this aggressive form of breast cancer [11]. Patients with TNBC are treated systemically with chemotherapeutic agents which include anthracyclines and taxanes [8,12]. Numerous obstacles compromise the clinical usefulness of these medications, and some of these obstacles are related to the disease itself; as cancerous cells should not only be treated for their uncontrolled cell proliferation, rather integration of cancer metastasis activity, the heterogeneity of the tumor microenvironment, and the complexity of the signaling pathways must be contemplated in choosing appropriate treatment [13]. Other hurdles are associated with the chemotherapeutic agents; lacking selectivity towards cancerous tissues, poor drug delivery, and accumulation leading to dose-limiting and long-term systemic toxicity [14,15,16,17]. Additionally, most cytotoxic agents were designed to destroy the cancerous cells rather than affecting the damaged and/or mutant oncogenes that cause aberrant growth and differentiation of tumor cells [18]. As a result, in most TNBC patients, these agents cause cancer exacerbation, resulting in relapse, cancer metastasis, and therapeutic resistance [19,20]. It is, therefore, a desirable approach to identify new treatment modalities that involves targeted delivery of agents and able to modulate the damaged genes that are involved in cancer pathways.
High diversification of genetic mutations in TNBC patients is the fundamental barrier for effective cancer treatment. Tumor initiation and development is driven by a group of genes that are normally present in healthy cells, in damaged or mutated condition [21]. Overexpression of one or more of these susceptible oncogenes favor uncontrolled cell division, inhibition of the cell growth, and avoidance of the immune system, blockage of cell death, and drug resistance [22]. Targeting suppression of the expressions of oncogenes is one of the paramount important strategies in the treatment of cancer. A number of methods were employed to knockdown the genes that are involved in cancer pathophysiology, but none of them succeeded in complete suppression of the gene [23]. In 1998, Fire and his colleagues revealed that short double-stranded RNA (dsRNA) causes selective suppression of the targeted gene expression. This novel method of controlling the gene expression was termed RNA interference (RNAi) [24]. In RNAi, short double stranded RNAs (dsRNAs) or micro RNAs (miRNAs) is processed into a short interfering RNAs (siRNAs), capable of silencing sequence-specific genes. The siRNA were loaded into the effector complex RNA-induced silencing complex (RISC) to unwound, where single-stranded RNA hybridizes with messenger RNAs (mRNA) target, resulting in gene silencing and down-regulation of certain proteins [25,26,27].
Successful gene silencing necessitates identification of the targeted genes involved in specific cancer signaling pathways and fabrication of the desired sequence of siRNA that is delivered specifically to its site of action in the targeted cells to selectively knockdown the expression of susceptible oncogene. In vivo systemic administration of naked siRNAs is limited due to their physicochemical and pharmacokinetics properties. Naked siRNA has an average diameter of <10 nm, which often undergo rapid renal clearance, causing inefficient accumulation in the targeted tissues. siRNA may be engulfed by tissue monophages, circulating monocytes, and phagocytic cells, which are part of reticuloendothelial systems (RES), to clear foreign bodies and eliminate cellular debris. Naked siRNAs are also vulnerable for biodegradation by serum nucleases and extracellular enzymes Negatively-charged siRNA from its phosphate backbone further prevents efficient anionic cell membrane from cellular internalization. The fate of successfully internalized siRNA were also greatly influenced by cell surface recycling, lysosomal degradation by lysozyme within cytosol and intracellular compartments release. All these criteria mentioned cause systemic delivery of siRNAs deemed challenging [28,29,30]. A growing number of studies have been implicated to develop safe and effective siRNA-targeted delivery systems with the means of utilizing viral and non-viral vehicles [31,32]. The potential immunogenicity, toxicity, poor cell targeting, and expensive production are among the most important challenges that limit the comprehensive application of the engineered viral carriers [33,34]. Alternatively, biocompatible and biodegradable non-viral vectors are promising gene transfer vehicles that shield siRNAs from biological degradation and rapid clearance and circumvent immune system activation. [35]. Diversity in the non-viral-based delivery system has been attributed to remarkable innovations in siRNA-based anti-cancer therapies including nanoparticles.
With the advancement of nanotechnology, nanoparticles are the vehicle of choice for naive siRNA targeted delivery. Nanocarriers have remarkable physicochemical features, including the surface functionality and the particle size that promote their rational development for siRNA delivery [36]. Neutral or negatively-charged nanoparticles are more liable for specific cellular uptake and have longer circulation time. The engineered delivery carriers are often optimized to be in the nanoscale from 5 nm to 100 nm [36,37]. Large nanoparticles with sizes greater than 100 nm are susceptible to non-specific accumulation in healthy organs causing unwanted side effects, whereas small nanoparticles with sizes less than 5 nm have lower circulation half-life as their size are below the renal threshold, so they are prone to renal elimination [37]. Nanoparticles within the range of 5–100 nm prolonged circulation time and reduce protein opsonization [37,38]. There are different studies performed on fabricating nanoparticles using many methods, including co-precipitation, microemulsion, and hydrothermal synthesis [39].
Accumulation of nanoparticles in the targeted tumor cell is enhanced by the permeability and retention effect (PRE), resulting from defective angiogenesis and dysfunctional lymphatic drainage [40,41]. Nanocarriers in the proposed range are passively accumulated in the tumor cells resulting in higher blood residence time, improved drug efficacy, and minimum toxicity [40]. The heightened nanosize is contributed by the penetration and distribution of the nanoparticles within the targeted tumor cell interstitium towards its predetermined site of action [42,43]. Physicochemical properties of nanoparticles were tuned and/or targeting moieties that employ one of the distinctive characteristics of the tumor microenvironment (TME), such as hypoxia, acidosis, or high interstitial fluid pressure (IFP), can be conjugated to the nanoparticles in order to enhance their penetration deeply in the tumor tissues [44,45]. Numerous nanoplatforms were designed to exploit TME characteristics, such as gelatin-coated lipid nanoparticles containing tyrosine kinase inhibitors and PAMAM dendrimer containing tertiary amino group. In the former platform, gelatin is degraded by matrix metalloproteinases (MMT) which is aberrantly upregulated in TME, whereas tyrosine kinase inhibitors suppress Bcr-Abl gene and platelet derived growth factor (PDGF) expression, hence reducing IFP [46]. The tertiary amino group in PAMAM dendrimer protonates in acidic media and enhance the drug release and cellular uptake [47]. Xu et al. developed an acidity-sensitive linkage-bridged copolymer of polyethylene glycol (PEG) and poly lactide-co-glycolide (PLGA) encapsulating siRNA, where PEG was seen detached at acidic TME and siRNA was released and internalized into tumor cells [48].
Owing to their diminutive-size, these nano-formulations improve the solubility and serum stability of siRNAs which, consequently, enhance their oral bioavailability, and siRNA nano-encapsulation modifies the pharmacokinetic properties of siRNAs protecting them from serum degradation, renal and hepatic elimination, improving distribution, and targeting siRNAs activity. Additionally, stimuli-mediated nano-therapeutics permits enhanced cellular internalization and intracellular drug release and decreasing the cancerous cells resistance toward siRNAs [49,50]. The most important goals of an ideal nanocarrier are to introduce siRNAs in a safe, biocompatible, biodegradable, and non-immunogenic manner [51,52].
Clinical application of siRNA-based nanotherapeutics for treatment of many health concerns, in general, and breast cancer, in particular (Table 1), poses many advantages. siRNA-based therapies demonstrate remarkable safety-effectiveness profiles: they are non-teratogenic and mutagenic, as they interfere with the late translational stage of the gene expression, and they are highly effective as they suppress targeted genes preferentially and selectively [26,28]. siRNA-based therapeutics are readily fabricated and modified to effectively knock down any gene in the targeted tumor cells with minimal off-target effects and immunogenicity [53]. Several siRNAs can be simultaneously-incorporated within the same carrier, affecting multiple genes’ expressions which, in turn, improves the antitumor effectiveness without increasing cytotoxicity.

Table 1.
Effectiveness of siRNA-loaded nanotherapeutics in patients with HR and/or HER2 positive breast cancer.
2. Pre-Clinical Activity of Engineered siRNA-Mediated Therapies for Treatment of TNBC
TNBC is characterized by inter- and intra-individual diversity of gene mutations. TNBC genes mutations that are concomitant with poor TNBC prognosis are involved in substantial signaling pathways including: cell proliferation and disease progression, cell survival and death, angiogenesis, cancer metastasis and drug resistance (Figure 1). Growing body of evidence revealed promising results concerning the effectiveness of targeted gene suppression using engineered siRNA mediated nanocarriers in the following major areas: Tumor cell proliferation and cell cycle regulation, tumor invasion and metastasis, angiogenesis, and tumor microenvironment and chemotherapeutics resistance (Table 2).
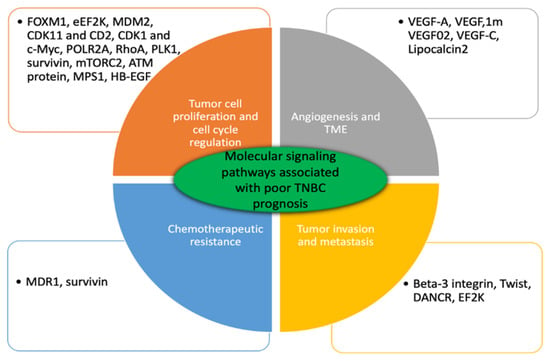
Figure 1.
Major genes involved in substantial molecular pathways affiliated with poor TNBC prognosis. These genes are upregulated in patients with TNBC. Bioengineered siRNA mediated anticancer therapeutics targeted single or multiple genes knockdown their suppression and effectively improve disease progression.

Table 2.
Preclinical studies of siRNA-based therapies mediated antitumor effect.
2.1. Tumor Cell Proliferation and Cell Cycle Regulation
Forkhead box protein M1 (FOXM1) is a key oncogenic transcription factor, which plays a crucial role in regulating the expression of genes involved in cancerous cell proliferation and cell cycle progression. It was found that FOXM1 is significantly overexpressed in patients with TNBC. Hamurcu et al. examined the effectiveness of liposomal FOXM1 siRNA in mice implanted with MDA-MB-231 cells and found that these gene therapeutics cause a pronounced downregulation of FOXM1 expression and consequently suppress TNBC cells growth significantly [66]. Wang et al. further revealed incorporation of anti FOXM1 siRNA into polyethylimine (PEI)-based cationic polymers enhances cellular uptake of the targeted therapy within the tumor cells for 24 h after its administration. Both in vitro and in vivo studies indicated that PEI-based anti-FOXM1 siRNA knockdown the expression of FOXM1 causing reduction in the protein levels associated with cell proliferation and disease progression [67].
Eukaryotic Elongation Factor 2 Kinase (eEF2K) is a principal regulator of tumor growth and progression. Patients with TNBC had significant elevation in eEF2K expression. As FOXM1 and eEF2K are involved in the same functions in TNBC cells, it was expected that FOXM1 regulates the expression of eEF2K. Hamurcu et al. demonstrated that the engineered siRNA liposomal nanoparticles were able to bind to the promoter region of eEF2K and regulates its expression [66]. Modified gold anti-eEF2K siRNA nanoparticles are highly effective for treating TNBC cells on xenografted mice resulting in a remarkable down-regulation of eEF2K expression and, thus, inhibiting tumor growth and progression [68].
MDM2 is an oncogene that inactivates the tumor suppressor p53, which are greatly overexpressed in TNBC patients. Overexpression of MDM2 leads to suppression of p53 affecting tumor cell proliferation and apoptosis. Single-walled carbon nanotubes (SWNTs) were functionalized with PEG for efficient delivery of siRNA for targeting MDM2. It was concluded that PEG-modified SWNTs are effective drug delivery system promoting major MDM2 silencing effect causing tumor cell proliferation suppression and induced apoptosis in breast cancer cells [69].
Cyclin dependent kinase (CDK) 11 and casein kinase 2 (CK2) are important protein kinases for cancerous cell survival, which are also highly expressed in TNBC cells. Kren et al. prepared novel Tenfibgen-coated nanocapsules (TBG nanocapsules) containing anti-CDK11 siRNA or anti-CK2 siRNA targeting Tenascein-C receptors that are found abundantly within the breast cancer stroma. Intravenous administration of these gene therapeutics suppresses mRNA and protein expression causing significant reduction in tumor cell proliferation and growth [70].
Several studies demonstrating molecular targeting of v-Myc myelocytomatosis viral oncogene homolog (c-Myc) that is highly expressed in TNBC. The significance of c-Myc overexpression arises when its suppression is concomitantly associated with inhibition of CDK1 expression. Downregulation of these two genes results in specific targeting and synthetic lethality in TNBC cells. As c-Myc, CDK1 plays a key role in cell death and apoptosis. Administration of anti-c-Myc siRNA-encapsulated nanoparticles causes significant c-Myc gene silencing that contributes to a slight therapeutic effect on TNBC cells whereas the delivery of siRNA targeting CDK1 results in silencing of CDK1 expression only in TNBC cells containing high levels of c-Myc expression. The combination is ideal not only for its pronounced decreased cell proliferation and cell apoptosis, but also for its selective and specific targeting of TNBC cells with no adverse events on normal breast cells [71].
Tumor suppression (TP53) mutation is one of the most frequent oncogenic mutations in TNBC. Remarkable preclinical studies were conducted to reestablish TP53’s effect but none of them were transformed into human clinical studies. Hemizygous loss of TP53 is the most recurrent TNBC mediated TP53 mutations and is associated with deletion of neighboring genes, most importantly, hemizygous deletion of POLR2A [69]. Antitumor-based silencing of POLR2A expression was investigated by Xu and his coworkers. A pH-sensitive nano-bomb was developed incorporating antiPOL siRNA and guanidine-CO2 functionalized chitosan (CG) at neutral pH. siRNA-nanoplatforms-containing CG-CO2 were not only efficaciously internalized into TNBC cancerous cells harboring hemizygous TP53 deletion, but also able to escape the intracellular trafficking and release siRNA at low pH. TNBC cell lines embracing hemizygous loss of POLR2A are more sensitive than those harboring the wild type for siRNA nanotherapeutics, as a result, knockdown of POLR2A expression cause selective TNBC cell death with no cytotoxicity. Inhibition of tumor growth by targeting POLR2A was not influenced with the status of TP53 loss [70].
Ras homologous A (RhoA) and Ras homologous C (RhoC) are low molecular weight GTPases of the Ras family. Significant high levels of RhoA and RhoC are reported in all subtypes of breast cancer especially TNBC. Overexpression of RhoA enhances tumor cell proliferation, and induces angiogenesis and tumor invasion and metastasis [72]. Li et al. studied the antitumor effect of anti-RhoA siRNA nanoparticles in vivo where chitosan-coated polyisohexylcyanoacrylate (PIHCA) nanoparticles encapsulating anti-RhoA siRNA were fabricated and given to MDA-MB-231 xenografted mice intravenously. Complete suppression and more than 90% inhibition of tumor growth were perceived after intravenous administration of anti-RhoA siRNA [73]. As RhoA and RhoC are expressed exclusively in cancerous cells only, specific and selective targeting of RhoA is a promising candidate for treatment patients with TNBC [72].
Polo-like kinase 1 (PLK1) is extensively upregulated in patients with either primary or metastatic TNBC. Inhibition of PLK1 expression is associated with ameliorating tumor cell death in a wide variety of metastatic cancer [74,75]. Targeted nanoplatforms incorporating anti-PLK1 siRNA encapsulated within antibody-conjugated bioreducible cross-linked with PEI and polyethylene glycol (PEG) layer-by-layer with coated mesoporous silica nanoparticles (MSNP) showed the following characteristics: PEI is highly effective for siRNA binding and escapes the intracellular trafficking. PEG is incorporated for siRNA protection by enzymatic degradation, reducing PEI toxicity, circumventing the immune system, and decreasing nanoparticles’ aggregation. In vitro and in vivo studies revealed that these nanoparticles were efficiently internalized into targeted TNBC metastatic cells overwhelming both cancer hallmarks: tumor cell proliferation and tumor metastasis. It is worth noting that reactive oxygen species’ (ROS) scavenging ability of MSNP contributes to the antimetastatic effect, whereas the internalized anti-PLK1 siRNA exhibits apoptotic cellular death activity [76].
MDM2 [77], cyclin-dependent kinase (CDK) 11 and casein kinase 2 (CK2) [78], c-Myc [79], survivin (also known as BIRC-5, an inhibitor of apoptosis protein) [80,81], mTORC 2 [82], Ataxia-telangiectasia mutated (ATM) protein [83], monopolar spindle 1 (MPS1, most commonly known TTK protein kinase) and cell division cycle protein 20 (CDC20) [84], Heparin-binding EGF-like growth factor (HB-EGF) [85], epidermal growth factor receptors (EGFR) [86,87,88], CXCR-4 [89], luciferase mRNA protein [90], and enhanced green fluorescent protein (eGFP) [91] are overexpressed in TNBC cells. Gene function studies showed that silencing any of these molecular factors using siRNA-mediated nanoparticles caused significant tumor growth suppression. Many other genes, such as the brother of the regulators of imprinted sites (BORIS) [92], HER2/neu gene [93], E2F3 [94], and Akt 1, 2, and 3 [95], are involved in tumor cell cycle regulation and cell proliferation. Pre-clinical in vitro studies confirmed that downregulation any of these genes’ expression using siRNA-based gene therapy showed encouraging results in inhibition tumor cell proliferation and cell survival.
2.2. Tumor Invasiveness and Metastasis
Tumor metastasis is the primary factor associated with cancer related deaths in patients with TNBC. Epithelial mesenchymal transition (EMT) is the driving force for tumor invasiveness, metastasis, drug resistance, and remodeling of TME [96]. The morphological alterations of the normal epithelial cells’ characteristics into the mesenchymal phenotype allow the cancerous cells to disseminate out into distant organs [97,98]. Central nervous system metastasis and lung metastasis are among the utmost fatal metastatic repercussions in patients with TNBC [20]. Upregulation of numerous transcriptional factors is among the foremost causes to induce EMT. Epidermal growth factor (EGF), fibroblast growth factor, hepatocyte growth factor, transforming growth factor (TGF)-β, and bone morphogenetic proteins are EMT master regulators that govern the development and progression of breast cancer metastasis [99,100].
Breast cancer metastasis and EMT are governed by the transmembrane protein β3 integrin. Upregulation of TGF-β in cancerous cells persuades overexpression of β3 integrin, therefore, it is expected that silencing β3 integrin expression inhibits TGF-β induced EMT, tumor invasion, and metastasis [101]. Parvani et al. prepared a cationic lipid nanocarrier (ECO) containing siRNA that are delivered specifically to the targeted β3 integrin gene. Treatment of TGF-β pre-stimulated TNBC cells with ECO/β3-siRNA nanoparticles reduces TGF-β-mediated EMT, tumor proliferation, and invasion as these engineered nanoparticles prompt inhibition of more than 70% of β3 integrin expression for approximately seven days. Downregulation of mesenchymal markers (N-cad and PAI-1) and upregulation of epithelial markers (E-cad and CK-19) attributes to inhibition of TGF-β induced EMT. Cellular uptake of β3-siRNA based nanoparticles into targeted cells was improved by surface modifications of the engineered nanoparticles with RGD peptide. In vivo analysis of the modified ECO/β3-siRNA nanoparticles decreased the primary tumor burden and tumor recurrence, designating that systemic administration of modified siRNA nanoparticles demonstrated significant suppression of tumor metastasis and invasion [102].
Patients with TNBC and ovarian cancers are characterized by elevated levels of TWIST. TWIST is a major transcription factor activates EMT, promotes stem like phenotyping, and inhibits apoptosis, increasing the risk of tumor recurrence and poor prognosis [103]. Inhibition of TWIST overexpression in TNBC cells was examined by the administration of poly(amidoamine) dendrimer (PAMAM) loaded with TWIST-siRNA. The modified siRNA-based dendrimers were efficiently internalized into TNBC cells causing significant reduction in TWIST expression accompanied by phenotyping changes associated with decreasing cancerous cells motility, decreasing TWIST-mediated upregulation mesenchymal markers (Vimentin and N-cad) and attenuating the dissemination and invasion of TNBC cells for approximately one week after dendrimer treatment. As TWIST protein is expressed only in cancerous cells and not in normal breast cells, silencing TWIST expression using siRNA-based dendrimer is a promising safe and biocompatible approach for treating breast cancer-associated metastasis [104].
A novel class of RNA consisting of more than 200 nucleotides, so-called long noncoding RNAs (lncRNAs), are vital regulators of gene expression associated with cellular differentiation and development [105]. The oncogenic lncRNAs have a pivotal role in inducing malignant cell proliferation, invasion, and metastasis [106]. BMP/OP-Responsive Gene (BORG) [107], HOX Antisense intergenic RNA (HOTAIR) [108] and Differentiation Antagonizing Non-Coding RNA (DANCR) [109] are lncRNAs concerned in tumor recurrence and progression, indicating poor cancer prognosis. As DANCR is highly expressed in TNBC cells, Vaidya et al. revealed targeting DANCR lncRNAs causes suppression of TNBC metastasis, and inhibition of cell proliferation and survival. Vaidya and his colleagues prepared RGD-PEG-ECO nanoparticles loaded with anti-DANCR siRNA. In vitro transfection of MDA-MB-231 and BT549 cells with anti-DANCR siRNA showed significant reduction of cancer cell proliferation and inhibition of tumor cell migration and invasion. Targeting DANCR in MDA-MB-231 xenografted mice using RGD-PEG-ECO/siRNA-engineered nanoparticles results in decreasing tumor volume and the suppression of tumor growth [109].
A recent study examined the silencing effect of EF2 kinase in tumor proliferation, apoptosis, invasion, and metastasis. EF2K is a typical kinase that is overexpressed in BRCA1-mutated breast cancer cells. Silencing EF2K was performed by treatment of HCC-1937 and MDA-MB-436 cells with cobalt-ferric anti-EF2K siRNA nanoparticles where internalization of anti-EF2k-mediated siRNA into TNBC cells were enhanced in comparison to normal breast cells. Cobalt-ferric anti-EF2K siRNA nanoparticles knocked-down the expression of EF2K in transfected TNBC cells and, hence, inhibited TNBC cells proliferation and colony formation and impaired cell migration and invasion. Targeting of EF2K in BRCA1-mutated MDA-MB-436 and HCC-1937 cell-xenografted mice inhibited tumor growth, reduced cancer cell proliferation, angiogenesis, and induced apoptosis. Remarkably, COFe-siRNA nanoparticles showed no obvious cytotoxicity [110].
2.3. Angiogenesis and Tumor Microenvironment
Both tumor growth and metastasis are driven by angiogenesis [111]. Tumor angiogenesis is characterized by high neovascular density, high lymphatic density, and vascular invasion. Numerous angiogenic factors are secreted in the tumor microenvironment stimulating new blood vessels sprout. Angiogenic factors overexpression is correlated with cancer development as vascular endothelial growth factors (VEGF) especially VEGF-A subtype, attributed for cancer progression such insulin like growth factors (IGF) and TGF-β1 and concerned with tumor invasion and metastasis like vascular endothelial growth factor receptors (VEGFRs) [112]. These proangiogenic factors are more pronounced in TNBC compared to other subtypes of breast cancer [111,113].
It was reported that inhibition of VEGF using monoclonal antibodies is not effective for treating patients with TNBC as theses anti-VEGF antibodies hinder VEGF interaction with their receptors at the endothelial cells and the plasma membrane of the tumor cells without affecting the internal VEGFR expressed at the nuclear envelope [114,115,116,117,118,119,120,121,122]. Salva et al. prepared chitosan nanoplexes encapsulating siRNA targeting VEGF-A, VEGFR-1, VEGFR-2, and neuropilin-1 (co-receptors for VEGF, NPR-1). Intratumoral administration of VEGF/siRNA nanoplexes into TNBC induced rats declined VEGF expression by more than 80% and reduced the tumor volume by more than 90%. Chitosan/siVEGF-mediated nanoplexes transfection did not increase interferon mediated immune response. Salve et al. demonstrated the silencing effect of the combination of VEGF genes cause potent and specific inhibition of breast cancer growth [117]. In another study, PLEGP nanocomplex containing anti-VEGF siRNA was developed. Different PLEGP nanocomplexes were investigated for their anti-tumor and cytotoxic activity. PLGEP1800 was shown to have a modest downregulation of VEGF expression with no significant cytotoxicity. In vitro studies revealed that transfection of MDA-MB-231 cells with PLGEP1800 nanocomplex suppressed cancerous cell migration and invasion. Vascular endothelial cell proliferation and tube formation were repressed in PLEGP1800/VEGF siRNA transfected HUVECs cells. Knock down of VEGF expression and subsequent inhibition of CD31 level in siVEGF treated MDA-MB-231 xenografted mice indicated suppression of tumor growth and impairment of angiogenesis [118].
Many identifiable factors attribute for lymphangiogenesis in breast cancer. Among them is VEGF-C that activates the lymph vascular cells and lymphatic cells growth. Upregulation of VEGF-C level indicates tumor metastasis particularly for the lymph nodes. The silencing of VEGF-C expression was studied by Chen and his colleagues using siRNA mammalian vectors. Anti-VEGF-C siRNA knocked down VEGF-C mRNA and VEGF-C protein production in C166 cells. Downregulation of VEGF-C expression leads to suppression lymphangiogenesis but not angiogenesis, suppression of regional and distant metastasis implicated by inhibition of lymph node metastasis and spontaneous lung metastasis, immunomodulating effect, and improving mice survival [119].
Lipocalin 2 (Lcn2), a subtype of lipocalin protein family, is upregulated in TNBC contributing to tumor progression, invasion and metastasis through its interaction with matrix metalloproteinase-9 (MMP-9) [119]. Lipocalin 2 play a striking role in regulating of angiogenesis, its overexpression in TNBC is accompanied by increased VEGF expression. It is suggested that Lcn 2 induced VEGF production through the Erk/HIF-1α signaling pathway [120]. Elevated urinary levels of Lcn 2 in patients with TNBC correlated with its poor progression representing that Lcn 2 is biomarker for both diagnosis and prognosis of breast cancer [119]. Anti-angiogenic engineered liposomes encapsulating both ICAM-1 and Lcn2 siRNA were prepared (ICAM-Lcn-LP). ICAM-Lcn-LP exposed MDA-MB-231 cells suppress Lcn expression significantly with no cytotoxicity. Ablation of Lnc 2 expression in ICAM-Lcn-LP transfected MDA-MB-231 harvested conditioned media knocked down VEGF expression in MDA-MB-231 cells. ICAM-Lcn-LP transfected MDA-MB-231 harvested conditioned media was co-cultured with HUVEC and HMVEC cells. Silencing of Lcn2 production minimized endothelial cells migration and tube formation in HUVEC and HMVEC cells. Inhibitory effect of TNBC-induced angiogenesis were seen both in vitro and in vivo [121].
Angiogenesis is a key driver for tumor growth and metastasis. Selective targeting, accessibility and low drug resistance liability are major advantages for targeting vascular endothelial cells in addition to cancerous cells [122]. Receptor mediated endocytosis is an innovative tactic targeting receptors available at many cell populations. Nucleolin is a receptor that is highly expressed at both cancerous cells and endothelial cells originating from blood vessels [91]. Novel fabrication of nucleolin targeted siRNA based nanoparticles is a promising approach employing gene delivery for dual therapy. Gomes-da-Silva et al. developed F3 targeted liposomes incorporating enhanced green fluorescent protein (eGFP) and transfected them into MDA-MB-231 cells and HMEC-1 cells. It was reported that F3 targeted liposomes enhanced the cellular uptake by both cancerous cells and endothelial cells but not by non-cancerous cells. Additionally, improved targeted siRNA liposomes internalization by MDA-MB-231 and HMEC-1 cells was accompanied by knock down eGFP expression in both cells [91].
Most TNBC cells exhibits mutation in the tumor suppressor p53 gene. p53 is an oncogene that is overexpressed in patients with TNBC and involved in multiple molecular pathways including tumorigenesis, apoptosis, metastasis and angiogenesis [123,124]. Braicu et al. demonstrated anti-angiogenic impact of silencing mutated p53 gene using p53-siRNA based approach where transfection of Hs578T cells with anti-p53 siRNA induced apoptosis, decreased cell survival and reduced angiogenesis. Treatment of TNBC cells with a combination of p53 siRNA and epigallocatechingallate (EGCG) demonstrated more pronounced anti-apoptotic and anti-angiogenic as compared for each treatment alone [125].
cAMP and its two receptors protein kinase A (PKA) cAMP dependent and exchange protein activated cAMP (EPAC1). EPAC1 is a cAMP activated guanine nucleotide exchange factor. cAMP and its receptors are exposed to be overexpressed in patients with breast cancer; associated with cell differentiation, cell migration and cell survival and death [126]. Cell culture studies revealed that suppression of EPAC1 expression in anti-cAMP siRNA treated MDA-MB-231 cells co-cultured HUVEC cells exhibited activities include cessation in the formation of the nodes and tubes in HUVEC cells, upregulation of the anti-angiogenic proteins involved in adhesion and angiogenesis, downregulation of pro-angiogenic proteins such as fibroblast growth factor (FGF), transforming growth factors (TGF) and many others attributed to cell migration and locomotion, inhibited vascular cells invasion mediated TNBC cells and downregulation of Paxillin and MENA molecules that are concerned with focal adhesion. It is suggested that EPAC1 have a remarkable role in regulating tumor microenvironment, neovascularization and invasiveness of TNBC cells contributing to vascular metastasis [127].
2.4. Chemotherapeutic Resistance
Cancerous breast cells are highly sensitive to chemotherapeutic agents with a response rate extent approximately 80% but unfortunately most of the cancerous cells develop resistance contributing to treatment failure and poor TNBC prognosis [128]. Emerged chemotherapeutics’ resistance is incongruent with the type; structure and the biological activity of the chemotherapeutics therefore substitution between different agents is no longer effective [129,130].
Though 90% of the initially chemo-responsive TNBC cells develop resistance, advanced research exploring the impact of siRNA-based nanotherapeutics as a targeted platform to overcome doxorubicin-based [131,132,133] and taxane-based resistance [134,135,136] exploiting non-TNBC cell lines, most commonly MCF-7 cells. Extensive research are exploring the mechanisms of multidrug resistance (MDR) evolved in breast cancerous cells including ATP-binding cassette (ABC) transporter, activation of anti-apoptotic pathways, enhancement chemotherapeutics detoxification, overexpression of β-tubulin III, KIF14, and many other signaling factors, alterations of DNA repair enzymes, and chemoresistance-induced hypoxia and NF-κB signaling pathway [137]. Among these different mechanisms, ABC transporter, alterations of the antiapoptotic genes and the role of hypoxia are major mechanisms of chemoresistance (Figure 2).
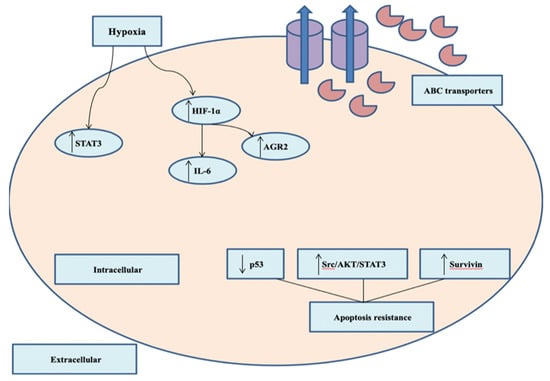
Figure 2.
Major mechanisms of chemotherapeutics resistance in TNBC. Overexpression of ABC transporters and extruding chemotherapeutic agents out of the cancerous cells. Hypoxia induces upregulation of HIF-1α and other signaling factors.
Extruding the internalized chemotherapeutics out of the cancerous cells is governed by ATP-binding cassette (ABC) transporter family. Two efflux pump members namely ABCB1 (P-glycoprotein) and ABCG2 (BCRP) transporters are identified [130]. ABCB1 and ABCG2 transporters are overexpressed in patients with TNBC [138]. Clinical studies failed to verify MDR phenotype modulated therapeutics either for their cytotoxicity or for their unwanted pharmacokinetics interactions, thus silencing gene expression mediating thwarting the synthesis of ABC transporters is the most accessible approach to circumvent MDR clinically [139,140]. It was earlier reported that multidrug resistance genes (lung resistance-related protein (LRP), multidrug resistance associated proteins 1 and 2 (MRP1, MRP2), multidrug resistance gene 1 (MDR1), and breast cancer associated proteins (BCRP)) mediated mRNA expressions in metastatic breast tumor cells specimens of patients receiving first line chemotherapy based regimens correlates inversely with treatment efficacious with elevated genes expression predicting poor prognosis in those patients [141].
Growing bodies of evidence revealed that knockdown of ABCB1 and ABCG2 expression mediated siRNA-based nanoparticles increased intracellular concentration and cancerous cells chemosensitivity of many cytotoxic drugs such as doxorubicin and paclitaxel [142,143,144]. Deng et al. prepared multi-layers nanoparticles constituting of doxorubicin-based nanoparticles encircled with alternating layers of siRNA targeted MDR1 and poly-l-arginine enclosed by an outer shell of hyaluronic acid (HA). The engineered drug delivery platform posed high siRNA loading capacity, stability and cellular internalization with high efficacy-toxicity profile. Administration of doxorubicin-siRNA nanoparticle complexes decreases the tumor volume significantly in comparison to the treatment with either anti-MDR1 siRNA or doxorubicin alone [145].
Targeting the anti-apoptotic genes/proteins is another major mechanism-induced chemoresistance. Survivin is one of the most attractive inhibitors of apoptosis (IOP) controlling tumor growth, angiogenesis, apoptosis, and recently drug resistance, in particular, paclitaxel (Figure 3) [146]. Therapeutic effectiveness of survivin to enhance TNBC cells sensitivity to chemotherapeutics agents were investigated using polymeric micelles of PEG2000-PE incorporating survivin siRNA. Treatment of anti-survivin siRNA PM transfected MDA-MB-231 cells with paclitaxel showed that knockdown survivin expression in resistant cancerous cell enhance paclitaxel cytotoxicity significantly and cause destabilization of microtubules. In addition, co-encapsulation of paclitaxel and anti-survivin siRNA into PM exhibited a significant suppression of tumor growth in comparison to the paclitaxel free anti-survivin siRNA PM [147]. Triple therapies of chemotherapy, gene therapy, and photothermal therapy revealed outstanding antitumor effect in vitro and in vivo in comparison to mono or dual therapies [148].
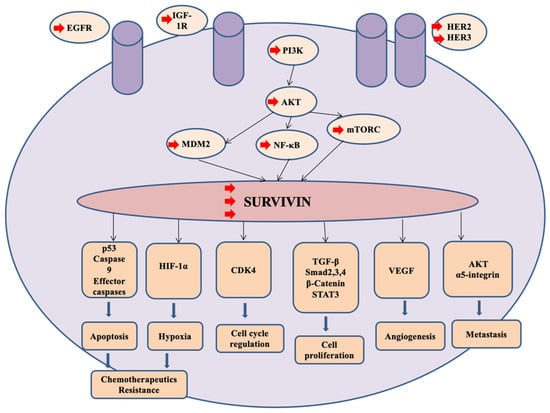
Figure 3.
Survivin is a potential target for treatment patients with TNBC. Activation of several kinases such as HER2, HER3, IGF-1R and EGFR elicits PI3K/AKT signaling pathways resulting in upregulation of survivin expression. Overexpression of survivin stimulates numerous genes and proteins associated with cancerous cell proliferation and survival, migration, angiogenesis, and promotes chemoresistance.
Chemoresistance-induced kinases in TNBC were identified by screening a kinome of siRNA into doxorubicin treated/untreated MDA-MB-231 cells. siRNA transfected MDA-MB-231 cells showed many potential kinases of doxorubicin-based resistance in TNBC cells. Src kinase is the most significant kinase for doxorubicin-induced chemoresistance not only in TNBC cell lines (MDA-MB-231, MDA-MB-468 and Hs578T) but also in non-TNBC cells (MCF-7 and T47D). Src kinase was highly expressed in TNBC cells rather than in normal breast cells. Src silenced MDA-MB-231 cells showed low protein levels of STAT3 and AKT proposing that Src/AKT/STAT3 signaling pathway play a role in chemoresistance [149]. These results are consistent with Moreira et al. findings that confirmed overexpression of STAT3 protein in doxorubicin-treated TNBC BT-549 cells suggesting that STAT3 is an essential EGF gene involved in doxorubicin-based resistance [150].
3. Clinical Trials of Anticancer siRNA-Mediated Nanotherapeutics
Current pre-clinical advances exploiting siRNA-based nanotherapeutics for treatment of cancer, in general, and TNBC, in particular, established the effectiveness of these therapeutics in inhibition cell proliferation and tumor growth, suppression tumor invasion and metastasis, pronounced anti-angiogenic effect, and enhancement of chemotherapeutics efficacy. Remarkably, several siRNA nanoparticles succeeded in being investigated in clinical trials. To date, eight siRNA-mediated nanoparticles have commenced phase I and II clinical trials (Figure 4) for treatment of a wide variety of solid cancers that could be employed for patients with TNBC (Table 3).
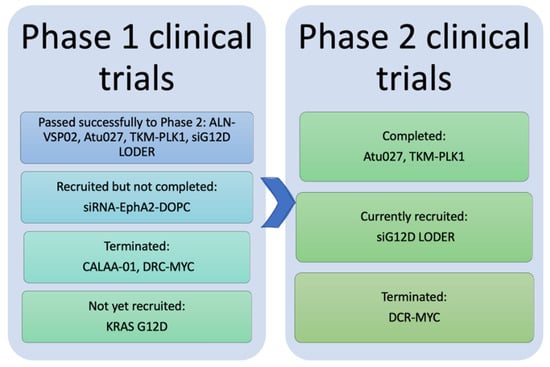
Figure 4.
Representative diagram for the results of phase I and phase II clinical trials of the eight anticancer nano-siRNAs. Phase I trials of CALAA-01 and DCR-MYC were terminated. The phase I trial of siRNA-EphA2-DOPC has recruited but not completed yet, whereas the mesenchymal stem cell-derived exosomes with KRAS G12D trial has not yet recruited. ALN-VSP02, Atu027, TKM-PLK1, and siG12D LODER passed phase I successfully. Four siRNA nanotherapeutics moved to phase II; Atu027 and TKM-PLK1 completed phase II while siG12D LODER is currently being recruited. The phase II trial of DCR-MYC was terminated.

Table 3.
Anticancer siRNA-mediated nanoparticles in clinical trials.
EphA2-siRNA-DOPC is the most recent anticancer siRNA-mediated nanotherapeutics investigated clinically. EphA2 is a protein member of tyrosine kinases receptors that are expressed abundantly in the embryos and expressed to a lesser extent in adults mainly at the surface of epithelial cells. Several studies reported that EphA2 is selectively upregulated in diverse arrays of cancer including, but not limited to, breast, pancreas, prostate, lung, and most importantly ovarian cancer affecting cancerous cells growth, invasion, and metastasis and angiogenesis and increasing the tumor burden. EphA2-siRNA was incorporated into liposomal nanoparticles (DOPC) called EPHARNA (EphA2-siRNA-DOPC) specifically targeting EphA2 expression in the epithelial mediated tumor [164,165,166]. In vitro and in vivo studies demonstrated that EPHARNA has anti-angiogenic effect and reduce tumor growth dramatically. Moreover, remarkable tumor growth inhibition was found with simultaneous administration of EphA2-siRNA-DOPC and paclitaxel [167]. In vivo toxicological studies showed that DOPC nanoliposomes exhibit no observed adverse events at dose range of 75–225 mcg/kg after single administration and twice administration results in no major toxicities [168]. In 2015, EphA2-siRNA-DOPC entered phase I trial recruiting patients with advanced metastatic solid cancer receiving intravenous EPHARNA twice weekly over two hours [169].
4. Conclusions
Despite comprehensive knowledge and advanced research on breast carcinoma, the cancer burden remains significantly high. High diversification of genomic transmutations and enormous phenotypic alterations attributed to traditional anticancer therapeutics’ failure, which contributes to relapse, recurrence, and cancer-related mortality, especially on TNBC. Most patients with TNBC exhibit drug resistance, invasion, and metastasis causing poor disease prognosis and poor overall patient survival rate. Inter- and intra-individual heterogeneity in TNBC limits intervention and efficacious treatment for TNBC. RNAi interference and, in particular, siRNA-based therapeutics technology attracted attention via salient physicochemical characteristics, and pharmacokinetics properties along with the systemic biodegradation liability mitigate the systemic administration of naked siRNA. Targeted delivery systems are needed to overcome the aforementioned obstacles attaining specific and selective gene-based therapeutic products’ internalization in the tumor cells, sufficient intracellular retention, and efficacious antitumor activity with no off-target side effects. Nanosized platforms aid in encapsulating siRNA protecting it from the external environment, targeting the tumor cells and evading the immune-stimulating effect. Most importantly, multiple drug modalities (chemotherapeutics, photothermal agent and siRNA-based therapeutics) may be incorporated in these nano-formulations. Integrations of different drug modalities in one nanocarrier posed significant antitumor effectiveness compared to the delivery of one agent or sequential administration of multiple agents. Several anticancer-based gene nanotherapies have commenced phase I and II clinical trials and have shown promising results. To date, there are no siRNA-anticancer therapies on the market, but the progression of siRNA-based therapy from the bench to the clinic will open the horizon for these advanced anticancer therapeutics to evolve.
Author Contributions
Both authors equally contributed to this article. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- You, J.S.; Jones, P.A. Cancer genetics and epigenetics: Two sides of the same coin? Cancer Cell 2012, 22, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Cancer Facts Figures 2019. American Chemical Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html (accessed on 18 December 2019).
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Prev. Biomark. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Facts Figures 2019–2020. American Chemical Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html (accessed on 20 December 2019).
- Qattan, A. Gene Silencing Agents in Breast Cancer. In Modulating Gene Expression—Abridging the RNAi and CRISPR-Cas9 Technologies; Singh, A., Khan, M.W., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Metzger-Filho, O.; Tutt, A.; De Azambuja, E.; Saini, K.S.; Viale, G.; Loi, S.M.; Bradbury, I.; Bliss, J.M.; Azim, H.A.; Ellis, P.A.; et al. Dissecting the heterogeneity of triple-negative breast cancer. J. Clin. Oncol. 2012, 30, 1879–1887. [Google Scholar] [CrossRef]
- Le Du, F.; Eckhardt, B.L.; Lim, B.; Litton, J.K.; Moulder, S.; Meric-Bernstam, F.; Gonzalez-Angulo, A.M.; Ueno, N.T. Is the future of personalized therapy in triple-negative breast cancer based on molecular subtype? Oncotarget 2015, 6, 12890–12908. [Google Scholar] [CrossRef]
- Kalimutho, M.; Parsons, K.; Mittal, D.; Lopez, J.A.; Srihari, S.; Khanna, K.K. Targeted therapies for triple-negative breast cancer: Combating a stubborn disease. Trends Pharmacol. Sci. 2015, 36, 822–846. [Google Scholar] [CrossRef]
- Crown, J.; O’Shaughnessy, J.; Gullo, G. Emerging targeted therapies in triple-negative breast cancer. Ann. Oncol. 2012, 23, vi56–vi65. [Google Scholar] [CrossRef]
- Saraswathy, M.; Gong, S. Recent developments in the co-delivery of siRNA and small molecule anticancer drugs for cancer treatment. Mater. Today 2014, 17, 298–306. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.; Song, G.; Liu, T.; He, B.; Zhang, H.; Wang, X.; Zhang, Q. Combination antitumor therapy with targeted dual-nanomedicines. Adv. Drug Deliv. Rev. 2017, 115, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Bennie, L.A.; McCarthy, H.O.; Coulter, J.A. Enhanced nanoparticle delivery exploiting tumour-responsive formulations. Cancer Nanotechnol. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Mirza, Z.; Karim, S. Nanoparticles-based drug delivery and gene therapy for breast cancer: Recent advancements and future challenges. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Pawar, A.; Prabhu, P. Nanosoldiers: A promising strategy to combat triple negative breast cancer. Biomed. Pharmacother. 2019, 110, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.M.; Meltzer, P.S. Cancer: Lucky draw in the gene raffle. Nature 2002, 417, 906–907. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Watts, J.K.; Corey, D.R. Silencing disease genes in the laboratory and the clinic. J. Pathol. 2012, 226, 365–379. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef]
- Am Hong, C.; Nam, Y.S. Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics 2014, 4, 1211–1232. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Balekar, N.; Mishra, P.K. Nanoengineered strategies for siRNA delivery: From target assessment to cancer therapeutic efficacy. Drug Deliv. Transl. Res. 2017, 7, 346–358. [Google Scholar] [CrossRef]
- Li, J.; Xue, S.; Mao, Z.W. Nanoparticle delivery systems for siRNA-based therapeutics. J. Mater. Chem. B 2016, 4, 6620–6639. [Google Scholar] [CrossRef]
- Moffatt, S. siRNA-based nanoparticles for cancer therapy: Hurdles and hopes. MOJ Proteom. BioInform. 2016, 4, 345–347. [Google Scholar] [CrossRef][Green Version]
- Babu, A.; Munshi, A.; Ramesh, R. Combinatorial therapeutic approaches with RNAi and anticancer drugs using nanodrug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1391–1401. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Alibakhshi, A.; Yaghoobi, H.; Hashemi, M.; Hejazi, M.; Ramezani, M. Recent advances on biocompatible and biodegradable nanoparticles as gene carriers. Expert Opin. Biol. Ther. 2016, 16, 771–785. [Google Scholar] [CrossRef]
- Chen, M.; Du, Q.; Zhang, H.Y.; Wahlestedt, C.; Liang, Z. Vector-based siRNA delivery strategies for high-throughput screening of novel target genes. J. RNAi Gene Silenc. Int. J. RNA Gene Target. Res. 2005, 1, 5–11. [Google Scholar]
- Zhou, Z.; Liu, X.; Zhu, D.; Wang, Y.; Zhang, Z.; Zhou, X.; Qiu, N.; Chen, X.; Shen, Y. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. Adv. Drug Deliv. Rev. 2017, 115, 115–154. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Kutty, R.V. Recent advances in nanotheranostics for triple negative breast cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 430. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Arranja, A.G.; Pathak, V.; Lammers, T.; Shi, Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol. Res. 2017, 115, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.H.; Luong, N.H. Nanoparticles: Synthesis and applications. Mater. Biomed. Eng. 2019, 1, 211–240. [Google Scholar]
- Teles, R.H.G.; Moralles, H.F.; Cominetti, M.R. Global trends in nanomedicine research on triple negative breast cancer: A bibliometric analysis. Int. J. Nanomed. 2018, 13, 2321–2336. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolcular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Chauhan, V.P.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Delivery of molecular and nanoscale medicine to tumors: Transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 281–298. [Google Scholar] [CrossRef]
- Kratz, F.; Warnecke, A. Finding the optimal balance: Challenges of improving conventional cancer chemotherapy using suitable combinations with nano-sized drug delivery systems. J. Control. Release 2012, 164, 221–235. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Huang, Z.; Zuo, T.; Lu, Q.; Wu, G.; Shen, Q. Reducing interstitial fluid pressure and inhibiting pulmonary metastasis of breast cancer by gelatin modified cationic lipid nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 29457–29468. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Du, J.-Z.; Liu, J.; Du, X.-J.; Shen, S.; Zhu, Y.-H.; Wang, X.; Ye, X.; Nie, S.; Wang, J. Smart superstructures with ultrahigh pH-sensitivity for targeting acidic tumor microenvironment: Instantaneous size switching and improved tumor penetration. ACS Nano 2016, 10, 6753–6761. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-F.; Zhang, H.-B.; Sun, C.-Y.; Liu, Y.; Shen, S.; Yang, X.; Zhu, Y.-H.; Wang, J. Tumor acidity-sensitive linkage-bridged block copolymer for therapeutic siRNA delivery. Biomaterials 2016, 88, 48–59. [Google Scholar] [CrossRef]
- Xu, C.-F.; Zhang, H.-B.; Sun, C.-Y.; Liu, Y.; Shen, S.; Yang, X.; Zhu, Y.-H.; Wang, J. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv. Drug Deliv. Rev. 2012, 64, 1363–1384. [Google Scholar]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Dawidczyk, C.M.; Russell, L.M.; Searson, P.C. Nanomedicines for cancer therapy: State-of-the-art and limitations to pre-clinical studies that hinder future developments. Front. Chem. 2014, 2, 69. [Google Scholar] [CrossRef]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic nanoparticles for cancer therapy: A transition from lab to clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Parvani, J.G.; Jackson, M.W. Silencing the roadblocks to effective triple-negative breast cancer treatments by siRNA nanoparticles. Endocr.-Relat. Cancer 2017, 24, R81–R97. [Google Scholar] [CrossRef]
- De Mello, L.J., Jr.; Souza, G.R.R.; Winter, E.; Silva, A.H.; Pittella, F.; Creczynski-Pasa, T.B. Knockdown of antiapoptotic genes in breast cancer cells by siRNA loaded into hybrid nanoparticles. Nanotechnology 2017, 28, 175101. [Google Scholar] [CrossRef]
- Li, X.Y.; Luo, Q.F.; Wei, C.K.; Li, D.F.; Fang, L. siRNA-mediated silencing of CDK8 inhibits proliferation and growth in breast cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 92–100. [Google Scholar] [PubMed]
- Kamaruzman, N.I.; Tiash, S.; Ashaie, M.; Chowdhury, E.H. siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways. Biomedicines 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Chuaa, M.; Tiasha, S.; Fatemiana, T.; Noordinb, M.; Kengc, C.; Chowdhurya, E. Carbonate apatite-facilitated intracellular delivery of c-ROS1 siRNA sensitizes MCF-7 breast cancer cells to cisplatin and paclitaxel. OA Cancer 2013, 1, 1–9. [Google Scholar]
- Subramanian, N.; Kanwar, J.R.; Athalya, P.K.; Janakiraman, N.; Khetan, V.; Kanwar, R.K.; Elchuri, S.V.; Krishnakumar, S. EpCAM aptamer mediated cancer cell specific delivery of EpCAM siRNA using polymeric nanocomplex. J. Biomed. Sci. 2015, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.; Shao, R.; Xu, Y.; Gao, J.; Liang, W. VEGF siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017, 12, 6075–6088. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Cheng, K. Silencing of the IKKε gene by siRNA inhibits invasiveness and growth of breast cancer cells. Breast Cancer Res. 2010, 12, R74. [Google Scholar] [CrossRef]
- Bakhtiar, A.; Kamaruzman, N.I.; Othman, I.; Zaini, A.; Chowdhury, E.H. Intracellular Delivery of p53 Gene and MAPK siRNA into Breast Cancer Cells Utilizing Barium Salt Nanoparticles. J. Breast Cancer Res. Adv. 2017, 1. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, H.; Lin, S.Y.; Goss, J.A.; Brunicardi, F.C.; Li, K. siRNA-based targeting of cyclin E overexpression inhibits breast cancer cell growth and suppresses tumor development in breast cancer mouse model. PLoS ONE 2010, 5, e12860. [Google Scholar] [CrossRef]
- Gu, S.; Ngamcherdtrakul, W.; Reda, M.; Hu, Z.; Gray, J.W.; Yantasee, W. Lack of acquired resistance in HER2-positive breast cancer cells after long-term HER2 siRNA nanoparticle treatment. PLoS ONE 2018, 13, e0198141. [Google Scholar] [CrossRef]
- Yao, Y.-D.; Sun, T.-M.; Huang, S.-Y.; Dou, S.; Lin, L.; Chen, J.-N.; Ruan, J.; Mao, C.; Yu, F.-Y.; Zeng, M.; et al. Targeted delivery of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and metastasis. Sci. Transl. Med. 2012, 4, ra48–ra130. [Google Scholar] [CrossRef]
- Cristofolini, T.; Dalmina, M.; Sierra, J.A.; Silva, A.H.; Pasa, A.A.; Pittella, F.; Creczynski-Pasa, T.B. Multifunctional hybrid nanoparticles as magnetic delivery systems for siRNA targeting the HER2 gene in breast cancer cells. Mater. Sci. Eng. C 2020, 109, 110555. [Google Scholar] [CrossRef] [PubMed]
- Hamurcu, Z.; Ashour, A.; Kahraman, N.; Ozpolat, B. FOXM1 regulates expression of eukaryotic elongation factor 2 kinase and promotes proliferation, invasion and tumorgenesis of human triple negative breast cancer cells. Oncotarget 2016, 7, 16619–16635. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gartel, A.L. The suppression of FOXM1 and its targets in breast cancer xenograft tumors by siRNA. Oncotarget 2011, 2, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Asik, E.; Kahraman, N.; Turk, M.; Ozpolat, B.; Ulubayram, K. Modified gold-based siRNA nanotherapeutics for targeted therapy of triple-negative breast cancer. Nanomedicine 2017, 12, 1961–1973. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Han, C.; Wan, G.; Huang, X.; Ivan, C.; Jiang, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Rao, P.H.; et al. TP53 loss creates therapeutic vulnerability in colorectal cancer. Nature 2015, 520, 697–701. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, Y.; Wang, H.; Stewart, S.; Van Der Jeught, K.; Agarwal, P.; Zhang, Y.; Liu, S.; Zhao, G.; et al. Precise targeting of POLR2A as a therapeutic strategy for human triple negative breast cancer. Nat. Nanotechnol. 2019, 14, 388–397. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar]
- Pillé, J.-Y.; Denoyelle, C.; Varet, J.; Bertrand, J.-R.; Soria, J.; Opolon, P.; Lu, H.; Pritchard, L.-L.; Vannier, J.-P.; Malvy, C.; et al. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol. Ther. 2005, 11, 267–274. [Google Scholar] [CrossRef]
- Pillé, J.Y.; Li, H.; Blot, E.; Bertrand, J.-R.; Pritchard, L.-L.; Opolon, P.; Maksimenko, A.; Lu, H.; Vannier, J.-P.; Soria, J.; et al. Intravenous delivery of anti-RhoA small interfering RNA loaded in nanoparticles of chitosan in mice: Safety and efficacy in xenografted aggressive breast cancer. Hum. Gene Ther. 2006, 17, 1019–1026. [Google Scholar]
- Maire, V.; Némati, F.; Richardson, M.; Vincent-Salomon, A.; Tesson, B.; Rigaill, G.; Gravier, E.; Marty-Prouvost, B.; De Koning, L.; Lang, G.; et al. Polo-like kinase 1: A potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer. Cancer Res. 2013, 73, 813–823. [Google Scholar] [CrossRef]
- Hu, K.; Law, J.H.; Fotovati, A.; Dunn, S.E. Small interfering RNA library screen identified polo-like kinase-1 (PLK1) as a potential therapeutic target for breast cancer that uniquely eliminates tumor-initiating cells. Breast Cancer Res. 2012, 14, R22. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Gu, S.; Reda, M.; Castro, D.J.; Sangvanich, T.; Gray, J.W.; Yantasee, W. Targeted treatment of metastatic breast cancer by PLK1 siRNA delivered by an antioxidant nanoparticle platform. Mol. Cancer Ther. 2017, 16, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, X.; Li, Z.; Shi, Q.; Zheng, W.; Liu, Y.; Wang, P. Functionalization of single-walled carbon nanotubes enables efficient intracellular delivery of siRNA targeting MDM2 to inhibit breast cancer cells growth. Biomed. Pharmacother. 2012, 66, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kren, B.T.; Unger, G.M.; Abedin, M.J.; Vogel, R.I.; Henzler, C.M.; Ahmed, K.; Trembley, J.H. Preclinical evaluation of cyclin dependent kinase 11 and casein kinase 2 survival kinases as RNA interference targets for triple negative breast cancer therapy. Breast Cancer Res. 2015, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.H.; Mao, C.Q.; Dou, S.; Shen, S.; Tan, Z.B.; Wang, J. Triple negative breast cancer therapy with CDK1 siRNA delivered by cationic lipid assisted PEG-PLA nanoparticles. J. Control. Release 2014, 192, 114–121. [Google Scholar] [CrossRef]
- Montazeri Aliabadi, H.; Landry, B.; Mahdipoor, P.; Uludag, H. Induction of apoptosis by survivin silencing through siRNA delivery in a human breast cancer cell line. Mol. Pharm. 2011, 8, 1821–1830. [Google Scholar] [CrossRef]
- Li, F.; Aljahdali, I.; Ling, X. Cancer therapeutics using survivin BIRC5 as a target: What can we do after over two decades of study? J. Exp. Clin. Cancer Res. 2019, 38, 368. [Google Scholar] [CrossRef]
- Werfel, T.A.; Wang, S.; Jackson, M.A.; Kavanaugh, T.E.; Joly, M.M.; Lee, L.H.; Hicks, D.J.; Sánchez, V.; I Gonzalez-Ericsson, P.; Kilchrist, K.V.; et al. Selective mTORC2 inhibitor therapeutically blocks breast cancer cell growth and survival. Cancer Res. 2018, 78, 1845–1858. [Google Scholar] [CrossRef]
- Xu, R.; Huang, Y.; Mai, J.; Zhang, G.; Guo, X.; Xia, X.; Koay, E.J.; Qin, G.; Erm, D.R.; Li, Q.; et al. Multistage Vectored siRNA Targeting Ataxia-Telangiectasia Mutated for Breast Cancer Therapy. Small 2013, 9, 1799–1808. [Google Scholar] [CrossRef]
- Parmar, M.B.; Ballesteros, B.E.A.; Fu, T.; K.C., R.B.; Aliabadi, H.M.; Hugh, J.C.; Löbenberg, R.; Uludağ, H. Multiple siRNA delivery against cell cycle and anti-apoptosis proteins using lipid-substituted polyethylenimine in triple-negative breast cancer and non-malignant cells. J. Biomed. Mater. Res. Part A 2016, 104, 3031–3044. [Google Scholar] [CrossRef]
- Okamoto, A.; Asai, T.; Hirai, Y.; Shimizu, K.; Koide, H.; Minamino, T.; Oku, N. Systemic administration of siRNA with anti-HB-EGF antibody-modified lipid nanoparticles for the treatment of triple-negative breast cancer. Mol. Pharm. 2018, 15, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Cheng, W.; Li, S.; Wu, B.; Leng, X.; Xu, S.; Tian, J. Novel cell-penetrating peptide-loaded nanobubbles synergized with ultrasound irradiation enhance EGFR siRNA delivery for triple negative Breast cancer therapy. Colloids Surf. B Biointerfaces 2016, 146, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, W.; Xia, Y.; Sun, J.; Chen, H.; Li, B.; Zhang, D.; Qian, W.; Meng, Y.; Li, W.; et al. The promotion of siRNA delivery to breast cancer overexpressing epidermal growth factor receptor through anti-EGFR antibody conjugation by immunoliposomes. Biomaterials 2011, 32, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Patil, R.; Portilla-Arias, J.; Ding, H.; Konda, B.; Espinoza, A.; Mongayt, D.; Markman, J.; Elramsisy, A.; Phillips, H.W.; et al. Nanobiopolymer for direct targeting and inhibition of EGFR expression in triple negative breast cancer. PLoS ONE 2012, 7, e31070. [Google Scholar] [CrossRef]
- Misra, A.C.; Luker, K.E.; Durmaz, H.; Luker, G.D.; Lahann, J. CXCR4-targeted nanocarriers for triple negative breast cancers. Biomacromolecules 2015, 16, 2412–2417. [Google Scholar] [CrossRef]
- Ho, E.A.; Osooly, M.; Strutt, D.; Masin, D.; Yang, Y.; Yan, H.; Bally, M. Characterization of long-circulating cationic nanoparticle formulations consisting of a two-stage PEGylation step for the delivery of siRNA in a breast cancer tumor model. J. Pharm. Sci. 2013, 102, 227–236. [Google Scholar] [CrossRef]
- Da Silva, L.C.G.; Santos, A.O.; Bimbo, L.M.; Moura, V.; Ramalho, J.S.; De Lima, M.C.P.; Simões, S.; Moreira, J.N. Toward a siRNA-containing nanoparticle targeted to breast cancer cells and the tumor microenvironment. Int. J. Pharm. 2012, 434, 9–19. [Google Scholar] [CrossRef]
- Dougherty, C.J.; Ichim, T.E.; Liu, L.; Reznik, G.; Min, W.; Ghochikyan, A.; Agadjanyan, M.G.; Reznik, B.N. Selective apoptosis of breast cancer cells by siRNA targeting of BORIS. Biochem. Biophys. Res. Commun. 2008, 370, 109–112. [Google Scholar] [CrossRef]
- Faltus, T.; Yuan, J.; Zimmer, B.; Kramer, A.; Loibl, S.; Kaufmann, M.; Strebhardt, K. Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia 2004, 6, 786–795. [Google Scholar] [CrossRef]
- Vimala, K.; Sundarraj, S.; Sujitha, M.V.; Kannan, S. Curtailing overexpression of E2F3 in breast cancer using siRNA (E2F3)-based gene silencing. Arch. Med. Res. 2012, 43, 415–422. [Google Scholar] [CrossRef]
- Santi, S.A.; Lee, H. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS ONE 2011, 6, e14614. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.N.; Bhowmick, N.A. Role of EMT in metastasis and therapy resistance. J. Clin. Med. 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Thiyagarajan, V.; Shen, P.C.; Mathew, D.C.; Lin, K.Y.; Liao, J.W.; Hseu, Y.C. Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J. Exp. Clin. Cancer Res. 2019, 38, 186. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef]
- Parvani, J.G.; Galliher-Beckley, A.J.; Schiemann, B.J.; Schiemann, W.P. Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β. Mol. Biol. Cell 2013, 24, 3449–3459. [Google Scholar] [CrossRef]
- Parvani, J.G.; Gujrati, M.D.; Mack, M.A.; Schiemann, W.P.; Lu, Z.R. Silencing β3 integrin by targeted ECO/siRNA nanoparticles inhibits EMT and metastasis of triple-negative breast cancer. Cancer Res. 2015, 75, 2316–2325. [Google Scholar] [CrossRef]
- Glackin, C.A. Nanoparticle Delivery of TWIST Small Interfering RNA and Anticancer Drugs: A Therapeutic Approach for Combating Cancer. Enzymes 2018, 44, 83–101. [Google Scholar]
- Finlay, J.; Roberts, C.M.; Lowe, G.; Loeza, J.; Rossi, J.J.; Glackin, C.A. RNA-Based TWIST1 Inhibition via Dendrimer Complex to Reduce Breast Cancer Cell Metastasis. BioMed. Res. Int. 2015, 2015, 382745. [Google Scholar] [CrossRef]
- Mathy, N.W.; Chen, X.M. Long non-coding RNAs (lncRNAs) and their transcriptional control of inflammatory responses. J. Biol. Chem. 2017, 292, 12375–12382. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.K.; Mueller, A.C.; Dutta, A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription 2014, 5, e944014. [Google Scholar] [CrossRef] [PubMed]
- Gooding, A.J.; Zhang, B.; Jahanbani, F.K.; Gilmore, H.L.; Chang, J.C.; Valadkhan, S.; Schiemann, W.P. The lncRNA BORG drives breast cancer metastasis and disease recurrence. Sci. Rep. 2017, 7, 12698. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Overstreet, A.-M.; Chen, M.-S.; Wang, J.; Zhao, H.-J.; Ho, P.-C.; Smith, M.A.; Wang, S.-C. Combined inhibition of EGFR and c-ABL suppresses the growth of triple-negative breast cancer growth through inhibition of HOTAIR. Oncotarget 2015, 6, 11150–11161. [Google Scholar] [CrossRef]
- Vaidya, A.M.; Sun, Z.; Ayat, N.; Schilb, A.; Liu, X.; Jiang, H.; Sun, D.; Scheidt, J.; Qian, V.; He, S.; et al. Systemic delivery of tumor-targeting siRNA nanoparticles against an oncogenic lncRNA facilitates effective triple-negative breast cancer therapy. Bioconjugate Chem. 2019, 30, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Asik, E.; Akpinar, Y.; Caner, A.; Kahraman, N.; Guray, T.; Volkan, M.; Albarracin, C.; Pataer, A.; Arun, B.; Ozpolat, B. EF2-kinase targeted cobalt-ferrite siRNA-nanotherapy suppresses BRCA1-mutated breast cancer. Nanomedicine 2019, 14, 2315–2338. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Ruggieri, S.; Tamma, R.; Simone, G.; Mangia, A. Angiogenesis and antiangiogenesis in triple-negative breast cancer. Transl. Oncol. 2016, 9, 453–457. [Google Scholar] [CrossRef]
- Braicu, C.; Chiorean, R.; Irimie, A.; Chira, S.; Tomuleasa, C.; Neagoe, E.; Paradiso, A.; Achimas-Cadariu, P.; Lazar, V.; Berindan-Neagoe, I. Novel insight into triple-negative breast cancers, the emerging role of angiogenesis, and antiangiogenic therapy. Expert Rev. Mol. Med. 2016, 18, e18. [Google Scholar] [CrossRef]
- Bender, R.J.; Mac Gabhann, F. Expression of VEGF and Semaphorin Genes Define Subgroups of Triple Negative Breast Cancer. PLoS ONE 2013, 8, e61788. [Google Scholar] [CrossRef]
- Lee, T.H.; Seng, S.; Sekine, M.; Hinton, C.; Fu, Y.; Avraham, H.K.; Avraham, S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007, 4, e186. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Boucher, Y.; Duda, D.G.; Martin, J.D.; Seano, G.; Ancukiewicz, M.; Barry, W.T.; Goel, S.; Lahdenrata, J.; Isakoff, S.J.; et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl. Acad. Sci. USA 2015, 112, 14325–14330. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Alto, P. Anti-vascular endothelial growth factor therapy in breast cancer: Game over. J. Clin. Oncol. 2015, 33, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Şalva, E.; Kabasakal, L.; Eren, F.; Özkan, N.; Çakalağaoğlu, F.; Akbuğa, J. Local delivery of chitosan/VEGF siRNA nanoplexes reduces angiogenesis and growth of breast cancer in vivo. Nucleic Acid Ther. 2012, 22, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Shukla, R.; Liu, H.; Jain, A.; Barve, A.; Cheng, K. Development of a biocompatible copolymer nanocomplex to deliver VEGF siRNA for triple negative breast cancer. Theranostics 2019, 9, 4508. [Google Scholar] [CrossRef]
- Hu, C.; Yang, K.; Li, M.; Huang, W.; Zhang, F.; Wang, H. Lipocalin 2: A potential therapeutic target for breast cancer metastasis. Oncotargets Ther. 2018, 11, 8099. [Google Scholar] [CrossRef]
- Yang, J.; McNeish, B.; Butterfield, C.; Moses, M.A. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J. 2013, 27, 45–50. [Google Scholar] [CrossRef]
- Guo, P.; Yang, J.; Di Jia, M.A.M.; Auguste, D.T. ICAM-1-targeted, Lcn2 siRNA-encapsulating liposomes are potent anti-angiogenic agents for triple negative breast cancer. Theranostics 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Gregorio, A.C.; Lacerda, M.; Figueiredo, P.; Simoes, S.; Dias, S.; Moreira, J.N. Meeting the needs of breast cancer: A nucleolin’s perspective. Crit. Rev. Oncol./Hematol. 2018, 125, 89–101. [Google Scholar] [CrossRef]
- Walerych, D.; Napoli, M.; Collavin, L.; Del Sal, G. The rebel angel: Mutant p53 as the driving oncogene in breast cancer. Carcinogenesis 2012, 33, 2007–2017. [Google Scholar] [CrossRef]
- Teodoro, J.G.; Evans, S.K.; Green, M.R. Inhibition of tumor angiogenesis by p53: A new role for the guardian of the genome. J. Mol. Med. 2007, 85, 1175–1186. [Google Scholar] [CrossRef]
- Braicu, C.; Pileczki, V.; Pop, L.; Petric, R.C.; Chira, S.; Pointiere, E.; Achimaş-Cadariu, P.; Berindan-Neagoe, I. Dual targeted therapy with p53 siRNA and Epigallocatechingallate in a triple negative breast cancer cell model. PLoS ONE 2015, 10, e0120936. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, S.; Dabral, S.; Singh, S.; Sehrawat, S. Role of exchange protein directly activated by cAMP (EPAC1) in breast cancer cell migration and apoptosis. Mol. Cell. Biochem. 2017, 430, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Prasad, P.; Jash, E.; Jayasundar, S.; Singh, I.; Alam, N.; Murmu, N.; Somashekhar, S.P.; Goldman, A.; Sehrawat, S. cAMP regulated EPAC1 supports microvascular density, angiogenic and metastatic properties in a model of triple negative breast cancer. Carcinogenesis 2018, 39, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.Z.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar]
- Modok, S.; Mellor, H.R.; Callaghan, R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr. Opin. Pharmacol. 2006, 6, 350–354. [Google Scholar] [CrossRef]
- Doyle, L.A.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340. [Google Scholar] [CrossRef]
- Navarro, G.; Sawant, R.R.; Biswas, S.; Essex, S.; Tros de Ilarduya, C.; Torchilin, V.P. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine 2012, 7, 65–78. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, W.; Sun, C.; Wu, J.; Tang, J. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids Surf. B Biointerfaces 2016, 138, 60–69. [Google Scholar] [CrossRef]
- Yu, M.; Han, S.; Kou, Z.; Dai, J.; Liu, J.; Wei, C.; Li, Y.; Jiang, L.; Sun, Y. Lipid nanoparticle-based co-delivery of epirubicin and BCL-2 siRNA for enhanced intracellular drug release and reversing multidrug resistance. Artif. Cells Nanomed. Biotechnol. 2018, 46, 323–332. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, M.; Gong, P.; Deng, J.; Yi, H.; Zhang, P.; Zhang, Y.; Liu, P.; Ma, Y.; Cai, L. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials 2013, 34, 3431–3438. [Google Scholar] [CrossRef]
- Zhu, C.; Jung, S.; Luo, S.; Meng, F.; Zhu, X.; Park, T.G.; Zhong, Z. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA–PCL–PDMAEMA triblock copolymers. Biomaterials 2010, 31, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Bi, X.; Yang, L.; Wu, S.; Yu, Y.; Jiang, B.; Zhang, A.; Lan, K.; Duan, S. Co-Delivery of Paclitaxel and PLK1-Targeted siRNA Using Aptamer-Functionalized Cationic Liposome for Synergistic Anti-Breast Cancer Effects In Vivo. J. Biomed. Nanotechnol. 2019, 15, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.-M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Li, Y.T.; Chua, M.J.; Kunnath, A.P.; Chowdhury, E.H. Reversing multidrug resistance in breast cancer cells by silencing ABC transporter genes with nanoparticle-facilitated delivery of target siRNAs. Int. J. Nanomed. 2012, 7, 2473–2481. [Google Scholar]
- Shi, Z.; Liang, Y.J.; Chen, Z.S.; Wang, X.W.; Wang, X.H.; Ding, Y.; Fu, L.W. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol. Ther. 2006, 5, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Sodani, K.; Chen, Z.S. Current advances in modulation of ABC transporter-mediated multidrug resistance in cancer. Int. J. Toxicol. Pharmcol. Res. 2009, 1, 1–6. [Google Scholar]
- Burger, H.; A Foekens, J.; Look, M.P.; Gelder, M.E.M.-V.; Klijn, J.G.M.; Wiemer, E.A.; Stoter, G.; Nooter, K. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: Correlation with chemotherapeutic response. Clin. Cancer Res. 2003, 9, 827–836. [Google Scholar]
- Chen, M.; Wang, L.; Wang, F.; Li, F.; Xia, W.; Gu, H.; Chen, Y. Quick synthesis of a novel combinatorial delivery system of siRNA and doxorubicin for a synergistic anticancer effect. Int. J. Nanomed. 2019, 14, 3557–3569. [Google Scholar] [CrossRef]
- Bai, M.; Shen, M.; Teng, Y.; Sun, Y.; Li, F.; Zhang, X.; Xu, Y.; Duan, Y.; Du, L. Enhanced therapeutic effect of Adriamycin on multidrug resistant breast cancer by the ABCG2-siRNA loaded polymeric nanoparticles assisted with ultrasound. Oncotarget 2015, 6, 43779–43790. [Google Scholar] [CrossRef]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, H.; Xu, P.; Yin, Q.; Zhang, Z.; Wang, S.; Yu, H.; Li, Y. Simultaneous inhibition of metastasis and growth of breast cancer by co-delivery of twist shRNA and paclitaxel using pluronic P85-PEI/TPGS complex nanoparticles. Biomaterials 2013, 34, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Riehle, R.; Navarro, G.; Perche, F.; De Rosa, G.; Torchilin, V.P. Polymeric micelles containing reversibly phospholipid-modified anti-survivin siRNA: A promising strategy to overcome drug resistance in cancer. Cancer Lett. 2014, 343, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Tian, Y.; Li, Y.; Ding, Y.; Ji, T.; Wu, M.; Wu, Y.; Nie, G. “Triple-punch” strategy for triple negative breast cancer therapy with minimized drug dosage and improved antitumor efficacy. ACS Nano 2015, 9, 1367–1378. [Google Scholar] [CrossRef]
- Tzeng, Y.-D.T.; Liu, P.-F.; Li, J.-Y.; Liu, L.-F.; Kuo, S.-Y.; Hsieh, C.-W.; Lee, C.-H.; Wu, C.-H.; Hsiao, M.; Chang, H.-T.; et al. Kinome-wide siRNA screening identifies Src-enhanced resistance of chemotherapeutic drugs in triple-negative breast Cancer cells. Front. Pharmacol. 2018, 9, 1285. [Google Scholar] [CrossRef]
- Moreira, M.P.; da Conceição Braga, L.; Cassali, G.D.; Silva, L.M. STAT3 as a promising chemoresistance biomarker associated with the CD44+/high/CD24−/low/ALDH+ BCSCs-like subset of the triple-negative breast cancer (TNBC) cell line. Exp. Cell Res. 2018, 363, 283–290. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Gritli, I.; Tolcher, A.; Heidel, J.D.; Lim, D.; Morgan, R.; Chmielowski, B.; Ribas, A.; Davis, M.E.; Yen, Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA 2014, 111, 11449–11454. [Google Scholar] [CrossRef]
- Tabernero, J.; Shapiro, G.I.; Lorusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Landen, C.N.; Chavez-Reyes, A.; Bucana, C.; Schmandt, R.; Deavers, M.T.; Lopez-Berestein, G.; Sood, A.K. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005, 65, 6910–6918. [Google Scholar] [CrossRef]
- Naing, A.; Lopez-Berestein, G.; Fu, S.; Tsimberidou, A.M.; Pant, S.; Piha-Paul, S.A.; Janku, F.; Hong, D.S.; Sulovic, S.; Meng, X.; et al. EphA2 gene targeting using neutral liposomal small interfering RNA (EPHARNA) delivery: A phase I clinical trial. J. Clin. Oncol. 2017, 35, TPS2604. [Google Scholar] [CrossRef]
- Aleku, M.; Schulz, P.; Keil, O.; Santel, A.; Schaeper, U.; Dieckhoff, B.; Janke, O.; Endruschat, J.; Durieux, B.; Röder, N.; et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008, 68, 9788–9798. [Google Scholar] [CrossRef] [PubMed]
- Strumberg, D.; Schultheis, B.; Traugott, U.; Vank, C.; Santel, A.; Keil, O.; Giese, K.; Kaufmann, J.; Drevs, J. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int. J. Clin. Pharmacol. Ther. 2012, 50, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, B.; Strumberg, D.; Santel, A.; Vank, C.; Gebhardt, F.; Keil, O.; Lange, C.; Giese, K.; Kaufmann, J.; Khan, M.; et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 4141–4148. [Google Scholar] [CrossRef] [PubMed]
- Demeure, M.J.; Armaghany, T.; Ejadi, S.; Ramanathan, R.K.; ElFiky, A.; Strosberg, J.R.; Smith, D.C.; Whitsett, T.; Liang, W.S.; Sekar, S.; et al. A phase I/II study of TKM-080301, a PLK1-targeted RNAi in patients with adrenocortical cancer (ACC). J. Clin. Oncol. 2016, 34, 2547. [Google Scholar] [CrossRef]
- Abou-Alfa, G.; Yoon, J.; Modiano, M.; Ryoo, B.; Yau, T.; Freilich, B.; Knox, J.; Ly, M.; Ahmad, H.; Gahir, S.; et al. An open-label, multi-center, phase I/II, dose escalation study of IV TKM-080301 in subjects with advanced hepatocellular carcinoma. Eur. J. Cancer 2016, 69, S22. [Google Scholar] [CrossRef]
- El Dika, I.; Lim, H.Y.; Yong, W.P.; Lin, C.; Yoon, J.-H.; Modiano, M.; Freilich, B.; Choi, H.J.; Chao, T.; Kelley, R.K.; et al. An Open-Label, Multicenter, Phase I, Dose Escalation Study with Phase II Expansion Cohort to Determine the Safety, Pharmacokinetics, and Preliminary Antitumor Activity of Intravenous TKM-080301 in Subjects with Advanced Hepatocellular Carcinoma. Oncologist 2019, 24, 747. [Google Scholar] [CrossRef]
- Golan, T.; Khvalevsky, E.Z.; Hubert, A.; Gabai, R.M.; Hen, N.; Segal, A.; Domb, A.; Harari, G.; Ben-David, E.; Raskin, S.; et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 2015, 6, 24560–24570. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Martinez, D.; Wood, D.L.; Fielman, B.; Sharma, M.; Janisch, L.A.; Brown, B.D.; et al. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J. Clin. Oncol. 2015, 33, 11006. [Google Scholar] [CrossRef]
- Ramot, Y.; Rotkopf, S.; Gabai, R.M.; Khvalevsky, E.Z.; Muravnik, S.; Marzoli, G.A.; Domb, A.J.; Shemi, A.; Nyska, A. Preclinical Safety Evaluation in Rats of a Polymeric Matrix Containing an siRNA Drug Used as a Local and Prolonged Delivery System for Pancreatic Cancer Therapy. Toxicol. Pathol. 2016, 44, 856–865. [Google Scholar] [CrossRef]
- Vakhshiteh, F.; Atyabi, F.; Ostad, S.N. Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. Int. J. Nanomed. 2019, 14, 2847–2859. [Google Scholar] [CrossRef]
- Whiteside, T.L. Therapeutic targeting of oncogenic KRAS in pancreatic cancer by engineered exosomes. Transl. Cancer Res. 2017, 6 (Suppl. 9), S1406–S1408. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Zatsepin, T.S.; Kotelevtsev, Y.V.; Koteliansky, V. Lipid nanoparticles for targeted siRNA delivery—Going from bench to bedside. Int. J. Nanomed. 2016, 11, 3077–3086. [Google Scholar]
- Wagner, M.J.; Mitra, R.; McArthur, M.J.; Baze, W.; Barnhart, K.; Wu, S.Y.; Rodriguez-Aguayo, C.; Zhang, X.; Coleman, R.L.; Lopez-Berestein, G.; et al. Preclinical Mammalian Safety Studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol. Cancer Ther. 2017, 16, 1114–1123. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).